To the Editor:
In a recent report in Blood, Seyama et al1described 28 unique mutations of the CD40 ligand(CD40L) gene in 45 X-linked hyper IgM syndrome (XHIM) patients from 30 unrelated families. Generally, peripheral blood mononuclear cells (PBMC) are screened for CD40L expression after activation with phorbol myristate acetate (PMA) and ionomycin to diagnose XHIM. Activated PBMC of most XHIM patients did not express functional CD40L (CD154): they failed to bind a CD40-Ig fusion protein (bCD40-Ig). The investigators discerned 5 different CD40L staining patterns on cultured T cells, using a polyclonal antiserum (pAb), 4 different monoclonal antibodies (McAb), and bCD40-Ig. The type 1 pattern showed weak staining of CD40L with all reagents mentioned; the type 2 through 5 patterns successively showed loss of functional activity (bCD40-Ig binding) and protein epitope expression (loss of 1 to 4 McAb and finally pAb binding). A relationship between genotype and phenotype was suggested, with “milder genotypes” (resulting in staining pattern type 1 or 2) showing milder clinical phenotypes: in 5 of 10 patients from 9 families with staining pattern type 1 or 2, symptoms started relatively late, and none of them suffered from opportunistic infections. Two patients with the 782C → T mutation, a missense mutation in exon 5, fit into this group.
We found the 782C → T XHIM mutation in 4 patients from 2 different families. Although they showed a favorable clinical course after Ig replacement therapy was started, the 2 index patients had symptoms from an early age onwards, including 1 who presented with Pneumocystis carinii pneumonia (PCP). The other 2 children were diagnosed shortly after birth because of the XHIM index patient in the family.
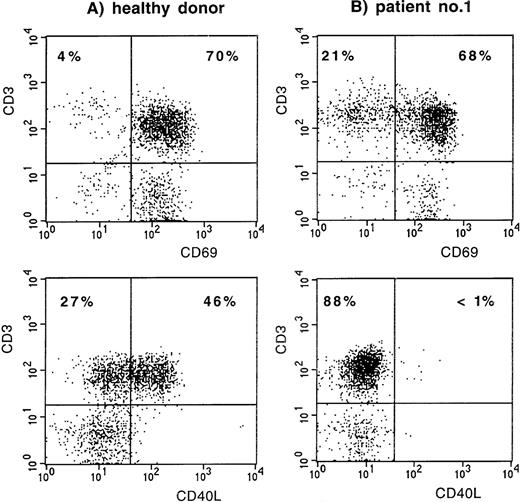
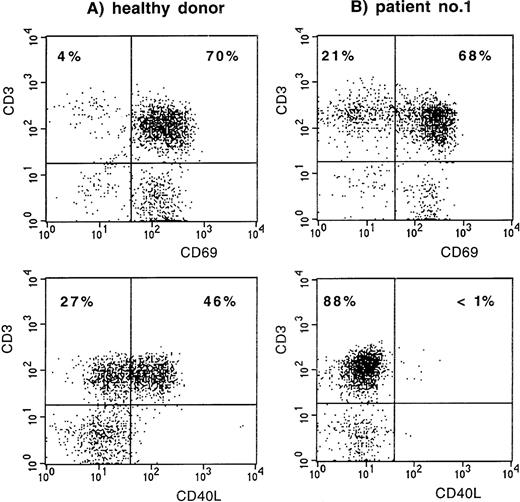
The first index patient, patient no. 1 (of family A), is a young Dutch man, now 19 years of age, who was treated with Ig replacement therapy from the age of 2 years onward because of hypogammaglobulinemia and frequent respiratory tract infections. Since then, the frequency of infections has normalized. He never had an infection with an opportunistic pathogen and never received prophylaxis with Co-trimoxazole. Recently, stimulation of his PBMC with PMA and ionomycin induced expression of the early activation marker CD69 (Leu-23; Becton Dickinson, San Jose, CA) on his CD3+ T lymphocytes, whereas CD40L (LL48; Schering-Plough, Dardilly, France) expression was absent (Fig 1). Fluorescent sequencing of the CD40L gene showed a 782C → T mutation (expected protein alteration T254M). The family was screened, and in patient no. 2, his cousin now 2 years of age, XHIM was identified shortly after birth (Fig 2). At 9 months of age, the serum IgG level had decreased to less than 0.5 g/L. Although there were no clinical symptoms, Ig replacement therapy was started because of the increased risk of serious infections.
