Abstract
Although proteolytic processing of pro-von Willebrand factor (pro-vWF) resulting in free propeptide and mature vWF is known to be initiated intracellularly, vWF released from endothelial cells may contain a high proportion of incompletely processed pro-vWF. Because pro-vWF is only rarely detectable in normal human plasma, we investigated whether extracellular processing of pro-vWF is possible. A recombinant preparation (rpvWF) containing both pro-vWF and mature vWF subunits was infused into 2 pigs and 1 dog with severe von Willebrand disease, 2 mice with a targeted disruption of the vWF gene, and 2 healthy baboons. Total vWF antigen (vWF:Ag), free propeptide, and pro-vWF were measured using enzyme-linked immunosorbent assay techniques in blood samples drawn before and after infusion. vWF:Ag increased promptly. No pro-vWF could be detected when the first postinfusion sample was drawn after 30 minutes (pigs) or 60 minutes (mice), but pro-vWF was detectable for short periods when postinfusion samples were drawn after 15 minutes (dog) or 5 minutes (baboons). In contrast, free propeptide was increased at the first timepoint measured, suggesting that it was generated from the pro-vWF in the rpvWF preparation. vWF multimers were analyzed in the rpvWF preparation and in plasma samples drawn before and after infusion of rpvWF using ultra-high resolution 3% agarose gels to allow separation of homo- and hetero-forms of the vWF polymers. Within 30 minutes after infusion in the pigs, 1 hour in the dog and the mice, and within 2 hours in the baboons, the multimer pattern had changed to that typically seen in mature vWF. These data indicate that propeptide cleavage from unprocessed vWF can occur extracellularly in the circulation. The enzyme or enzymes responsible for this cleavage in plasma remain to be identified.
VON WILLEBRAND FACTOR (vWF) is a large multimeric adhesive glycoprotein that circulates in plasma and is also found in platelets, megakaryocytes, endothelial cells, and the subendothelial matrix.1,2 The molecule has a dual function in hemostasis because it mediates platelet adhesion at sites of vascular injury, which is necessary for primary hemostasis, and it stabilizes factor VIII (FVIII) in the circulation.3 The pathophysiological significance of the different biological functions of this protein is demonstrated by von Willebrand disease, in which severe von Willebrand deficiency results in defective platelet adhesion, secondary FVIII deficiency, and prolonged bleeding time.4 5
vWF is synthesized in endothelial cells and megakaryocytes as pre-pro-vWF, a 2,813–amino acid precursor protein that consists of a signal peptide of 22 amino acid residues, a 741-amino acid propeptide (also called von Willebrand antigen II6), and the 2,050–amino acid mature vWF subunit. During biosynthesis, pre-pro-vWF is subjected to a series of intracellular posttranslational modifications.3,7,8 After cleavage of the signal peptide, the remaining pro-vWF undergoes glycosylation, C-terminal dimerization, sulfation and carbohydrate processing, aminoterminal multimerization, and proteolytic removal of the propeptide from the aminoterminal end of the vWF subunit. Intracellular propeptide cleavage coincides with aminoterminal multimerization in the trans-Golgi network.9This posttranslational endoproteolysis is accomplished by furin (PACE),10-13 a membrane-associated, calcium-dependent endoprotease belonging to a family of subtilisin-like serine proteases. Propeptide cleavage is necessary for the binding of FVIII to vWF,14-16 but not for multimerization.17,18When cleaved, the propeptide remains noncovalently associated with the mature vWF multimers,9 and both are stored in intracellular granules such as the Weibel-Palade bodies in endothelial cells for release through the regulated pathway.19 However, the majority of vWF produced by endothelial cells is secreted via the constitutive pathway. After secretion, the free propeptide circulates in normal human plasma as a homodimer6 at a concentration of about 1 μg/mL with a half-life of 2 to 3 hours, while mature vWF multimers circulate at a concentration of about 10 μg/mL with a half-life of about 12 hours.20 21
Constitutively secreted vWF may contain a high proportion of incompletely processed pro-vWF.22,23 However, pro-vWF is detectable in normal human plasma only on rare occasions, and even then only trace amounts are observed. In a study of healthy human subjects, administration of 1-deamino-8-d-arginine vasopressin (DDAVP) or endotoxin induced an increase in pro-vWF.21 Even after stimulation, pro-vWF levels were still approximately 1,000-fold lower than free propeptide levels. The absence of significant amounts of pro-vWF in plasma might be explained if rapid cleavage of pro-vWF occurred after secretion from endothelial cells. To investigate whether extracellular processing of pro-vWF can indeed take place in vivo, we studied the structure and properties of vWF in plasma before and after infusion of a hetero-multimeric recombinant preparation consisting of both pro-vWF and mature vWF in canine, porcine, and murine models of von Willebrand disease and in healthy baboons.
MATERIALS AND METHODS
Assays
Total vWF antigen (vWF:Ag), consisting of mature and pro-vWF, was measured by enzyme-linked immunosorbent assay (ELISA) using polyclonal rabbit anti-human vWF antibodies (Asserachrom vWF; Boehringer-Mannheim, Mannheim, Germany). vWF:Ag was expressed in human plasma-equivalent units per milliliter (U/mL) using the standard preparation from the test kit. For comparison with propeptide and pro-vWF, 1 U/mL was assumed to be equivalent to 50 nmol/L.21
Free propeptide was measured as antigen by ELISA with murine antibodies directed against vWF-propeptide as described by Borchiellini et al.21 (CLB-Pro 35 for capture; CLB-Pro 14.3 for detection.) A pool of normal human plasma (Reference Plasma 100%; Immuno, Vienna, Austria) containing 5.5 nmol/L vWF propeptide (calibrated against purified recombinant propeptide21) served as a reference. Pro-vWF antigen was determined in an ELISA with the same capture antibody directed against propeptide (CLB Pro-35) as in the ELISA for propeptide, but using a horseradish peroxidase–labeled polyclonal rabbit anti-human vWF antibody (Dakopatts, Glostrup, Denmark) for detection. Recombinant pro-vWF calibrated against an uncleavable pro-vWF mutant (pro-vWFgly763)21 was used as a reference. The specificity of this assay system for pro-vWF was shown by its lack of cross-reactivity with a recombinant preparation containing only mature vWF.24 The propeptide ELISA poorly recognized pro-vWF, yielding values of approximately 0.05 nmol/L propeptide per nmol/L pro-vWF. This 20-fold difference in reactivity allowed us to monitor the quantitative increase of the propeptide in plasma samples.
