Abstract
Eosinophil granule major basic protein (MBP) is expressed exclusively in eosinophils and basophils in hematopoietic cells. In our previous study, we demonstrated a major positive regulatory role for GATA-1 and a negative regulatory role for GATA-2 in MBP gene transcription. Further analysis of the MBP promoter region identified a C/EBP (CCAAT/enhancer-binding protein) consensus binding site 6 bp upstream of the functional GATA-binding site in the MBP gene. In the cell line HT93A, which is capable of differentiating towards both the eosinophil and neutrophil lineages in response to retinoic acid (RA), C/EBP mRNA expression decreased significantly concomitant with eosinophilic and neutrophilic differentiation, whereas C/EBPβ expression was markedly increased. Electrophoretic mobility shift assays (EMSAs) showed that recombinant C/EBPβ protein could bind to the potential C/EBP-binding site (bp −90 to −82) in the MBP promoter. Furthermore, we have demonstrated that both C/EBPβ and GATA-1 can bind simultaneously to the C/EBP- and GATA-binding sites in the MBP promoter. To determine the functionality of both the C/EBP- and GATA-binding sites, we analyzed whether C/EBPβ and GATA-1 can stimulate the MBP promoter in the C/EBPβ and GATA-1 negative Jurkat T-cell line. Cotransfection with C/EBPβ and GATA-1 expression vectors produced a 5-fold increase compared with cotransfection with the C/EBPβ or GATA-1 expression vectors individually. In addition, GST pull-down experiments demonstrated a physical interaction between human GATA-1 and C/EBPβ. Expression of FOG (riendATA), which binds to GATA-1 and acts as a cofactor for GATA-binding proteins, decreased transactivation activity of GATA-1 for the MBP promoter in a dose-dependent manner. Our results provide the first evidence that both GATA-1 and C/EBPβ synergistically transactivate the promoter of an eosinophil-specific granule protein gene and that FOG may act as a negative cofactor for the eosinophil lineage, unlike its positively regulatory function for the erythroid and megakaryocyte lineages.
DIFFERENTIATION OF eosinophils from myeloid progenitors is regulated by several different cytokines, including interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-5.1-3 Of these cytokines, IL-5 is eosinophil-lineage specific and plays a principal role in the terminal differentiation of eosinophils and development of eosinophilia.3-6 However, the molecular basis for the commitment of hematopoietic stem cells to the eosinophil lineage remains to be elucidated. We have been characterizing the regulatory regions of genes encoding eosinophil-specific proteins, including eosinophil peroxidase (EPO),7 Charcot-Leyden crystal (CLC) protein,8 and eosinophil major basic protein (MBP),9 to identify transcription factors involved in regulating the commitment and terminal differentiation of myeloid progenitors to the eosinophil lineage. In analyses of the MBP promoter region, we have identified a major positive regulatory role for GATA-1 and a negative regulatory role for GATA-2 in MBP gene transcription, providing the first evidence for a GATA-regulated gene in the eosinophil lineage.9 Further analysis of the MBP promoter region identified a C/EBP (CCAAT/enhancer-binding protein) consensus binding site 6 bp upstream of the functional GATA-binding site in a positively acting region of the MBP promoter.9 The C/EBP family belongs to the larger family of basic leucine zipper (bZip) transcription factors. To date, 6 members have been cloned and characterized, including C/EBPα,10,11C/EBPβ,12-15 C/EBPγ,16C/EBPδ,17-20 C/EBPε,21,22 and CHOP.23 C/EBP proteins have been shown to regulate a number of hepatic10,14,24,25 and adipocyte17,26,27genes. Expression of C/EBP proteins in the hematopoietic system, particularly C/EBPα, C/EBPβ, and C/EBPε, is limited to myeloid cells, suggesting that they play an important role in the differentiation of myeloid cells.21,22,28-37 Recent studies from other laboratories have shown that PU.1 (Spi-1) and the C/EBP family, in particular C/EBPα, may serve as master regulators of myeloid development, in part through their regulation of lineage-specific growth factor receptor genes, including macrophage colony-stimulating factor receptor (M-CSFR),29,30granulocyte colony-stimulating factor receptor (G-CSFR),31,32 and GM-CSF receptor α (GM-CSFRα).38 In addition, in the avian hematopoietic system, it has been reported that NF-M, the chicken homolog of C/EBPβ, induces eosinophil differentiation in a multipotent progenitor cell line transformed by the Myb-Ets oncoprotein.39 However, the target promoter(s) of eosinophil-associated genes for the C/EBP transcription factor family remains unknown.
We have recently demonstrated that GATA-1 is one of the transcription factors involved in regulating expression of eosinophil genes during the differentiation of myeloid progenitors to the eosinophil lineage.9,40 However, GATA-1 plays a crucial role in the differentiation of hematopoietic progenitors to the erythroid and megakaryocyte lineages.41-43 Differences in the role of GATA-1 in eosinophil versus erythroid/megakaryocyte development and gene expression remain to be elucidated.
We report here that C/EBPβ can physically interact with GATA-1 and synergistically transactivate the MBP P2 promoter with GATA-1. In addition, we provide evidence that riendf ATA (FOG), a cofactor for GATA-binding proteins, may act as a negative cofactor in the eosinophil lineage, unlike its role as a positive cofactor for erythroid and megakaryocyte gene expression.
MATERIALS AND METHODS
Cell cultures.
The eosinophil-committed subline of the HL-60 promyelocytic leukemic cell line, HL-60-C15 (a gift of Dr Steven Fishkoff),44 and T-lymphocytic Jurkat cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS) and 2 mmol/L L-glutamine and passaged twice weekly. Another eosinophil cell line, HT93A cells, which are able to differentiate towards the eosinophil and neutrophil lineages in response to retinoic acid (RA), was also maintained in RPMI 1640 supplemented with 8% FBS, 2 mmol/L L-glutamine, 5 × 10−5M2-mercaptoethanol (2ME), and 1 mmol/L sodium pyruvate.45
Northern blot analysis.
Total RNA was prepared from HT93A cells stimulated with 2 μmol/L RA by TRizol Reagent (GIBCO BRL, Rockville, MD). Poly (A)+mRNA was then separated by Oligotex-dT30 (Takara Shuzo, Kyoto, Japan). The poly (A)+ mRNA was transferred to Hybond-N nylon membranes (Amersham International plc, Buckinghamshire, UK) and the blots were probed sequentially with full-length cDNAs for MBP,46 gp91phox,47 neutrophil elastase,48 GATA-1,41 GATA-2,49GATA-3,50 C/EBPα,51 C/EBPβ,12PU.1 (Spi-1),52 and β-actin. Hybridization with the random-primed DNA probes was performed at 42°C in 50% formamide, 6× SSC, 0.2% Ficoll-polyvinylpyrrolidone (PVP), and 0.1% sodium dodecyl sulfate (SDS). Filters were washed twice in 2× SSC with 0.2% SDS at 53°C for 30 minutes and twice in 0.2× SSC with 0.2% SDS at 55°C for 30 minutes. Autoradiography was performed at −80°C with Kodak XAR-5 film (Eastman Kodak, Rochester, NY).
Plasmids for transient transfections.
The promoterless luciferase plasmid pXP2 containing the sequence between positions bp −117 to +47 of MBP gene P2 promoter region9 was used for all promoter studies.
Expression vectors for human GATA-1 and human C/EBPβ under control of the elongation factor-1α promoter, pEF-GATA1 and pEF-C/EBPβ, were prepared by cloning the human GATA-1 or C/EBPβ cDNAs inserted into pEF-BSSHII, a modified version of the expression vector pEF-BOS.53
Transient transfections.
