To the Editor:
Deletion of a single G nucleotide (ΔG) in exon 3 of the thrombopoietin (TPO) gene was found to cosegregate with thrombocytosis and elevated TPO serum levels in a Japanese kindred with thrombocythemia.1 The ΔG mutation affects the 5′-untranslated region (5′-UTR) of the TPO mRNA. Cells transfected with a cDNA carrying this mutation produced more TPO protein than controls transfected with the normal TPO cDNA, strongly suggesting that the ΔG mutation is responsible for thrombocythemia in this family.1 However, the molecular mechanism of how this mutation causes TPO overproduction was not elucidated, and in particular, no evidence for increased RNA stability or more efficient translation of the mutant TPO mRNA was found.1
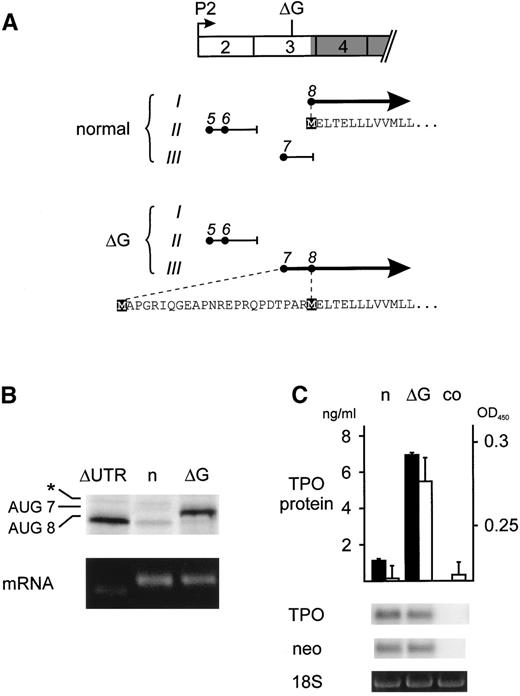
We have previously shown that the 5′-UTR of TPO mRNA exerts a strong inhibitory effect on translation.2 This inhibition is mediated by the presence of several upstream open reading frames (uORFs) that prevent efficient translation of TPO.2 Loss of this inhibition due to a splice donor mutation in TPO intron 3 was shown to be the cause of hereditary thrombocythemia (HT) in a Dutch family.3 We noticed that the ΔG mutation described by Kondo et al1 results in a frameshift in the TPO 5′-UTR changing the composition of the inhibitory uORFs (Fig1A). Only transcripts originating from the TPO promoter 2 (P2), which account for greater than 90% of total TPO mRNAs, are shown. The frameshift specifically affects uORF7, which consequently is placed into the same reading frame as the TPO protein, extending the N-terminus of the TPO signal peptide by 23 amino acids (Fig 1A). Because uORF7 was previously shown to have a very strong inhibitory effect on translation,2 we reasoned that this frameshift should relieve the physiologic block on translation and thereby might cause TPO overproduction. We present here experimental evidence in support of this model.
Analysis of the translational efficiency of normal and mutant TPO transcripts in reticulocyte lysates. (A) Exon composition and ORFs of the normal and mutant TPO mRNAs originating from promoter 2 (P2). Exons are drawn as numbered boxes and the TPO protein coding region is shaded. The position of the single G nucleotide deletion is indicated (▵G). The patterns of uORFs are drawn separately for normal and mutant TPO mRNA. The uAUG codons (•) are placed in the 3 possible reading frames (Roman numbers) and numbered in the order in which they appear in the full-length P1 transcript. The resulting uORFs are shown as horizontal lines and the position of stop codons is indicated by short vertical lines. Thick solid lines with arrowheads represent the normal and ▵G-mutant TPO-ORFs, with the corresponding amino acid sequences indicated below. Initiator methionines are highlighted by black boxes. (B) In vitro transcription-translation analysis of mRNAs originating from P2. Equal amounts of in vitro-transcribed TPO mRNA variants (lower panel) were translated in vitro in reticulocyte lysate in the presence of 35S-methionine (upper panel). ▵UTR, mRNA with deletion of the entire 5′-UTR; n, normal mRNA; ▵G, ▵G-mutant mRNA. The protein bands in the upper panel were AUG8, the normal TPO protein initiated at the physiological start site; AUG7, mutant TPO protein originating from AUG7; asterisk, cryptic non-AUG initiation in exon 3. (C) TPO secretion by COS cells transfected with either the normal (n) or ▵G-mutant (▵G) TPO cDNA. TPO protein concentration in COS cell supernatants was determined by ELISA (▪) and by bioassay (□). Error bars represent the standard deviations. co, supernatant from nontransfected COS cells. The abundance of transfected TPO mRNA was assessed by Northern blot. To confirm equal transfection efficiencies, mRNA for vector-derived neomycin resistance gene (neo) was determined by reprobing of the Northern blot and equal RNA loading was verified by visualizing the 18S ribosomal RNA with ethidium bromide staining (lower panels).