Ristocetin cofactor activity (RCoF) was determined by measuring the ristocetin-induced agglutination of formaldehyde-fixed human platelets25 under conditions described in the literature26 using a 570-VS whole blood aggregometer (Chrono-Log, Havertown, PA) equipped with a chart recorder. FVIII activity was measured with a 2-stage clotting method performed as described by Austen and Rhymes27 using the reagents from the 2-stage FVIII assay kit from Immuno. A reference plasma calibrated against the 3rd International Standard 91/666 was used in the determination of FVIII and RCoF, and the results were expressed in human plasma-equivalent units as IU/mL.
Test Substance and Electrophoretic Analyses
The human recombinant preparation containing pro-vWF (rpvWF) was produced by expression in a rodent cell line, Chinese hamster ovary (CHO) cells.28 rpvWF was purified from cell culture supernatant by ion-exchange chromatography followed by immuno-affinity chromatography on an immobilized monoclonal antibody directed against mature vWF as described by Mejan et al.29 Because of the specificity of the final purification step, the preparation contained only a minute quantity of free propeptide (<4% on a molar basis). For the initial characterization of the test substance, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed under reducing conditions.30 Samples were incubated with buffer containing dithiothreitol at 96°C for 5 minutes. Aliquots of 20 μL of these samples were subjected to electrophoresis on 8% gels in the presence of SDS. Protein bands were stained with Coomassie brilliant blue by a standard method.31
For analysis before and after the rpvWF infusion, plasma samples were reduced at 70°C for 15 minutes, applied to gradient polyacrylamide gels (4% to 12%; Novex, San Diego, CA) in the presence of SDS, and subjected to standard blotting procedures on PVDF membrane (Pall Gelman Sciences, Ann Arbor, MI). A polyclonal rabbit antibody against vWF (Dakopatts, Glostrup, Denmark), which cross-reacts with porcine, canine, and baboon vWF, and reacts with both mature and pro-vWF, was used as the primary antibody, and an alkaline phosphatase–labeled goat anti-rabbit IgG (Accurate, Westbury, NY), as secondary antibody. The blots were developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue-tetrazolium substrate (Bio-Rad, Richmond, CA).
For the mouse experiment, in which 125I-labeled rpvWF was administered, the samples were separated by SDS-PAGE and the gels were dried and exposed to x-ray films (Hyperfilm MP; Amersham-Pharmacia, Uppsala, Sweden) to obtain autoradiograms. The “Full Range Rainbow Marker” (Amersham-Pharmacia) ranging from 10 to 250 kD was used to determine the molecular mass.
To analyze the size distribution of vWF multimers in the rpvWF preparation and in plasma before and after infusion of rpvWF, high-density horizontal SDS agarose gel electrophoresis was performed as previously described,32 except that 3% agarose was used to obtain ultra-high resolution of the vWF multimers. This method allowed separation of the homo- and hetero-forms of the vWF polymers. The same anti-human vWF antibody as described above was used for immunodetection.
Radioiodination of rpvWF
Radioiodination of rpvWF was performed by labeling 100 μg of rpvWF with 125I (Amersham-Pharmacia) according to the iodobeads method of Markwell.33 The 125I-labeled rpvWF had a specific activity of 6.3 × 105 Bq/vWF:Ag unit.
Animal Studies
We previously used the porcine model of severe von Willebrand disease for infusion studies with a recombinant preparation of mature human vWF (rvWF).34 35 In the present study, we administered the pro-vWF–containing rpvWF intravenously to 2 previously untreated vWF-deficient pigs from this colony, 1 pig receiving 17 and the other receiving 70 RCoF IU/kg body weight. With citrate for anticoagulation, blood samples were withdrawn from the venous catheters before infusion and 30 minutes, 1, 2, 3, 6, 8, 24, 32, 48, 72, and 96 hours after infusion of the rpvWF. The timing of the sampling was based on the results of the previous studies. Plasma was separated by immediate centrifugation at 1,000g for 20 minutes at 15°C and deep frozen in aliquots for analysis of vWF parameters (total vWF antigen, pro-vWF antigen, propeptide antigen, RCoF and FVIII activity, and multimers) as described above.
We had also tested recombinant human mature vWF in an earlier study in Dutch Kooiker dogs with severe vWF deficiency.32 We used similar techniques in the present study, infusing 1 previously untreated dog from the same colony with rpvWF intravenously at a dose of 35 RCoF IU/kg. Samples of citrated plasma obtained by venous puncture before infusion and at timepoints 15, 30, and 45 minutes and 1, 2, 3, 6, 24, 48, 72, and 95 hours after the administration of the rpvWF were then subjected to in vitro analyses as described for the pigs, and in addition were analyzed by SDS-PAGE under reducing conditions and Western blotting.
Mice with a targeted disruption of the vWF gene36 (breeding pair for establishment of a colony kindly provided by Denisa Wagner, Center for Blood Research, Boston, MA) were used for infusion studies with 125I labeled rpvWF. Two homozygous vWF knockout mice from this colony received 70 RCoF U/kg containing 1.3 × 105 Bq/vWF:Ag U rpvWF. Citrated samples were drawn by heart puncture 60 minutes after intravenous administration and subjected to SDS-PAGE under reducing and nonreducing conditions. Autoradiograms of the dried gels were then evaluated.
