Abstract
Endotoxic lipopolysaccharide (LPS) is a proinflammatory agonist produced by gram-negative bacteria and a contributor to the majority of the 400,000 septic shock cases recorded annually in US hospitals. The primary target cells for LPS are monocytes and macrophages. Their response consists of massive production of proinflammatory cytokines, reactive oxygen- and nitrogen-intermediates, procoagulants, and cell adhesion molecules. In turn, expression of these LPS-responsive factors contributes to collapse of the circulatory system, to disseminated intravascular coagulation, and to a 30% mortality rate. A common intracellular mechanism responsible for the expression of septic shock genes in monocytes and macrophages involves the activation of NF-κB. This transcription factor is regulated by a family of structurally related inhibitors including IκB, IκBβ, and IκBɛ, which trap NF-κB in the cytoplasm. In this report, the investigators show that LPS derived from different gram-negative bacteria activates cytokine-responsive IκB kinases containing catalytic subunits termed IKK (IKK1) and IKKβ (IKK2). The kinetics of IKK and IKKβ activation in LPS-stimulated human monocytic cells differ from that recorded on their stimulation with tumor necrosis factor-, thereby implying a distinct activation mechanism. LPS-activated IKK complexes phosphorylate all 3 inhibitors of NF-κB: IκB, IκBβ, and IκBɛ. Moreover, LPS activates IKKβ preferentially, relative to IKK. Thus, IKK complex constitutes the main intracellular target for LPS-induced NF-κB signaling to the nucleus in human monocytic cells to activate genes responsible for septic shock.
PROINFLAMMATORY STIMULI signal to the nucleus via transcription factor NF-κB, which activates a set of genes responsive to inflammatory, immune, and oxidant stress. These stimuli encompass biologic agents such as endotoxic lipopolysaccharide (LPS) and bacterial superantigens (eg, toxic shock syndrome toxin and streptococcal pyrogenic exotoxin), which are responsible for the majority of septic shock cases. In addition, intracellular bacteria (eg, Mycobacterium tuberculosis), viruses (eg, human immunodeficiency virus, human T-cell lymphotropic virus, and cytomegalovirus), cytokines (tumor necrosis factor-α [TNF-α], interlukin-1 [IL-1]), and lipid peroxides induce nuclear import of NF-κB.1-3 In response to these agents, the cytoplasmic ankyrin motif-rich inhibitors of NF-κB, IκBα, IκBβ, and IκBε are phosphorylated, ubiquitinated, and then degraded by adenosine triphosphate (ATP)-dependent 26S proteosomes.4-9Thus, phosphorylation-dependent proteolysis of IκB proteins releases NF-κB for subsequent import to the nucleus. In turn, NF-κB stimulates transcription of a number of genes containing cognate κB sites in their enhancer/promoter regions. These NF-κB–responsive genes encode cytokines (TNF-α, IL-1, IL-6, IL-8, and IL-12), procoagulant molecules (tissue factor and plasminogen activator inhibitor 1), signal transducers (inducible nitric oxide synthase and cyclooxygenase 2), cell adhesion molecules (endothelial selectin, intracellular cell adhesion molecule-1, and vascular cell adhesion molecule-1), and growth factors (granulocyte-colony-stimulating factor).3,6,10 The products of these NF-κB–regulated genes expressed in monocytes, macrophages, and vascular endothelial cells contribute to the development of septic shock and disseminated intravascular coagulation, leading to multiple organ failure and death.1,3 These findings highlight NF-κB as a major intracellular mediator of the systemic inflammatory response syndrome known as sepsis and septic shock.11
The fact that multiple proinflammatory stimuli, LPS, TNF-α, and IL-1, involved in induction and mediation of septic shock syndrome, evoke activation of NF-κB indicates that the signals generated by these stimuli and their cognate receptors converge at the common step of NF-κB activation.6 This step appears to be specific phosphorylation of inhibitory proteins, such as IκB, by their kinase(s). A primary target for IκB kinases is IκBα. This inhibitor is characterized by a signal response domain in the amino-terminal segment, ankyrin repeats in the midsection, and a PEST domain in the carboxyl terminal segment.12 The signal response domain contains two serines at positions 32 and 36, which are targeted for specific phosphorylation by IκB kinases.5,13 The PEST domain bears serines phosphorylated by casein kinase II involved in constitutive turnover of IκBα.14
The IκBα kinase that catalyzes inducible phosphorylation of serines 32 and 36 in response to cytokines was initially reported by Chen et al15 as part of a 700-kD complex requiring ubiquitin for full activity. Subsequently, two serine/threonine kinases termed IκB kinase α (IKKα or IKK1) and IκB kinase β (IKKβ or IKK2) of molecular weight 85 kD and 87 kD, respectively, were identified.16-19 Both of these kinases are homologous to a previously reported conserved helix-loop-helix ubiquitous kinase with a unique structure containing a kinase domain linked to a leucine zipper domain and a helix-loop-helix domain. Both IKKα and IKKβ phosphorylate serines 32 and 36 in IκBα, suggesting functional homology as well.19
Although it is well known that primary target cells for LPS are monocytes and macrophages,3 20 the mechanism by which LPS induces NF-κB in this cellular setting remained unknown. Therefore, we hypothesized that the recently discovered IκB kinases constitute an intracellular target for LPS. Our results provide evidence that IκB kinase in human monocytic THP-1 cells is targeted by LPS, thereby assigning this multiunit kinase complex a primary role as the signal transducer responsible for NF-κB mobilization in response to septic-shock inducers.