DNA was prepared and cells were transfected by electroporation as previously described using 1.5 × 107 cells in 500 μL with minor modifications.7 Briefly, the HL-60-C15 cells were electroporated at 280 V, 960 μF, and the Jurkat cells were electroporated at 250 V, 960 μF, respectively, conditions previously optimized for these lines.7-9 Luciferase activity in cell lysates was measured as relative light units (RLU) using a Lumat LB9501 luminometer (Berthold GmbH & Co KG, Bad Wildbad, Germany). Cell extracts were prepared in 500 μL of 1% Triton X-100, 25 mmol/L Gly-gly, 15 mmol/L MgSO4, 4 mmol/L EGTA, and 1 mmol/L dithiothreitol (DTT), and 100-μL aliquots of these extracts (equivalent to 1.5 × 106 cells) were analyzed for luciferase activity. Cotransfection with a cytomegalovirus (CMV)-β galactosidase (Gal) plasmid (CMV-βGal) was used for normalization of transfection efficiency among the different cell lines, different plasmid DNA preparations, and individual transfection experiments. β-Galactosidase activity was measured by a β-galactosidase enzyme assay kit (Promega Co, Madison, WI). Individual transfection experiments were repeated at least 3 times, and the results are shown as mean RLU per milliunit of β-galactosidase per milliliter (±SEM).
Electrophoretic mobility shift assay (EMSA).
COS cells transfected with the GATA-1 expression vector, pEF-GATA-1, were harvested 24 hours after electroporation with this plasmid. Nuclear extracts were prepared as described,54 and protein concentrations were determined using the Bradford assay (Bio-Rad, Richmond, CA). Purification of recombinant C/EBPβ protein was performed as described previously.55 Human C/EBPβ cDNA was subcloned into the maltose-binding protein (MBP) expression plasmid, pMALc-2 (New England Biolabs, Boston, MA), yielding the plasmid designated pMBP-C/EBPβ. This plasmid was transferred to the Escherichia coli strain PS1, and the transformed cells were grown and harvested after induction with 0.3 mmol/L isopropyl-1-thio-β-galactopyranoside for 3 hours at 37°C. The MBP-C/EBPβ fusion protein was then purified by amylose resin affinity chromatography according to the manufacturer’s instructions and was diluted with a buffer containing 25 mmol/L HEPES/KOH, pH 7.6, 40 mmol/L KCl, 0.1 mmol/L EDTA, 1 mmol/L dithiothreitol, 10% glycerol, and 1 mg/mL bovine serum albumin (BSA) for further use in a gel-shift assay. Nuclear extracts or recombinant proteins were incubated with radiolabeled oligonucleotides (0.1 to 1 ng) and then subjected to electrophoresis as described previously.9 Reactions were electrophoresed at 14 V/cm on 6% polyacrylamide gels cast in 0.5× TBE at 4°C.
EMSAs were performed with the following oligonucleotides (mutated sites are underlined): (A) MBP WT (−93/−58 bp) (5′-AAGTGATGAAATGGTCCTTATCAGCCTTGCTATCTC-3′); (B) MBP mut GATA (5′-AAGTGATGAAATGGTCCGGCGACGCCTTGCTATCTC-3′); (C) MBP mut C/EBP (5′-AAGGTATTACCGGGTTCCTTATCAGCCTTGCTATCTC-3′); and (D) MBP mut GATA/C/EBP (5′-AAGGTATTACCGGGTTCCGGCGACGCCTTGCTATCTC-3′).
GST pull-down assays with [35S] methionine-labeled proteins.
DNA was transcribed and translated in vitro using the TNT T7-coupled reticulocyte lysate system (Promega), following the instructions of the manufacturer. The reticulocyte lysate containing the [35S]-labeled protein was then incubated with GST or GST-hGATA-1 in a buffer containing 50 mmol/L Tris-HCl (pH 7.8), 150 mmol/L KCl, 0.1% (vol/vol) Nonidet P-40, 0.1 % (vol/vol) Triton X-100, 5 mmol/L MgCl2, 0.5 mmol/L EDTA, 10% (vol/vol) glycerol, 50 μmol/L ZnCl2, 0.1 mmol/L sodium orthovanadate, 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), leupeptin (5 μg/mL), aprotinin (10 μg/mL), and pepstatin A (5 μg/mL) in a total volume of 500 μL at 4°C for overnight. The resin was subsequently washed 4 times with 1 mL of binding buffer. Bound proteins were released in an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and then analyzed by electrophoresis under denaturing conditions and autoradiography.
RESULTS
Identification of a C/EBP consensus site in the functional promoter region of the MBP gene.
There are 2 promoters, P1 and P2, in the MBP gene. The distal promoter, which exists 5′ upstream to exon 1 and drives expression of the 1.6-kb transcript, and the proximal promoter, which exists 5′ upstream to exon 9 and drives expression of the 1.0-kb transcript, have been designated the P1 and P2 promoters, respectively.56 We previously reported the specificity of P2 promoter for the eosinophil lineage and identified a positively acting region in the bp −117 to −67 segment of the MBP P2 gene.9 In addition, we showed that GATA-1 could bind to a GATA consensus site, bp −76 to −71, in this region and could transactivate the MBP P2 promoter.9 As shown in Fig 1, we have identified a C/EBP consensus binding site at bp −90 to −82, 6 bp upstream of the functional GATA-binding site in this promoter.
The positive acting cis-elements of MBP P2 promoter. Sequence of the MBP gene between bp −108 and −70, showing the GATA consensus site (bp −76 to −71) and C/EBP consensus site (bp −90 to −82).
The positive acting cis-elements of MBP P2 promoter. Sequence of the MBP gene between bp −108 and −70, showing the GATA consensus site (bp −76 to −71) and C/EBP consensus site (bp −90 to −82).
Induction of C/EBPβ mRNA expression in a cell line capable of differentiating towards both the eosinophil and neutrophil lineages.
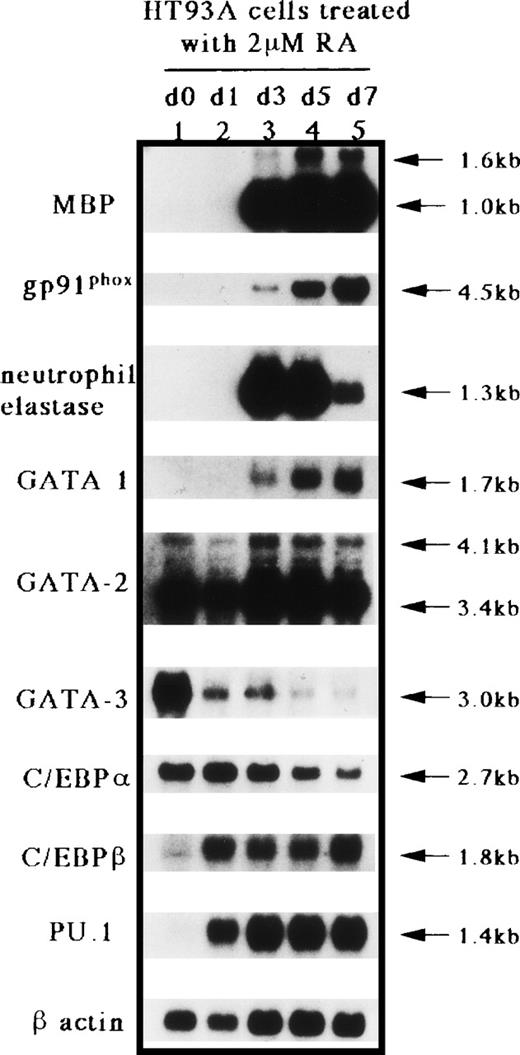
We have established a cell line capable of differentiating towards the eosinophil and neutrophil lineages in response to RA, HT93A.45 As shown by Northern blot analysis (Fig 2), no mRNA expression for MBP, gp91phox, and neutrophil elastase, which are markers for eosinophil and neutrophil lineages, GATA-1, or PU.1 (Spi-1), was observed in untreated HT93A cells (Fig 2, lane 1). This result shows that the uninduced HT93A cell line does not express genes characteristic of the eosinophil and neutrophil lineages. For the GATA-binding proteins, GATA-1 expression was induced 3 days after induction of HT93A cells with RA, whereas GATA-2 was expressed constitutively before and during the eosinophilic and neutrophilic differentiation of the cell line, consistent with our previous studies.9 40 In contrast, expression of GATA-3, which plays an important role in lymphoid development, was markedly downregulated during the eosinophilic and neutrophilic differentiation of HT93A cells. For the C/EBPs, expression of C/EBPα decreased somewhat after 7 days of culture with RA, whereas the expression of C/EBPβ markedly increased from day 1 to 7 during differentiation towards the eosinophil and neutrophil lineages. The expression of PU.1 (Spi-1), an ETS-domain transcription factor essential for the development of myeloid and B-lymphoid cells, also increased considerably along with the eosinophilic and neutrophilic differentiation of HT93A cells. These results suggest that GATA-1, C/EBPβ, and PU.1 (Spi-1) play an important role in eosinophilic and neutrophilic differentiation.