We also treated 2 colony-bred 4-year-old healthy male baboons (papio papio hamadryas), weighing 8.5 kg, with rpvWF intravenously at a dose of 50 RCoF IU/kg each. The animals were anesthetized by intramuscular injection of 10 mg/kg ketamine (Ketavet; Parke Davis, Munich, Germany). Citrated blood was drawn by venous puncture before infusion and 5, 10, 15, 30 minutes and 2, 4, 24, 48, and 72 hours postinfusion and subjected to analyses as described for the pigs.
The dog, baboon, and mouse studies were performed in accordance with the Austrian Animal Experiments Act (BG 501/1989). The dog was housed in the Animal Care Facility of the Medical Faculty of the University of Vienna in Himberg, Austria. The baboon study was performed in the Hans Popper Primate Center in Orth/Donau, Austria, which is fully certified by the American Association of Accreditation of Laboratory Animal Care (AAALAC). The study was in full compliance with guidelines for the care and use of laboratory animals (Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council, Washington, DC, 1996). The pig study was performed at the Institut de la Recherche Agronomique (INRA) in Jouy en Josas, France, according to the ethical guidelines established by the Institut National de la Recherche Medical (INSERM) and INRA institutional policies.
Biometrical Methods
Half-life calculations were performed using the method described by Lee et al.37
RESULTS
rpvWF Preparation
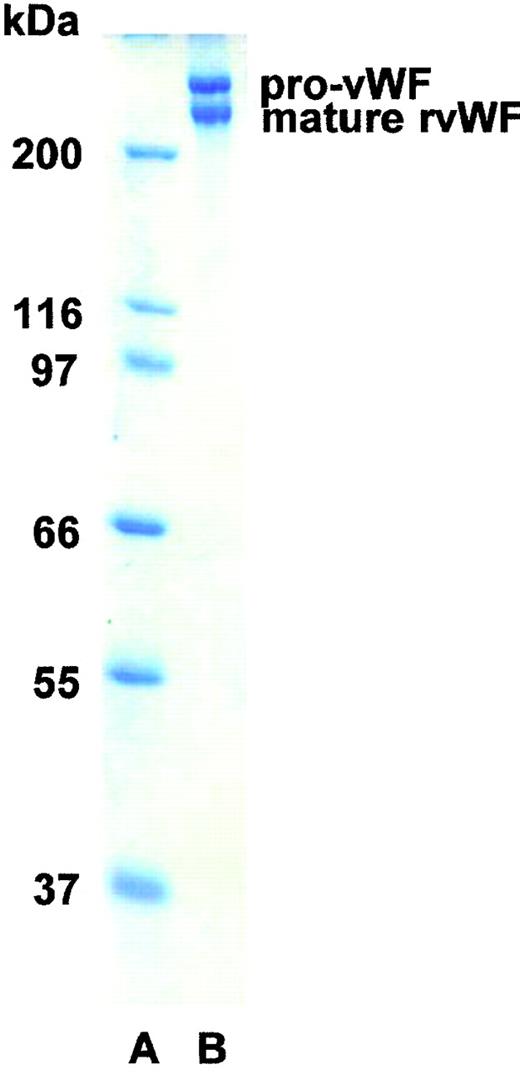
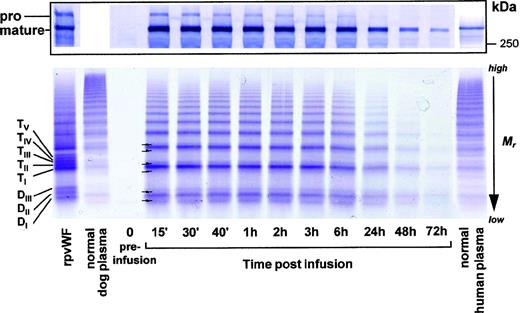
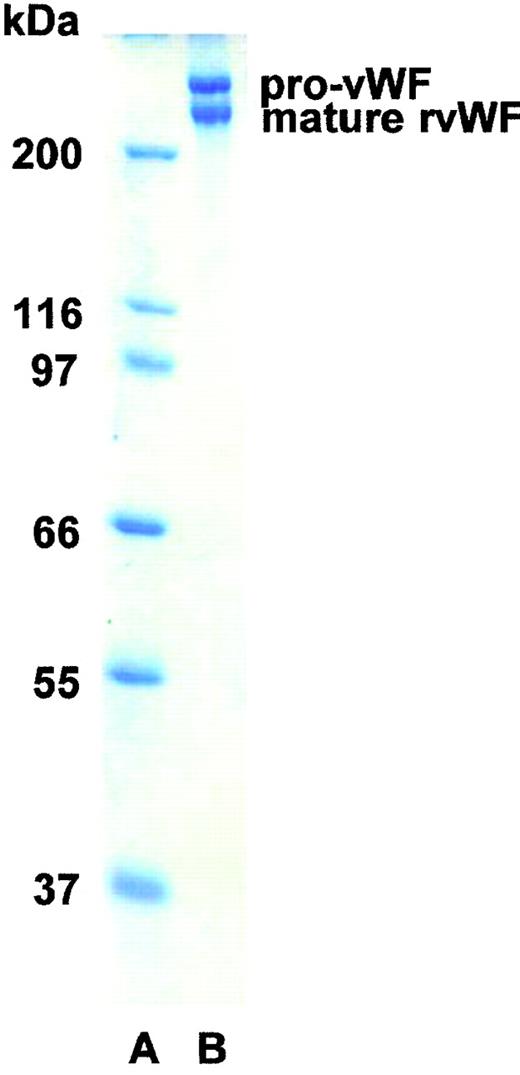
SDS-PAGE analysis showed the rpvWF preparation to be composed of approximately 50% pro-vWF and 50% mature vWF, with a specific activity of 50 RCoF IU/mg protein (Fig 1). SDS agarose gel electrophoresis revealed that the homo- and hetero-dimers of pro-vWF and mature vWF monomers were multimerized to high-molecular-weight forms (see Figs 3, 6, and 9, rpvWF lanes). Trace amounts of free propeptide (<2% of pro-vWF on a molar basis) could be detected in the rpvWF preparation with the ELISA method. However, no propeptide was visible on the autoradiogram (see control lane A of Fig7) of the 125I-labeled rpvWF.