MATERIALS AND METHODS
Reagents.
Rabbit polyclonal antibodies were generated by immunizing rabbits with synthetic peptides corresponding to amino acids 1-28 (NH2-terminal peptide) and 731-744 (COOH-terminal peptide) of human IKKα and a synthetic peptide corresponding to amino acids 743-756 of human IKKβ. The peptides were covalently coupled to keyhole limpet hemocyanin before injection together with complete Freund adjuvant. The antibody lgG fraction of each antiserum was purified by absorption to and elution from Protein A Sepharose (Zymed, San Francisco, CA). IκBα-specific (amino acids 289-317) antipeptide rabbit antibodies were prepared as described previously.9Glutathione S-transferase (GST)-IκBα (1-54), IκBβ (1-44), and IκBε (1-61) fusion proteins (wild type and IκBα mutant S32A and S36A) were prepared as described.17,19 21
LPS preparations were derived from: (1) Escherichia coli0127:B8 (extracted by Boivin method; DIFCO, Detroit, MI), (2) E coli 0127:B8 (prepared by phenol extraction and gel filtration chromatography), (3) Pseudomonas aeruginosa serotype 10 (chromatographically purified by gel filtration), and (4)Salmonella minnesota (prepared by phenol extraction and gel filtration chromatography). The latter three LPS preparations were obtained from Sigma Chemical Company (St. Louis, MO).
Cell culture.
Human monocytic THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum containing no detectable LPS (<0.006 ng/mL in Limulus Amebocyte Gel Clot as determined by the manufacturer, Atlanta Biologicals, Norcross, GA), 2 mmol/L L-glutamine, and antibiotics as described.4 Where indicated, 5 mL of THP-1 cells (106/mL) were stimulated with E coli LPS O127:B8 (DIFCO) or TNF-α with the potency of 32,000 U per μg (Mallinckrodt, St Louis, MO) at concentrations and times specified in the text.
Immunoprecipitation and immunoblotting.
Cytoplasmic extracts were prepared from THP-1 cells by detergent lysis (0.1 % Nonidet P40 in 10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 300 mmol/L sucrose) in the presence of phosphatase inhibitors (12.5 mmol/L β-glycerophosphate, 2 mmol/L NaF, 100 μmol/L Na3VO4) and protease inhibitors (0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mg/mL pefabloc SC, 50 μg/mL antipain, 1 mg/mL aprotinin, 20 μg/mL chymostatin, 5 μg/mL E64, 1 μg/mL leupeptin, 1 μg/mL pepstatin, and 20 μg/mL phosphoramidon). Lysates were adjusted to a final concentration of 50 mmol/L HEPES (pH 7), 250 mmol/L NaCl, and 5 mmol/L EDTA before addition of specific IKKα or IKKβ antibody IgG (20 μg added to reaction mixture). Specific IκBα antipeptide antibody was used as described previously.9
Immunoprecipitations were typically performed in 400 μL reaction mixtures containing 100 to 500 μg of total cytoplasmic protein and 10 μL of protein A-Sepharose beads (Zymed). In some immunoprecipitation experiments, protein A-Sepharose beads were precoated with specific antibodies and washed once with ELB buffer (50 mmol/L HEPES, 250 mmol/L NaCl, 5 mmol/L EDTA, and 0.1% Nonidet P-40).5 Immunoprecipitates were washed three times with ELB buffer in the presence of phosphatase inhibitors, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidine difluoride membranes. Immunoreactive polypeptides were detected with anti-IKK antibodies and an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ).