CD69 and CD40L expression on CD3+ T lymphocytes after stimulation of PBMC with PMA and ionomycin. (A) Normal control. (B) Patient no. 1.
CD69 and CD40L expression on CD3+ T lymphocytes after stimulation of PBMC with PMA and ionomycin. (A) Normal control. (B) Patient no. 1.
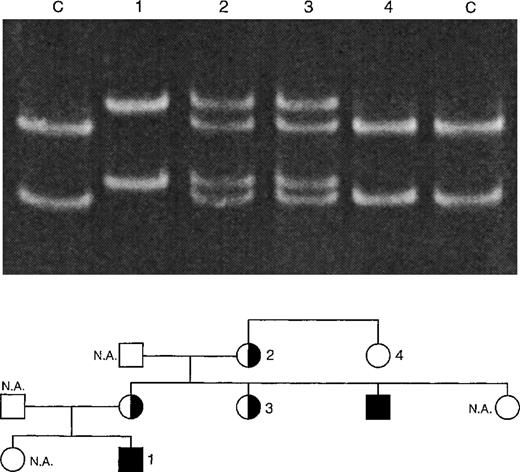
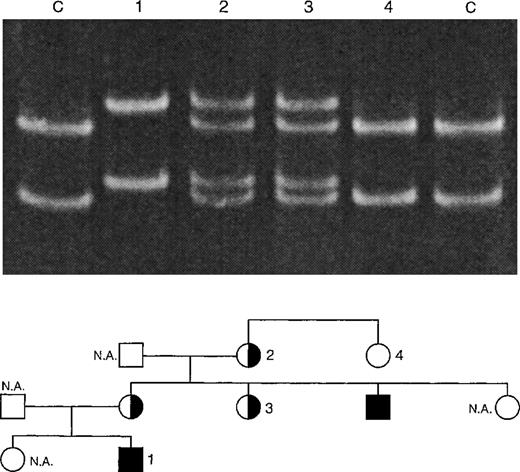
Single-strand conformation polymorphism (SSCP) analysis of XHIM family A with T254M mutation. PCR products (198 bp) of exon 5 of the CD40L gene were denatured and run on a 12.5% polyacrylamide gel at 150 V for 14 hours. The 2 control (C) lanes show the positions of the DNA strands of the unaffected X-chromosome. The C → T mutation leads to a shift in the position of these bands, as is shown in lane 1 (XHIM patient no. 2). Carriers (lanes 2 and 3) show both mutated and unmutated DNA strands. Noncarriers (lane 4) only show the unmutated DNA strands.
Single-strand conformation polymorphism (SSCP) analysis of XHIM family A with T254M mutation. PCR products (198 bp) of exon 5 of the CD40L gene were denatured and run on a 12.5% polyacrylamide gel at 150 V for 14 hours. The 2 control (C) lanes show the positions of the DNA strands of the unaffected X-chromosome. The C → T mutation leads to a shift in the position of these bands, as is shown in lane 1 (XHIM patient no. 2). Carriers (lanes 2 and 3) show both mutated and unmutated DNA strands. Noncarriers (lane 4) only show the unmutated DNA strands.
