Abstract
Cross-linking of Fc receptors for IgA, FcR (CD89), on monocytes/macrophages is known to enhance phagocytic activity and generation of oxygen free radicals. We provide evidence here that the FcR signals through the γ subunit of FcɛRI in U937 cells differentiated with interferon γ (IFNγ). Our results provide the first evidence that FcR-mediated signals modulate a multimolecular adaptor protein complex containing Grb2, Shc, SHIP, CrkL, Cbl, and SLP-76. Cross-linking of FcRI using anti-FcRI induces the phosphorylation of the γ subunit as detected by mobility retardation on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Stimulation of FcRI induced the tyrosine phosphorylation of Shc and increased the association of Grb2 with Shc and CrkL. Grb2 associates constitutively with Sos, and the latter undergoes mobility shift upon FcRI stimulation. The complex adapter proteins, Cbl and SLP-76, are physically associated in myeloid cells and both proteins undergo tyrosine phosphorylation upon FcR stimulation. These data indicate that the stimulation of FcR results in the modulation of adaptor complexes containing tyrosine-phosphorylated Cbl, Shc, SHIP, Grb2, and Crkl. Experiments performed with the Src kinase inhibitor, PP1, provide the first evidence that Src kinase activation is required for FcRI-induced production of superoxide anions and provide insight into the mechanism for FcR-mediated activation of downstream oxidant signaling in myeloid cells.
IgA IS THE PRIMARY Ig in body secretions and plays a critical role in antibody-dependent cell-mediated immunity against continual threats from microbes at mucosal surfaces.1 IgA plays a central role in secretory immunity, and the receptor for Fc portion of IgA, FcαR (CD89), has been cloned.2 The FcαR is expressed primarily on monocytes/macrophages, neutrophils, and eosinophils and a subpopulation of lymphocytes.1,3-5 FcαR contains sequence homology with Fc receptors for IgG and IgE in the Ig-like extracellular domains.2 The transmembrane regions of Fc receptors (FcRs), including FcγRI, FcγRII, FcγRIII, and FcεRI, also share 2 hydrophobic residues; however, there is minimal sequence homology in the cytoplasmic tails of these FcRs, suggesting a certain degree of divergent structure and function. FcαR is known to share functional characteristics with other FcRs of the Ig gene superfamily.2 Cross-linking of FcαR triggers phagocytosis, antibody-dependent cell-mediated cytotoxicity (ADCC), tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) release, and oxygen free radical generation in myeloid cells.5-9Recent evidence suggests that FcαRI activation may be a very potent effector mechanism for delivering a cell-mediated cytotoxic response against tumor targets in vivo.10 11
The γ subunit originally described as part of FcεRI receptor is known to associate with certain FcγRs, FcαRI, and the T-cell receptor (TCR)-CD3 complex.12,13 The FcεRI γ chain contains a functional motif coupling these receptors to intracellular signaling cascades.14,15 Recent evidence generated in the FcεRIγ knockout mouse model provides definitive proof that the γ subunit is critically important in mediating inflammation and autoimmunity.14,16,17 Signaling through FcγRs is initiated by the phosphorylation of tyrosine residues present in the γ chain, termed the immunoreceptor tyrosine-based activation motif (ITAM).14,18,19 The current model for FcR signaling is that the tyrosine phosphorylation of the ITAM is mediated by nonreceptor protein tyrosine kinases belonging to the Src-family, which leads to the activation of downstream signaling cascades.20,21 Tyrosine phosphorylation of the ITAM provides a docking site for Src-homology domain 2 (SH2)-containing proteins, including Syk, ZAP-70, and Shc.22-25 In turn, this upstream signaling event is coupled through adaptor molecules to Ras/Raf-1/MAP kinases pathway and to activation of phosphoinositol-3 (PI-3) kinase and AKT.25 26 From these combined results, we conclude that the elucidation of FcεRIγ specific signaling events may identify important pharmacologic and biologic targets for control of inflammation and autoimmunity.