IKK assay.
IκB kinase activity in immunoprecipitates obtained with anti-IKKα and anti-IKKβ antibodies in cytoplasmic lysate of THP-1 cells was measured as described previously.17, 22 Briefly, cytoplasmic extracts from 5 × 106 THP-1 cells were prepared and immunoprecipitated as described above. They were immunoprecipitated with anti-IKKα antibody IgG (10 μg added to 100 to 500 μg of total protein), unless specified otherwise. Immunoprecipitates were washed thrice with ELB buffer and once with kinase buffer (10 mmol/L HEPES pH 7.4, 1 mmol/L MnCl2, 5 mmol/L MgCl2, 12.5 mmol/L β-glycerophosphate, 50 μmol/L Na3VO4, 2 mmol/L NaF, 0.5 mmol/L dithiothrietol, and 10 μmol/L ATP). Immunoprecipitates were resuspended in kinase buffer (20 μL) containing 1 μg IκBα (residues 1-54), GST fusion protein, and 5μCi of32P-γ-ATP (ICN cat no. 38101x, 4,500 Ci/mmol). The kinase reaction was done at 30°C for 30 minutes and was stopped by addition of Laemmli buffer containing 2.5% β-mercaptoethanol. After a 5-minute incubation at 100°C, the mixture was spun down and the supernatant applied to SDS-PAGE (10%). Dried gels were applied to Biomax film (Eastman Kodak, Rochester, NY). In some experiments, dried gels were analyzed in Fuji FLA 2000 Fluorescent Image Analyzer (Fuji Medical Systems, Stamford, CT).
Electrophoretic mobility shift assay (EMSA).
RESULTS
Expression of endogenous IKKα and IKKβ in human monocytic THP-1 cells.
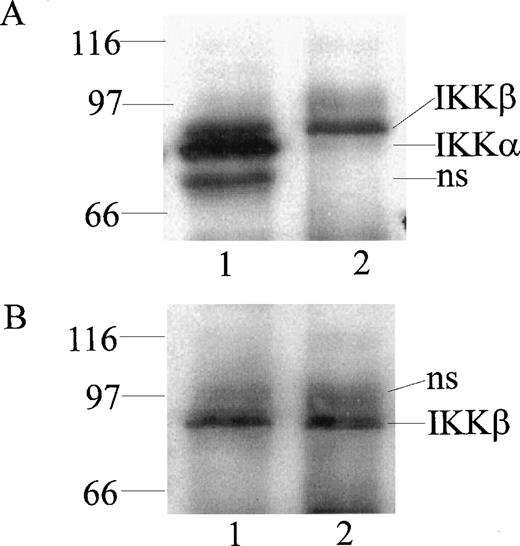
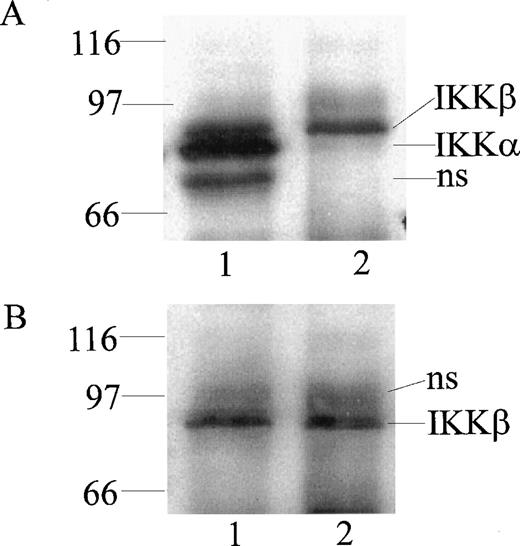
At least 2 kinases specific for IκBα and IκBβ were recently identified in Hela and 293 EBNA cells and named IKKα and IKKβ.16-19 To monitor their expression in human monocytic cells, we prepared rabbit polyclonal antibodies directed against peptides derived from the NH2-terminal segment (residues 1-28) and the COOH-terminal segment (residues 731-744) of IKKα and the COOH-terminal segment of IKKβ (residues 743-756). The antibodies were monospecific for homologous peptides in an enzyme-linked immunosorbent assay using peptide-coated wells (results not shown). When cytoplasmic extracts from monocytic THP-1 cells were immunoprecipitated and then probed with these antibodies by immunoblotting, 2 bands of 85 kD and 87 kD, apparent molecular weights, were discerned with anti-IKKα antibody (Fig 1A, lane 1). In contrast, anti-IKKβ antibody precipitated and reacted in immunoblotting with the 87-kD band representing IKKβ (Fig 1B, lane 2). Thus, the anti-IKKβ antibody was monospecific for IKKβ and did not cross-react with IKKα (Fig1B, lane 1). These bands were not visualized when antibodies were preincubated with homologous peptides before the immunoblotting (results not shown).