The second index patient, patient no. 3 (of family B), is a British boy, now 11 years of age, who was diagnosed as having XHIM at 6 months of age when he presented with PCP. After successful treatment of this infection, he has remained asymptomatic on Ig replacement therapy and Co-trimoxazole prophylaxis except for 1 episode of a diarrhoeal illness at 7 years of age that was of unknown etiology, lasted for 2 months, and was associated with weight loss. Recent studies show no evidence of cholangiopathy on liver function tests, liver histology, and endoscopic retrograde cholangio-pancreatogram. His younger brother (patient no. 4), now 8 years of age, was diagnosed soon after birth based on the family history. He has remained completely well on Ig replacement therapy and Co-trimoxazole prophylaxis. His liver is also normal. The mutation in these boys has been previously reported (case 13 in Katz et al2).
In our opinion, the issue of a phenotype-genotype relation in XHIM remains debatable. Not all patients with a staining pattern type 1 or 2 described by Seyama et al1 showed a mild clinical phenotype. Comparison of our 2 index patients with the 782C → T mutation with the 2 mild patients described by Seyama et al1 does not support a tight phenotype-genotype relation in XHIM patients, although our 4 patients all showed a favorable course after Ig replacement therapy. We agree that symptomatic PCP may occur in nonimmunocompromised infants, although this is very unusual. Nevertheless, we do not believe that the life-threatening episode of PCP in our patient no. 3 is compatible with a label of mild phenotype.
Furthermore, our XHIM cases also show that a favorable clinical course on Ig replacement therapy is compatible with a diagnosis of XHIM. This implies that testing of CD40L expression on activated PBMC should be considered in all patients with unexplained hypogammaglobulinemia.
Response
In a recent report,1-1 we have described 27 unique mutations of the CD40 ligand (CD40L) gene in 30 unrelated families with X-linked hyper IgM syndrome (XHIM). Assessment of CD40L expression by activated lymphocytes identified a subgroup of XHIM patients whose lymphocytes could be stained with all monoclonal antibodies to CD40L tested and with a CD40-Ig construct (type I) or only with the monoclonal antibodies but not with CD40Ig (type II). We further reported that 5 of the 9 patients with type I or type II staining had a mild disease with late onset, few infections, and no history of Pneumocystis carinii pneumonia (PCP). Two of these XHIM patients with mild disease had a T254M mutation. In their letter, de Vries et al report 2 pairs of unrelated brothers with the same T254M mutation. They point out that the older boy of family A, now doing well at 19 years of age, presented with PCP. The others, now 2, 8, and 11 years of age, are on IVIG and have also shown a consistently benign course. The observation by de Vries et al confirms our findings that, although mild, some of the patients with type I or type II staining (2 of 9 in our group) may develop PCP. As we reported in a subsequent communication,1-2 3 of the 5 mild cases initially presented with parvovirus B19-induced anemia at 8, 14, and 17 years of age, respectively. All were asymptomatic before parvovirus B19 infection and again after IVIG therapy was initiated. Thus, patients with type I and type II staining may develop opportunistic infections such as PCP and persistent parvovirus B19 infection, suggesting that any mutation affecting the function of CD40L1-3 renders T cells less efficient. In addition, abnormal T-helper function was demonstrated in every patient with type I or II staining immunized with the T-cell–dependent antigen, bacteriophage φX174. However, antibody titers to phage were higher in patients with type I/II staining than in classic XHIM.1-1
Long-term follow-up of XHIM patients with mutations affecting CD40L suggests that classic XHIM is not a benign disease.1-1,1-4,1-5Many patients report chronic diarrhea, progressive liver disease often associated with cryptosporidium infection, ascending cholangiolitis, chronic neutropenia, autoimmunity, lymphohyperplasia, and increased incidents of neoplasms, including lymphomas and biliary tract tumors. A recent European study found that 23% of their study population with XHIM had died (mean age at death, 11.7 years).1-4
Based on the grim diagnosis of classical XHIM, bone marrow transplantation has been advocated.1-6 Long-term follow-up of patients with mild and classic phenotype is required to determine if those patients with type I and type II staining have a better long-term prognosis. Two registries have begun collecting data, one in Europe and one in the United States, with emphasis on mutation analysis and clinical phenotype. These long-term observations will provide the details needed for optimal clinical management of mild and classic XHIM phenotypes.