Adaptor proteins, including Grb2, Nck, CrkL, and Shc, relay signals from upstream protein tyrosine kinases to small GTPases by coupling aggregated receptors with guanidine nucleotide exchange factors such as Sos and C3G.27 These adaptor proteins containing SH2 and SH3 domains provide binding sites for proteins having phosphotyrosine or proline-rich regions, respectively.28 The complex adaptor protein, p120c-Cbl, is tyrosine phosphorylated upon stimulation of growth factor receptors, cytokine receptors, and other receptors lacking intrinsic catalytic activity, such as TCR, B-cell receptor (BCR), or Fc receptors.29,30 Cbl binds to the SH3 domains of a number of proteins, including Fyn, Grb2, Lck, Fgr, Nck, Crk, PLCγ1, and PI-3 kinase.31 It also interacts with SH2-containing proteins, including Fyn, Lck, or Blk, after tyrosine phosphorylation.32
Little is known about how the FcαR mediates signals induced by IgA immune complexes leading to its effector functions. Recent work has shown that the FcαR associates with the FcεR1γ chain and that the γ subunit is tyrosine phosphorylated upon FcαR stimulation.12,13 Other data have recently implicated the Src family kinase, Lyn, in FcαRI signaling in THP-1 cells, and Launay et al33 demonstrated that the nonreceptor protein tyrosine kinases, Syk and Btk, are activated after FcαRI aggregation.33,34 Despite this progress, the FcαRI signaling events downstream of FcεRγ, Src, and nonreceptor kinases have not been elucidated. We postulated that FcαR signaling requires the upstream activation of Src family kinases, resulting in the tyrosine phosphorylation of adaptor proteins that then mediate specific signaling events (oxidant signaling) in myeloid cells. We provide evidence here in our myeloid system that cross-linking of FcαRI induces the tyrosine phosphorylation of the γ subunit of FcεRIα as indicated by an induction of mobility shift. FcαR induces the association of Grb2 with Shc and CrkL. Sos, which is constitutively bound to Grb2, is mobility shifted in response to FcαR cross-linking. Shc is heavily phosphorylated on tyrosine residues and associated with Grb2 and the SH2 domain-containing inositol polyphosphate 5-phosphatase (SHIP)35 36 upon FcαR stimulation. Cbl and SLP-76 are observed to interact constitutively, but their level of tyrosine phosphorylation increases dramatically upon FcαR stimulation. Experiments using the specific Src-kinase inhibitor, PP1, provide the first evidence that Src kinases are required for the tyrosine phosphorylation of Shc and SHIP, the formation of adaptor protein-protein complexes, and FcαR signaling leading to the activation of the myeloid respiratory burst.
MATERIALS AND METHODS
Antibodies.
The FcαRI (A77)- and FcγRI (32.2)-specific antibodies (both Fab′2 fragments) were kindly provided by Medarex Inc (Annandale, NJ). The cross-linking antibody was a rabbit antimouse F(ab′)2 fragment (RαM) obtained from Organon Teknika Corp (West Chest, PA). Antiphosphotyrosine [anti-Tyr(p)] and anti-Shc were purchased from Upstate Biotechnology Inc (Lake Placid, NY). Anti-Grb2, anti-Cbl, anti-Sos, and anti-CrkL were obtained from Santa Cruz Co (Santa Cruze, CA). Dr Gary A. Koretzky (University of Iowa, Iowa City, IA) generously provided anti–SLP-76 antisera. Anti-SHIP antisera used in these experiments was kindly provided by Dr K. Mark Coggeshall36 (Ohio State University, Columbus, OH).
Differentiation and cross-linking of U937 cells.
U937 cells were maintained in RPMI 1640 with 10% fetal bovine serum (FBS) and differentiated with 250 U/mL human recombinant interferon γ (IFNγ) for 4 days; these cells were then termed U937IF cells. U937IF cells were cultured at a concentration of 5 × 105cells and the medium was replenished with fresh IFNγ every 2 days. For cross-linking of FcαR or FcγRI receptors of U937IF cells, the cells were washed twice in cold Hanks’ balanced salt solution (HBSS) and adjusted to a concentration of 2 × 107 cells/mL. Cells in a volume of 0.5 mL were incubated on ice for 30 minutes with anti-FcαR (A77; 1.0 μg/sample). We then added RαM (5 μg/sample) at 37°C for different periods. Stimulated cells were rapidly cooled with 0.8 mL of cold HBSS and centrifuged at 500g at 4°C for 5 minutes. The cell pellet was lysed with 0.8 mL of Triton X-100 extraction buffer on ice for 30 minutes.
Immunoprecipitation.
Cell lysates were prepared in a Triton X-100 extraction buffer containing 1% Triton X-100, 10 mmol/L Tris, pH 7.6, 50 mmol/L NaCl, 0.1% bovine serum albumin (BSA), 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), 1% aprotinin, 5 mmol/L EDTA, 50 mmol/L NaF, 10 μmol/L phenylarsine oxide, and 2 mmol/L sodium orthovanadate. Lysates were cleared by centrifugation at 15,000g at 4°C for 30 minutes. For immunoprecipitation of protein, 1 μg of anti-γ subunit, anti-Cbl, anti-Shc, anti-Grb2, or anti–SLP-76 antibody was added to clarified cell lysates. After incubation on ice for 1 hour, 100 μL of a 10% solution of formalin-fixed Staphylococcus aureus was added to immunoprecipitates and incubated on ice for 1 hour. The absorbed immune complexes were washed 3 times in Triton X-100 extraction buffer and resuspended with 25 μL of 1× sample buffer. After boiling at 98°C for 5 minutes, immune complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Electrophoresis and immunoblotting.