Northern blot analysis for MBP, gp91phox, neutrophil elastase, GATA-1, GATA-2, GATA-3, C/EBP, C/EBPβ, PU.1 (Spi-1), and β-actin mRNA expression in HT93A cells treated with RA. Lane 1, untreated HT93A cell; lanes 2 through 5, HT93A cells treated with 2 μmol/L RA for 1, 3, 5, and 7 days, respectively. Each lane contained 4 μg of poly(A)+ RNA. The blot was probed sequentially with each cDNA probe and the β-actin probe to control for equivalent loading.
Northern blot analysis for MBP, gp91phox, neutrophil elastase, GATA-1, GATA-2, GATA-3, C/EBP, C/EBPβ, PU.1 (Spi-1), and β-actin mRNA expression in HT93A cells treated with RA. Lane 1, untreated HT93A cell; lanes 2 through 5, HT93A cells treated with 2 μmol/L RA for 1, 3, 5, and 7 days, respectively. Each lane contained 4 μg of poly(A)+ RNA. The blot was probed sequentially with each cDNA probe and the β-actin probe to control for equivalent loading.
C/EBPβ binds to the C/EBP consensus site in the MBP P2 promoter region.
Northern blot analysis in HT93A cells treated with RA indicated that C/EBPβ was induced during the eosinophilic and neutrophilic differentiation. Müller et al39 have reported that NF-M, the chicken homolog of C/EBPβ, induces eosinophil differentiation in a multipotent progenitor cell line transformed by the Myb-Ets oncoprotein. Furthermore, we have recently shown that MBP promoter activity is increased 10-fold by cotransfecting the plasmids expressing C/EBPα or C/EBPδ, whereas cotransfection with the C/EBPβ expression vector produced a 20-fold increase.9From the above-noted findings, it was suggested that C/EBPβ might be involved in the regulation of MBP gene expression. Accordingly, to determine whether C/EBPβ binds to the potential C/EBP-binding site, bp −90 to −82, in the MBP P2 promoter region, EMSAs were performed (Fig 3). The gel-shift analysis was performed using a purified recombinant C/EBPβ protein, maltose-binding protein (MBP)-C/EBPβ, and nuclear extracts from COS7 cells transiently transfected with the GATA-1 expression vector, as described previously.9 Full-length murine C/EBPβ fused to the maltose-binding protein (MBP) was expressed in E coli and then purified by amylose resin affinity chromatography.55 A DNA probe spanning bp −93 to −58 of the MBP promoter formed a specific protein-DNA complex when incubated with MBP-C/EBPβ (Fig 3, lane 2), but not with maltose-binding protein alone (Fig 3, lane 1). The complex formed with the specific probe was inhibited by an excess amount of the wild-type (competitor DNA A) and GATA site-mutated (competitor DNA B) oligonucleotides (Fig 3, lanes 3 and 4) but not by an oligonucleotide mutated in only the C/EBP consensus site (competitor DNA C) or an oligonucleotide mutated in both sites (competitor DNA D; Fig 3, lanes 5 and 6). An EMSA with nuclear extracts from COS7 cells transfected with a GATA-1 expression vector and oligonucleotide spanning the MBP promoter from position bp −93 to −58 showed the formation of a specific GATA-1 complex (Fig 3, lane 7). Incubation of both GATA-1 and C/EBPβ proteins with the labeled probe showed 3 specific complexes, GATA-1 alone, C/EBPβ alone, and combined GATA-1 and C/EBPβ (Fig 3, lane 8). The C/EBPβ-DNA complex band was competed by unlabeled oligonucleotides A and B (Fig 3, lanes 9 and 10), but not by unlabeled oligonucleotides C and D containing a mutated C/EBP site (Fig3, lanes 11 and 12). The GATA-1-DNA complex band was also competed by oligonucleotides A and C (Fig 3, lanes 9 and 11), but not by oligonucleotides B and D (Fig 3, lanes 10 and 12). To confirm the results shown in Fig 3, we performed the EMSA with various labeled oligonucleotide probes (Fig 4). When incubated with labeled wild-type oligonucleotide (probe A) and MBP-C/EBPβ or GATA-1 protein, the probe spanning position bp −93 to −58 (probe A) yielded 1 DNA-protein complex with C/EBPβ or GATA-1 protein (Fig 4, lanes 2 and 3). Incubation of both C/EBPβ and GATA-1 proteins with the wild-type probe (probe A) formed 3 specific bands, GATA-1, C/EBPβ, and GATA-1/CEBPβ complexes (Fig4, lane 4). In contrast, incubation of both proteins with the GATA-site mutated probe (probe B) or C/EBP-site mutated probe (probe C) did not yield the 3 complexes (Fig 4, lanes 5 and 6). Also, the incubation of both proteins with C/EBP and GATA site-mutated probes formed no DNA-protein complex (Fig 4, lane 7). These results demonstrate that C/EBPβ binds to the C/EBP consensus site, bp −90 to −82, and that both C/EBPβ and GATA-1 can bind to the C/EBP- and GATA-binding sites simultaneously.
Binding of recombinant C/EBPβ fusion protein and GATA-1 protein to the MBP promoter. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 1 μg maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 through 6 and 8 through 12) and 8 μg nuclear protein from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 7 through 12). Unlabeled double-stranded competitor oligonucleotides, shown schematically in the lower panel, were added at a 100-fold molar excess over the labeled probe oligonucleotide, MBP bp −93 to −58 (competitor A; lanes 3 and 9), mutated GATA-consensus site oligonucleotide (competitor B; lanes 4 and 10), mutated C/EBP-consensus site oligonucleotide (competitor C; lanes 5 and 11), and mutated GATA-1 and C/EBP-consensus site oligonucleotide (competitor D; lanes 6 and 12).
Binding of recombinant C/EBPβ fusion protein and GATA-1 protein to the MBP promoter. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 1 μg maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 through 6 and 8 through 12) and 8 μg nuclear protein from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 7 through 12). Unlabeled double-stranded competitor oligonucleotides, shown schematically in the lower panel, were added at a 100-fold molar excess over the labeled probe oligonucleotide, MBP bp −93 to −58 (competitor A; lanes 3 and 9), mutated GATA-consensus site oligonucleotide (competitor B; lanes 4 and 10), mutated C/EBP-consensus site oligonucleotide (competitor C; lanes 5 and 11), and mutated GATA-1 and C/EBP-consensus site oligonucleotide (competitor D; lanes 6 and 12).
Demonstration of C/EBPβ and GATA-1 binding to the MBP promoter by gel-shift assay. Double-stranded oligonucleotides (lower panel) containing MBP promoter sequence from bp −93 to −58 (probe A), with mutated GATA-consensus site (probe B), mutated C/EBP-consensus site (probe C), and mutated GATA- and C/EBP-consensus sites (probe D) were end-labeled with [γ-32P] ATP and incubated with 1 μg double-stranded poly (dI-dC) in the presence of 1 μg purified maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 and 4 through 7) and 8 μg nuclear proteins from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 3 through 7).
