Abstract
CD22 is a B-cell–specific adhesion molecule that modulates BCR-mediated signal transduction. Ligation of human CD22 with monoclonal antibodies (MoAbs) that block the ligand binding site triggers rapid tyrosine phosphorylation of CD22 and primary B-cell proliferation. Because extracellular signal-regulated kinases (ERKs) couple upstream signaling pathways to gene activation and are activated by B-cell antigen receptor (BCR) signaling, we examined whether CD22 ligation also activated ERKs and/or modified BCR-induced ERK activation. Ligation of CD22 on either primary B cells or B-cell lines failed to significantly activate the mitogen activated protein kinase (MAPK) ERK-2, but did activate the stress-activated protein kinases (SAPKs; c-jun NH2-terminal kinases or JNKs). In contrast, BCR ligation resulted in ERK-2 activation without significant SAPK activation. Concurrent ligation of CD22 and BCR enhanced BCR-mediated ERK-2 activation without appreciably modulating CD22-induced SAPK activation. Consistent with its induction of SAPK activity, there was a marked increase in nuclear extracts of activator protein-1 (AP-1) and c-jun levels within 2 hours of exposure of primary B cells to the CD22 MoAb. Despite their differences in ERK activation, both CD22 and BCR ligation triggered several Burkitt lymphoma cell lines to undergo apoptosis, and the 2 stimuli together induced greater cell death than either signal alone. The pro-apoptotic effects were CD22-blocking MoAb-specific and dose-dependent. Examination of expression levels of Bcl-2 protoncogene family members (Bcl-2, Bcl-xL, Mcl-1, and Bax) showed a downregulation of Bcl-xL and Mcl-1 after CD22 ligation. This study provides a plausible mechanism to explain how CD22 and BCR signaling can costimulate B-cell proliferation and induce apoptosis in Burkitt lymphoma cell lines.
CD22 IS A MEMBER OF the sialoadhesin subclass of the Ig superfamily whose members function as mammalian lectins.1-3 Although the physiologic significance of the adhesive properties of CD22 remains unknown, the extracellular portion of CD22 mediates interactions with sialic acid-bearing ligands expressed by lymphocytes, neutrophils, monocytes, erythrocytes, and some nonhematopoietic cell types.2-6 In vitro and in vivo studies have demonstrated that CD22 ligation regulates B-cell function, and CD22 binding to its ligand on T cells provides a costimulatory signal (reviewed in Tedder et al3).
The intracellular portion of CD22 contains 6 tyrosines that are potential targets for tyrosine phosphorylation. Two pairs of tyrosines are within regions that are structurally homologous to the tyrosine-based activation motif (TAM).7 TAMs are found in many hematopoietic signaling receptors and are thought to serve as platforms for docking of src family tyrosine kinases.8-10The physiologic relevance of these motifs in CD22 had been demonstrated by showing that the intracellular portion of CD22 physically associates with several effector proteins, including the src kinase Lyn, the src-related kinase Syk, PI-3 kinase, and phospholipase Cγ (PLCγ).7,11-13 The TAM motifs and the effector proteins with which they associate are implicated in the delivery of potent mitogenic signals. Indeed, CD22 cross-linking triggers B-cell proliferation and augments the proliferative response induced by lipopolysaccharide, CD40, or anti-Ig.12-16 The intracellular portion of CD22 also contains 4 tyrosine-based inhibitory motifs (TIMs), which provide docking sites for the SH2 domains of SHP1 protein tyrosine phosphatase.17 TIMs have been found in transmembrane proteins involved in the inhibition of signals through other receptors and include the FcγRIIB and the natural killer (NK) cell-inhibitory receptors p58 and p70.17CD22 has been shown to associate with SHP1 and the recruitment of CD22 into the antigen receptor complex likely inhibits antigen receptor signaling.11 18-20
Recently, 4 groups independently generated targeted deletions of the mouse CD22 gene.21-24 Although the observed CD22−/− phenotype resolved some of the questions related to the role of CD22 in B-cell function, many questions remained unanswered. Each of the groups demonstrated a downregulation of surface IgM expression on peripheral CD22−/− B cells and an augmented calcium flux after BCR cross-linking, results consistent with an inhibitory role for CD22 in BCR signaling. However, some significant differences in the observed CD22−/− phenotype were also reported. For example, the response to thymus-independent antigen was found to be impaired by 1 group,22 but unaffected by another.23 One group found that the lifespan of B cells in the periphery of CD22−/− mice was shortened,22 perhaps secondary to enhanced apoptosis. Another group found expansion of long-lived CD5+ B cells in the peritoneum.23 Two groups reported impaired proliferation after BCR cross-linking,22,23 whereas a third group found the converse.21 Although the basis of these differences remains elusive, the impaired response to T-independent antigen, reduced number of long-lived cells, and impaired proliferative response after BCR cross-linking would all be consistent with a positive role for CD22 in B-cell physiology. A recent comparison of BCR-mediated signaling events in CD19−/− and CD22−/− mice showed a reciprocal change in Vav phosphorylation.25 The CD19−/− B cells had decreased and the CD22−/− B cells had increased Vav phosphorylation. However, this study also showed that, after BCR cross-linking, CD22−/− B cells had reduced levels of several tyrosine phosphorylated proteins, including Ig-α, Ig-β, SH2-containing inositol phosphatase (SHIP), and PLCγ, a result perhaps inconsistent with CD22 solely being an inhibitor of BCR-mediated signal transduction. Furthermore, this study failed to see an alteration in BCR-mediated mitogen-activated protein kinase (MAPK) activation in the CD22−/− B cells. This contrasts with a recent study in which the use of a CD22 immobilization technique suggested that the recruitment of CD22 into the BCR complex inhibited BCR-mediated MAPK activation.26
Thus, although CD22 can inhibit BCR-mediated signaling events, it may also play a positive role in B-cell function. Dissection of the intermediate and late signaling events that occur after CD22 ligation may help to better define the role of CD22 in normal B-cell physiology. In this study, we used monoclonal antibodies (MoAbs) that are highly efficient at initiating early CD22-mediated signaling events to identify the downstream signaling cascades used by this receptor. Many recent reports have identified the importance of stress-activated protein kinase (SAPK; also JUN kinase) and MAPK in the propagation of proliferative signals in B cells. Specifically, ERK-2 has been identified as the principal MAPK involved in BCR-mediated signals in primary B cells,27 whereas the SAPK signaling cascade has been implicated in mediating the mitogenic effects after CD40 ligation.28 We show here that CD22 ligation activates the SAPK pathway and amplifies the ERK-2 activation observed after BCR cross-linking.
MATERIALS AND METHODS
Reagents.
