Abstract
Serine proteinase inhibitors (serpins) play a vital regulatory role in a wide range of biological processes, and serpins from viruses have been implicated in pathogen evasion of the host defence system. For the first time, we report a functional serpin gene from nematodes that may function in this manner. This gene, named Bm-spn-2, has been isolated from the filarial nematode Brugia malayi, a causative agent of human lymphatic filariasis. Polymerase chain reaction (PCR) and Western blot experiments indicate that Bm-spn-2 is expressed only by microfilariae (Mf), which are the long-lived blood-dwelling larval stage. A survey of the greater than 14,000 expressed sequence tags (ESTs) from B malayi deposited in dbEST shows that greater than 2% of the ESTs sequenced from Mf cDNA libraries correspond to Bm-spn-2. Despite its abundance in the microfilarial stage, Bm-spn-2 has not been found in any other point in the life cycle. The predicted protein encoded byBm-spn-2 contains 428 amino acids with a putative signal peptide. Antibodies to recombinant Bm-SPN-2 protein react specifically with a 47.5-kD native protein in Mf extract. Bm-SPN-2 is one of the largest of the 93 known serpins, due to a 22 amino acid carboxy-terminal extension, and contains the conserved serpin signature sequence. Outside these regions, levels of homology are low, and only a distant relationship can been seen to a Caenorhabditis elegansserpin. The Bm-spn-2 gene contains 6 introns, 2 of which appear to be shared by both nematode species. The B malayi introns have an extended and conserved 3′ splice site and are relatively large compared with C elegans. A panel of mammalian serine proteinases were screened and Bm-SPN-2 protein was found to specifically inhibit enzymatic activity of human neutrophil cathepsin G and human neutrophil elastase, but not a range of other serine proteinases. It is possible that Bm-SPN-2 could function as a stage-specific serpin in the blood environment of the microfilarial parasite in protection from human immunity and thus may be a good candidate for protective vaccine.
SERINE PROTEINASE inhibitors (serpin) genes comprise a large gene family,1 and their protein products regulate a wide variety of proteinase-dependent physiological functions, such as blood coagulation,2fibrinolysis,3 activation of complement, and the inflammatory response.4 In addition to these fluid-phase reactions, serpins have critical roles in cell interactions and mobility, eg, maspin suppresses invasion and motility of mammary tumor cells5; plasminogen activator inhibitor 1 (PAI-1) blocks migration of smooth muscle cells,6 whereas PAI-2 can prevent apoptosis in cells exposed to tumor necrosis factor-α (TNF-α).7
Infectious organisms have evolved an array of specific adaptations to evade the host defence system.8.9 In view of the potency of serpin regulation of biological processes, it seems likely that pathogens may themselves encode serpins and use them to block host defense functions. For example, virus-encoded serpins prevent the proteolytic activation of interleukin-1β (IL-1β),10-12block granzyme activity,13 and protect infected cells from Fas- and TNF-induced apoptosis.12,14 Less is known of serpins from parasitic agents, but protease inhibitors have been described that inhibit a diverse range of host functions, such as neutrophil chemotaxis,15 blood coagulation,16-18 and antigen processing.19
Lymphatic filariasis is one of the most important human tropical diseases, with an estimated 120 million people infected and a further 900 million at risk of infection.20 21 It is caused by a mosquito-transmitted nematode, Brugia malayi, which migrates to the lymphatic vessels, where sexually reproducing adult worms produce millions of microfilariae (Mf) that migrate into the bloodstream, where they can reside for long periods (≥1 year). The mechanisms by which circulating Mf avoid host immune defenses are poorly understood.
Most serpin family members so-far characterized are from mammals, insects, and viruses. Although a few serpin cDNA sequences have been reported from helminth parasites,22,23 only in the free-living nematode Caenorhabditis elegans has the genomic sequence of a serpin been determined.24 As part of our ongoing research on identification of vaccine candidates from the human filarial nematode B malayi, we have found a novel serpin gene from the Mf stage of B malayi. We report here the isolation, sequencing, and expression of this novel serpin gene, Bm-spn-2. Moreover, we show that recombinant Bm-SPN-2 inhibits the enzymatic activity of 2 serine proteinases from human neutrophils, cathepsin G and elastase, suggesting a functional interaction between parasites and host cells in the same blood environment.
MATERIALS AND METHODS
Antigens and cDNA expression libraries.
Mf were obtained from B malayi-infected jirds (Meriones unguiculatus) purchased from TRS Laboratories (Athens, GA). Mf obtained by extensive lavage of the peritoneal cavities of infected jirds were separated from host cells over lymphocyte separation media and then passed through a Sephadex G-25 column.25 Fractions containing Mf without host cells were pooled. Mf proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein fraction of molecular weight 33-55 kD was recovered by electro-elution. Protein concentrations were measured using the Bradford assay (Pierce, Rockford, IL).
Six B malayi stage-specific cDNA expression libraries from L3, adult males, adult females, and Mf were supplied by the Filarial Genome Project (Williams et al, unpublished data; and Scott et al, unpublished data).26 27 Further details on construction of these libraries and their availability are posted athttp://helios.bto.ed.ac.uk/mbx/fgn/net/fgpresource.html.
Mice and immunization.
Eight-week-old female BALB/c mice were injected with 50 μg of either Mf proteins with molecular weight 33-55 kD or Bm-SPN-2 recombinant protein (see below) in complete Freund’s adjuvant and boosted 4 weeks later. Mice were killed at 6 weeks and antisera were collected.
Isolation and sequencing of Bm-spn-2 cDNA clone.
A B malayi Mf cDNA expression library was screened using immune sera, preadsorbed against Escherichia coli lysate, obtained from BALB/c mice immunized with Mf proteins of molecular weight 33-55 kD. The immunoreactive plaques, detected using peroxidase-conjugated rabbit antimouse IgG (DAKO, Glostrup, Denmark) and the TMB membrane peroxidase substrate system (KPL, Gaithersburg, MD), were purified to homogeneity by 2 subsequent rounds of screening. Bluescript phagemids were then excised from positive clones using the manufacturer’s protocols and sequenced.
Isolation and characterization of Bm-spn-2 gene.