Immunoprecipitates were resolved on 7.5% to 12.5% acrylamide and 0.193% bisacrylamide gels by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane (11 mAh/cm2) using semidry blotting transfer system (Ellard Inc, Seattle, WA). The membrane was incubated with blocking solution (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 5% powered milk) at room temperature for 1 hour and then incubated with specific anti-Tyr(p), anti-Shc, anti-Grb2, anti-CrkL, anti-Sos, anti-Cbl, or anti–SLP-76 with continuous agitation. After 3 washes in rinse solution (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl), the membrane was incubated at room temperature for 1 hour with antimouse or antirabbit conjugated with horseradish peroxidase for enhanced chemiluminescence detection (Amersham Co, Arlington Heights, IL) or conjugated with alkaline phosphatase for colorimetric development. For reprobing, the membrane was stripped with 0.1 mol/L glycine, pH 2.5, at room temperature for 30 minutes and then reblotted with primary antibody.
Measurement of respiratory burst response.
U937IF cells were pretreated with the Src-specific protein tyrosine kinase inhibitor, PP137(4-amino-5-(4-methyphenyl-7-(t-butyl)pyrazolo(3,4-d)pyrimidine) (Calbiochem Co, La Jolla, CA; the PP1 concentration was 10 μmol/L) or dimethyl sulfoxide (DMSO) as control for 45 minutes at 37°C in the presence of A77 antibody. Cross-linking antibody was then added to the cells (RαM, Fab′2), and measurement of respiratory burst was performed as described previously.25 Briefly, this involves the quantitation of superoxide anions as measured by the reduction in ferricytochrome c as determined by absorbtion at 550 nm wavelength. Data were expressed in nanomoles of superoxide liberated from 2 × 106 cells over 30 minutes.
RESULTS
FcαRI signals through the FcεRIγ chain in myeloid cells.
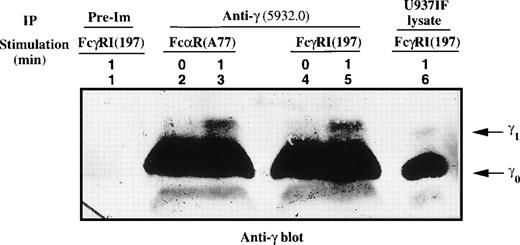
Flow cytometry has demonstrated that the FcαRI and FcγRI receptors are expressed in equivalent levels in U937IF cells (data not shown). We previously demonstrated that the γ subunit of FcεRIα was retarded in mobility upon FcγRI activation.38 Metabolic labeling experiments followed by phosphoamino acid analysis performed on the γ1 isoform of FcεRIγ showed that γ1 is phosphorylated on serine, threonine, and tyrosine upon FcγR cross-linking in U937IF cells.38 It is known that the FcαR is functionally related to the FcγRI receptor, a concept that led us to examine whether FcαR-mediated signaling may involve the γ subunit of the FcεRI receptor in our system. To test this hypothesis, U937IF lysates were prepared from resting and stimulated cells using anti-FcαRI (A77) or anti-FcγRI (197) specific antibodies followed by cross-linking and immunoprecipitated with anti-γ (5932.0) antibody; these IPs were then subjected to Western blot analysis. Anti-γ blots showed that γ subunit underwent a significant retardation in mobility as assessed by SDS-PAGE in U937IF cells (as indicated by the marked mobility shift in γ1 isoform) upon stimulation with anti-FcαR or anti-FcγRI antibodies (Fig 1, compare lanes 2 to 3 and 4 to 5). Antiphosphotyrosine blot showed that γ1 subunit was phosphorylated on tyrosine residues in U937IF cells upon stimulation with anti-FcαRI, similar to previous observations for anti-FcγRI stimulation38 39 (data not shown).
Mobility shift of the γ subunit of FcɛRI after cross-linking of FcRI. The γ subunit was immunoprecipitated from lysates of FcRI (lanes 2 and 3) or FcγRI (lanes 4 and 5) cross-linked U937IF cells as described in Materials and Methods. Lane 1 represents immunoprecipitation using rabbit IgG of U937IF cells stimulated with anti-FcγRI and rabbit antimouse F(ab′)2 antibody (RM) for 1 minute. Resting U937IF cells were immunoprecipitated with anti-γ antibodies (lanes 2 and 4). Anti-γ immunoprecipitates of U937IF cells stimulated with anti-FcR (A77) after RM (both Fab′2 fragments) for 1 minute (lane 3) or with anti-FcγRI after RM for 1 minute (lane 5). Lane 6 is a whole cell lysate prepared from U937IF cells stimulated with anti-FcγRI antibody after RM for 1 minute. γ0 and γ1 represent the baseline and mobility shifted isoforms of FcɛRIγ subunit, respectively.