The blocking (HB22.7, HB22.23, and HB22.33) and nonblocking (HB22.27) MoAbs were purified from ascites and previously characterized.12 The goat antihuman IgM F(ab′)2 was purchased from Jackson Immunoresearch (West Grove, PA). The biotinylated goat antirabbit MoAb and horseradish peroxidase (HRP)-conjugated streptavidin used for immunoblotting were purchased from Dako (Carpinteria, CA). Antimouse IgG-coated magnetic beads were used for immobilization and cross-linking CD22, as well as for immunoprecipitation for kinase assays (Dynal, Oslo, Norway). The anti–c-Jun antisera used for the mobility shift assay (MSA) and immunoblotting was a kind gift of Kevin Johnson (National Cancer Institute [NCI], National Institutes of Health [NIH], Bethesda, MD). The anti-JNK antisera used for the JNK/SAPK kinase assay was purchased from Pharmacia (Piscataway, NJ). The anti–ERK-2 antiserum used for the ERK-2 kinase assay was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The GST–c-Jun and myelin basic protein (MBP) used as a substrate for the in vitro kinase assays were purchased from Santa Cruz Biotechnology and Sigma (St Louis, MO), respectively. The anti–Bcl-xL, anti–Bcl-2, anti–Mcl-1, and anti-Bax used for intracytoplasmic flow cytometery were purchased from Santa Cruz Biotechnology. The p38/JNK2 inhibitor SB203580 was purchased from Calbiochem (San Diego, CA).
B-cell preparations, cell culture, and cell lines.
Human tonsillar B cells were isolated as described.12 These cell preparations are routinely greater than 98% CD20+ as assessed by flow cytometry. The Burkitt lymphoma cell line Ramos and the plasmacytoma cell line HS-Sultan were obtained from the American Type Culture Collection (Rockville, MD). The Burkitt lymphoma cell lines ST486 and BL41 were kind gifts of E. McGrath (NCI, NIH). The cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum at 0.5 × 106/mL. The anti-CD22 MoAbs were immobilized by incubating 20 μg of MoAb with 16 × 106 Dynabeads for 20 minutes at 4°C. The complex was washed with phosphate-buffered saline (PBS) and used to stimulate 2 × 107 tonsillar, 107 Ramos, or 107 HS-Sultan B cells for various periods of time, as indicated.
In vitro kinase assays.
Tonsillar, Ramos, or HS-Sultan B cells were stimulated with immobilized MoAb or media for the indicated periods of time. Cells were then lysed in kinase lysis buffer (20 mmol/L HEPES, pH 7.4, 2 mmol/L EGTA, 50 mmol/L β-glycerol phosphate, 1 mmol/L dithiothreitol [DTT], 1 mmol/L sodium orthovanidate, 1% Triton X-100, 10% glycerol, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF]) for 1 hour at 4°C. Cleared lysates were incubated with (5 μL) anti-JNK or anti–ERK-2 MoAb for 1 hour at 4°C. Immunoprecipitates were captured with antimouse IgG Dyna beads for 2 to 3 hours at 4°C. The immunoprecipitates were washed twice with lysis buffer, once with LiCl wash buffer (500 mmol/L LiCl, 100 mmol/L Tris-HCl, pH 7.6, 0.1% Triton X-100, and 1 mmol/L DTT), and twice with kinase assay buffer (20 mmol/L MOPS, pH 7.2, 2 mmol/L EGTA, 10 mmol/L MgCl2, 1 mmol/L DTT, 0.1% Triton X-100, and 100 μmol/L sodium orthovanidate). GST-c-Jun (1 μg) and MBP (10 μg) were used as substrates for the SAPK and ERK-2 kinase assays, respectively. Immunoprecipitates were resuspended in 80 μL of kinase assay buffer (50 mmol/L MgCl2, 500 μmol/L ATP, 5 μCi [γ32P]ATP), with either GST-c-Jun or MBP as a substrate. Assays were incubated at 30°C for 20 minutes and the reactions were stopped by adding 25 μL of 4× sodium dodecyl sulfate (SDS)-sample buffer. Subsequently, the immunoprecipitates were boiled, size-fractionated on a 10% SDS-polyacrylamide gel, and visualized by autoradiography. The amount of 32P incorporation into MBP or GST-c-Jun was determined by excising the appropriate band and liquid scintillation counting.
Mobility shift assays and immunoblot analysis.
Nuclear extracts were prepared using a standard protocol. Oligonucleotides containing an AP-1 binding site and its complementary strand were annealed and gel-purified. A 32P-labeled AP-1 probe was incubated with 5 μg of nuclear extracts at room temperature for 20 minutes in a binding reaction followed by electrophoresis on 4.5% polyacrylamide gels in 0.25% TBE buffer.29 For the analysis of c-Jun, expression nuclear extracts (50 μg) were size-fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose, and the membrane was blocked with TTBS (150 mmol/L NaCl, 50 mmol/L Tris-Cl, pH 7.4, and 0.05% Tween) plus 10% milk. The membranes were then incubated with anti–c-Jun or anti-p52 NF-κB MoAb (1:1,000) in TTBS plus 5% milk overnight at 4°C. Subsequently, they were then incubated with biotinylated goat antimouse antibodies (1:5,000) in 5% bovine serum albumin (BSA) and HRP-Streptavidin (1:10,000). Detection was performed by enhanced chemiluminescence (ECL; Amersham, Little Chalfont, UK).
Flow cytometry, cell cycle, and apoptotic analysis.
Ramos cells were stimulated with media, blocking CD22 MoAbs (HB22.7, HB22.23, and HB22.33), nonblocking MoAb HB22.27, or antihuman IgM (all at 20 μg/mL) for 24 or 48 hours. Cells were examined for viability by their ability to exclude trypan blue. Apoptotic analysis was performed by propidium iodide (PI) uptake, as previously described,30and also by using the APO-BRDU kit (Pharmagen, San Diego, CA) and following the manufacturer’s recommendations. Briefly, 106 cells were fixed in 1% paraformadehyde (wt/vol) and stored in 70% ethanol. Fragmented DNA was labeled with BR-dUTP and TdT, followed by detection with anti-BRDU-fluorescein isothiocyanate (FITC) (Becton Dickinson, San Jose, CA). Data from 50,000 events were acquired using a Becton Dickinson FACstar flow cytometer and analyzed using Multicycle cell cycle analysis software (Phoenix Systems, San Diego, CA). Intracytoplasmic florescence-activated cell sorting (FACS) analysis was performed as previously described.31 32 Briefly, after appropriate stimulation, 106 Ramos cells were fixed with 1:1 4% paraformaldehyde/lysing solution (Becton Dickinson). After 2 washes with PBS/0.5% Tween 20, cells were blocked with 10% heat-inactivated human serum/PBS for 30 minutes, stained with indicated antisera at 1:1,000, and detected with goat-antirabbit-FITC (Becton Dickinson). Fifty thousand nonapoptotic events were analyzed by gating out PI staining cells.
RESULTS
CD22 cross-linking with the blocking MoAb HB22.7 activates the SAPK pathway in tonsillar B cells and in several B-cell lymphoma cell lines.