Demonstration of C/EBPβ and GATA-1 binding to the MBP promoter by gel-shift assay. Double-stranded oligonucleotides (lower panel) containing MBP promoter sequence from bp −93 to −58 (probe A), with mutated GATA-consensus site (probe B), mutated C/EBP-consensus site (probe C), and mutated GATA- and C/EBP-consensus sites (probe D) were end-labeled with [γ-32P] ATP and incubated with 1 μg double-stranded poly (dI-dC) in the presence of 1 μg purified maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 and 4 through 7) and 8 μg nuclear proteins from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 3 through 7).
C/EBPβ transactivates the MBP promoter synergistically with GATA-1.
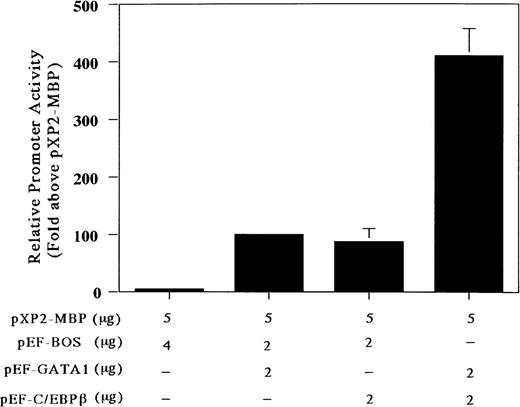
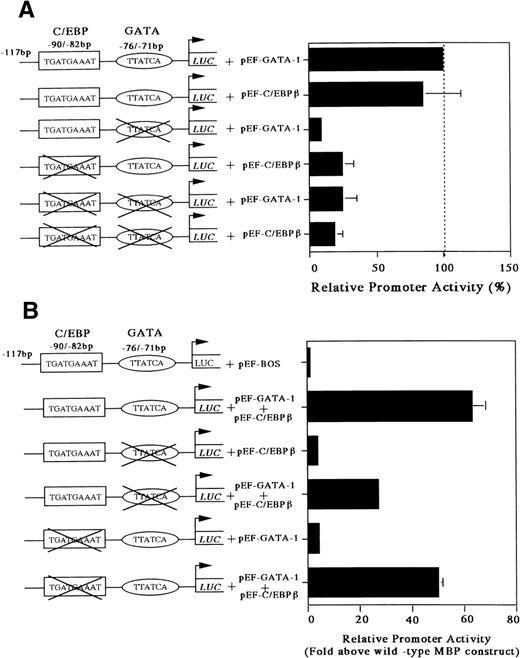
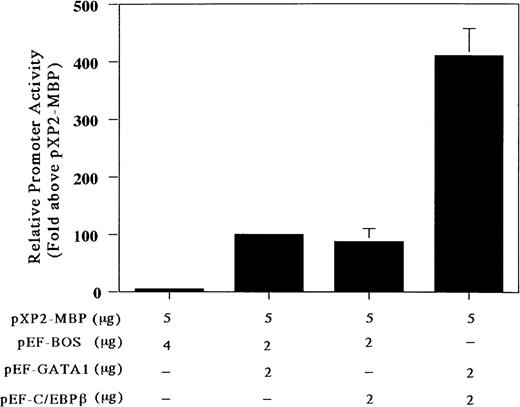
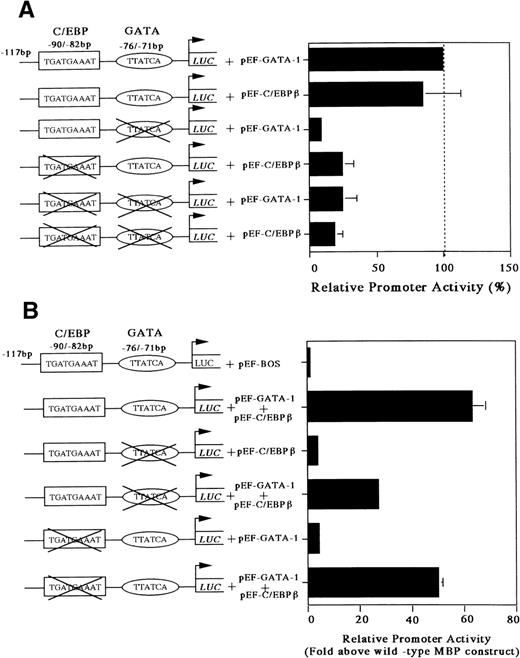
Our gel-shift analysis showed that C/EBPβ can bind to the C/EBP consensus site of the MBP promoter. Accordingly, to determine whether the C/EBP-binding site is functional, we analyzed the ability of C/EBPβ and GATA-1 either individually or in combination to transactivate the MBP promoter in the C/EBPβ and GATA-1 negative Jurkat cell line. Cotransfection with the GATA-1 or C/EBPβ expression vector produced a 5-fold increase in MBP promoter activity (Fig 5). When both C/EBPβ and GATA-1 expression vectors were transfected together, there was a 5-fold increase in MBP promoter activity above that obtained with these vectors individually (Fig 5). To obtain more detailed information on the relative function of the GATA- versus C/EBP-binding sites, we constructed mutant reporter plasmids in which the GATA- and/or the C/EBP-binding sites were disrupted by multiple nucleotide sequence substitutions (Fig 6A and B). As shown in Fig 6A, the constructs with the mutated C/EBP- and/or GATA-binding sites decreased the promoter activity compared with those shown in the wild-type construct. When GATA-1 and C/EBPβ expression vectors were added together with a wild-type construct, there was a 60-fold induction of MBP promoter activity (Fig 6B). When C/EBPβ or GATA-1 expression vector was added individually with constructs containing mutated GATA- or C/EBP-binding sites, a 4-fold induction of MBP promoter activity could be detected, confirming a synergistic effect of GATA-1 and C/EBPβ for MBP promoter activity (Fig 6B). On the other hand, when both factors, GATA-1 and C/EBPβ, were used together with the constructs containing mutated GATA- or C/EBP-binding sites, MBP promoter activity was induced by 30-fold or 50-fold, respectively (Fig6B). When GATA-1 and C/EBPβ expression vectors were added simultaneously together with the construct containing mutations in both the GATA- and C/EBP-binding sites, the promoter activity was the same as that obtained with these vectors individually for the construct of both sites’ mutants (data not shown). This suggests that GATA-1 and C/EBPβ may interact physically. However, the mechanisms by which C/EBPβ synergistically transactivates the MBP promoter in association with GATA-1 remain to be determined.
C/EBPβ and GATA-1 synergistically transactivate the MBP P2 promoter. Transactivation of the MBP promoter (bp −117/pXP2-MBP) in Jurkat cells, in which neither GATA-1 nor C/EBPβ transcription factors are expressed. The T-lymphocytic Jurkat cell line was transfected by the electroporation method with 5 μg of the MBP promoter construct (bp −117/pXP2-MBP) along with the following expression constructs: pXP2-MBP + pEF-BOS, pXP2-MBP (control) and 4 μg of pEF-BOS (the control plasmid containing the elongation factor promoter without cDNAs); pXP2-MBP + pEF-GATA1, 2 μg of pEF-GATA-1, and 2 μg of pEF-BOS; pXP2-MBP + pEF-C/EBPβ; 2 μg of pEF-C/EBPβ and 2 μg of pEF-BOS, pXP2-MBP + pEF-GATA-1 + pEF-C/EBPβ; and 2 μg of pEF-GATA-1 and 2 μg of pEF-C/EBPβ. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (CMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM).