Analysis of the translational efficiency of normal and mutant TPO transcripts in reticulocyte lysates. (A) Exon composition and ORFs of the normal and mutant TPO mRNAs originating from promoter 2 (P2). Exons are drawn as numbered boxes and the TPO protein coding region is shaded. The position of the single G nucleotide deletion is indicated (▵G). The patterns of uORFs are drawn separately for normal and mutant TPO mRNA. The uAUG codons (•) are placed in the 3 possible reading frames (Roman numbers) and numbered in the order in which they appear in the full-length P1 transcript. The resulting uORFs are shown as horizontal lines and the position of stop codons is indicated by short vertical lines. Thick solid lines with arrowheads represent the normal and ▵G-mutant TPO-ORFs, with the corresponding amino acid sequences indicated below. Initiator methionines are highlighted by black boxes. (B) In vitro transcription-translation analysis of mRNAs originating from P2. Equal amounts of in vitro-transcribed TPO mRNA variants (lower panel) were translated in vitro in reticulocyte lysate in the presence of 35S-methionine (upper panel). ▵UTR, mRNA with deletion of the entire 5′-UTR; n, normal mRNA; ▵G, ▵G-mutant mRNA. The protein bands in the upper panel were AUG8, the normal TPO protein initiated at the physiological start site; AUG7, mutant TPO protein originating from AUG7; asterisk, cryptic non-AUG initiation in exon 3. (C) TPO secretion by COS cells transfected with either the normal (n) or ▵G-mutant (▵G) TPO cDNA. TPO protein concentration in COS cell supernatants was determined by ELISA (▪) and by bioassay (□). Error bars represent the standard deviations. co, supernatant from nontransfected COS cells. The abundance of transfected TPO mRNA was assessed by Northern blot. To confirm equal transfection efficiencies, mRNA for vector-derived neomycin resistance gene (neo) was determined by reprobing of the Northern blot and equal RNA loading was verified by visualizing the 18S ribosomal RNA with ethidium bromide staining (lower panels).
To compare the translational efficiencies of normal and mutant TPO mRNAs, the ΔG mutation was introduced into the 5′-UTR of TPO cDNA by recombinant polymerase chain reaction (PCR) and subcloned into the pcDNA3 vector, as previously described.2 Mutant and control TPO mRNAs were transcribed in vitro using T7 polymerase and translated in reticulocyte lysate in the presence of 35S-methionine (Fig 1B). Translation of normal TPO mRNA was strongly repressed, as previously described.2 In contrast, the ΔG-mutant mRNA was translated with high efficiency, producing amounts of TPO protein comparable to an artificial construct with a deletion of all but the last 7 nucleotides of the 5′-UTR (ΔUTR). Consistent with presence of an extended N-terminus resulting from translation initiation at AUG7, the translation product of the ΔG-mutant migrated higher than the normal TPO protein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Previous mutational analysis demonstrated that AUG7 is used very efficiently, whereas AUG5 and AUG6 are weak inhibitors of translation.2 Thus, the ΔG-mutation improves the efficiency of TPO translation in reticulocyte lysates by eliminating the inhibitory effect normally exerted by uORF7. The ΔG mutation also improved translation of TPO transcripts originating from promoter 1 (P1) and of a rare P1-variant that lacks exon 2 (not shown).
To demonstrate that the addition of 23 amino acids to the TPO signal peptide resulting from the ΔG frameshift in uORF7 does not interfere with secretion of a biologically active TPO protein, we transfected COS cells with TPO cDNA expression constructs and measured TPO protein concentrations in tissue culture supernatants (Fig 1C). Cells transfected with a construct carrying the ΔG mutation secreted 7-fold more TPO protein, as determined by enzyme-linked immunosorbent assay (ELISA), than cells transfected with the corresponding normal construct (solid bars). A similar increase was detected using a TPO bioassay (open bars), demonstrating that the extended signal peptide can ensure secretion of biologically active TPO.
Our results show that derepression of translation is responsible for overproduction of TPO protein in individuals carrying the ΔG mutation in this Japanese HT family. A different TPO gene mutation, which also results in derepression of TPO mRNA translation, was previously described as the cause of HT in a Dutch family.3 These data suggest that increased efficiency of TPO mRNA translation might be a common mechanism in the pathogenesis of HT and illustrate the importance of translational repression for normal platelet homeostasis.