Mobility shift of the γ subunit of FcɛRI after cross-linking of FcRI. The γ subunit was immunoprecipitated from lysates of FcRI (lanes 2 and 3) or FcγRI (lanes 4 and 5) cross-linked U937IF cells as described in Materials and Methods. Lane 1 represents immunoprecipitation using rabbit IgG of U937IF cells stimulated with anti-FcγRI and rabbit antimouse F(ab′)2 antibody (RM) for 1 minute. Resting U937IF cells were immunoprecipitated with anti-γ antibodies (lanes 2 and 4). Anti-γ immunoprecipitates of U937IF cells stimulated with anti-FcR (A77) after RM (both Fab′2 fragments) for 1 minute (lane 3) or with anti-FcγRI after RM for 1 minute (lane 5). Lane 6 is a whole cell lysate prepared from U937IF cells stimulated with anti-FcγRI antibody after RM for 1 minute. γ0 and γ1 represent the baseline and mobility shifted isoforms of FcɛRIγ subunit, respectively.
FcαRI signals through Grb2 and CrkL adaptor proteins.
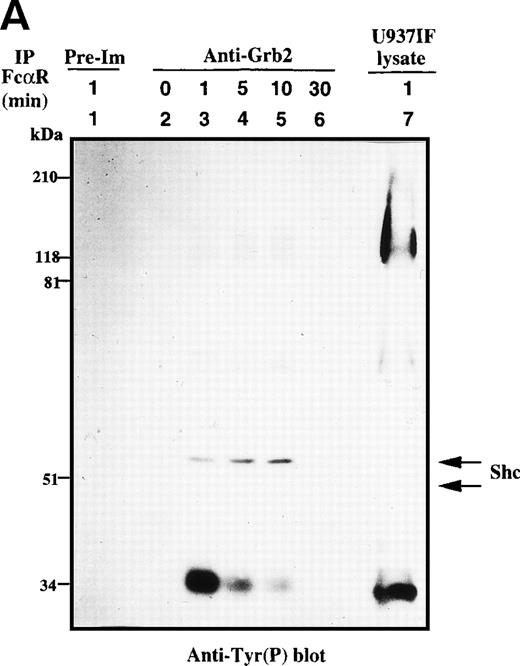
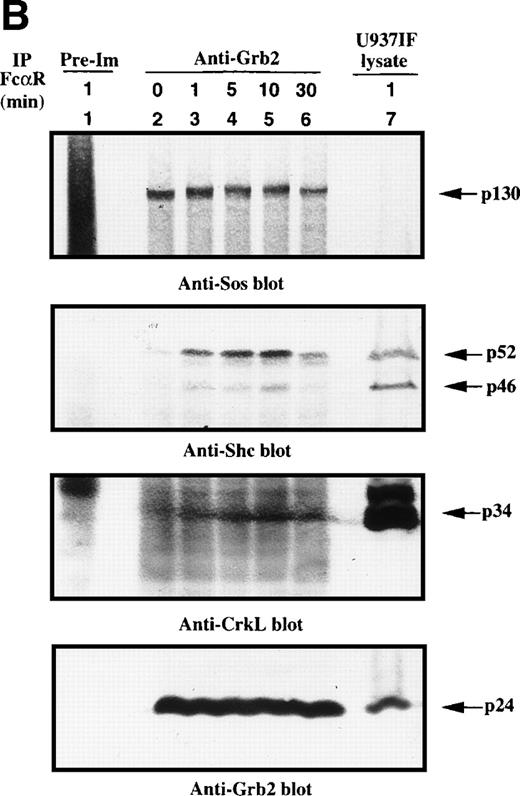
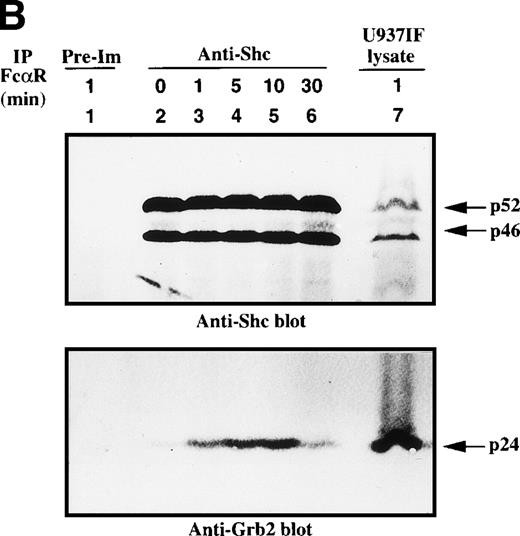
Because the tyrosine phosphorylation of the ITAM motif in the γ subunit provides a docking site to nonreceptor tyrosine kinases or adaptor proteins after FcγRI stimulation,24-26 we examined whether FcαR mediates signals through adaptor proteins, including Grb2, Shc, Cbl, and SLP-76. Immunoprecipitation studies of Grb2 demonstrated that Grb2 recruited tyrosine-phosphorylated Shc, mainly the p52 isoform, 1 minute after FcαR stimulation and peaked at 5 to 10 minutes (Fig 2A). We then tested whether the Grb2/Shc adaptor complex contains other proteins, including Sos and CrkL. Sos was associated with Grb2 to form a Grb2/Shc/Sos complex (Fig 2B). Mobility shift of Sos appeared by 1 minute and reached a maximum by 5 to 10 minutes after FcαR stimulation (Fig 2B, first panel). Interestingly, the mobility shift of Sos was coincident with the pattern of interaction of Grb2 with tyrosine-phosphorylated Shc (Fig 2B, second panel). Grb2 inducibly recruited Shc 1 minute after FcαR stimulation and lost this interaction with tyrosine-phosphorylated Shc when the mobility of Sos returned to its baseline in resting U937IF cells around 30 minutes after stimulation of FcαR. CrkL was induced to bind to Grb2 in U937IF cells stimulated with anti-FcαR antibody (Fig 2B, third panel). Other data