SDS-PAGE (8% T) under reducing conditions of rpvWF (lane B), consisting of 50% pro-vWF and 50% mature vWF. Molecular-weight standard (lane A). The purified rpvWF in the gel was visualized by Coomassie blue staining.
SDS-PAGE (8% T) under reducing conditions of rpvWF (lane B), consisting of 50% pro-vWF and 50% mature vWF. Molecular-weight standard (lane A). The purified rpvWF in the gel was visualized by Coomassie blue staining.
Fate of rpvWF After Infusion
vWF-deficient pigs.
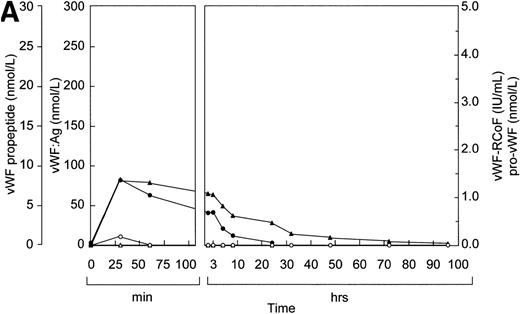
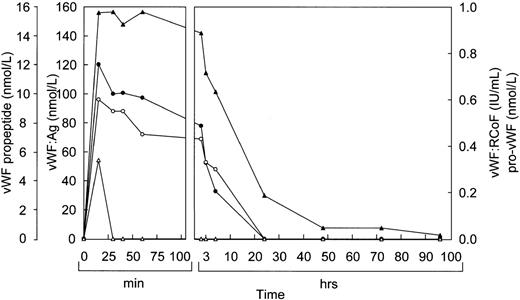
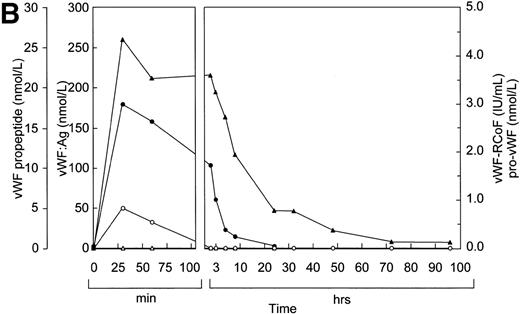
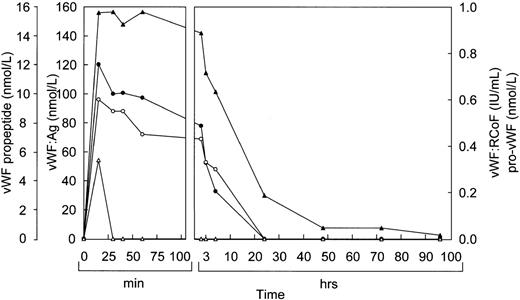
Before infusion of the rpvWF preparation, levels of vWF:Ag, RCoF activity, free propeptide, and pro-vWF were below the limits of detection in each pig (Fig 2A and B). Plasma concentrations of vWF:Ag, RCoF, and free propeptide increased promptly after infusion of the rpvWF preparation and reached maximum values within 30 minutes, rising higher in the pig that received the larger dose. Because no free propeptide was present in the rpvWF preparation, this propeptide likely appeared due to proteolytic processing of pro-vWF after infusion. The vWF:Ag remained in the circulation for at least 96 hours in both pigs, with a half-life of about 22 hours, which was comparable to our previous observations after infusion of mature rvWF.33 34 The free propeptide disappeared within 24 hours, with a half-life of 3.3 hours in the pig treated with 17 RCoF IU/kg, and 1.7 hours in the pig treated with 70 RCoF IU/kg. Pro-vWF was not detectable in any of the samples tested.