Detection of IKK and IKKβ in human monocytic THP-1 cells by immunoprecipitation and immunoblotting. Cytoplasmic lysates from unstimulated cells were immunoprecipitated with anti-IKK NH2-terminal peptide antibody (lane 1) and anti-IKKβ COOH-terminal peptide antibody (lane 2). Immunoprecipitated proteins were resolved by (A) SDS-PAGE and immunoblotted with anti-IKK NH2-terminal peptide antibody, with (B) anti-IKKβ COOH-terminal peptide antibody (NS represents nonspecific band).
Detection of IKK and IKKβ in human monocytic THP-1 cells by immunoprecipitation and immunoblotting. Cytoplasmic lysates from unstimulated cells were immunoprecipitated with anti-IKK NH2-terminal peptide antibody (lane 1) and anti-IKKβ COOH-terminal peptide antibody (lane 2). Immunoprecipitated proteins were resolved by (A) SDS-PAGE and immunoblotted with anti-IKK NH2-terminal peptide antibody, with (B) anti-IKKβ COOH-terminal peptide antibody (NS represents nonspecific band).
Stimulus-dependent activation of IKKα and IKKβ in human monocytic THP-1 cells.
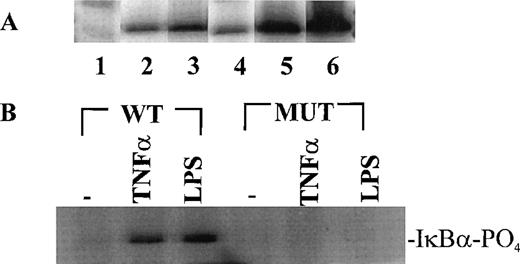
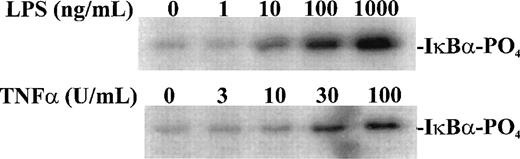
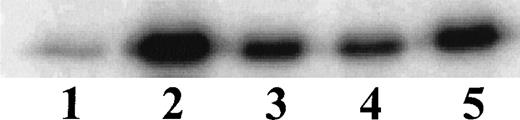
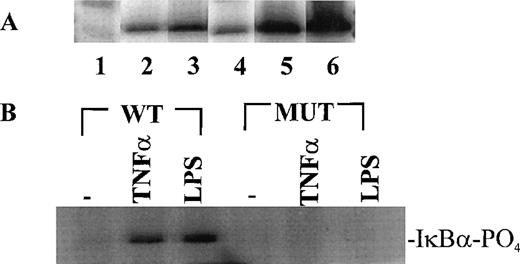
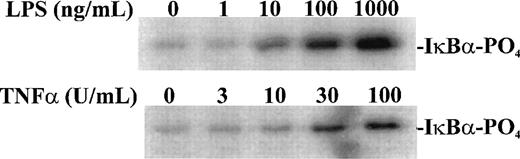
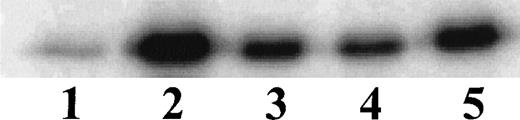
Having established the presence of IKKα and IKKβ in the cytoplasmic fraction of human monocytic THP-1 cells, we examined the kinase activity in immunoprecipitates obtained from LPS-stimulated cells as compared with those from TNF-α–stimulated cells (Fig 2A). Stimulated and nonstimulated THP-1 cells were lysed, and the cytoplasmic fraction was prepared by differential centrifugation. Immunoprecipitated IKKα and IKKβ were assayed for kinase activity with 32P-γ-ATP and a fusion protein made of IκBα residues 1-54 attached to GST.17 22 Nonstimulated THP-1 cells had no detectable kinase activity in immunoprecipitates obtained with anti-IKKα antibodies (Fig 2A, lane 1). Low-level basal kinase activity was detectable in anti-IKKβ antibody immunoprecipitates (Fig2A, lane 4). When cells were stimulated with LPS from E coli0127:B8 (Difco) (1 μg/mL for 30 minutes), kinase activity was readily detectable by phosphorylation of GST-IκBα (residues 1-54) fusion protein in immunoprecipitates produced by 2 antibodies (Fig 2A, lanes 3 and 6). Phosphoimager analysis of these bands indicated that the LPS-responsive kinase activity immunoprecipitated by monospecific anti-IKKβ antibody exceeds the activity precipitated by anti-IKKα antibody. Similar, albeit weaker, kinase activity of immunoprecipitated IKKα and IKKβ was evoked by TNF-α (100 U/mL for 5 minutes) (Fig2A, lanes 2 and 5). Kinase activity of immunoprecipitated IKKα and IKKβ was specific for serines 32 and 36 of IκBα because a fusion protein containing mutant IκBα segment with