C/EBPβ and GATA-1 synergistically transactivate the MBP P2 promoter. Transactivation of the MBP promoter (bp −117/pXP2-MBP) in Jurkat cells, in which neither GATA-1 nor C/EBPβ transcription factors are expressed. The T-lymphocytic Jurkat cell line was transfected by the electroporation method with 5 μg of the MBP promoter construct (bp −117/pXP2-MBP) along with the following expression constructs: pXP2-MBP + pEF-BOS, pXP2-MBP (control) and 4 μg of pEF-BOS (the control plasmid containing the elongation factor promoter without cDNAs); pXP2-MBP + pEF-GATA1, 2 μg of pEF-GATA-1, and 2 μg of pEF-BOS; pXP2-MBP + pEF-C/EBPβ; 2 μg of pEF-C/EBPβ and 2 μg of pEF-BOS, pXP2-MBP + pEF-GATA-1 + pEF-C/EBPβ; and 2 μg of pEF-GATA-1 and 2 μg of pEF-C/EBPβ. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (CMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM).
Mutations of the GATA- or C/EBP-binding sites diminish MBP promoter activity (A) and mutations of the GATA- or C/EBP-sites does not prevent synergy (B). Jurkat cells were cotransfected with pXP2-MBP containing wild-type GATA-1 and C/EBP-sites, pXP2-MBP containing a mutated GATA-binding site, and/or pXP2-MBP containing a mutated C/EBP-binding site, along with 2 μg of pEF-GATA-1 and/or pEF-C/EBPβ expression vectors. The error bar represents the SEM for 3 independent experiments. Luciferase activity was normalized to β-galactosidase activity from a cotransfected CMV-βGal plasmid.
Mutations of the GATA- or C/EBP-binding sites diminish MBP promoter activity (A) and mutations of the GATA- or C/EBP-sites does not prevent synergy (B). Jurkat cells were cotransfected with pXP2-MBP containing wild-type GATA-1 and C/EBP-sites, pXP2-MBP containing a mutated GATA-binding site, and/or pXP2-MBP containing a mutated C/EBP-binding site, along with 2 μg of pEF-GATA-1 and/or pEF-C/EBPβ expression vectors. The error bar represents the SEM for 3 independent experiments. Luciferase activity was normalized to β-galactosidase activity from a cotransfected CMV-βGal plasmid.
Physical interaction between GATA-1 and C/EBPβ.
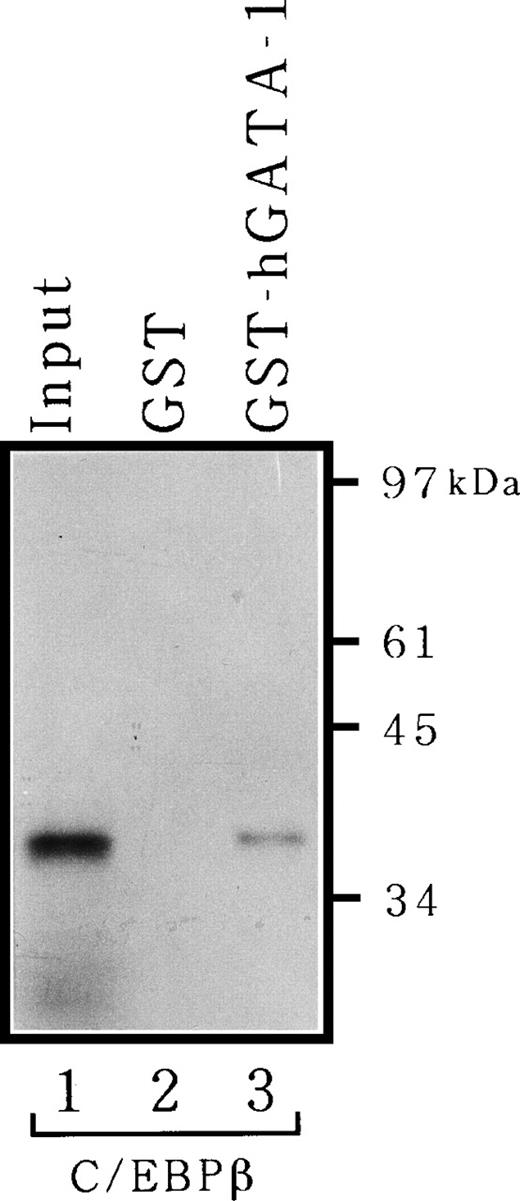
Because the data in Fig 6B suggested that GATA-1 may be able to bind C/EBPβ through protein-protein interactions, we proceeded to test this possibility directly in vitro. 35S-labeled C/EBPβ produced by translation in vitro was allowed to bind to hGATA-1 fused to GST or GST alone and was immobilized onto a glutathione-Sepharose matrix, after which the bound proteins were analyzed by SDS-PAGE. GATA-1 associated clearly with C/EBPβ in vitro, and as much as 15% of the input protein was recovered as complexes with GATA-1 (Fig 7). However, C/EBPβ did not adhere to GST resin devoid of GATA-1. Thus, there is a physical interaction between GATA-1 and C/EBPβ.
Physical interaction between C/EBPβ and GATA-1 in vitro. 35S-labeled full-length C/EBPβ was synthesized by translation in vitro and incubated with GST alone (lane 2) or GST-hGATA-1 (lane 3) adsorbed to glutathione-Sepharose, after which the matrix was washed and bound proteins were analyzed as described in Materials and Methods. Lane 1 represents 15% of the amount of labeled protein incubated with matrices.
Physical interaction between C/EBPβ and GATA-1 in vitro. 35S-labeled full-length C/EBPβ was synthesized by translation in vitro and incubated with GST alone (lane 2) or GST-hGATA-1 (lane 3) adsorbed to glutathione-Sepharose, after which the matrix was washed and bound proteins were analyzed as described in Materials and Methods. Lane 1 represents 15% of the amount of labeled protein incubated with matrices.
FOG decreases the ability of GATA-1 to transactivate the MBP promoter.