Fate of rpvWF after injection of a single bolus into vWF-deficient pigs: (A) 17 RCoF IU/kg body weight; (B) 70 RCoF IU/kg). vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
Fate of rpvWF after injection of a single bolus into vWF-deficient pigs: (A) 17 RCoF IU/kg body weight; (B) 70 RCoF IU/kg). vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
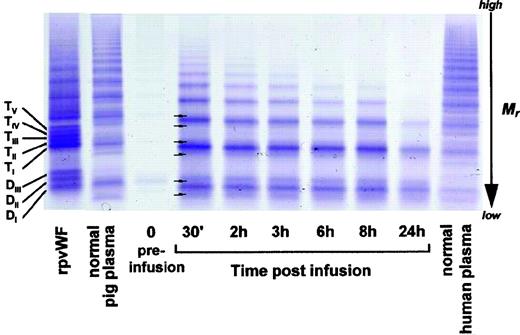
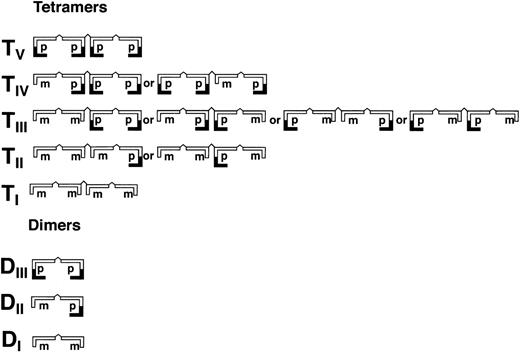
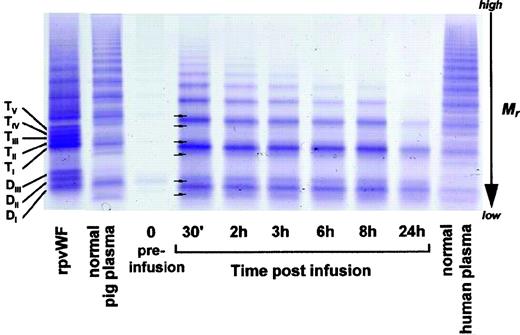
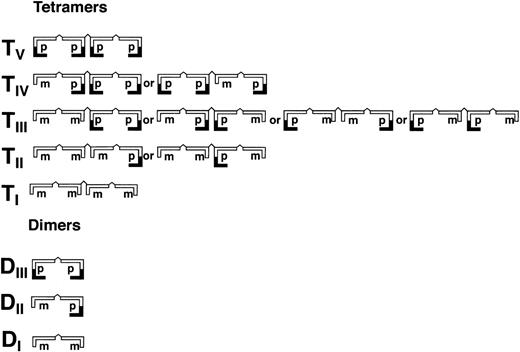
As shown in Fig 3 (see page 1639), vWF multimers were virtually absent before infusion of the rpvWF preparation. Comparative analysis of the multimers of the rpvWF preparation and of vWF multimers in the plasma samples taken after infusion of rpvWF (70 RCoF IU/kg) indicated that the pro-vWF had undergone proteolytic processing (Fig 3). The high-density agarose gel used for this analysis allowed separation of the homo- and hetero-forms of the vWF polymers. The resolution was sufficient to identify 3 and 5 bands on the dimer and the tetramer levels, respectively (D I-III and T I-V in Fig 3; schematic illustration of the composition of the vWF oligomers in Fig 4). Thirty minutes after infusion of the rpvWF into the pigs, we could detect only 1 dominating band at each oligomer and multimer of the vWF. This band had the same molecular weight as the fastest migrating band of the oligomer equivalent to the homopolymer of mature vWF and also correlated in molecular size with the central band of the human vWF triplet or quintuplet structure.38 All multimeric forms of vWF were then gradually removed from the circulation.
Fate of rpvWF after injection of a single bolus (70 RCoF IU/kg) into a vWF-deficient pig (see Fig 2B): vWF multimer pattern over time. Plasma samples taken before and at specified intervals after injection were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species. Control lanes: recombinant vWF preparation before injection (rpvWF), normal pig plasma, normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Arrows indicate satellite band formation. Mr, apparent relative molecular mass.
Fate of rpvWF after injection of a single bolus (70 RCoF IU/kg) into a vWF-deficient pig (see Fig 2B): vWF multimer pattern over time. Plasma samples taken before and at specified intervals after injection were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species. Control lanes: recombinant vWF preparation before injection (rpvWF), normal pig plasma, normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Arrows indicate satellite band formation. Mr, apparent relative molecular mass.
Schematic illustration of homo- and hetero-dimers (DI-III) and tetramers (TI-V) of partially processed recombinant vWF (rpvWF) consisting of pro (p) and mature (m) subunits at an equal ratio.
Schematic illustration of homo- and hetero-dimers (DI-III) and tetramers (TI-V) of partially processed recombinant vWF (rpvWF) consisting of pro (p) and mature (m) subunits at an equal ratio.
In addition to the degradation of the pro-vWF multimers, we observed satellite bands that were similar but not completely equivalent to those of the human triplet structure (see satellite band formation of dimers and tetramers in Fig 3). These satellites had a different molecular size than the satellite bands of human plasma-derived vWF multimers (Fig 3). Although the central band was equal in size to the central band of each multimer of human vWF, the faster and slower migrating satellite bands generated from the infused rpvWF had higher and lower molecular masses, respectively, than those found in normal human plasma, resulting in a more compact triplet structure than that seen in normal human plasma.
vWF-deficient dog.
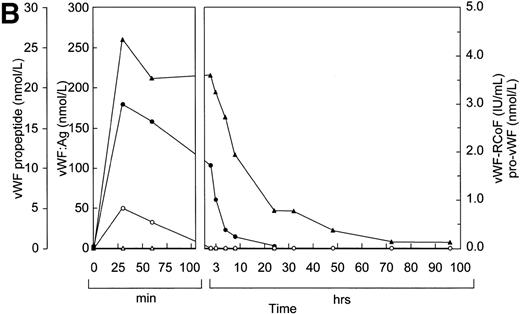
The results of the study in pigs prompted us to study the effects of rpvWF in a Dutch Kooiker dog with type 3 von Willebrand disease.32 39 In this study, we increased the frequency of blood drawing to every 15 minutes. As in the pigs, vWF:Ag, RCoF, and free propeptide levels increased rapidly in the dog from below the limit of detection to peak levels within 15 minutes after infusion of rpvWF (35 RCoF IU/kg) (Fig 5). The calculated half-life was 9 hours for the vWF antigen and 5 hours for RCoF activity. In contrast to the pigs, a small amount of pro-vWF could be measured in the dog, but only in the blood sample taken 15 minutes after injection of pro-rvWF. The free propeptide showed a peak 15 minutes after injection of the vWF preparation and decreased by 20% in the next 15 minutes, but stayed at this level for an additional 30 minutes. Thereafter it declined gradually, with a half-life of 3 hours.