serines 32 and 36 replaced by alanine was not phosphorylated (Fig 2B). Activation of IκB kinase complex containing IKKα and IKKβ by LPS in THP-1 cells was concentration dependent. At least 10 ng/mL of LPS from E coli 0127:B8 (DIFCO, Detroit, MI) was sufficient to induce detectable IKKα and IKKβ activity (Fig3). By comparison, the lowest concentration of TNF-α inducing similar activation was 30 U/mL or approximately 1 ng/mL. All preparations of LPS tested induced activation of IKKα and IKKβ. These included LPS derived from E coli 0127:B8, P aeruginosa, and S minnesota (Fig 4). The activation of IKKα and IKKβ by structurally diverse LPS from different gram-negative bacterial species indicates that IKK complex responds to all endotoxic LPS tested. However, the method of extraction of LPS derived from the same E coli 0127:B8 serotype results in different IKK complex activating potency of LPS (Fig 4, lanes 2 and 3).
Stimulus-dependent activation of IKK and IKKβ in human monocytic THP-1 cells. (A) Nonstimulated cells (lanes 1, 4), TNF-–stimulated (100 U/mL)(5 minutes) cells (lanes 2, 5), and LPSE coli 0127:B8 (1 μg/mL)-stimulated (30 minutes) cells (lanes 3, 6). Lanes 1-3 represent immunoprecipitates obtained with anti-IKK antibody; and lanes 4-6 represent immunoprecipitates obtained with anti IKKβ antibody. The IKK kinase assay was done as described in Materials and Methods by using IκB-GST fusion protein as the substrate. (B) IKK and IKKβ activity toward wild type IκB substrate and IκB mutant (S32A and S36A) substrate prepared as GST fusion proteins. The cells were stimulated with TNF- (100 U/mL) for 5 minutes and with LPS (1 μg/mL) for 30 minutes.
Stimulus-dependent activation of IKK and IKKβ in human monocytic THP-1 cells. (A) Nonstimulated cells (lanes 1, 4), TNF-–stimulated (100 U/mL)(5 minutes) cells (lanes 2, 5), and LPSE coli 0127:B8 (1 μg/mL)-stimulated (30 minutes) cells (lanes 3, 6). Lanes 1-3 represent immunoprecipitates obtained with anti-IKK antibody; and lanes 4-6 represent immunoprecipitates obtained with anti IKKβ antibody. The IKK kinase assay was done as described in Materials and Methods by using IκB-GST fusion protein as the substrate. (B) IKK and IKKβ activity toward wild type IκB substrate and IκB mutant (S32A and S36A) substrate prepared as GST fusion proteins. The cells were stimulated with TNF- (100 U/mL) for 5 minutes and with LPS (1 μg/mL) for 30 minutes.
Concentration-dependent activation of IKK and IKKβ by LPS and TNF-. Human monocytic THP-1 cells were stimulated with increasing concentrations of LPS for 30 minutes or TNF- for 5 minutes and cytoplasmic lysates were prepared with anti-IKK antibody. The IKK kinase assay was done as described in Materials and Methods; the substrate was a fusion protein made of wild-type IκB (amino acids 1-54) and GST.