Genomic DNA was isolated from B malayi parasites. Mf were lysed and digested in lysis buffer (100 mmol/L NaCl, 50 mmol/L EDTA, 1% SDS, 1% 2-ME, 10 μg/mL RNase, 100 μg/mL proteinase K, 100 mmol/L Tris-Cl, pH 8.5) at 65°C for 30 minutes and then extracted with phenol, phenol/chloroform, and chloroform. The DNA was precipitated and washed with ethanol, and then resuspended in TE buffer. Genomic DNA (100 μg/mL) was heated at 100°C for 10 minutes, put on ice for 10 minutes, and then used as template for polymerase chain reaction (PCR) amplification using the following primers: SF1 (nt 1-26 ofBm-spn-2 cDNA), 5′-AATATTGGCAATTCGCAATTATCCTC-3′; and SR4 (nt 780-761), 5′-ACGGTAGCGACACCGCTTGC-3′; or SF4 (nt 564-584), 5′-GGAGCCCGTAATATCGCTAGC-3′; and SR1 (nt 1418-1391), 5′-AAATTAATGCATTTTTTATTCAACATCA-3′. The PCR reactions were performed 94°C for 5 minutes and 30 cycles of 94°C for 40 seconds, 55°C for 40 seconds, and 65°C for 2 minutes, with a final 10-minute extension period at 65°C. The resulting products (SF1/SR4, 1,524 bp; SF4/SR1, 1,456 bp) were purified (GENECLEAN II; BIO 101, La Jolla, CA) and subcloned into pMOSBlue T-vector (Amersham, Buckinghamshire, UK). At least 4 independent clones of each target fragment were picked and sequenced. Introns and exons were identified by comparing genomic sequence and cDNA sequence.
Library PCR.
PCRs were performed on 1-μL aliquots of each cDNA library from B malayi L3, adult males, adult females, and Mf, with the following gene-specific primers: SF3 (nt 261-280), 5′-GCCAGAGGTGAAACTGAGCG-3′; and SR4 (as above). Primers for the B malayi tumor protein homolog-1 (tph-1) corresponded to the published sequence28: 5′-AATGTTGATCTTCAAGGGATGCATTCAC-3′ and 5′-TTTGTTTTTCTTCAATGAGTGCCTCCTT-3′. Forty cycles of amplification were performed under the following cycling conditions: 94°C for 1 minute, 50°C for 1 minute, and 72°C for 50 seconds.
Reverse transcriptase-PCR (RT-PCR).
Total RNA from L3, adult males, adult females, and Mf was isolated using RNAzol B Isolation of RNA kits (AMS, Witney, Oxfordshire, UK). Reverse transcription was performed using oligo(dT) as the first primer and 1 μg of total RNA according to the GeneAmp RT-PCR kit protocol (Perkin-Elmer, Branchburg, NJ). Amplification of Bm-spn-2 cDNA was performed using 1 μL of first-strand cDNA, with the following gene-specific primers: SF5 (nt 886-906), 5′-GTGCACTTGACTCATCTCACG-3′; and SR2 (nt 1310-1283), 5′-CTAACCTTTGTCTTTTTTTCGGTGTTTCC-3′. Primers for thetph-1 were used as described above. Thirty-five cycles of amplification were performed under the following cycling conditions: 94°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes.
5′ rapid amplification of cDNA ends (5′ RACE).
Total RNA from Mf was isolated using RNAzol B. Reverse transcription was performed using SR4 (as described above) as the first primer and 2 μg of total RNA according to the 5′/3′ RACE kit protocol (Boehringer Mannheim, Mannheim, Germany). Amplification of the 5′ end of Bm-spn-2 cDNA was performed using 5 μL of dA-tailed first-strand cDNA, with oligo dT-anchor primer and a gene-specific primer SR5 (nt 571-553) 5′-CGGGCTCCATAATTCATAC-3′. The resulting products were purified (Microcon, Beverly, MA), subcloned into pGEM T-vector (Promega, Madison, WI), and then sequenced.
Expression and purification of Bm-SPN-2 protein.
A cDNA fragment encoding Bm-SPN-2 without the putative N-terminal signal peptide (amino acid residues 1-20) was amplified by PCR from the Mf cDNA library using the following primers: SF2 5′-CAACAGTACTTTAAACCATTGTTCTG-3′ and SR2 (as described above). These primers were designed with an additional 5′ nucleotide for insertion and in-frame expression with the pET-29 T-Vector (Novagen, Madison, WI). PCR conditions were 35 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes. The resulting 1,226-bp product was purified (GENECLEAN II; BIO 101), ligated into pET-29 T-Vector, sequenced completely, and then transformed into BL21(DE3) strain of E coli.
Transformed BL21(DE3) cells were incubated in LB containing kanamycin (50 μg/mL) at 37°C for 4 hours and then 1 mmol/L isopropyl-1-β-D-galactopyranoside (IPTG; Stratagene, La Jolla, CA) was added. The cells were incubated for an additional 4 hours at 37°C and then at 4°C overnight. Cells were harvested and sonicated. The recombinant Bm-SPN-2 containing 6 C-terminal histidine residues (His-Tag) was purified by affinity column chromatography over His-Bind resin (Novagen) under native conditions.
Genomic Southern blot.
Amplification of a B malayi genomic fragment was performed by PCR using genomic DNA and gene-specific primers SF4 (as described above) and SR4 (as described above). A 408-bp PCR product, spanning exon 4, intron 4, and exon 5, was 32P-labeled by random hexanucleotide priming and then used as probe. Five micrograms of B malayi genomic DNA was digested with BamHI, EcoRI,Xba I, and Xho I. Digested DNA was fractionated on a 0.8% agarose gel, transferred to nylon membranes (Hybond-N), and hybridized with 32P-labeled probe. Blots were hybridized for 24 hours at 65°C in a rotary hybridization oven (Hybaid) in 3× standard sodium citrate (SSC), 1% SDS, and 0.2% skimmed milk containing 100 μg/mL sonicated herring sperm DNA. Washes were performed at 65°C in 2× SSC, 1% SDS for 30 minutes and then at room temperature in 0.1× SSC for 30 minutes, before autoradiography at −70°C.