Fate of rpvWF after injection of a single bolus (35 RCoF IU/kg) into a vWF-deficient dog: vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
Fate of rpvWF after injection of a single bolus (35 RCoF IU/kg) into a vWF-deficient dog: vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
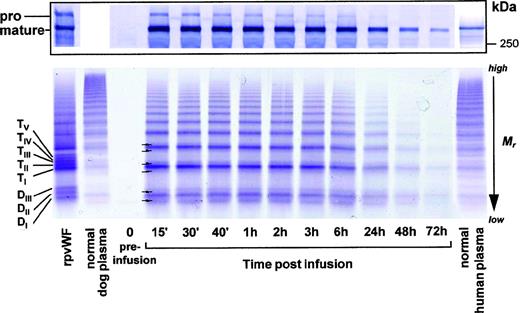
Consistent with these findings, electrophoretic analysis under reducing conditions (Fig 6, top) showed that the signal corresponding to that of the pro-vWF of the infused preparation was still slightly visible after 15 minutes, but gradually disappeared over time.
Fate of rpvWF after injection of a single bolus (35 RCoF IU/kg) into a vWF-deficient dog (see Fig 5): (Top) SDS-PAGE under reducing conditions/Western blot. (Bottom) vWF multimer pattern over time. Plasma samples taken before and at specified intervals after injection were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species. Control lanes: recombinant vWF preparation before injection (rpvWF), normal dog plasma, normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Arrows indicate satellite band formation. Mr, apparent relative molecular mass.
Fate of rpvWF after injection of a single bolus (35 RCoF IU/kg) into a vWF-deficient dog (see Fig 5): (Top) SDS-PAGE under reducing conditions/Western blot. (Bottom) vWF multimer pattern over time. Plasma samples taken before and at specified intervals after injection were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species. Control lanes: recombinant vWF preparation before injection (rpvWF), normal dog plasma, normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Arrows indicate satellite band formation. Mr, apparent relative molecular mass.
The analysis of the vWF multimers (Fig 6, bottom) for the timepoints up to 72 hours postinfusion showed that the pro-vWF multimers had disappeared completely after the first 15 minutes, resulting in a single banded multimer picture. All vWF multimers were gradually removed from the circulation over time. The formation of triplet bands was evident but not as clear here as it was in the pig studies.
vWF knockout mice.
Before the injection of rpvWF, the vWF knockout mice had zero levels of vWF antigen, vWF propeptide, and pro-vWF. The plasma sample taken from mice 60 minutes after injection of 70 RCoF U/kg rpvWF mixed with125I-labeled rpvWF contained 0.86 U/mL total vWF:Ag, 3.14 nmol/L propeptide and 0.43 U/mL RCoF. No pro-vWF:Ag was detected by the ELISA. On the autoradiograms of both the nonreducing and reducing SDS-PAGE (Fig 7), a new band appeared that was not present in the 125I-labeled rpvWF preparation and had a molecular mass of 73 kD (nonreduced) or 96 kD (reduced), corresponding to the molecular mass of the propeptide. No additional bands were observed. The 125I-labeled rpvWF showed only 1 band with a molecular mass greater than 250 kD, even when the gel was overloaded with 1,600 Bq per lane (data not shown).
Autoradiogram of a plasma sample from a vWF-deficient mouse treated with 125I-labeled rpvWF (B) after separation on SDS-PAGE under reducing and nonreducing conditions; control lane (A): 125I-labeled radiolabeled rpvWF preparation.
Autoradiogram of a plasma sample from a vWF-deficient mouse treated with 125I-labeled rpvWF (B) after separation on SDS-PAGE under reducing and nonreducing conditions; control lane (A): 125I-labeled radiolabeled rpvWF preparation.
Healthy baboons.
We then administered rpvWF to 2 healthy baboons at a dose of 50 RCoF IU/kg. Blood sampling was now performed every 5 minutes during the first 15 minutes after the injection. As shown for 1 baboon in Fig 8, rpvWF induced an immediate increase in vWF:Ag and free propeptide levels. vWF:Ag decreased gradually to baseline levels within 48 hours, with a half-life of 22 hours. Pro-vWF also showed a sharp increase and remained detectable during the first 30 minutes in both animals, but had disappeared by the 2-hour timepoint. Free propeptide levels began to decrease only after the pro-vWF had disappeared from the circulation, with a half-life of 5 hours in each baboon. The pretreatment levels of vWF:Ag and RCoF were around 5 U/mL, which is normal for baboons, but 5 times higher than the level of our human reference plasma. After injection of the rpvWF, RCoF increased by 50% in 1 baboon (Fig 8), but by only 10% in the other animal (not shown). Other parameters were almost identical in the 2 animals.
Fate of rpvWF upon injection of a single bolus (50 RCoF IU/kg) into a normal baboon (K6): vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
Fate of rpvWF upon injection of a single bolus (50 RCoF IU/kg) into a normal baboon (K6): vWF:Ag (▴), pro-vWF (▵), free propeptide (•), and vWF:RCoF (○).
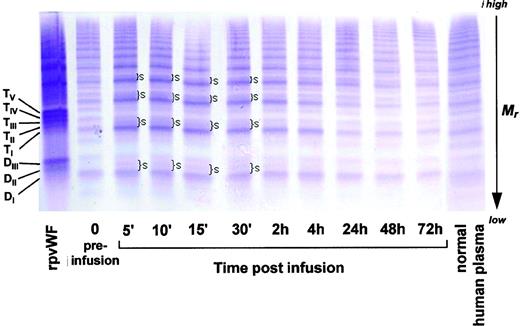
Multimer analysis of the plasma samples from the baboons (Fig 9, see page 1642) also showed the typical triplet structure similar to that observed in humans. After injection of rpvWF, a smear could be observed in the samples taken up to 2 hours after injection at the positions where the slower-migrating, higher-molecular-weight bands of each pro-vWF oligomer were seen in the recombinant preparation (Fig 9, DII-DIII, TII-TV). However, the changes in the baboons were not as visible as those in the pigs and the dog, and at the later timepoints no difference to the multimer pattern prior to infusion could be seen.