Concentration-dependent activation of IKK and IKKβ by LPS and TNF-. Human monocytic THP-1 cells were stimulated with increasing concentrations of LPS for 30 minutes or TNF- for 5 minutes and cytoplasmic lysates were prepared with anti-IKK antibody. The IKK kinase assay was done as described in Materials and Methods; the substrate was a fusion protein made of wild-type IκB (amino acids 1-54) and GST.
Activation of IKK and IKKβ in human monocytic THP-1 cells by LPS derived from different gram-negative bacteria. Lane 1, Unstimulated cells; Lane 2, LPS from E coli 0127:B8 extracted by Boivin method (DIFCO); Lane 3, LPS from E coli 0127:B8 prepared by phenol extraction and gel filtration chromatography (Sigma); Lane 4, LPS from P aeruginosa serotype 10. LPS fromS minnesota. Human monocytic THP-1 cells were stimulated with different preparations of LPS at 1 μg/mL for 30 minutes and the IKK kinase assay was done as specified in Materials and Methods.The kinase complex was immunoprecipitated with anti-IKKβ antibody.
Activation of IKK and IKKβ in human monocytic THP-1 cells by LPS derived from different gram-negative bacteria. Lane 1, Unstimulated cells; Lane 2, LPS from E coli 0127:B8 extracted by Boivin method (DIFCO); Lane 3, LPS from E coli 0127:B8 prepared by phenol extraction and gel filtration chromatography (Sigma); Lane 4, LPS from P aeruginosa serotype 10. LPS fromS minnesota. Human monocytic THP-1 cells were stimulated with different preparations of LPS at 1 μg/mL for 30 minutes and the IKK kinase assay was done as specified in Materials and Methods.The kinase complex was immunoprecipitated with anti-IKKβ antibody.
IKKα and IKKβ are differentially activated by LPS and TNF-α.
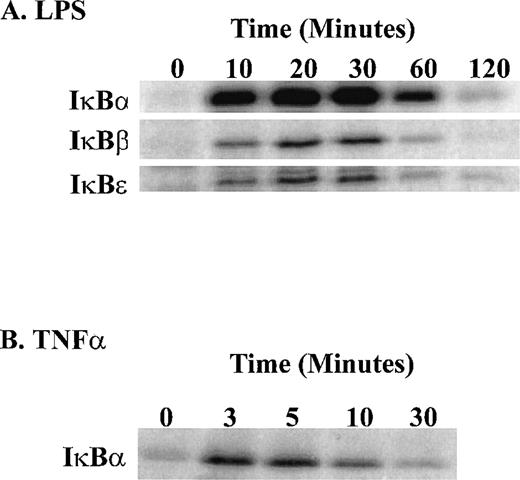
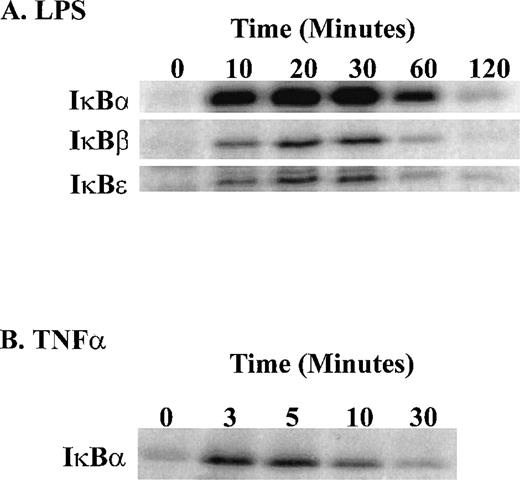
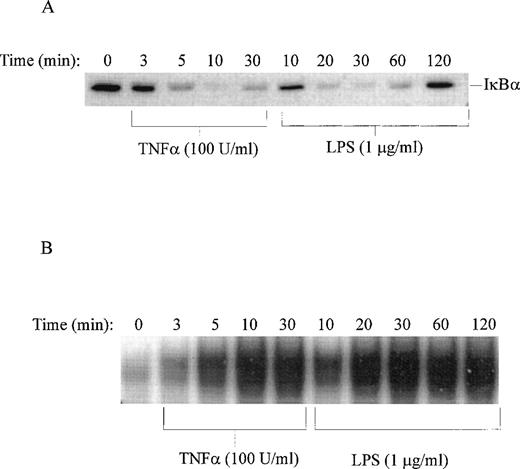
Stimulation of human monocytic THP-1 cells by LPS or TNF-α was followed by determination of enzymatic activity of IKKα and IKKβ in cytoplasmic extracts of stimulated cells prepared at different time intervals. Three NF-κB inhibitors, IκBα, IκBβ, and IκBε, presented as GST fusion proteins were tested as phosphorylation substrates in standard in vitro kinase assays with immune complex containing IKK. LPS stimulation of THP-1 cells for 10 minutes was sufficient to induce measurable kinase activity, which peaked at 30 minutes (Fig 5). The significant increase in IκBα kinase activity at 10 minutes is consistent with phosphorylation and degradation of IκBα in LPS-treated