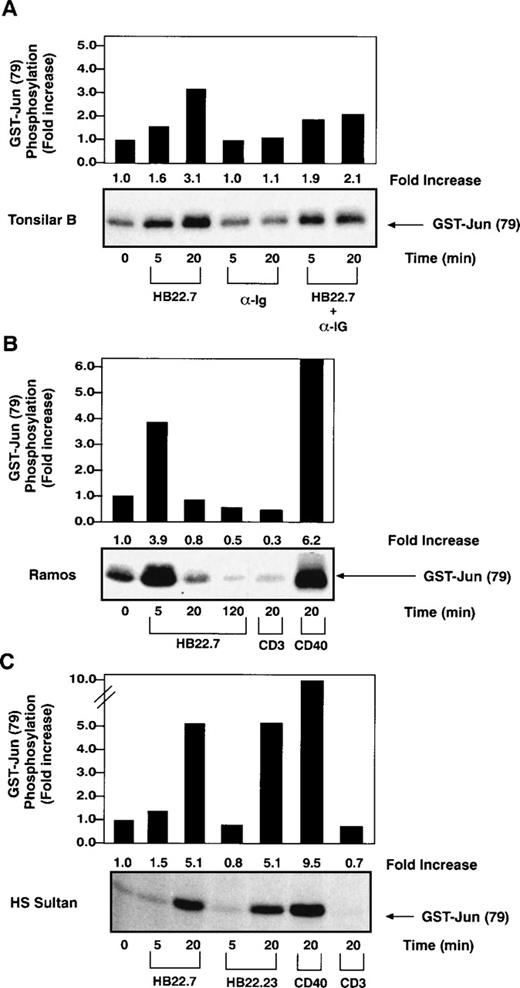
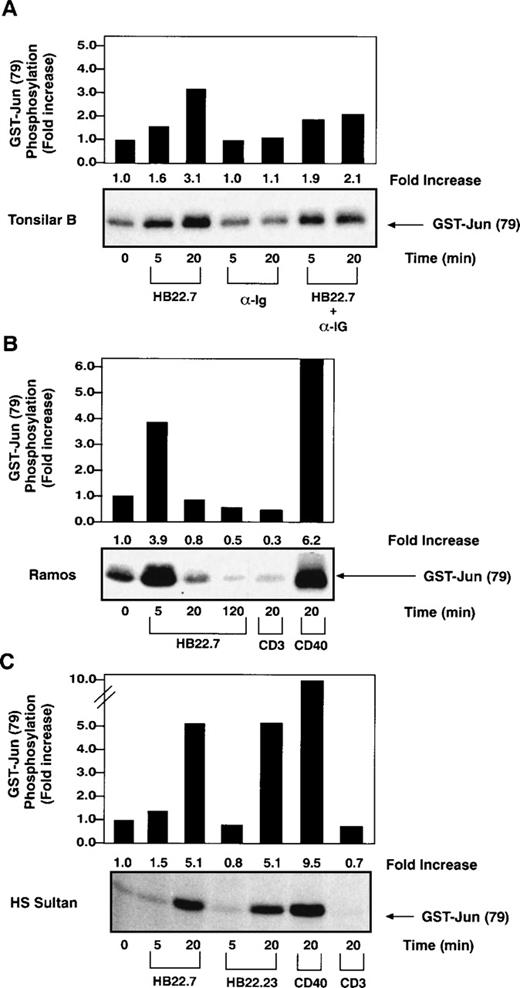
Recently, SAPK has been implicated as a critical mediator of c-jun phosphorylation after CD40 ligation in B cells28 as well as a pathway used for BCR-mediated apoptosis in neoplastic B-cell lines.33 To test whether CD22 cross-linking might also activate SAPK, we immobilized the CD22 MoAbs HB22.7, HB22.23, and HB22.33, which are known to block the binding of CD22 to its ligand, on magnetic beads and used them to cross-link CD22 on primary tonsillar B cells. SAPK activity was measured by performing immune complex kinase assays with GST-c-jun as a substrate.28 CD22 cross-linking induced a consistent 3-fold increase in SAPK activity after 20 minutes of ligation (Fig 1A). In contrast, cross-linking of the BCR with F(ab′)2fragments of anti-IgM failed to significantly induce SAPK. Concurrent cross-linking of CD22 and the BCR resulted in a similar, albeit slightly less pronounced increase in SAPK activity (Fig 1A). We extended these studies to analyze the effects of CD22 cross-linking on 2 B-lymphocyte cell lines, Ramos and HS-Sultan. The Ramos cell line is derived from a Burkitt lymphoma, has a germinal center phenotype, and is CD77+, CD22+, and sIgM+. HS-Sultan cells have features of a plasmacytoma cell line and, although the cells are CD22+, they are surface Ig−. We observed a similar induction of SAPK activity in both cell lines after CD22 ligation with the blocking MoAbs (Fig 1B and C). Similar levels of SAPK activation were seen after cross-linking with either of the blocking MoAbs, HB22.7 or HB22.23 (Fig 1C). Also, there was no significant difference in SAPK induction observed when CD22 and sIgM were cross-linked concurrently on the surface of Ramos cells (data not shown). By way of comparison, CD40 cross-linking induced a 6- and 9-fold induction of SAPK activity in Ramos and HS-Sultan cells, respectively (Fig 1B and C). No significant SAPK induction was observed with the control anti-CD3 MoAb in either cell line. All studies were performed in at least triplicate, with those shown being representative.
Increased SAPK activity after CD22 ligation on primary B cells and several B-cell lines. (A) SAPK kinase assays performed with lysates obtained after stimulating primary human tonsillar B cells (2 × 107/lane) with media, immobilized CD22 MoAb HB22.7 (20 μg/mL), F(ab′)2 fragments of goat MoAb to BCR (20 μg/mL), or both. Immune complex kinase assays were performed using GST-c-Jun as a substrate. (B) SAPK kinase assays performed as described in (A) after stimulation of the B-cell line Ramos (107/lane) with HB22.7 (20 μg/mL), CD40 (2 μg/mL), or CD3 (10 μg/mL). (C) SAPK kinase assays performed as described in (A) after stimulation of the B-cell line HS Sultan (107/lane) with the CD22 MoAbs HB22.7 and HB22.23 (20 μg/mL) or CD40 (2 μg/mL).
Increased SAPK activity after CD22 ligation on primary B cells and several B-cell lines. (A) SAPK kinase assays performed with lysates obtained after stimulating primary human tonsillar B cells (2 × 107/lane) with media, immobilized CD22 MoAb HB22.7 (20 μg/mL), F(ab′)2 fragments of goat MoAb to BCR (20 μg/mL), or both. Immune complex kinase assays were performed using GST-c-Jun as a substrate. (B) SAPK kinase assays performed as described in (A) after stimulation of the B-cell line Ramos (107/lane) with HB22.7 (20 μg/mL), CD40 (2 μg/mL), or CD3 (10 μg/mL). (C) SAPK kinase assays performed as described in (A) after stimulation of the B-cell line HS Sultan (107/lane) with the CD22 MoAbs HB22.7 and HB22.23 (20 μg/mL) or CD40 (2 μg/mL).