Fate of rpvWF upon injection of a single bolus (50 RCoF IU/kg) into a normal baboon (K6): vWF multimer pattern over time. Plasma samples were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species, particularly baboons. Braces (}) indicate rpvWF homo-and hetero-oligomer smearing on the agarose gel. Control lanes: recombinant vWF preparation before injection (rpvWF), normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Mr, apparent relative molecular mass.
Fate of rpvWF upon injection of a single bolus (50 RCoF IU/kg) into a normal baboon (K6): vWF multimer pattern over time. Plasma samples were analyzed electrophoretically on a 3% SDS agarose gel. Multimers were visualized using an anti-human polyclonal vWF antibody that cross-reacts with vWF from other species, particularly baboons. Braces (}) indicate rpvWF homo-and hetero-oligomer smearing on the agarose gel. Control lanes: recombinant vWF preparation before injection (rpvWF), normal human plasma. DI-III, TI-V: variants of homo- and hetero-dimers and tetramers (D, dimer; T, tetramer) as illustrated in Fig 4. Mr, apparent relative molecular mass.
Effects of rpvWF on FVIII
A substantial increase in FVIII levels was observed after infusion of rpvWF in all studies performed in vWF-deficient animals, reaching peaks between 20 and 30 hours posttreatment (Fig 10). The FVIII levels in the pigs remained higher than the starting levels for 96 hours, in the dog for 72 hours. No effect on FVIII could be observed in the healthy baboons; FVIII remained constant over the entire observation period despite the 2-fold increase in circulating levels of vWF.
Effect of single-bolus injection of human rpvWF on FVIII plasma levels in 2 vWF-deficient pigs: 17 RCoF IU/kg (▪), 70 RCoF IU/kg (□); 1 vWF-deficient dog: 35 RCoF IU/kg (•); and 2 normal baboons: 50 RCoF IU/kg each (K5 ▴, K6 ▵).
Effect of single-bolus injection of human rpvWF on FVIII plasma levels in 2 vWF-deficient pigs: 17 RCoF IU/kg (▪), 70 RCoF IU/kg (□); 1 vWF-deficient dog: 35 RCoF IU/kg (•); and 2 normal baboons: 50 RCoF IU/kg each (K5 ▴, K6 ▵).
DISCUSSION
The availability of a recombinant preparation containing unprocessed, multimerized pro-vWF in addition to mature vWF allowed us to study the fate of pro-vWF in plasma, both in porcine and canine models of von Willebrand disease as well as in vWF knockout mice and normal nonhuman primates. The preparation used in our previous canine and porcine infusion studies32,34,35 consisted of fully processed and polymerized rvWF obtained from CHO cells manipulated to overexpress recombinant furin in addition to rvWF.24 The rpvWF used in the experiments described here was expressed from CHO cells without coexpression of recombinant furin.28 Because the amount of endogenous furin is limited, these cells were only capable of partially processing the expressed rvWF without additional recombinant furin, which resulted in the formation of rvWF heteromultimers. Therefore, the preparation contained a mixture of mature, completely processed, propeptide-free subunits and unprocessed forms still containing the covalently linked propeptide.
The infusion studies presented here indicate that propeptide cleavage from unprocessed vWF can occur in the circulation. The infused pro-vWF could not be detected at all in the experiments with pigs and mice, in which the first postinfusion sample was not drawn until 30 and 60 minutes, respectively, and could be detected only for brief periods in the dog and baboon experiments, in which the first postinfusion sample was drawn after 15 and 5 minutes, respectively. In contrast, vWF:Ag and free propeptide levels (which before infusion of rpvWF were below the limit of detection in the pigs, dog, and mice) increased rapidly in all animals tested, indicating that the pro-vWF was quickly metabolized. The minute quantity of free propeptide contained in the rpvWF preparation was too small to explain the rapid increase in free propeptide seen after infusion. After the initial increase, the free propeptide remained at relatively high levels for periods of about 1 to 3 hours in the pigs, the dog, and the mice, and 4 hours in the baboons, then began to decrease, dropping below the limit of detection within 10 to 24 hours. This was consistent with continued propeptide release as long as pro-vWF remained in the circulation. In the pigs, the dog, and the mice, the levels of vWF:Ag paralleled those of the free propeptide, and in the baboons vWF:Ag remained high for longer periods.
These observations were confirmed by analyses of the multimeric composition of the infused vWF as shown on a 3% agarose gel that allowed separation of homo- and hetero-forms of the vWF polymers. Within 30 minutes after infusion in the pigs and the dog, and within 2 hours in the healthy baboons, the multimer pattern had changed to that typically seen in mature vWF. Electrophoretic analysis under reducing conditions of the composition of vWF after infusion of rpvWF into the vWF-deficient dog led to the same conclusions as those drawn from the multimer analysis. Because the Western blotting did not allow us to visualize the cleavage product of pro-vWF (ie, the propeptide), we used experiments with 125I-labeled rpvWF injected into vWF knockout mice to show the liberation of the propeptide in plasma. Under reducing conditions, autoradiograms from plasma samples obtained 1 hour after the injection showed bands of mature vWF as well as a band with a molecular mass of 96 kD, which corresponds to free propeptide. Under nonreducing conditions, the propeptide had a lower molecular mass of 73 kD, consistent with that previously described.21 These observations argue against a preferential clearing of pro-vWF as an explanation for the loss of pro-vWF from the circulation. Although the possibility that the infused pro-vWF is taken up into the trans-Golgi network and processed intracellularly cannot be completely ruled out, this also appears unlikely, considering the rapid time course of propeptide generation in the present study.
In fact, this is not the first observation of extracellular processing of vWF. Although previous studies13 had suggested that furin can mediate the processing of vWF only intracellularly, in a recent study performed in vitro,40 recombinant furin was shown to convert the heterogeneous multimeric pattern of incompletely processed rvWF into homogeneous and structurally intact multimers, even in the absence of cells, showing that vWF precursor-processing can occur extracellularly upon furin coexpression.