Western blot.
Lysates of L3, adult males, adult females, Mf, and BL21(DE3) cells containing the pET-29/Bm-spn-2 plasmid after IPTG induction were prepared by resuspending pellets in denaturing SDS-PAGE sample buffer and boiling for 10 minutes. Insoluble cellular debris was removed by centrifugation at 13,000 rpm for 30 minutes, and SDS-soluble proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blot strips were incubated with mouse antisera to recombinant Bm-SPN-2 protein, diluted 1/4,000, and then with peroxidase-conjugated rabbit antimouse IgG (DAKO). The bound antibodies were detected by chemiluminescence on the addition of the luminol-based ECL substrate (Amersham).
Sequence analysis.
All sequencing was performed on a Applied Biosystems 377 automated sequencer by the ABI PRISM Dye Terminator cycle sequencing method (Perkin-Elmer). Sequence analysis was performed using MacVector program version 6.0 (Oxford Molecular Group, Oxford, UK). Sequence searching was performed with the BLAST algorithm29 using the NCBI BLAST server. The GCG program PILEUP (Genetics Computer Group, University of Wisconsin, Madison, WI) and BOXSHADE were used to perform multiple alignments. The tree reflecting evolutionary relationships was constructed by PAUP 3.1.130 using maximum parsimony, excluding constant, and uninformative sites.
Inhibitory activity assay.
To investigate the inhibitory activities of Bm-SPN-2, 5 μL of each individual serine proteinase was incubated with 10 μL of Bm-SPN-2 (0.4 μg/μL) in TBS buffer (150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.8) or bovine serum albumin (0.4 μg/μL) in TBS buffer as negative control. After 10 minutes of incubation at room temperature, 0.7 mL of an appropriate peptidyl-p-nitroanilide substrate solution (100 μmol/L in 50 mmol/L Tris-HCl, pH 7.8, 50 mmol/L NaCl) was added to the mixture, and the residual enzyme activity was measured by monitoring the absorbance change at 405 with time.31 The enzymes and their substrates used in the tests were bovine pancreatic trypsin (50 ng/μL; Sigma, St Louis, MO) and D-Phe-L-pipecolyl-Arg-p-nitroanilide (Sigma); human plasmin (0.1 μg/μL; Athens Research & Technology, Athens, GA) and Z-D-Phe-Pro-Arg-p-nitroanilide (where Z stands for benzyloxycarbonyl; a gift from Dr J. Tomich, Microchemical Core Laboratory, Kansas State University); bovine pancreatic α-chymotrypsin (0.1 μg/μL; Sigma) andN-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (Sigma); human neutrophil cathepsin G (1 μg/μL; Athens Research & Techology, Inc) and N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (Sigma); porcine pancreatic elastase (0.1 μg/μL; Worthington, Lakewood, NJ) andN-succinyl-Ala-Ala-Pro-Leu-p-nitroanilide (Sigma); and human neutrophil elastase (1 μg/μL; Athens Research & Techology, Inc) and N-succinyl-Ala-Ala-Pro-Leu-p-nitroanilide (Sigma). Where the proteinase activities in the presence of the Bm-SPN-2 were lower than the control, a possible serpin-enzyme interaction was further examined. Aliquots of the proteinase (1.0 μg/μL, 4.0 μL) were mixed with 0, 5, 10, 15, and 20 μL of the concentrated Bm-SPN-2 (5.0 μg/μL). Different amounts of bovine serum albumin (0.4 μg/μL) were also included to stabilize the enzyme and to adjust the final volume up to 25 μL. After incubation, residual enzyme activities were determined as described above.
Clotting assays were performed with an activated partial thromboplastin time (APTT) kit (Sigma) to test whether Bm-SPN-2 inhibits the activity of serine proteinases in the human coagulation pathway. Pooled normal plasma (0.1 mL) and 0.1 mL of various concentrations of Bm-SPN-2 or TBS buffer alone were incubated at 37°C for 5 minutes, and then 0.1 mL of prewarmed APTT reagent was added. After 3 minutes, 0.1 mL of prewarmed 0.020 mol/L calcium chloride solution was added and the clotting time was recorded.
Assays of macrophage antigen processing and presentation were performed to investigate whether Bm-SPN-2 inhibits the activity of serine proteinases involved in the major histocompatibility complex (MHC) class II pathway within macrophages. The murine macrophage cell line J774A.1 was treated with various concentrations of Bm-SPN-2 for 30 minutes, and then viable Streptococcus pyogenesor synthetic peptides aa 17-31 or aa 308-319 of the S.pyogenesM5 protein were added and incubated for 3 hours. The macrophages were fixed and incubated with the T-cell hybridoma, HX17 recognizing peptides aa 17-31, and HY2 recognizing peptides aa 308-319 for 24 hours. The culture supernatants were collected for IL-2 assay.32
RESULTS
Isolation and characterization of a cDNA for Bm-spn-2.
Studies of B malayi Mf proteins were undertaken to identify prominent antigens recognized by the host immune system’s T lymphocytes. One fraction from Mf, containing proteins of 35-55 kD, proved highly potent at inducing antigen-specific T-cell proliferation and cytokine production (Zang et al, unpublished data). Antibodies were raised against this fraction by immunizing BALB/c mice and were used to screen an Mf cDNA expression library to identify the principal antigenic proteins. Three positive clones were isolated, one of which was found to be a novel cDNA insert and was analyzed as described below.
The sequence of the full-length cDNA was determined and is shown in Fig 1A. The cDNA insert contained 1,438 bp and a single open reading frame (ORF) starting at nt 27 and ending at nt 1311, which was followed by two more stop codons and a potential polyadenylation signal sequence (AATAAA, nt 1398-1404) 15 to 20 nt upstream from a poly A tail. Like most B malayicDNAs, the GC content was low (35.1%). The ORF encoded a 428 amino acid protein with 2 potential N-glycosylation sites at asparagines 21 and 360 of the deduced amino acid sequence. Residues 1 through 20 formed a very strong hydrophobic region consistent with a signal peptide function. The predicted mature protein, without this signal sequence, would have a molecular mass of 47.5 kD and an isoelectric point of 6.9.