reported from our laboratory suggest that the induced interaction between Grb2 and CrkL occurs through the capacity of Grb2 to bind to Cbl.26,40 When Cbl becomes tyrosine phosphorylated after FcγRI stimulation, it binds the CrkL-SH2 domain directly.40 Our Grb2 immunoblots showed that an equivalent amount of Grb2 was precipitated in Fig 2B, lanes 2 through 6 (panel 4). These data suggest that FcαRI modulates Grb2 to form a multimolecular complex containing tyrosine-phosphorylated Shc, CrkL, and the mobility-shifted form of Sos.
Grb2-Sos complex recruits tyrosine-phosphorylated Shc and CrkL after FcRI aggregation. We studied Grb2-bound phosphoproteins in U937IF cells after FcR stimulation. (A) Antiphosphotyrosine blot performed on anti-Grb2 immunoprecipitates. Lane 1 is a control immunoprecipitation with rabbit IgG. Anti-Grb2 immunoprecipitates from resting U937IF cells (lane 2) and from U937IF cells stimulated with anti-FcR and RM (both Fab′2 fragments) for 1 minute (lane 3), for 5 minutes (lane 4), for 10 minutes (lane 5), or for 30 minutes (lane 6), respectively. Lane 7 represents a whole cell lysate of U937IF cells stimulated with anti-FcR after RM antibodies for 1 minute. (B) The same membrane was stripped and reprobed with anti-Sos (first panel), anti-Shc (second panel), anti-CrkL (third panel), and anti-Grb2 (fourth panel), respectively. Lanes are identical to those in (A).
Grb2-Sos complex recruits tyrosine-phosphorylated Shc and CrkL after FcRI aggregation. We studied Grb2-bound phosphoproteins in U937IF cells after FcR stimulation. (A) Antiphosphotyrosine blot performed on anti-Grb2 immunoprecipitates. Lane 1 is a control immunoprecipitation with rabbit IgG. Anti-Grb2 immunoprecipitates from resting U937IF cells (lane 2) and from U937IF cells stimulated with anti-FcR and RM (both Fab′2 fragments) for 1 minute (lane 3), for 5 minutes (lane 4), for 10 minutes (lane 5), or for 30 minutes (lane 6), respectively. Lane 7 represents a whole cell lysate of U937IF cells stimulated with anti-FcR after RM antibodies for 1 minute. (B) The same membrane was stripped and reprobed with anti-Sos (first panel), anti-Shc (second panel), anti-CrkL (third panel), and anti-Grb2 (fourth panel), respectively. Lanes are identical to those in (A).
Kinetics of Shc-Grb2 interaction after FcαRI aggregation.