Cross-linking CD22 with the blocking MoAb HB22.7 fails to significantly activate ERK-2 but prolongs BCR-mediated ERK-2 activation.
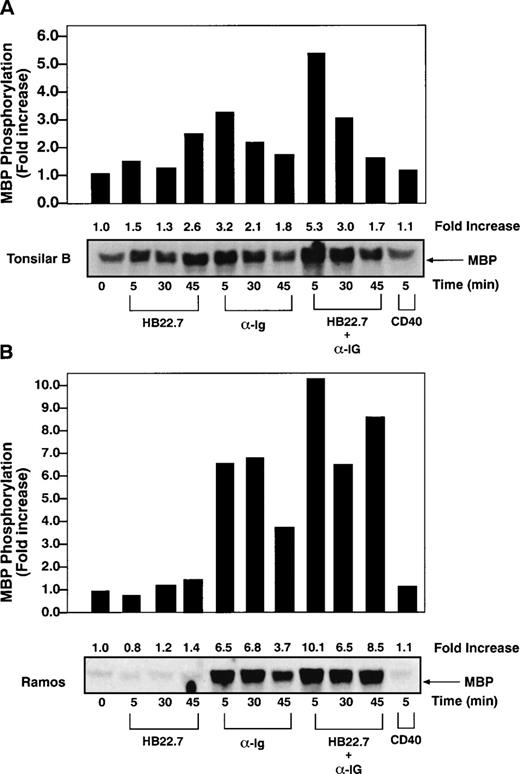
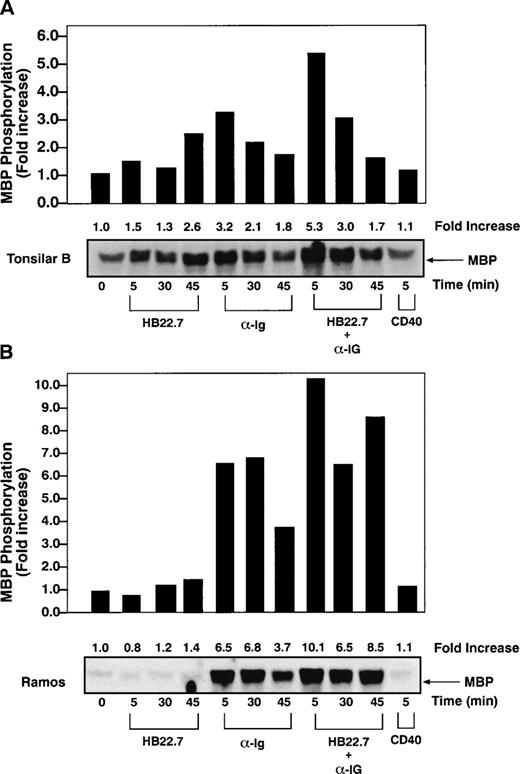
We next examined the effects of CD22 cross-linking on MAPK activation. The best characterized MAPKs are the extracellular signal-regulated protein kinases (ERKs) 1 and 2.34 Previous studies have demonstrated that BCR cross-linking activates MAPKs27,35 and increases c-fos expression and AP-1 activity.36 Because ERK-2 is the principal MAPK isoform activated after BCR cross-linking,27 we measured ERK-2 kinase activity after cross-linking CD22 on human tonsillar B cells. However, the HB22.7 MoAb failed to significantly induce ERK-2 activity (Fig 2A). As expected, anti-IgM cross-linking resulted in a 3- to 4-fold increase in ERK-2 activity. Maximal activity was seen after ligating for 5 minutes, with a significant decrease observed after 30 minutes and a return to near baseline after 45 minutes. Concurrent cross-linking of CD22 and IgM resulted in a slight increase in ERK-2 activity above that seen with anti-IgM alone. Similar experiments using Ramos cells again showed an additive effect on ERK-2 activation after concurrent CD22 and anti-IgM cross-linking; however, more strikingly, ERK-2 activity failed to decline at the rate seen with anti-IgM alone (Fig 2B). All studies were performed in at least triplicate, with those shown being representative. Significant heterogeneity of baseline ERK activity was seen in tonsilar B cells, which likely represents the nonhomogenous activation state of tonsilar B cells, which may explain the difference in ERK-2 kinase induction between tonsilar and Ramos B cells.
CD22 and BCR coligation prolongs BCR-mediated ERK-2 activation. (A) ERK-2 kinase assays were performed on lysates obtained after stimulating primary human tonsil B cells (2 × 107/lane) with media, immobilized CD22 MoAb HB22.7 (20 μg/mL), F(ab′)2 fragments of goat antihuman IgM (20 μg/mL), both, or CD40 (2 μg/mL). Immune complex kinase assays were performed using MBP as a substrate. (B) ERK-2 kinase assays of lysates obtained from Ramos cells (107/lane) stimulated as described in (A).
CD22 and BCR coligation prolongs BCR-mediated ERK-2 activation. (A) ERK-2 kinase assays were performed on lysates obtained after stimulating primary human tonsil B cells (2 × 107/lane) with media, immobilized CD22 MoAb HB22.7 (20 μg/mL), F(ab′)2 fragments of goat antihuman IgM (20 μg/mL), both, or CD40 (2 μg/mL). Immune complex kinase assays were performed using MBP as a substrate. (B) ERK-2 kinase assays of lysates obtained from Ramos cells (107/lane) stimulated as described in (A).
CD22 cross-linking results in increased AP-1 activity.