In addition to the rapid disappearance of the pro-vWF-containing multimers, satellite bands with molecular masses lower and higher than the central band appeared in both the pigs and the dog. This structure resembled the triplet structure of multimers seen in human plasma-derived vWF,38 although the satellite bands were less widely separated than in plasma-derived vWF. The appearance of a satellite band structure suggests that further proteolytic cleavage of the mature vWF subunit occurred in addition to the propeptide removal. We had not observed any satellite band formation after infusion of recombinant mature vWF without the pro-moiety in our previous study in dogs.32 However, we were not then using ultra-high-resolution 3% agarose gels, but standard electrophoretic techniques with 2% agarose gels, which were not sufficient to resolve all homo- and hetero-multimers of the rpvWF. Therefore, we may have missed any further proteolytic degradation that might have occurred in the mature vWF. The present finding of satellite band formation is consistent with the results of a recent study showing that a plasma-derived protease known to be specific for vWF cleavage at Tyr842Met843 in the vWF subunit41-43 can cleave recombinant vWF in vitro.44 It remains to be investigated whether the same protease is responsible for the in vivo degradation of mature vWF observed in the present study.
As shown in the medium of cultured endothelial cells, vWF propeptide and mature vWF are released in nearly equimolar amounts with both constitutive and stimulated secretion.19,21 Because of its shorter half-life, the concentration of the propeptide in normal human plasma (≈5 nmol/L) is 1 order of magnitude lower than that of mature vWF (≈50 nmol/L).21,45 After administration of DDAVP or endotoxin in healthy volunteers or patients with von Willebrand disease or acquired von Willebrand syndrome, plasma propeptide concentrations increased 5- to 20-fold, while mature vWF increased 4- to 5-fold.20,21,46,47 Based on our findings that propeptide covalently linked to mature vWF in the rpvWF preparation can be cleaved off after infusion, the possibility exists that, rather than resulting from a concerted release of free propeptide and mature vWF molecules, increases in the plasma concentrations of propeptide and mature vWF might be caused at least in part by increased secretion of pro-vWF, which is processed in the circulation immediately after its release. This would be consistent with the finding of increased pro-vWF levels in normal subjects after stimulation with DDAVP or endotoxin.21 The extent to which the extracellular processing of circulating pro-vWF actually occurs under physiological conditions remains to be clarified.
The significance of the propeptide in the intracellular multimerization process of human vWF has been clearly shown.48,49 The propeptide is essential for promoting the multimerization of vWF, possibly by promoting interchain disulfide bond formation, because specific sequences in the D1 and D2 domain of the propeptide resemble functional sites of the thiol-disulfide oxidoreductases.50In contrast, an extracellular function for the propeptide has not yet been clearly established. The propeptide has been shown to bind to type I collagen and to cross-link to laminin in the presence of FXIIIa, suggesting that it could play a role as a matrix protein after release from endothelial cells.51 Furthermore, the propeptide inhibits collagen-induced platelet aggregation.52 Taking into consideration the finding that mature vWF supports platelet adhesion and platelet aggregation, free propeptide found in the circulation could attenuate or regulate excess platelet aggregation by collagen in the presence of vWF. Recently, a ligand function of the propeptide for very late antigen-4 integrin present on lymphocytes and leukocytes was described, suggesting a link between vWF and inflammation.53 Propeptide deposition into the subendothelium could play a role in leukocyte recruitment at sites of vascular injury. Further studies are necessary to investigate whether the proteolytic cleavage of pro-vWF generates two polypeptide species with distinct physiological functions and whether the circulating propeptide plays a role in hemostasis.
Proteolytic removal of the propeptide is required for high-affinity binding of FVIII to the D-domain of vWF, and the uncleaved propeptide is believed to inhibit binding of FVIII sterically by preventing access to the FVIII binding site.14,16 Alternatively, removal of the propeptide may result in a conformational change in the binding region of each vWF subunit, leading to appropriate exposure of the FVIII binding site.15 We did not determine the precise site of cleavage of the propeptide from pro-vWF after infusion of rpvWF. Whether the new aminoterminus generated after the in vivo processing of pro-vWF is identical to that obtained after intracellular processing remains to be investigated.
In our earlier studies in vWF-deficient dogs and pigs, infusion of the rvWF preparation containing only completely processed mature subunits was associated with increased FVIII levels that were maintained even after vWF antigen had returned to very low levels.32,34 35In the present studies, comparable effects were achieved by infusion of rpvWF containing both mature subunits and pro-vWF. The infused pro-vWF was metabolized immediately, and the resulting mature vWF appears to have acted in the same way as intracellularly processed mature vWF to bind and stabilize FVIII. To test this hypothesis, however, an in vivo study would have to be performed with a preparation containing only pro-vWF without mature vWF subunits. In the present studies, the free propeptide at least did not appear to inhibit FVIII binding.
In conclusion, these studies show that processing of pro-vWF to a molecular size similar to that generated intracellularly occurs in the circulation of 4 different species by an as yet unknown enzyme, suggesting that the vWF propeptide, besides being derived from the Weibel-Palade bodies or other stores after stimulation, can also be cleaved from pro-vWF in the circulation.
ACKNOWLEDGMENT
We are grateful to Dr Jürgen Siekmann, Herbert Gritsch, Günter Richter, Manfred Billwein, Jutta Schreiner, and Ingrid Neunteufel for their skilled contributions to the analyses, to Christine Eder for expertise in performing the baboon study, and to Sylvia C. Maurer for graphical assistance and Kathryn Nelson for editorial assistance.
Supported in part by the Dutch Thrombosis Foundation (Grant No. 96.001).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H.P. Schwarz, MD, Baxter Hyland Immuno, Industriestrasse 67, A-1221 Vienna, Austria; e-mail:schwarh@baxter.com.