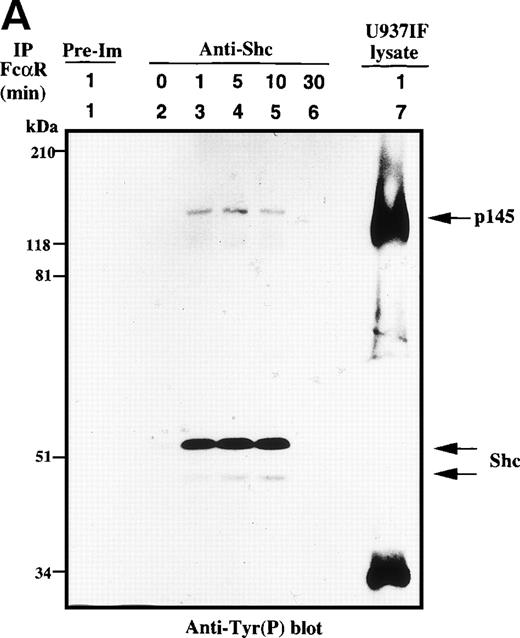
We next assessed the kinetics of the Shc interaction with Grb2. Shc was immunoprecipitated from U937IF cells stimulated with anti-FcαRI antibody and immunoblotted for phosphotyrosine (Fig 3A). Shc, mainly p52, was heavily tyrosine phosphorylated by 1 minute after FcαR stimulation and remained tyrosine phosphorylated for 10 minutes. Tyrosine-phosphorylated Shc coprecipitated with a p140-145 phosphoprotein only in U937IF cells stimulated with anti-FcαR antibody for 1 to 10 minutes. The same membrane was stripped and reprobed for Shc and Grb2. Grb2 was inducibly associated with Shc in a phosphorylation-dependent manner by 1 to 30 minutes after stimulation (Fig 3B, lower panel). To confirm the identity of the p46 and p52 phosphoproteins, the membrane was immunoblotted for Shc. All lanes except immunoprecipitates with preimmune (normal rabbit) serum brought down an equivalent amount of p46 and p52 isoforms of Shc (Fig 3B, upper panel). These data provide the first evidence that FcαRI signals through the Shc-Grb2-Sos interaction, suggesting a role for Ras and PI-3 kinase in FcαR signaling.
Shc is tyrosine phosphorylated and associated with Grb2 upon FcR stimulation. (A) Antiphosphotyrosine blot of anti-Shc immunoprecipitates. U937IF lysates from resting cells and those stimulated with anti-FcR (A77) and RM antibodies (both Fab′2 fragments) for different periods were immunoprecipitated with rabbit anti-Shc antibody and immunoblotted for phosphotyrosine. Lane 1 represents immunoprecipitation performed with preimmune antisera of U937IF cells stimulated with anti-FcR and RM for 1 minute. Anti-Shc immunoprecipitates from resting U937IF cells (lane 2) and from U937IF cells stimulated with anti-FcR and RM for 1 minute (lane 3), for 5 minutes (lane 4), for 10 minutes (lane 5), and for 30 minutes (lane 6), respectively. Lane 7 represents a whole cell lysate of U937IF cells stimulated with anti-FcR and RM antibodies for 1 minute. The p145 protein was determined to be SHIP. (B) Anti-Shc (upper panel) and anti-Grb2 (lower panel) immunoblots performed on the same membrane of (A) after stripping with 0.1 mol/L glycine, pH 2.5, at room temperature for 30 minutes. Lanes are identical to those in (A).
Shc is tyrosine phosphorylated and associated with Grb2 upon FcR stimulation. (A) Antiphosphotyrosine blot of anti-Shc immunoprecipitates. U937IF lysates from resting cells and those stimulated with anti-FcR (A77) and RM antibodies (both Fab′2 fragments) for different periods were immunoprecipitated with rabbit anti-Shc antibody and immunoblotted for phosphotyrosine. Lane 1 represents immunoprecipitation performed with preimmune antisera of U937IF cells stimulated with anti-FcR and RM for 1 minute. Anti-Shc immunoprecipitates from resting U937IF cells (lane 2) and from U937IF cells stimulated with anti-FcR and RM for 1 minute (lane 3), for 5 minutes (lane 4), for 10 minutes (lane 5), and for 30 minutes (lane 6), respectively. Lane 7 represents a whole cell lysate of U937IF cells stimulated with anti-FcR and RM antibodies for 1 minute. The p145 protein was determined to be SHIP. (B) Anti-Shc (upper panel) and anti-Grb2 (lower panel) immunoblots performed on the same membrane of (A) after stripping with 0.1 mol/L glycine, pH 2.5, at room temperature for 30 minutes. Lanes are identical to those in (A).
Role of Cbl and SLP-76 in FcαRI signaling.