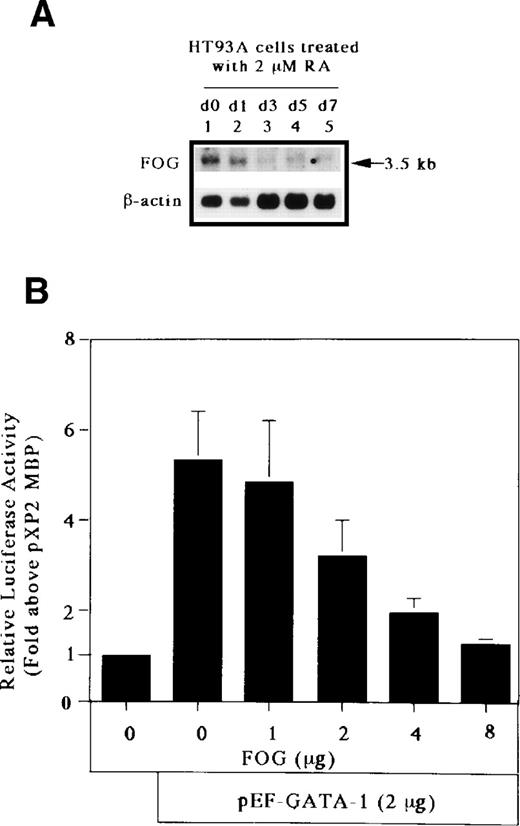
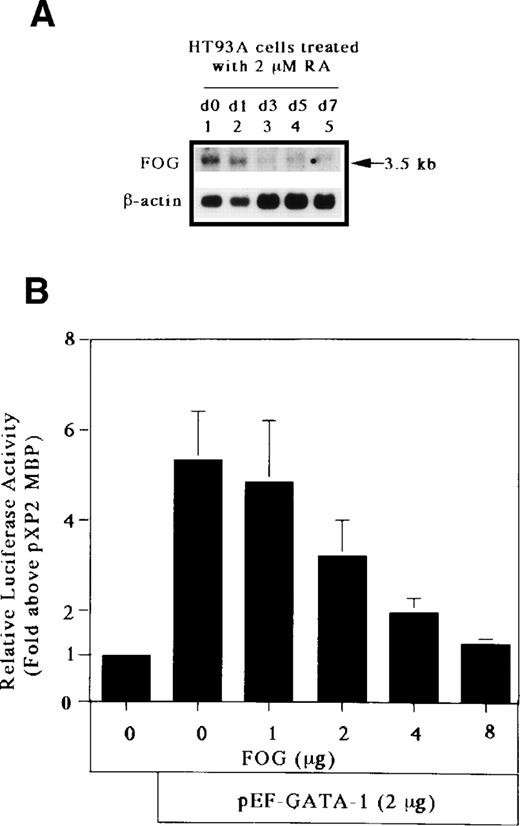
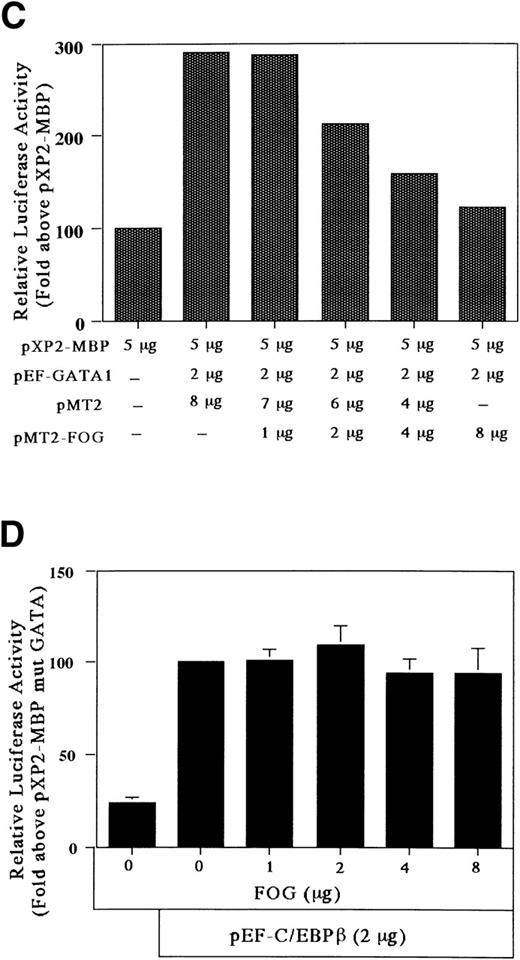
The GATA-1 protein has 2 zinc fingers, a carboxyl-terminal zinc finger (C-f) and an amino-terminal zinc finger (N-f). The C-f of GATA-1 provides the essential DNA binding domain necessary for GATA-1 binding to GATA motifs, whereas the N-f of GATA-1 is not necessary for DNA binding. Recently, Tsang et al57 identified a novel, multitype zinc finger protein, FOG, that binds to the N-f of GATA-1 and acts as a cofactor for GATA-binding proteins. To determine the effect of FOG on GATA-1 transactivation of the MBP promoter, we analyzed the expression of FOG mRNA in HT93A cells capable of differentiating towards eosinophil and neutrophil lineages. As shown in Fig 8A, the transcript for FOG was rapidly downregulated during the RA-induced eosinophilic and neutrophilic differentiation of the HT93A cell line. It has been shown that FOG and GATA-1 synergistically activate transcription of an erythroid- and megakaryocyte-associated p45NF-E2 gene promoter.57 We addressed whether FOG can synergize with GATA-1 to activate transcription of the eosinophil-specific MBP P2 promoter. A fixed amount of GATA-1 and increasing amounts of FOG were transiently coexpressed in Jurkat cells together with pXP2-MBP. Surprisingly, expression of FOG decreased the transactivation activity of GATA-1 for the MBP P2 promoter in a dose-dependent manner (Fig 8B). To examine the effect of the pMT2 vector used to express FOG on GATA-1 transactivation of the MBP promoter, a constant amount (8 μg) of pMT2 plus pMT2-FOG was used in the transactivation study. As shown in Fig 8C, a pMT2 vector did not affect the negative regulatory role of FOG for GATA-1 transactivation of the MBP promoter. In addition, to analyze the specificity of the inhibitory effect of FOG on GATA-1 transactivation of the MBP promoter, a fixed amount of FOG-unrelated expression vector, pEF-C/EBPβ, and increasing amounts of FOG expression vectors were coexpressed in Jurkat cells together with the construct containing mutated GATA-binding site. As shown in Fig 8D, expression of FOG did not affect the transactivation activity of C/EBPβ for the MBP P2 promoter, indicating the specificity of the inhibitory effect of FOG for GATA-1–dependent transactivation of the MBP promoter. These results suggest an inhibitory role of FOG for GATA-1–regulated genes in the eosinophil lineage, unlike that of FOG activity in the erythroid and megakaryocyte lineages.
FOG acts as a negative (inhibitory) cofactor for GATA-1 transactivation of the MBP promoter. (A) Northern blot analysis of mRNA for FOG in uninduced HT93A cells (lane 1) and HT93A cells induced with RA for 1, 3, 5, and 7 days (lanes 2 through 5, respectively). Control hybridization with β-actin cDNA is shown in the lower panel. (B) FOG inhibits transactivation of the MBP promoter by GATA-1. Jurkat cells were transiently transfected with 5 μg of pXP2-MBP, 2 μg of pEF-GATA-1, increasing amounts of pMT2-FOG as shown, and 1 μg of β-galactosidase expression vector (pCMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM). (C) Jurkat cells were transfected by the electroporation method with 2 μg of the pXP2-MBP containing wild-type GATA- and C/EBP-binding sites along with the following expression vectors: 4 μg of pEF-BOS, 2 μg of pEF-GATA-1 plus 8 μg of pMT2, 2 μg of pEF-GATA-1 plus 7 μg of pMT2 plus 1 μg of pMT2-FOG, 2 μg of pEF-GATA-1 plus 6 μg of pMT2 plus 2 μg of pMT2-FOG, 2 μg of pEF-GATA-1 plus 4 μg of pMT2 plus 4 μg of pMT2-FOG, and 2 μg of pEF-GATA-1 plus 8 μg of pMT2-FOG. pBluescriptIIKS(−) plasmids were added to maintain total DNA constant at 22 μg. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of cotransfected β-galactosidase expression vector (pCMV-βGal). (D) Jurkat cells were transfected with 5 μg of the pXP2-MBP containing a mutated GATA-binding site (pXP2-MBP mutGATA) along with the following expression vectors: 2 μg of pEF-BOS, 2 μg of pEF-C/EBPβ, 2 μg of pEF-C/EBPβ plus 7 μg of pMT2 plus 1 μg of pMT2-FOG, 2 μg of pEF-C/EBPβ plus 6 μg of pMT2 plus 2 μg of pMT2-FOG, 2 μg of pEF-C/EBPβ plus 4 μg of pMT2 plus 4 μg of pMT2-FOG, and 2 μg of pEF-C/EBPβ plus 8 μg of pMT2-FOG. pBluescriptIIKS(−) plasmids were added to maintain total DNA constant at 22 μg. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (pCMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM).