THP-1 cells after 10 minutes stimulation as documented by immunoblotting with specific IκBα antipeptide antibody. Concurrently, the nuclear import of NF-κB monitored by the electrophoretic mobility shift assay shows progressive increase reaching maximum at 30 minutes (Fig 6A and B). Significantly, all three inhibitory proteins, IκBα, IκBβ, and IκBε, used as GST fusion proteins at equivalent concentrations were phosphorylated with similar kinetics in response to LPS. In contrast, TNF-α stimulation of THP-1 cells resulted in much faster kinetics of phosphorylation of IκBα-GST fusion protein reaching peak at 3 to 5 minutes and declining after 20 minutes (Fig 5). This pattern of rapid activation by TNF-α is consistent with much faster degradation of endogenous IκBα and nuclear import of NF-κB reaching maximum at 10 minutes in TNF-α–stimulated THP1 cells (Fig 6A and B). The 5 times lower concentration of TNF-α (20 U/mL) did not change the rapid course of IKKα/IKKβ activation (results not shown). Thus, the differences in activation kinetics of IκB kinase complex induced by LPS and TNF-α suggest that these proinflammatory agonists evoke different signaling pathways resulting in activation of IKKα-containing and IKKβ-containing complex in human monocytic cells.
Time course of LPS-induced activation of IKK and IKKβ as compared with that induced by TNF-. (A) LPS-induced phosphorylation of 1μg IκB-GST, IκBβ-GST, and IκBɛ-GST fusion proteins; (B) TNF-–induced phosphorylation of IκB-GST fusion protein. The IKK kinase assay was done as specified in Materials and Methods and the kinase complex was immunoprecipitated with anti-IKK antibody.
Time course of LPS-induced activation of IKK and IKKβ as compared with that induced by TNF-. (A) LPS-induced phosphorylation of 1μg IκB-GST, IκBβ-GST, and IκBɛ-GST fusion proteins; (B) TNF-–induced phosphorylation of IκB-GST fusion protein. The IKK kinase assay was done as specified in Materials and Methods and the kinase complex was immunoprecipitated with anti-IKK antibody.
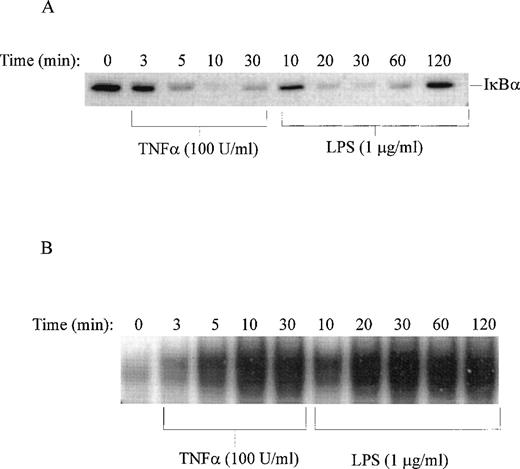
Time course of LPS-induced degradation of IκB and nuclear import of NF-κB. (A) Immunoblotting of IκB in nonstimulated (time 0) and TNF-–stimulated or LPS-stimulated THP-1 cells. (B) Electrophoretic mobility shift assay of NF-κB in nuclear fractions isolated from nonstimulated (time 0) and TNF-–stimulated or LPS-stimulated THP-1 cells. LPS from E coli 0127:B8 was used in concentration of 1 μg/mL and TNF- at 100 U/mL.