(A) Nucleotide and deduced amino acid sequences of theBm-spn-2 cDNA. Inferred amino acid numbering is in boldface. The putative hydrophobic signal peptide (amino acids 1-20) is underlined and 2 potential N-glycosylation sites (amino acids 21 and 360) are circled. The serpin motif (amino acids 353-357) and serpin signature (amino acids 377-387) are boxed. The position of a potential polyadenylation signal (nucleotide 1398-1404) is in boldface. Primers used in PCRs are indicated with solid arrow. The nucleotide sequence has been deposited in GenBank with the accession no. AF009825. (B) Schematic of the gene structure of Bm-spn-2. The gene spans 2.7 kb and is split into 7 exons and 6 introns, as depicted. The nucleotide sequence has been deposited in GenBank with the accession no. AF009826.
(A) Nucleotide and deduced amino acid sequences of theBm-spn-2 cDNA. Inferred amino acid numbering is in boldface. The putative hydrophobic signal peptide (amino acids 1-20) is underlined and 2 potential N-glycosylation sites (amino acids 21 and 360) are circled. The serpin motif (amino acids 353-357) and serpin signature (amino acids 377-387) are boxed. The position of a potential polyadenylation signal (nucleotide 1398-1404) is in boldface. Primers used in PCRs are indicated with solid arrow. The nucleotide sequence has been deposited in GenBank with the accession no. AF009825. (B) Schematic of the gene structure of Bm-spn-2. The gene spans 2.7 kb and is split into 7 exons and 6 introns, as depicted. The nucleotide sequence has been deposited in GenBank with the accession no. AF009826.
In GenBank searches for similar amino acid sequences, 85 significant matches were found by the BLAST algorithm,29 all of which belonged to the serine proteinase inhibitor, or serpin, superfamily. One member of this superfamily from B malayi, previously described by Yenbutr and Scott,23 encodes a distantly related gene we now refer to as Bm-spn-1. In light of this similarity, the novel protein was designated Bm-SPN-2, and its gene was designated Bm-spn-2.
Isolation and characterization of the Bm-spn-2 gene.
The Bm-spn-2 cDNA clone contained a 26-bp 5′-untranslated region (UTR) and a 105-bp 3′-UTR, which allowed us to isolate the genomic copy of Bm-spn-2 by PCR. The Bm-spn-2gene spanned 2572 bp (Fig 1B) and, on comparison to the cDNA sequence, was found to consist of 7 exons and 6 introns with the intron/exon boundaries conforming to the normal pattern (exon/GT…AG/exon) of splice acceptor and splice donor sites. Intron lengths were remarkably uniform, varying from 182 to 210 bp. At position −5 at the 3′ end of the introns, T was present in all 6 introns. Digests ofB malayi genomic DNA with BamH I, EcoRI,Xba I, and Xho I all yielded single bands that hybridized with the 32P-labeled probe (data not shown), indicating that Bm-spn-2 is either a single copy gene or is represented by copies in identical genomic environments.
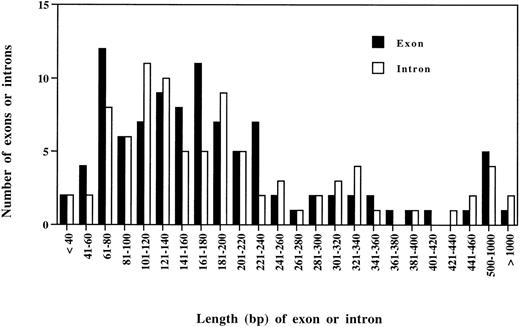
A comparison was made between Bm-spn-2 and the 12 genes fromB malayi for which full genomic sequence information is available in GenBank. These contained a total of 99 exons and 86 introns, with each gene containing between 2 and 27 exons. The majority of both exons and introns range from 60 to 240 bp, with only 3/86 introns being smaller than 60 bp (Fig 2). In this respect, Bm-spn-2 appears to be a typical B malayi gene, in marked contrast to C elegans in which more than half of the introns are shorter than 60 bp.33 Although other lower eukaryotes show a marked shift towards AT abundance in intronic sequences,34 the AT-richness of exons in the 13 known B malayi genes attenuates this effect. B malayiexons and introns are 62% and 69% AT, respectively, whereas the corresponding figures for C elegans are 54% and 70%.33 Although B malayi introns conform to the GT-AG rule, in rare instances, GC or TT were used as 5′ splice sites and AC used as 3′ splice site (Table 1). B malayi introns have an extended and conserved 3′ splice site consensus sequence, TTTCAG, in which the −5 position is T in 93% of the cases (Table 1).
Length distribution of exons and introns of all 13 B malayi genes so far characterized. The survey included 99 exons and 86 introns. Each bar represents the number of exons and introns in each size class.
Length distribution of exons and introns of all 13 B malayi genes so far characterized. The survey included 99 exons and 86 introns. Each bar represents the number of exons and introns in each size class.
Mf-specific abundant transcription of Bm-spn-2 gene.
Two Bm-spn-2 gene-specific primers, SF3 in exon 2 and SR4 in exon 5, were designed to amplify a 520-bp region of cDNA. Using 4 B malayi stage-specific cDNA expression libraries from L3, Mf, adult males, and adult females as templates, transcription ofBm-spn-2 was shown to be restricted to the Mf stage (data not shown). The identity of a 520-bp PCR product from the Mf cDNA library was confirmed by sequencing. First-strand cDNA were then prepared from these 4 stages and RT-PCR was performed with 2 gene-specific primers, SF5 in exon 5 and SR2 in exon 7, and generated a product of the expected length (425 bp) only in Mf-stage (Fig 3). The absence of a Bm-spn-2product in the adult female implies that embryonic Mf within the uterus do not transcribe this gene. Parallel PCRs were performed with primers specific for another B malayi gene, tph-1, which is known to be expressed in all stages.28 Atph-1–specific 575-bp band was found in all 4 stages from cDNA expression libraries or RNAs. In this respect, Bm-spn-2 differs quite markedly from Bm-spn-1, which is expressed in all life-cycle stages.23
Transcription of Bm-spn-2 at different development stages of B malayi. RNAs isolated from L3, Mf, adult males (lane M), and adult females (lane F) were processed to obtain first-strand cDNA. The cDNAs were then used as templates for PCR amplification of a 425-bp fragment from Bm-spn-2 or a 575-bp fragment from B malayi tph-1.