FOG acts as a negative (inhibitory) cofactor for GATA-1 transactivation of the MBP promoter. (A) Northern blot analysis of mRNA for FOG in uninduced HT93A cells (lane 1) and HT93A cells induced with RA for 1, 3, 5, and 7 days (lanes 2 through 5, respectively). Control hybridization with β-actin cDNA is shown in the lower panel. (B) FOG inhibits transactivation of the MBP promoter by GATA-1. Jurkat cells were transiently transfected with 5 μg of pXP2-MBP, 2 μg of pEF-GATA-1, increasing amounts of pMT2-FOG as shown, and 1 μg of β-galactosidase expression vector (pCMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM). (C) Jurkat cells were transfected by the electroporation method with 2 μg of the pXP2-MBP containing wild-type GATA- and C/EBP-binding sites along with the following expression vectors: 4 μg of pEF-BOS, 2 μg of pEF-GATA-1 plus 8 μg of pMT2, 2 μg of pEF-GATA-1 plus 7 μg of pMT2 plus 1 μg of pMT2-FOG, 2 μg of pEF-GATA-1 plus 6 μg of pMT2 plus 2 μg of pMT2-FOG, 2 μg of pEF-GATA-1 plus 4 μg of pMT2 plus 4 μg of pMT2-FOG, and 2 μg of pEF-GATA-1 plus 8 μg of pMT2-FOG. pBluescriptIIKS(−) plasmids were added to maintain total DNA constant at 22 μg. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of cotransfected β-galactosidase expression vector (pCMV-βGal). (D) Jurkat cells were transfected with 5 μg of the pXP2-MBP containing a mutated GATA-binding site (pXP2-MBP mutGATA) along with the following expression vectors: 2 μg of pEF-BOS, 2 μg of pEF-C/EBPβ, 2 μg of pEF-C/EBPβ plus 7 μg of pMT2 plus 1 μg of pMT2-FOG, 2 μg of pEF-C/EBPβ plus 6 μg of pMT2 plus 2 μg of pMT2-FOG, 2 μg of pEF-C/EBPβ plus 4 μg of pMT2 plus 4 μg of pMT2-FOG, and 2 μg of pEF-C/EBPβ plus 8 μg of pMT2-FOG. pBluescriptIIKS(−) plasmids were added to maintain total DNA constant at 22 μg. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (pCMV-βGal). Data are shown as the mean of 3 independent experiments (±SEM).
DISCUSSION
We previously reported that GATA-1 can independently transactivate the MBP P2 promoter, but that GATA-2 has the capacity to compete for GATA-1 binding and to inhibit GATA-1 transactivation.9Furthermore, we have also shown that C/EBP family members, in particular C/EBPα, C/EBPβ, and C/EBPδ, are able to transactivate the MBP promoter individually.9 In the present study, we have shown that C/EBPβ can bind to the C/EBP consensus site (bp −90 to −82) of the MBP P2 promoter and can transactivate MBP promoter activity synergistically with GATA-1. In the hematopoietic system, C/EBPα, -β, and -ε play an important role in myeloid differentiation. Scott et al28 have reported that the expression of C/EBPα predominates in immature myelomonocytic cells and that C/EBPβ expression predominates in terminally differentiated cells, consistent with our Northern blot analysis of RA-induced HT93A cells. C/EBPα is crucial to the activity of the G-CSFR promoter,31 and C/EBPα knock out (KO) mice have impaired G-CSFR expression and signaling through this lineage-specific cytokine receptor.32 Expression of C/EBPβ was found to parallel the differentiation of myeloid cells to macrophages, and C/EBPβ KO mice display impaired macrophage function.58 Recently, Chih et al37 have shown that C/EBPε shows a restricted pattern of expression in cells of the myeloid lineage during their differentiation toward granulocytes. In particular, they suggest that C/EBPε plays an important role in the differentiation of granulocytic progenitors to myeloblasts/promyelocytes.37 In this study, we have not analyzed the effect of C/EBPε on eosinophilic differentiation, but C/EBPε null mice have been shown to have impaired eosinophil development.59 Further studies on the role of C/EBPε in the eosinophilic differentiation are required. Although these studies indicate that C/EBP family members are closely associated with the differentiation of myeloid cells, the C/EBP transcription factors that play key roles in the differentiation of eosinophil lineage are not known. We found that C/EBPβ expression increased along with the eosinophilic and neutrophilic differentiation of HT93A cell line and that C/EBPα mRNA was downregulated in these cells (Fig 2). This result suggested that C/EBPβ played an important role in the eosinophilic and neutrophilic differentiation. Additionally, granulocyte-committed cells express little C/EBPα compared with C/EBPβ, whereas in more primitive myeloid progenitors, only C/EBPα is present.28 These findings suggest that C/EBPβ plays a more important role in the terminal differentiation of the eosinophil lineage than C/EBPα.
Cell-type specific gene expression often involves interaction with different transcription factors. In particular, C/EBP family members can form homodimers or heterodimers with each other and also interact with other transcription factors such as the myb oncogene.39 Attempts to demonstrate direct interactions between two transcription factors by gel-shift analysis, eg, C/EBPβ and the glucocorticoid receptor for the rat α1-acid glycoprotein gene60 and C/EBPα and PU.1 for the murine neutrophil elastase gene,61 have been performed without success. Williams et al62 have shown that C/EBPβ contains a negative regulatory region composed of 2 elements, RD1 and RD2. They suggested that the inactive form of C/EBPβ adopts a tightly folded conformation that masks the activation and DNA-binding domains located at the N-and C-termini, respectively, and that activation of C/EBPβ requires some event, which causes the protein to unfold and unmask the activation and DNA-binding domains.
Transcription factors activate promoter activity by interacting with the basal transcriptional machinery. The CREB-binding protein (CBP) has been suggested to bridge c-Myb and NF-M, the chicken homolog of C/EBPβ.63 Recently, it has been reported that CBP acts as a coactivator of GATA-1.64 These observations suggest that CBP functions as a bridging protein between C/EBPβ, GATA-1, and the basal transcriptional complex in the MBP promoter. It has been reported that FOG acts as a cofactor for GATA-binding proteins and that FOG and GATA-1 synergize in activating transcription of the erythroid/megakaryocytic-expressed p45 NF-E2 gene.57However, in our study, FOG acted as a negative cofactor for transcription of the eosinophil-specific MBP P2 promoter. FOG is expressed in both erythroblasts and megakaryocytes, but not in mast cells.57 In our experiment, the expression of FOG mRNA was downregulated during the RA-induced eosinophilic and neutrophilic differentiation of the HT93A cells. These findings suggest that FOG acts as a negative regulator for the eosinophil, neutrophil, or mast cell lineages, in contrast to its positive regulatory function for the erythroid and megakaryocytic lineages. One of the mechanisms by which FOG decreases the transactivation activity of GATA-1 for the MBP P2 promoter may be to block the interaction between GATA-1 and the basal transcriptional complex in the MBP gene.
To date, it has been reported that C/EBPβ has the capacity to act synergistically to transactivate promoters with a number of other factors, including NF-κB,65 c-Jun,66glucocorticoid receptor,60 and v-Myb.67 In the present study, physical interaction between GATA-1 and C/EBPβ was demonstrated by a GST pull-down assay. It has been reported that C/EBPβ can bind to the c-fos serum response element binding protein (SRE BP),68 NF-κB,69 glucocorticoid receptor,60 and AML1.30 However, to date, there are no reports of a physical interaction between GATA-1 and C/EBPβ. Our report is the first to describe both a physical interaction and cooperativity between GATA-1 and C/EBPβ. However, these data are not definitive. We have to perform the coimmunoprecipitation experiment using extracts from eosinophilic cell lines for demonstrating that endogenous GATA-1 and C/EBPβ interact in vivo. The extent of the enhancement of C/EBPβ-GATA-1–mediated transcription for the eosinophil-specific MBP P2 promoter by cotransfected CBP was less than that of GATA-1–dependent transcription for the erythroid-specific EKLF promoter by cotransfected CBP (data not shown). The role of FOG and CBP in the eosinophil lineage may be different from that in the erythroid/megakaryocytic lineages, with the difference in commitment towards eosinophilic versus erythroid/megakaryocytic differentiation due to the differential functions of these cofactors.