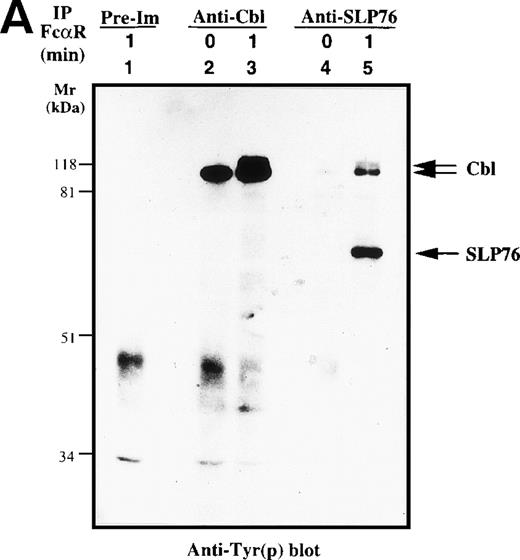
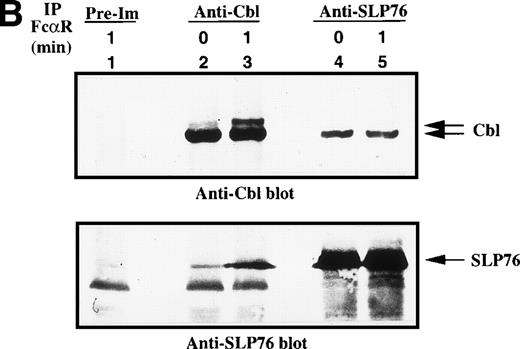
c-Cbl, a complex adaptor protein, is known to function in the signaling pathways of the TCR, BCR, FcγR1, FcεRI, and receptors for growth factor.26,32 41-43 To determine if Cbl or SLP-76 is involved in the FcαR-mediated signaling cascade, we performed in vivo immunoprecipitation studies with anti-Cbl and anti–SLP-76 antibodies. Cbl was tyrosine phosphorylated in resting and FcαR-stimulated U937IF cells (Fig 4A). Tyrosine phosphorylation of Cbl was significantly increased by 1 minute after stimulation of FcαR. Cross-linking of FcαRI also induced the tyrosine phosphorylation of SLP-76 only in U937IF cells stimulated with anti-FcαR antibody for 1 minute. Furthermore, SLP-76 coprecipitated tyrosine-phosphorylated Cbl in FcαR-stimulated U937IF cells. To delineate the nature of interaction between Cbl and SLP-76, we stripped the membrane and reblotted for Cbl and SLP-76. Anti-Cbl blots demonstrated that Cbl was constitutively associated with SLP-76 in resting and FcαRI-stimulated U937IF cells (Fig 4B, upper panel). Cross-linking of FcαR induced the Cbl-SLP-76 interaction in U937IF cells as measured by immunoprecipitation of Cbl (Fig 4B, lower panel). Western blot analysis confirmed that the anti-Cbl and anti–SLP-76 antibodies brought down the same amount of Cbl or SLP-76 proteins (Fig4B). We interpret these results to suggest that Cbl forms a stable complex with SLP-76 and other proteins, ie, Grb2, Shc, etc (Fig 4B, and data not shown), in resting U937IF cells and that more SLP-76 binds to the signaling complex upon FcαR aggregation. No increase in Cbl binding was seen in SLP-76 immunoprecipitates, a result we suggest may be an effect of anti–SLP-76 antisera on the capacity to detect a subgroup of augmented SLP-76-Cbl complexes in vivo. FcαR aggregation induced tyrosine phosphorylation of both SLP-76 and Cbl, and these 2 complex adaptor proteins are associated in vivo. These data provide the first evidence that Cbl and SLP-76 adaptor proteins function in FcαRI signaling.
Characterization of Cbl-SLP-76 interaction in U937IF cells. We examined the tyrosine phosphorylation of Cbl and SLP-76 in U937IF cells under conditions of anti-FcR (A77) stimulation. (A) Antiphosphotyrosine blot performed on anti-Cbl and anti–SLP-76 immunoprecipitates. Lane 1 is a preimmune immunoprecipitate. Anti-Cbl immunoprecipitation was performed from resting U937IF cells (lane 2) and from U937IF stimulated with anti-FcR and RM (lane 3). Anti–SLP-76 immunoprecipitates of resting (lane 4) and U937IF cells stimulated with anti-FcR and RM for 1 minute (lane 5). (B) Anti-Cbl (upper panel) and anti–SLP-76 (lower panel) immunoblots performed on the same membrane of (A) after stripping with 0.1 mol/L glycine, pH 2.5, at room temperature for 30 minutes. Lanes are identical to those in (A).
Characterization of Cbl-SLP-76 interaction in U937IF cells. We examined the tyrosine phosphorylation of Cbl and SLP-76 in U937IF cells under conditions of anti-FcR (A77) stimulation. (A) Antiphosphotyrosine blot performed on anti-Cbl and anti–SLP-76 immunoprecipitates. Lane 1 is a preimmune immunoprecipitate. Anti-Cbl immunoprecipitation was performed from resting U937IF cells (lane 2) and from U937IF stimulated with anti-FcR and RM (lane 3). Anti–SLP-76 immunoprecipitates of resting (lane 4) and U937IF cells stimulated with anti-FcR and RM for 1 minute (lane 5). (B) Anti-Cbl (upper panel) and anti–SLP-76 (lower panel) immunoblots performed on the same membrane of (A) after stripping with 0.1 mol/L glycine, pH 2.5, at room temperature for 30 minutes. Lanes are identical to those in (A).