Transcription of Bm-spn-2 at different development stages of B malayi. RNAs isolated from L3, Mf, adult males (lane M), and adult females (lane F) were processed to obtain first-strand cDNA. The cDNAs were then used as templates for PCR amplification of a 425-bp fragment from Bm-spn-2 or a 575-bp fragment from B malayi tph-1.
To date, the Filarial Genome Project has sequenced a total of approximately 14,351 expressed sequence tags (ESTs), including 2,334 ESTs from Mf. Of these, 49 ESTs corresponded to Bm-spn-2, all of which were isolated from the Mf cDNA library. This suggests an abundance level of 2.1% of Mf mRNAs. These data are consistent with our PCR results and confirm that Bm-spn-2 is an unusually highly expressed transcript in the Mf stage.
The trans-spliced leader (SL) primer and a reverse primer in exon 5 were designed for PCR using the Mf cDNA library or first-strand cDNA from Mf as templates. Even after 40 rounds of amplification, no band was detected in ethidium-stained gels (data not shown). Moreover, 2 reverse primers in exon 5 and 4 were designed for 5′ RACE and no SL was present at the 5′ end of Bm-spn-2 cDNA, suggesting that Bm-spn-2 mRNA is not trans-spliced. This forms another contrast to Bm-spn-1, which is known to contain the spliced leader sequence in its mRNA.23
Expression of Bm-SPN-2 in E coli and purification of the recombinant protein.
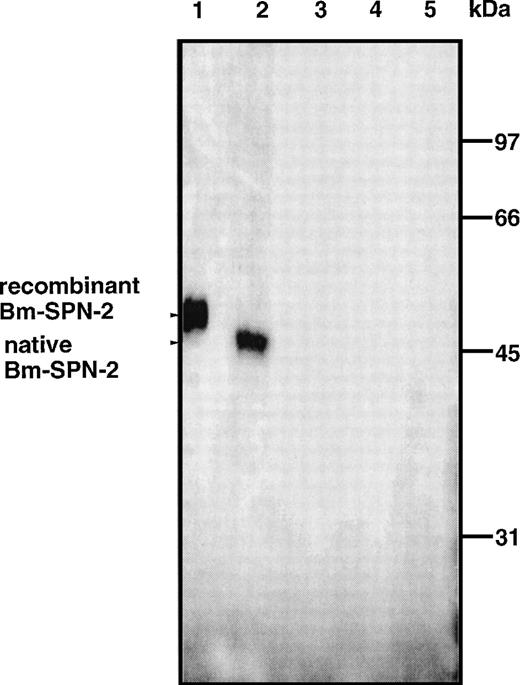
The Bm-spn-2 cDNA ORF without the signal peptide was subcloned into the pET-29 E coli expression vector, and recombinant Bm-SPN-2 was expressed as a fusion protein containing a C-terminal hexahistidine-tag for affinity purification. The recombinant fusion protein was expressed at very high levels on induction with IPTG, and soluble material was purified by affinity chromatography. The purified recombinant Bm-SPN-2 had an apparent molecular mass of 52 kD on SDS-PAGE (Fig 4).
Recombinant and native Bm-SPN-2 protein. Western blot of lysates of BL21(DE3) cells containing the pET-29/Bm-spn-2plasmid after IPTG induction (lane 1) and extracts of Mf (lane 2), L3 (lane 3), adult males (lane 4), and adult females (lane 5) probed with mouse antirecombinant Bm-SPN-2. Recognition of a 52-kD band in lane 1 and of a 47.5-kD band in lane 2 is arrowed.
Recombinant and native Bm-SPN-2 protein. Western blot of lysates of BL21(DE3) cells containing the pET-29/Bm-spn-2plasmid after IPTG induction (lane 1) and extracts of Mf (lane 2), L3 (lane 3), adult males (lane 4), and adult females (lane 5) probed with mouse antirecombinant Bm-SPN-2. Recognition of a 52-kD band in lane 1 and of a 47.5-kD band in lane 2 is arrowed.
Native protein.
Specific antibodies obtained from mice immunized with recombinant Bm-SPN-2 bound the recombinant Bm-SPN-2 protein and also recognized a single endogenous Bm-SPN-2 protein in Western blots of Mf proteins as antigens (Fig 4, lane 2); no reactivity was seen with normal mouse sera. The native Bm-SPN-2 had an apparent molecular mass of 47.5 kD. This result was in good agreement with the predicted molecular weight of the mature protein encoded by Bm-spn-2 ORF. Western blot analyses were also performed with proteins from L3, adult males, and adult females, but no reactivity was found (Fig 4, lanes 3 through 5). These data confirm that Bm-spn-2 is expressed only in the Mf stage.
Homology with other serpins.