It has been shown that GATA-1 and PU.1, an ets transcription factor, stimulate activity of the IL-4 intronic enhancer70 and that both GATA-1 and the ets family transcription factors increase promoter activity of the c-mpl (the thrombopoietin receptor) gene.71In addition, it was reported that GATA-1 could convert myeloid cells in part to eosinophils in Myb-Ets–transformed chicken myeloblasts.72 Recently, McNagny et al73 have reported that low levels of GATA-1 stimulated the eosinophil-specific EOS47 promoter moderately in the presence of Ets-1, c-Myb, and C/EBPα in the chicken hematopoietic system. Also, Nerlov et al74have shown that chicken C/EBPα and C/EBPβ can induce both myeloid and eosinophil lineage commitment. However, as far as we know, it has not been previously reported that the GATA-1 and C/EBP transcription factors have the capacity to act coordinately or interactively to stimulate promoter activity in a synergistic fashion. Our results provide the first evidence that GATA-1 and C/EBPβ have the capacity to act synergistically to affect the transcriptional activity of an eosinophil-specific granule protein gene.
ACKNOWLEDGMENT
The authors thank Dr Michio Nakamura, Dr Ronald G. Crystal, Dr Masayuki Yamamoto, Dr Kleanthis G. Xanthopoulos, Dr Akira Shizuo, Dr Francoise Moreau-Gachelin, and Stuart H. Orkin for providing the gp91phox; neutrophil elastase; GATA-1, GATA-2, and GATA-3; C/EBPα; C/EBPβ; PU.1 (Spi-1) cDNAs; and a FOG expression vector, respectively. We are also grateful to Dr Masaki Takiguchi for providing the MBP-C/EBPβ fusion protein.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan (to Y.Y.) and the National Institutes of Health, NIAID, Grant No. AI33043 (to S.J.A.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yuji Yamaguchi, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto, 860 Japan; e-mail: yujiya@gpo.kumamoto-u.ac.jp.


![Fig. 3. Binding of recombinant C/EBPβ fusion protein and GATA-1 protein to the MBP promoter. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 1 μg maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 through 6 and 8 through 12) and 8 μg nuclear protein from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 7 through 12). Unlabeled double-stranded competitor oligonucleotides, shown schematically in the lower panel, were added at a 100-fold molar excess over the labeled probe oligonucleotide, MBP bp −93 to −58 (competitor A; lanes 3 and 9), mutated GATA-consensus site oligonucleotide (competitor B; lanes 4 and 10), mutated C/EBP-consensus site oligonucleotide (competitor C; lanes 5 and 11), and mutated GATA-1 and C/EBP-consensus site oligonucleotide (competitor D; lanes 6 and 12).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1429/4/m_blod41613003w.jpeg?Expires=1767814621&Signature=wdxsFMKhT4V~J7JEzjvrLVHdQ4tJpMgcwDrrjkonH-fY9FukqMmxQacWF4pLrcrrWtvLA6aKfsTFKMbDFswms1XLwyhs~Llp~AMsmbZ11heC7~V5Z2Ch78kSH~4R7k5rdCVL-KUt~hSiOgG4Lnq20wp6XxVbebfkarCyXq3nCuZCzVmV-XMGlnSoyLMFZFlkYiIVKdGm~Ic5M579pqQq4FBmocNqSBGCMIKTWmHbysMU~u7AEaZFW9Mxv0iw90CAKV-atdV0p4l6ZSsRACc~PIepXLql89w3oi7tR4BBN3LbdimknlZkKYJKRRGoVx1IttbEHZzHZYMr2~yZFLI8MA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Demonstration of C/EBPβ and GATA-1 binding to the MBP promoter by gel-shift assay. Double-stranded oligonucleotides (lower panel) containing MBP promoter sequence from bp −93 to −58 (probe A), with mutated GATA-consensus site (probe B), mutated C/EBP-consensus site (probe C), and mutated GATA- and C/EBP-consensus sites (probe D) were end-labeled with [γ-32P] ATP and incubated with 1 μg double-stranded poly (dI-dC) in the presence of 1 μg purified maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 and 4 through 7) and 8 μg nuclear proteins from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 3 through 7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1429/4/m_blod41613004w.jpeg?Expires=1767814621&Signature=LS4bg5yqNloUO2QAUtluaXKEjzDGyq66WnVgAh8WGvBNSlkRnheLTOBn8xflEMBoJtwZS-wT6z7ZCHixrMMflLwPKrUEiW9-0X-ZMUv1IAC0DzkpT7Z3vXYB4P0PHA3N7k1Gd-oeHCg6L0qe62S~pYOdi9Hgb334WZubMwRdHVweLcJvgW0YVgDaFu-9SytTcUiOZI3Y7WYPAw7HDjfHs6lrYRIuntNXW8NBQUnFIBdDUQIP0fEdTASPvrILahcx2V3fe7Lo3NzzVDOTthVYpaX3rUGgY1ROVkbjvPG3oe~MIQa3hCyqdVHc-TPVssCZKCYh9f0Ms04DXG6rhcZAPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 3. Binding of recombinant C/EBPβ fusion protein and GATA-1 protein to the MBP promoter. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 1 μg maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 through 6 and 8 through 12) and 8 μg nuclear protein from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 7 through 12). Unlabeled double-stranded competitor oligonucleotides, shown schematically in the lower panel, were added at a 100-fold molar excess over the labeled probe oligonucleotide, MBP bp −93 to −58 (competitor A; lanes 3 and 9), mutated GATA-consensus site oligonucleotide (competitor B; lanes 4 and 10), mutated C/EBP-consensus site oligonucleotide (competitor C; lanes 5 and 11), and mutated GATA-1 and C/EBP-consensus site oligonucleotide (competitor D; lanes 6 and 12).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1429/4/m_blod41613003w.jpeg?Expires=1768040865&Signature=GzsAnl1gaGxmOEoPMqW7DxubY6EzqhB28i4d4p3egee~ShiKvLL2IRPvneNO2EFLNB-OLlryA2siAEQtKlblMIAVdB-TYX78J0o3K3h3QvYChxSSfsQlNZkWUS2EmN8CRUackBDwuEPNKsKVFl3uWx5td2kE1xBuwt78KPnJIMY1F9cBfKc6VYU~Fuqx9LpaviMgwjrJTlGi7q2yeVvFUrYNDOPEre3B1Qnqkqmorf9WqMVjekOl-CVqtLSZoo-DbmRqNQEOBb3emyRK8cssZ44XU-mGyZoJE91AgGygBe~IfwH~M2NqymwNzloYpKdlRKoEiB2-cpoHhWcqOc3AHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Demonstration of C/EBPβ and GATA-1 binding to the MBP promoter by gel-shift assay. Double-stranded oligonucleotides (lower panel) containing MBP promoter sequence from bp −93 to −58 (probe A), with mutated GATA-consensus site (probe B), mutated C/EBP-consensus site (probe C), and mutated GATA- and C/EBP-consensus sites (probe D) were end-labeled with [γ-32P] ATP and incubated with 1 μg double-stranded poly (dI-dC) in the presence of 1 μg purified maltose-binding protein (MBP)-C/EBPβ fusion protein (lanes 2 and 4 through 7) and 8 μg nuclear proteins from COS7 cells that were transiently transfected with a GATA-1 expression vector (lanes 3 through 7).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1429/4/m_blod41613004w.jpeg?Expires=1768040865&Signature=nZDosGGz4qMpCOko7iNFcA-idSQCq1qG7bewfvf-ADhRnK6mBH3D3DMu1yXqBqENfGqFlXJgadTj1ewYw~22KbloRKbZNgT0T506gG2V96U3ReBlQxNIKw79TSLG3wE1WwOS0Ip3UOm8wDBAkTp05Nm~b4oyC1Ve7to7tu-F2PWfkd7n47BvY36UmvTmHhqjMzhxZJR9shUA2G4K30CyyPnaCzPTS-tIbxVNY~BTS2lnw275PlRSC1IAUD41fREPtKslh0-hlV8ud8Km-x~BaJx~clJqqHRN48zWWWzFL9zORDuLrM6uZQrveQf6aoKEAIZUvB-xiHyGV~OVV2MPHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)