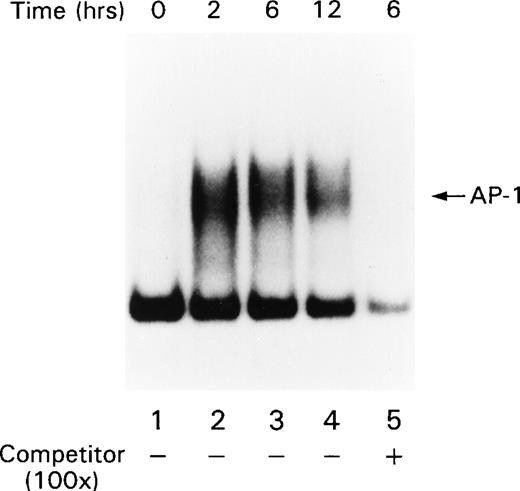
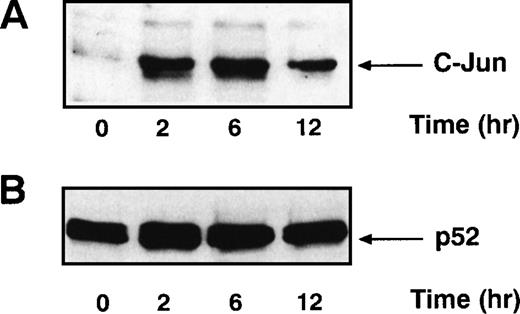
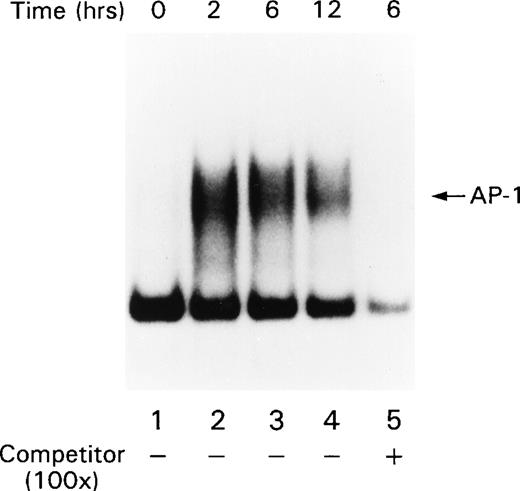
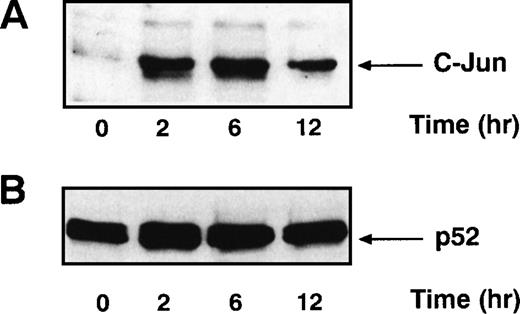
Signals that lead to B-cell activation are often initiated by receptor ligation and propagated by signaling cascades that eventually modify the transcriptional activity of AP-1 and NF-κB. AP-1 is a heterodimer that consists of c-jun paired with a member of either the Fos or ATF family of transcription factors. Full AP-1 activation requires phosphorylation of residues Ser 63 and Ser 73 in c-jun (reviewed in Karin and Hunter37), which is catalyzed by the SAPKs. Because the ligation of CD22 with the blocking MoAbs resulted in increased SAPK activity, we examined AP-1 activity in B cells after CD22 cross-linking using MSAs. Human tonsillar B cells were stimulated with either beads alone or the MoAb HB22.7 immobilized on beads. Nuclear extracts were examined by MSA with an oligonucleotide probe containing an AP-1 site. There was a substantial induction of AP-1 binding activity after 2 hours of stimulation (Fig 3). This activity diminished significantly after 12 hours of stimulation. Detection of the AP-1 complex was eliminated when the binding reactions for the gel shift assay were performed in the presence of a c-jun antisera (data not shown), indicating that the AP-1 complex contained c-jun. Preincubating the reaction mixture with 100-fold excess of cold probe also abolished the AP-1 band, demonstrating that the pattern is AP-1–specific (Fig3). Replicate MSAs were performed in triplicate, with those shown being representative. To confirm these results, we also examined the nuclear extracts for the presence of c-jun by immunoblotting. Consistent with the results of the MSAs, 2 hours of CD22 ligation resulted in a significant increase in c-jun levels in the nucleus (Fig 4A). The immunoblot was stripped and reprobed with antibody that recognizes the transcription factor p52 NF-κB. The levels of p52 NF-κB were found to be uniform at all time points examined, suggesting equal loading of the nuclear extracts (Fig4B).
AP-1 activity increases after stimulating primary human tonsil B cells with the CD22 blocking MoAb HB22.23. Tonsillar B cells were stimulated with media alone or with immobilized HB22.23 (20 μg/mL) for 2, 6, and 12 hours. Nuclear extracts were prepared and AP-1 activity was assayed by MSA.
AP-1 activity increases after stimulating primary human tonsil B cells with the CD22 blocking MoAb HB22.23. Tonsillar B cells were stimulated with media alone or with immobilized HB22.23 (20 μg/mL) for 2, 6, and 12 hours. Nuclear extracts were prepared and AP-1 activity was assayed by MSA.
c-Jun protein levels increase after stimulating primary human tonsillar B cells with the CD22 MoAb HB22.23. (A) Tonsil B cells were stimulated as described in Fig 3. Nuclear extracts were prepared, analyzed by immunoblotting with anti–c-Jun antisera, and detected by ECL. (B) The immunoblot described above was stripped and reprobed with anti-p52 NF-κB MoAb and detected by ECL.
c-Jun protein levels increase after stimulating primary human tonsillar B cells with the CD22 MoAb HB22.23. (A) Tonsil B cells were stimulated as described in Fig 3. Nuclear extracts were prepared, analyzed by immunoblotting with anti–c-Jun antisera, and detected by ECL. (B) The immunoblot described above was stripped and reprobed with anti-p52 NF-κB MoAb and detected by ECL.
CD22 ligation with HB22.7 causes cell cycle arrest and apoptosis in the cell line Ramos.
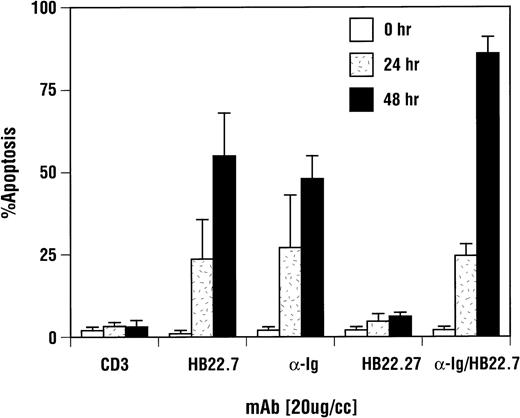
Immune development and function are dependent on eupeptic elimination of dysfunctional or autoreactive lymphocytes. Suicide signals initiated by the CD95/Fas receptor, tumor necrosis factor (TNF) receptor, and, in specific cases, the BCR are propagated via the SAPK/JNK signaling cascade.33,38,39 Whereas BCR cross-linking in primary B cells generates a proliferative signal, cross-linking the BCR on several B-cell lymphoma cell lines initiates the apoptotic process.40-42 Whether this represents standard elimination of germinal center B cells or a dysfunction specific for these malignant clones remains unclear, but it does provide a functional endpoint to examine CD22 and anti-Ig signaling. One study has demonstrated that, upon CD22 ligation and cross-linking, apoptosis could be induced in the Burkitt B-cell lymphoma cell line, Ramos.42 However, this was dependent on extensive cross-linking, and the amount of B-cell apoptosis was modest. The effect of CD22 MoAbs, which block the interaction with its ligands and induce SAPK activation, on Ramos cells has not previously been examined. We found that addition of the blocking anti-CD22 MoAbs, HB22.7 and HB22.33, to Ramos cell cultures for 48 hours resulted in apoptosis in 48% and 82% of the cells, respectively (Fig 5). A more complete analysis of the effects of HB22.7 alone and in combination with anti-IgM after 24 and 48 hours of incubation yielded similar results (Fig 6). In contrast, the nonblocking CD22 MoAb HB22.27 induced the apoptosis of only 7% to 10% of the cells (Fig 6). Ramos cell cultures treated the control MoAb CD3 contained a similar number of apoptotic cells as did those cultures treated with HB22.27 (Fig 6). After 48 hours of treatment with HB22.7, nearly 505 to 60% of the cells had undergone apoptosis, compared with 10% with HB22.27 (Fig 6). The extent of apoptosis after ligation with HB22.7 was similar to that observed with anti-IgM (Fig 6). Ligation of CD22 with the blocking MoAb resulted in a modest increase in G1 cell cycle arrest, which was not seen with the nonblocking MoAb or anti-Ig (data not shown). The further addition of a cross-linking MoAb did not affect the changes in cell cycle or degree of apoptosis observed with CD22 alone (data not shown). Recently, a pyridinyl imidizole (SB203580) has been identified that specifically and effectively blocks p38 and JNK2 kinases, but not JNK3, JNK4, or ERK kinases.43 44 To elucidate the role of these kinases on CD22-mediated apoptosis in Ramos cells, we preincubated Ramos cells with 25 and 50 μmol/L SB203580 before stimulation with HB22.7 and anti-IgM. No change in the extent of apoptosis was observed (data not shown), which is consistent with JNK1 and ERK kinases mediating the effects of CD22 and sIgM ligation, respectively.