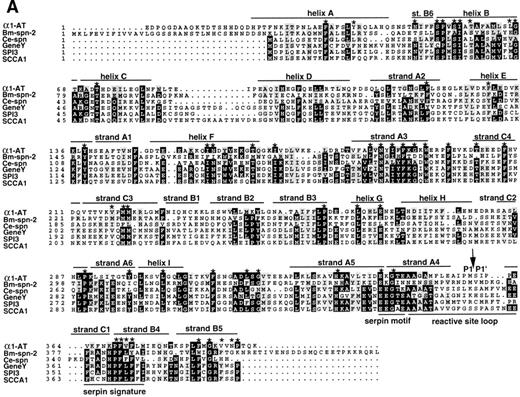
The amino acid sequence of Bm-SPN-2 shows a low level of overall similarity with the other identified serpins at the level of 20% to 26%. However, certain characteristic features of serpins are evident, and of the 51 residues identified as highly conserved within the serpin superfamily,35 33 are identical in Bm-SPN-2 and several more show conservative substitutions (Fig5A). Figure 5A compares Bm-SPN-2 with 5 known serpins, namely human α1-antitrypsin (α1-AT),35 human squamous cell carcinoma antigen 1 (SCCA1),36,C elegans serpin (Ce-spn),24 chicken ovalbumin-like gene Y protein (gene Y),37 and mouse proteinase inhibitor 3 (SPI3).38
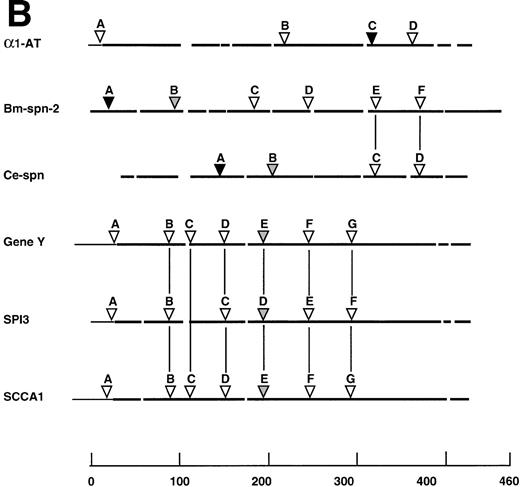
Comparison of Bm-SPN-2 with other serpins: human 1-antitrypsin (1-AT), C elegans serpin (Ce-spn), chicken gene Y protein (gene Y), mouse proteinase inhibitor 3 (SPI3), and human squamous cell carcinoma antigen 1 (SCCA1). (A) Amino acid sequences: the alignment of amino acid sequences using PILEUP and BOXSHADE programs was based on the 1-AT crystal structure35 40 in which the conserved helices and strands are defined and was manually adjusted to give the best fit. Gaps were introduced in sequences for optimal alignment. The 1-AT sequence is given without the signal sequence (MPSSVSWGILLLAGLCCLVPVSLA). Identical residues are highlighted in black and similar residues in gray shading. The 51 starred residues are those categorized as conserved in the larger serpin superfamily. The scissile bond is marked with an arrow. (B) Intron/exon positions in serpins. Thick lines indicate coding regions, thin lines indicate untranslated regions, and gaps have been introduced to optimize alignment; arrows marked by uppercase letters indicate positions of introns. Sequences were aligned on the basis of amino acid similarity with the scale showing amino acid positions beginning with the first methionine of Bm-SPN-2. Introns are shaded according to their phase: open (phase 0), shaded (phase 1), and solid (phase 2). Vertical lines connect introns considered to be in identical positions.
Comparison of Bm-SPN-2 with other serpins: human 1-antitrypsin (1-AT), C elegans serpin (Ce-spn), chicken gene Y protein (gene Y), mouse proteinase inhibitor 3 (SPI3), and human squamous cell carcinoma antigen 1 (SCCA1). (A) Amino acid sequences: the alignment of amino acid sequences using PILEUP and BOXSHADE programs was based on the 1-AT crystal structure35 40 in which the conserved helices and strands are defined and was manually adjusted to give the best fit. Gaps were introduced in sequences for optimal alignment. The 1-AT sequence is given without the signal sequence (MPSSVSWGILLLAGLCCLVPVSLA). Identical residues are highlighted in black and similar residues in gray shading. The 51 starred residues are those categorized as conserved in the larger serpin superfamily. The scissile bond is marked with an arrow. (B) Intron/exon positions in serpins. Thick lines indicate coding regions, thin lines indicate untranslated regions, and gaps have been introduced to optimize alignment; arrows marked by uppercase letters indicate positions of introns. Sequences were aligned on the basis of amino acid similarity with the scale showing amino acid positions beginning with the first methionine of Bm-SPN-2. Introns are shaded according to their phase: open (phase 0), shaded (phase 1), and solid (phase 2). Vertical lines connect introns considered to be in identical positions.
The serpin signature of FRANHPFLYAI (aa 377-387, Fig 1A) corresponds well to that, for example, of chicken gene Y, FRADHPFLFFI. The serpin motif or hinge is less well conserved, with only E353 and G355 conforming to the consensus. Between these two motifs is the reactive site loop of serpins, a stretch of approximately 14 amino acids containing the cleavable bait attacked by proteases. Serpin sequences here show extensive variation,31,39 and Bm-SPN-2 is dissimilar to any known serpin in this region. Other novel structural features of Bm-SPN-2 include the absence of a highly conserved dipeptide Ile-Asn from within helix F and the proximity of a potentialN-glycosylation site between the serpin motif and the reactive loop. Most interestingly, of all 93 serpin sequences deposited in public databases, Bm-SPN-2 is one of the largest and has the longest C-terminus other than antiplasmin.35 All of these features would be more clearly understood in the light of a structure for the protein. The 3-dimensional structures of serpins such as α1-AT,40 PAI-1,41 and antithrombin III,42 have been solved, and these provide a useful framework for analyzing other members of the superfamily.
At the level of gene structure, Bm-spn-2, which contains 6 introns, also made an interesting comparison with 5 other serpins (Fig5B). Introns E and F of Bm-spn-2 are positioned exactly as introns C and D in Ce-spn, supporting a close relationship between the nematode genes, but no vertebrate serpins contained introns at the same point. The genes for chicken gene Y protein, murine SPI3 and human SCCA1, had 5 identical intron positions, but these were not found either in the mammalian α1-AT gene or in either of the nematode sequences.
Phylogenetic analysis.
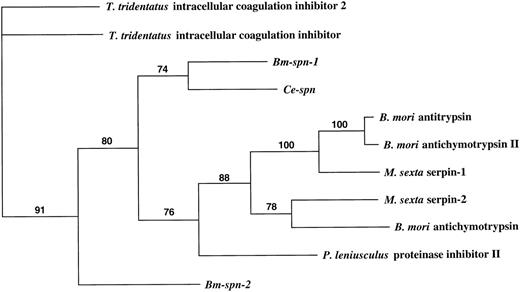
Of the 93 members of serpin superfamily currently in accessible databases, 62 are vertebrate (mainly mammalian) and 11 are from invertebrates, including insects, crayfish, horseshoe crabs, and nematodes, whereas 5 are from plants and 15 are from viruses. A phylogenetic comparison of all invertebrate serpins was performed using PAUP to indicate the likely evolutionary relationship ofBm-spn-2 to known genes (Fig 6). This analysis divides invertebrate serpins into 2 groups (91% bootstrap confidence): group I comprising serpins from Tachypleus tridentatus (horseshoe crab), and group II containing serpins fromB malayi, C elegans, Bombyx mori (Silkworm), Manduca sexta (tobacco hornworm), and Pacifastacus leniusculus (signal crayfish). Within the latter group,Bm-spn-1 and Ce-spn form a subgroup that is quite distant from Bm-spn-2.