Time course of LPS-induced degradation of IκB and nuclear import of NF-κB. (A) Immunoblotting of IκB in nonstimulated (time 0) and TNF-–stimulated or LPS-stimulated THP-1 cells. (B) Electrophoretic mobility shift assay of NF-κB in nuclear fractions isolated from nonstimulated (time 0) and TNF-–stimulated or LPS-stimulated THP-1 cells. LPS from E coli 0127:B8 was used in concentration of 1 μg/mL and TNF- at 100 U/mL.
DISCUSSION
The results presented here have several important implications for the cellular and molecular pathogenesis of septic shock induced by LPS. First, our studies provide evidence that IκB kinase complex containing IKKα and IKKβ is an intracellular target for LPS produced by different gram-negative bacteria (enterobacteriaceae and nonenterobacteriaceae).
Second, IKKβ appears to be a more LPS-responsive signal transducer than IKKα based on observed kinase activity (Fig 2). This is consistent with recent demonstration that IKKα is not required for signaling induced by proinflammatory cytokines, IL-1, or TNF-α.23-25 The lowest concentration of LPS that induces detectable activation of IKKα and IKKβ in human monocytic THP-1 cells was 10 ng/mL. Treatment of human peripheral blood mononuclear cells (“adherent monocytes”) with 100 ng/mL of E coli0111:B4 lipopolysaccharide also induces IKK activity, thus validating THP-1 cells as a model for studying LPS-induced IKK activation in human cells of the myelomonocytic lineage.26 Moreover, LPS-stimulated and TNF-α–stimulated IKK complex can phosphorylate not only inhibitory proteins IκBα and IκBβ, but also IκBε. The IκB kinases provide a pivotal checkpoint in NF-κB–mediated signaling to the nucleus. Thus, activation of IκB kinases by LPS in human monocytic cells constitutes an important link in understanding the NF-κB recruitment in molecular pathogenesis of septic shock. Whether continuing activation of IκB kinases in vivo by LPS derived from different gram-negative bacteria results in persistent nuclear import of NF-κB demonstrated in nonsurvivors of septic shock11 remains to be established with in vivo models. Importantly, kinetics of phosphorylation of all three inhibitory proteins, IκBα , IκBβ, and IκBε, as demonstrated herein by IKK complex activated by LPS can contribute to progressive activation of the nuclear import of NF-κB.
Third, the distinct activation kinetics of IKKα-containing and IKKβ-containing complex by LPS and TNF-α in human monocytic cells is consistent with different rate of the nuclear import of NF-κB in these cells (Fig 6).4,9,27 These agonists signal through distinct receptors (toll-like receptor 2 and 4 vTNF-α receptor 1 and 2).28-31 Receptor-proximal steps in intracellular signaling induced by TNF-α that interacts with its cognate receptor, TNFR1, and by LPS interacting with its cognate receptor TLR2 differ.29 Whereas TNF-α–induced signaling requires TRAF2 in mammalian cell transfectants, LPS-induced signaling via TLR2 seems to require TRAF6.29,32 The latter is required for IL-1 induced signaling, which also uses an adaptor protein MyD88.33-35 Dominant-negative mutants of MyD88 and TRAF6 expressed in TLR2- and IL-1R–transfected cells selectively blocked signaling evoked by LPS and IL-1 but remained ineffective toward TNF-α–induced signaling.29 32 These differences in receptor-proximal signaling may explain the different kinetics of IKK complex activation by LPS and TNF-α in human monocytic THP-1 cells used in our experiments. Further dissection of LPS-stimulated and TNF-α–stimulated specific pathways of IKK complex activation will help in the development of new inhibitors designed to interrupt potentially lethal lipopolysaccharide-induced intracellular mechanism of septic shock.
ACKNOWLEDGMENT
The authors thank Traci Tidwell, Susan Rowlinson, and Erica Holleran for assistance in preparation of the manuscript, Zhi-Liang Chu and Min Dai for preparation of GST fusion proteins, and Hiroyasu Nakano, Juntendo University School of Medicine, Tokyo, Japan, for plasmid pGEX-4T-IκBε (1-61).
Supported in part by the National Institutes of Health, Grant Nos. R37HL30647, HL45994, and AI33839.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jacek Hawiger, MD, PhD, Department of Microbiology & Immunology, Vanderbilt University School of Medicine, A-5321 MCN, 1161 21st Ave South, Nashville, TN 37232-2363; e-mail:jacek.hawiger@mcmail.vanderbilt.edu.