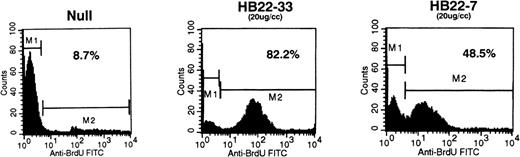
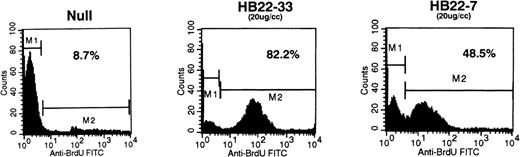
Ligation of CD22 on the Burkitt lymphoma cell line Ramos results in apoptosis. Ramos B cells were incubated with media (Null) or with 20 μg/mL of the CD22 blocking MoAbs HB22.7 or HB22.33. Apoptosis was assessed by BRDU incoporation by DNA fragments and detecting apoptotic cells with anti-BRDU-FITC via FACS.
Ligation of CD22 on the Burkitt lymphoma cell line Ramos results in apoptosis. Ramos B cells were incubated with media (Null) or with 20 μg/mL of the CD22 blocking MoAbs HB22.7 or HB22.33. Apoptosis was assessed by BRDU incoporation by DNA fragments and detecting apoptotic cells with anti-BRDU-FITC via FACS.
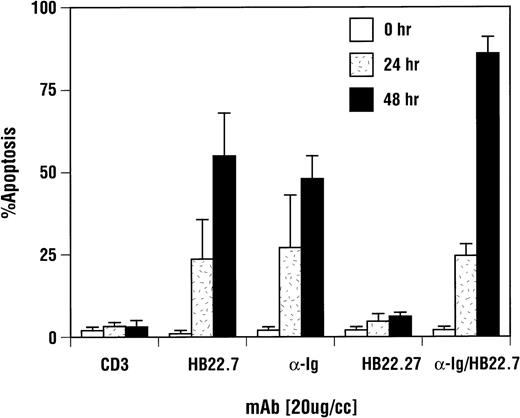
CD22-mediated apoptosis induction was CD22 blocking MoAb-specifc and equivalent to ligation of sIgM. Ramos B cells were incubated with media, the CD22 MoAb HB22.7 (20 μg/mL), the nonblocking CD22 MoAb HB22.27 (20 μg/mL), F(ab′)2fragments of goat MoAb to BCR (20 μg/mL), or CD3 (10 μg/mL) for 24 and 48 hours. Apoptosis was assayed by trypan blue exclusion and confirmed via PI staining and BRDU incorporation as described in Fig 5. The results shown are an average of 4 replicate experiments, with the error bars representing the standard deviation.
CD22-mediated apoptosis induction was CD22 blocking MoAb-specifc and equivalent to ligation of sIgM. Ramos B cells were incubated with media, the CD22 MoAb HB22.7 (20 μg/mL), the nonblocking CD22 MoAb HB22.27 (20 μg/mL), F(ab′)2fragments of goat MoAb to BCR (20 μg/mL), or CD3 (10 μg/mL) for 24 and 48 hours. Apoptosis was assayed by trypan blue exclusion and confirmed via PI staining and BRDU incorporation as described in Fig 5. The results shown are an average of 4 replicate experiments, with the error bars representing the standard deviation.
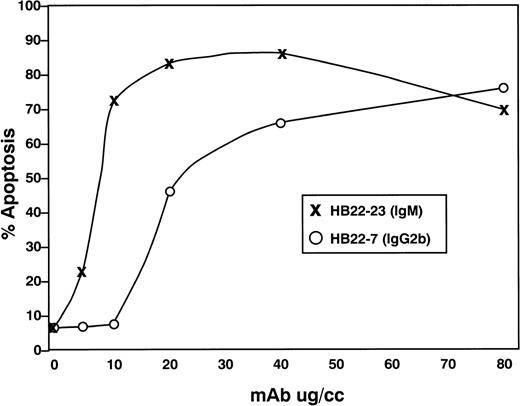
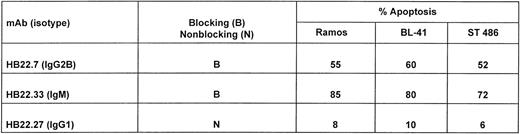
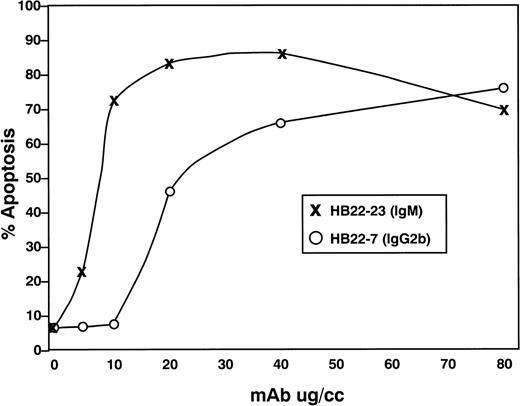
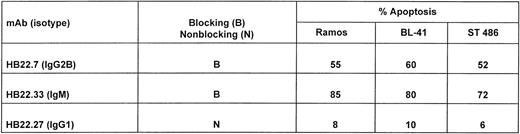
We next examined the dose-dependency of the pro-apoptotic effects of the 2 MoAbs HB22.7 and HB22.33. Forty-eight hours of incubation with 5, 10, 20, 40, and 80 μg/mL of each MoAb induced a similar degree of apoptosis, but at significantly different concentrations (Fig 7). Eighty-two percent of Ramos cells were apoptotic with 80 μg/mL of HB22.7, with a similar degree observed with only 10 μg/mL of HB22.33 (Fig 7). The effects of these MoAb were also examined in the Burkitt lymphoma cell lines BL41 and ST486. Both BL41 and ST486 are CD22+ and sIgM+(J. Tuscano, unpublished observation) and have been previously described.45 46 Incubating these cell lines with the blocking (HB22.7 and HB22.33) and nonblocking (HB22.27) MoAb induced 72% to 80% and 6% to 10% apoptosis, respectively (Fig 8).