Phylogenetic trees showing the relationship between the predicted protein encoded by Bm-spn-2 and all other recorded invertebrate serpins. Numbers above branches show the percentage of bootstrap support for each clade. GenBank, Protein Identification Resource, and SWISS-PROT accession numbers of the sequences used were as follows: T tridentatus intracellular coagulation inhibitor 2, A55533; T tridentatus intracellular coagulation inhibitor,D14483; Bm-spn-1 (Bm-SERPIN), U04206; Ce-spn (C elegansserpin), U50301; B mori antitrypsin, D00738; B moriantichymotrypsin II, P80034; M sexta serpin-1, U58361; M sexta serpin-2, U79184; B mori antichymotrypsin, D13895;P leniusculus proteinase inhibitor II, X82642;Bm-spn-2, AF009825.
Phylogenetic trees showing the relationship between the predicted protein encoded by Bm-spn-2 and all other recorded invertebrate serpins. Numbers above branches show the percentage of bootstrap support for each clade. GenBank, Protein Identification Resource, and SWISS-PROT accession numbers of the sequences used were as follows: T tridentatus intracellular coagulation inhibitor 2, A55533; T tridentatus intracellular coagulation inhibitor,D14483; Bm-spn-1 (Bm-SERPIN), U04206; Ce-spn (C elegansserpin), U50301; B mori antitrypsin, D00738; B moriantichymotrypsin II, P80034; M sexta serpin-1, U58361; M sexta serpin-2, U79184; B mori antichymotrypsin, D13895;P leniusculus proteinase inhibitor II, X82642;Bm-spn-2, AF009825.
Inhibitory activity of recombinant Bm-SPN-2.
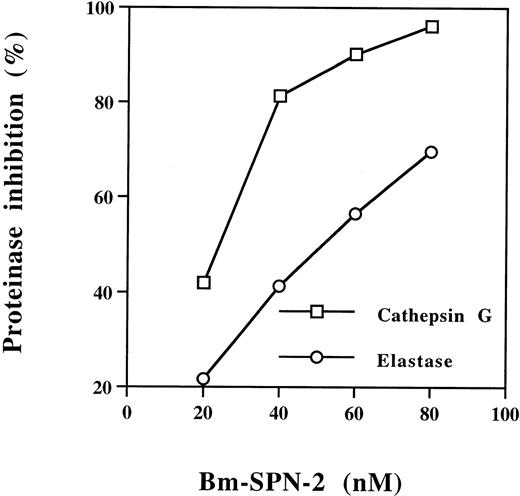
To screen for potential inhibitory function of Bm-SPN-2, we tested its ability to inhibit a panel of mammalian serine proteinases with different substrate specificities. Bm-SPN-2 inhibited the enzymatic activity of the human neutrophil cathepsin G and human neutrophil elastase in a dose-dependent manner (Fig7), but showed no inhibitory activity against bovine pancreatic trypsin, human plasmin, bovine pancreatic α-chymotrypsin, and porcine pancreatic elastase. The inhibition of the neutrophil proteinases by Bm-SPN-2 is strikingly specific, considering that human neutrophil cathepsin G and bovine pancreatic α-chymotrypsin hydrolyze the same substrates as do human neutrophil elastase and porcine pancreatic elastase.
Concentration-dependent inhibition of human neutrophil cathepsin G and human neutrophil elastase activation by Bm-SPN-2. Recombinant Bm-SPN-2 at different concentrations was mixed with human neutrophil cathepsin G or human neutrophil elastase before the addition of specific substrates. Changes in the rate of substrate cleavage were used to calculate the percentage of inhibition at each Bm-SPN-2 concentration.
Concentration-dependent inhibition of human neutrophil cathepsin G and human neutrophil elastase activation by Bm-SPN-2. Recombinant Bm-SPN-2 at different concentrations was mixed with human neutrophil cathepsin G or human neutrophil elastase before the addition of specific substrates. Changes in the rate of substrate cleavage were used to calculate the percentage of inhibition at each Bm-SPN-2 concentration.
To test whether Bm-SPN-2 inhibits the activity of serine proteinases in human coagulation pathway, the APTT was measured in the presence of Bm-SPN-2, but no prolongation of the APTT was observed (data not shown). This suggests Bm-SPN-2 dose not inhibit the serine proteinases of the clotting pathway such as factor X and Hageman factor.
Serine proteinases are involved in processing and presentation of 2 epitopes of the S pyogenes M5 protein to CD4+ T lymphocytes.32 To investigate whether Bm-SPN-2 inhibits this antigen-processing pathway, the ability of macrophages to present these epitopes from S pyogenes to T cells was measured in the presence of Bm-SPN-2. No change in stimulation was observed (Delvig et al, unpublished observation). This suggests that Bm-SPN-2 does not inhibit the activity of serine proteinases involved in antigen processing within macrophages.
DISCUSSION
The wide distribution of serpins and their ability to regulate a variety of divergent proteinases show that they play a major regulatory role in a host of biological processes. We describe here the isolation, characterization, expression, and inhibitory function of a new serpin gene, Bm-spn-2, from the human lymphatic filarial nematodeB malayi. The Bm-spn-2 gene, which appears to be a single copy in the genome and consists of 7 exons, was found to have Mf stage-specific expression. The 2 serpins that have been found in B malayi, Bm-spn-1 and Bm-spn-2, are quite different. They share low homology and are not closely related, as shown by phylogenetic analysis. Bm-spn-1 is expressed in most life stages and its mRNA contains the 22 nt nematode 5′ SL, whereasBm-spn-2 is expressed only in the Mf stage and its mRNA does not contain SL. At present, the gene structure of Bm-spn-1 is not available. It will be of interest to compare the gene structure, chromosome position, and physiological roles of these two serpins.