Titration of apoptotic effects of HB22.7 and HB22.33 in the Burkitt lymphoma cell line Ramos. Ramos B cells were incubated with 5, 10, 20, 40, or 80 μg/mL of MoAb for 48 hours. Apoptosis was assessed by PI staining or BRDU incororation as described in Fig 5.
Titration of apoptotic effects of HB22.7 and HB22.33 in the Burkitt lymphoma cell line Ramos. Ramos B cells were incubated with 5, 10, 20, 40, or 80 μg/mL of MoAb for 48 hours. Apoptosis was assessed by PI staining or BRDU incororation as described in Fig 5.
Ligation of CD22 with blocking MoAbs induces apoptosis in several Burkitt lymphoma cell lines. Twenty micrograms per milliliter of the blocking (HB22.7 and HB22.33) or nonblocking (HB22.27) MoAbs were incubated with the Burkitt lymphoma cell lines Ramos, ST486, and BL41 for 48 hours. Apoptosis was assessed by propidium iodide staining and BRDU incorporation as described in Fig 5.
Ligation of CD22 with blocking MoAbs induces apoptosis in several Burkitt lymphoma cell lines. Twenty micrograms per milliliter of the blocking (HB22.7 and HB22.33) or nonblocking (HB22.27) MoAbs were incubated with the Burkitt lymphoma cell lines Ramos, ST486, and BL41 for 48 hours. Apoptosis was assessed by propidium iodide staining and BRDU incorporation as described in Fig 5.
Ligation of CD22 results in downregulation of Bcl-xL and Mcl-1.
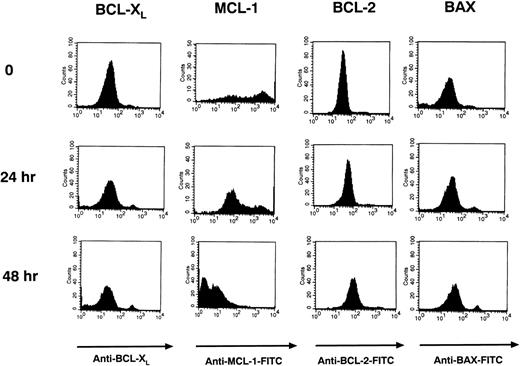
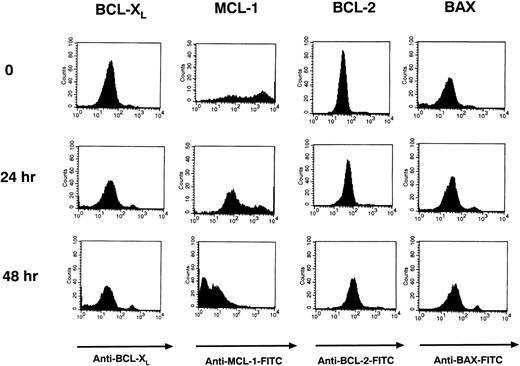
The Bcl-2 protoncogene family of proteins has been found to be important in regulating apoptosis in B cells. These proteins include the anti-apoptotic proteins Bcl-2, Mcl-1, and Bcl-xL and the pro-apoptotic protein Bax.47-51 Using intracytoplasmic flow cytometry (FACS), we examined the levels of these proteins in Ramos cells before and after stimulation with HB22.33 for 24 and 48 hours. Use of intracytoplasmic FACS and gating out PI-staining or apoptotic cells allowed for examination of protein levels in pre-apoptotic cells only. After 48 hours of stimulation, only Bcl-xL and Mcl-1 were significantly downregulated (Fig 9). Bcl-2 levels did not change appreciably, and Bax levels increased slightly (Fig 9).
Bcl-xL and Mcl-1 levels are downregulated in Ramos cells after CD22 ligation. Ramos B cells were stimulated with 20 μg/mL of HB22.33 for 0, 24, and 48 hours. Cells were assessed for Bcl-2, Bcl-xL, Mcl-1, and Bax levels via intracytoplasmic FACS. Only nonapoptotic cells were assessed by gating out PI staining cells.
Bcl-xL and Mcl-1 levels are downregulated in Ramos cells after CD22 ligation. Ramos B cells were stimulated with 20 μg/mL of HB22.33 for 0, 24, and 48 hours. Cells were assessed for Bcl-2, Bcl-xL, Mcl-1, and Bax levels via intracytoplasmic FACS. Only nonapoptotic cells were assessed by gating out PI staining cells.
DISCUSSION
In this report, we demonstrate that CD22 ligation with MoAbs that specifically block the interaction of CD22 with its ligand lead to CD22 tyrosine phosphorylation and result in activation of the SAPK signaling cascade. The induction of SAPK by CD22 ligation in the surface Ig-negative B-cell line HS-Sultan implies a BCR-independent mechanism of activation of this pathway. Likely as a consequence of SAPK activation, CD22 cross-linking leads to the translocation of AP-1 into the nucleus. Although CD22 cross-linking did not activate the ERK pathway, concurrent ligation of CD22 and the BCR resulted in prolonged ERK-2 activation.
The mechanism by which CD22 cross-linking results in SAPK activation remains to be elucidated. Treatment of B cells with the CD22 MoAbs induces TNF-α production,52 a known inducer of SAPK activation; however, this is unlikely to account for the rapid induction of SAPK observed after CD22 cross-linking. Recently, a subfamily of serine/threonine protein kinases has been identified that activate the SAPK pathway. Each of the subfamily members triggers SAPK activation without significant p38 or MAPK activation. Three of them, germinal center kinase (GCK), germinal center kinase related (GCKR), hematopoietic progenitor kinase (HPK), are known to be expressed by B lymphocytes. However, the upstream signals that activate these kinases are largely unknown. One signal known to activate them is TNF-α. TNF-α increases the catalytic activity of GCK, GCKR, and that of another GCK family member, germinal center like kinase (GLK).53-55 Directly implicating GCKR in TNF-α–induced SAPK activation is the observation that a catalytically impaired form of GCKR blocks TNF-α–induced SAPK activation.54 We are currently examining the effects of CD22 cross-linking on the activity of GCKR and GCK in B cells and have evidence that indeed GCKR is involved in CD22-mediated SAPK activation (Tuscano and Kehrl, unpublished observations). In addition, we are testing whether catalytically inactive forms of GCK and GCKR interfere with CD40- and CD22-induced SAPK activation.
The adhesive properties of CD22 allow B cells to interact with other hematopoietic cells and even some nonhematopoietic cells. Although the importance of these interactions in modulating B-cell function remains obscure, it seems unlikely that their only function would be to immobilize CD22, thereby preventing its association with the BCR. Several recent studies that manipulated the BCR/CD22 association using either antibody-based immobilization or co–cross-linking techniques have concluded that the function of CD22 is to inhibit BCR signaling and that the immobilization of CD22 by its ligands impairs the recruitment of CD22 into the BCR.26 It was argued that the apparent positive signaling function observed with CD22 MoAbs was the result of sequestration of SHP-1 from the BCR, leading to enhanced signaling by the derepressed BCR. Although these studies offer a compelling demonstration that co–cross-linking CD22 and the BCR impairs BCR signaling, for several reasons we think that CD22 is an independent signaling molecule capable of transmitting a positive signal to the B cells rather than simply serving as a platform for the recruitment of SHP-1. First, we have screened a panel of CD22 MoAbs and found significant differences in their agonistic activities.12,13 Several of these antibodies costimulate B-cell proliferation with anti-IgM and with CD40 and cytokines.13 Although it can be argued that the CD22 MoAbs recruit CD22 away from the BCR, thus increasing BCR signaling, the results with CD22 MoAb and cytokines or CD40 cannot be explained in that way. Also, other CD22 MoAbs that should recruit CD22 from the BCR complex as efficiently have little or no costimulatory effect. Second, we find that the exposure of HS-Sultan cells, a cell line that lacks surface Ig to agonistic CD22 MoAbs, activates SAPK, a result consistent with a direct signaling role for CD22 independently of the BCR. Third, the agonistic CD22 MoAbs are as efficient as anti-IgM in inducing the apoptosis of Ramos cells, whereas nonagonistic CD22 MoAbs are no better than a control antibody. In addition, the combination of the CD22 MoAbs and anti-IgM is better than either antibody alone. Fourth, CD22 cross-linking not only triggers the association of CD22 with Lyn and SHP-1, but also with Syk, PI-3 kinase, and phospholipase C-γ.11,12 18-20 The last 3 effectors are usually regarded as positive effectors in signaling pathways.
So, if CD22 has a positive role in B-cell function, why is the phenotype of the CD22−/− mice most consistent with CD22 downregulating BCR signaling. We would argue that, in fact, the CD22−/− B cells are only mildly compromised compared with wild-type cells. Perhaps this is because losing CD22’s inhibitory effects is in part tempered by the loss of its stimulatory effects. In fact, the CD22-deficient mice are fully capable of generating an antibody response of normal magnitude to both thymus-dependent and -independent antigens. Because significant BCR cross-linking does not occur during the response to thymus-dependent antigens, but rather the processing of antigen by B cells and direct T-B–cell collaborations, it seems unlikely that the recruitment of CD22 into the BCR complex would play a significant role in inhibiting B-cell function. In this context, an interaction between CD22 ligands on T cells and CD22 on B cells could promote B-cell activation. Clearly, a more complete understanding of the consequences of the loss of CD22 will require the identification of the ligands for CD22 and an examination of the consequences of their interactions with CD22 on B-cell function.
Although the consequences of treating primary B cells with the agonistic CD22 MoAbs have been examined, their effects on neoplastic B cells have not. We have demonstrated here that these MoAbs are highly efficient at inducing apoptosis in multiple Burkitt lymphoma cell lines. The extent of this effect is similar to that observed after surface Ig cross-linking and even to a greater extent when the IgM MoAb (HB22.33) is used. This latter effect is likely to be secondary to the pentameric structure of HB22.33 and a enhanced ability to cross-link CD22. However, despite their similar effect on Burkitt lymphoma cells, CD22 and surface Ig cross-linking result in distinctive patterns of ERK activation. CD22 cross-linking predominantly induces SAPK activation, whereas surface Ig cross-linking predominantly induces MAPK. Whereas SAPK activation has been implicated in the induction of apoptosis in certain cell types, CD40 is a potent SAPK activator yet prevents apoptosis induced by anti-Ig.56 A potential explanation for this dichotomy is that CD40 signaling also induces the translocation of NF-κB to the nucleus, which has been implicated in preventing apoptosis.57 However, making this explanation less likely, surface Ig cross-linking also induces the translocation of NF-κB. Alternatively, CD40 may induce a unique signaling pathway, which results in rescue of the Burkitt cells. Whether these Burkitt cell lines have relevance to normal B-cell physiology remains unclear; however, they do provide a functional readout of surface Ig and CD22 signaling. The inability of the p38/JNK inhibitor SB203580 to block CD22-mediated apoptosis in Ramos B cells is consistent with previous reports that demonstrate that, whereas SB203580 effectively and specifically inhibits p38 and JNK2, it does not inhibit JNK1, JNK3, or JNK4.43 44
Examination of Bcl-2 protoncogene family member protein levels in pre-apoptotic Ramos B cells before and after CD22 ligation showed a significant decrease in Bcl-xL and Mcl-1 and suggests a regulatory role for these proteins in CD22-mediated apoptosis in Ramos B cells. However whether this pattern is CD22-specific or not, and its relevance to normal B-cell physiology remains undefined, it is consistent with previous reports that demonstrate a significant role for these protein in regulating B-cell apoptosis.48,49 51
We also demonstrated that, although the engagement of CD22 does not increase ERK-2 activity, it does enhance and prolong BCR-mediated ERK-2 induction. Although it may be hypothesized that this is secondary to sequestration of SHP1 away from the BCR, the mechanism remains undefined. Recently, the physiologic significance of the duration of ERK activation has been explored in PC12 cells.58 These studies have suggested that the duration of ERK activation may be critical for determining cellular responses. Whereas a sustained activation and nuclear accumulation of ERK induce cell differentiation, a more transient activation leads to cell proliferation. The significance of these effects in B cells has yet to be examined; however, they may begin to explain the ultimate physiologic consequence of the ability of CD22 to modulate BCR-mediated signaling events.
ACKNOWLEDGMENT
The authors thank Mary Rust for her editorial assistance and Dr Anthony Fauci for his support.
Supported by National Institutes of Health Grants No. CA-54464, AI-26872, and HL50985.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John H. Kehrl, MD, B Cell Molecular Immunology Section, Laboratory of Immunoregulation, NIAID, NIH, Bldg 10, Room 11B08, Bethesda, MD 20892-1876.