We analyzed 13 B malayi genes for which complete sequence is available and found that Bm-spn-2 was typical of this set.B malayi genes have relatively large introns compared with the nematode model C elegans33 and, like C elegans, the introns are markedly AT-rich. This is a property shared with introns of other invertebrates and plants34,43that is possibly related to effective recognition and splicing of introns.43 It is interesting to note that B malayiintrons have an extended and conserved 3′ splice site in which T at −5 position is very highly conserved, a feature noted previously only in C elegans.33 In contrast, introns from most other organisms have only a combination of an upstream polypyrimidine tract and a YAG consensus at their 3′ boundaries. This suggests that the 3′ intron boundary may be a more important element in B malayi intron recognition than in most other organisms. Unusually, the exon sequences in these 13 B malayi genes are also AT-rich, much more so than C elegansexons, and no explanation is yet available for this finding.
The serpin gene family predates the common ancestor of nematodes, arthropods, and chordates and may consequently be more than 1,200 million years old.44 Based on vertebrate serpin gene organization, it has been proposed that the ancestral gene would have had a large number of introns and that modern serpin genes derived from this by duplication have undergone varied intron deletion.45 This model, consistent with the general introns-early hypothesis,46 contrasts with a gain-and-loss explanation, which would envisage an ancestral serpin gene with between 0 and 3 introns. A comparison of the intron/exon structure ofBm-spn-2 with 5 other serpins show sets of introns found only in nematode genes and others present only in vertebrate genes. Several serpin genes also possessed unique introns. Under the introns early mechanism, the ancestral serpin gene would have needed nearly 20 introns to account for the pattern observed in just 7 extant genes. Therefore, it seems that intron gain-and-loss is a more likely explanation of serpin gene evolution, as postulated for other gene families.47 We hope that, as more genes encoding serpin become available, the serpin family can be divided into subgroup on the basis of gene organization and position, and this may provide an alternate way to advance our fundamental knowledge of evolutionary relatedness.
The serpin superfamily has been constructed on the basis of sequence similarities at the protein level.48 All members of the serpin superfamily are thought to share a common highly ordered tertiary structure, defined by x-ray diffraction for the prototype molecule α1-AT35,40 which consists of 9 α-helices and 3 β-sheets. Although the predicted amino acid sequence of Bm-SPN-2 has a low overall homology to the sequences of serpins from other species, its sequence is identical or conserved at most of the characteristic amino acid positions. The serpin signature, which includes the hinge distal to the reactive loop, is, for example, well conserved. However, analysis of the serpin motif (in the proximal hinge) is ambiguous. Two residues in Bm-SPN-2 (E353 and G355) are those found in 100% of functional serpins.49 Few of the other residues in Bm-SPN-2 follow the consensus for small nonpolar sidechains, which are thought to be necessary to allow the conformational change associated with inhibitory function.49,50 Further work will establish whether the consensus sequence requires redefinition in the light of phylogenetically removed genes such as those from nematodes and trematodes.22
Genes that are expressed in a stage-specific manner have important functional roles in the parasite life cycle and thus may provide targets for the development for novel immunoprophylactic or chemoprophylactic agents. It is significant that Bm-spn-2 is an abundant transcript restricted to the Mf stage, and the presence of a putative signal peptide (amino acids 1-20) indicates that that the mature protein could be released as a secretory product to perform some function in parasite survival. In several systems, serpins from infectious organisms have been identified as factors that help infectious organisms to evade the host defence system.10,12,15,18 The ability of Bm-SPN-2 to inhibit 2 human neutrophil-derived serine proteinases, cathepsin G and elastase, may be important in this context. Genes encoding cathepsin G, cathepsin G-like lymphocyte granzymes B and H, and α/δ chains of the T-cell receptor are closely linked on human chromosomal band 14q11.2.51 Stimulated polymorphonuclear neutrophils release cathepsin G concomitantly with neutrophil elastase, and these proteinases mediate antimicrobial activity,52 degradation of extracellular matrix, vasoregulation, and IL-8 processing.53 Furthermore, cathepsin G binds to T lymphocytes and NK cells54,55 and exerts a mitogenic effect on T lymphocytes, which are dependent on retaining enzymatic activity.56 Cathepsin G is also known to be a chemokinetic stimulant for T lymphocytes and chemoattractant for monocytes.55 We suggest that neutrophils, representing 55% of human blood leukocytes, would be the primary cell type to interact with B malayi Mf and that release of Bm-SPN-2 neutralizes the stimulating properties of cathepsin G. It is interesting to note a study that identified a human squamous cell carcinoma antigen, SCCA2, as a novel serpin that also inhibits cathepsin G.57 These data suggest that a possible common strategy between parasites and tumors is the production of a cathepsin G inhibitor to counteract immune activation.
One of the remarkable features of the Mf stage of B malayi is its longevity (>1 year in the bloodstream), which is testimony to the effectiveness of immune evasion by this parasite. The survival of Mf provides not only a reservoir of infection in an endemic community, but also a clear target for intervention. Bm-SPN-2 seems an important component of parasite survival strategies, so it may prove to be an equally appropriate target for vaccination or pharmaceutical attack to clear infection both from afflicted individuals and endemic communities. Our preliminary results show that specific immune responses against Bm-SPN-2 do indeed mediate the clearance of B malayi Mf, supporting the contention that this serpin may be a critical component of the host-parasite relationship.
ACKNOWLEDGMENT
The authors thank the Filarial Genome Project for cDNA libraries; Drs Alexei Delvig and John Robinson (University of Newcastle) for testing Bm-SPN-2 in an antigen processing assay; and Alex Loukas, Bill Gregory, Andrew MacDonald, Franco Falcone, and Mark Dorris for invaluable assistance. We also thank Drs Judith Allen and Mark Blaxter for advice and critical review of the manuscript.
Supported by grants from the European Union (INCO-DC IC18-CT95-0014) and from the National Institutes of Health (GM41247).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Rick M. Maizels, PhD, Institute of Cell, Animal and Population Biology, University of Edinburgh, Edinburgh EH9 3JT, UK; e-mail:r.maizels@ed.ac.uk.