Role of Src kinases in Shc phosphorylation, Shc adaptor protein interactions, and FcαRI oxidant signaling.
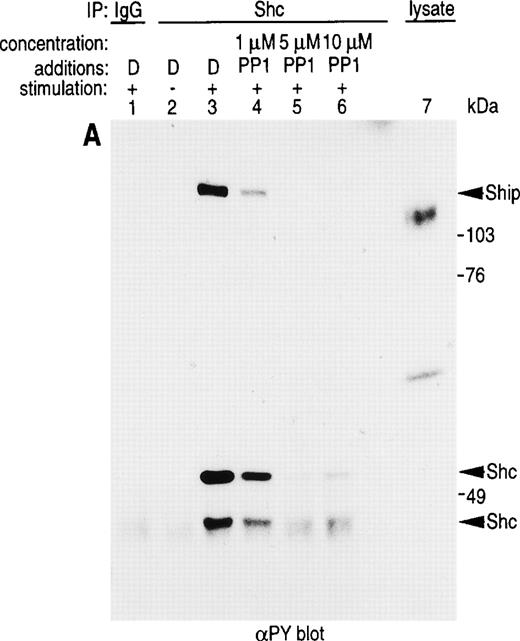
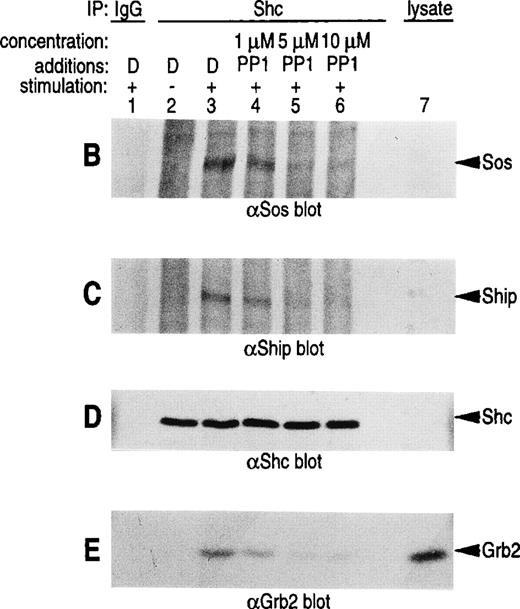
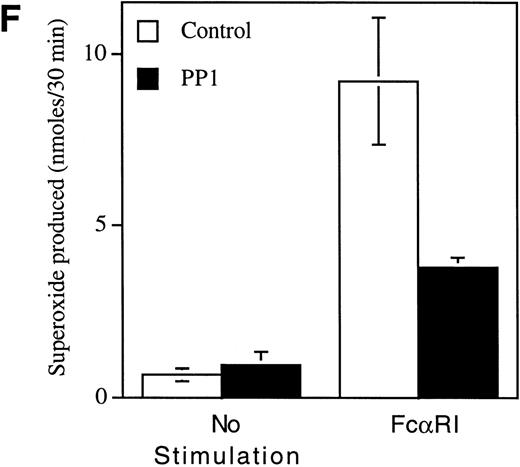
Pretreatment of U937IF cells with the Src-specific protein tyrosine kinase inhibitor, PP1, resulted in a dose-dependent inhibition of FcαRI-induced tyrosine phosphorylation of Shc (Fig 5A, compare lane 3 with lanes 4 through 6). Anti-Shc immunoblots (Fig 5D) performed on anti-Shc IP confirmed that an identical quantity of Shc was immunoprecipitated in lanes 2 through 6 and that the preimmune IP did not contain Shc (lane 1). We demonstrate the induced coimmunoprecipitation of Shc with Sos, SHIP, and Grb2 (Fig 5B, C, and E, lane 3) and that PP1 inhibits the interaction between Shc and Sos, SHIP, and Grb2 in a dose-dependent manner (compare lane 3 with lanes 4 through 6). The Src kinase inhibitor, PP1, was observed to inhibit the FcαRI-induced assembly of NADPH oxidase in U937IF cells as measured by a 59% decrease in superoxide generation (Fig 5F). This result was correlated with the inhibition of the myeloid-specific Src kinase, Hck (95% inhibition), in U937IF cells stimulated with FcαRI cross-linking (data not shown). In control experiments, PP1 did not inhibit the phorbol myristate acetate (PMA)-induced assembly of NADPH oxidase (data not shown), suggesting a specific requirement for Src in ITAM-induced oxidant signaling in myeloid cells. Similar results were observed for the effects of the Src kinase inhibitor, PP2, on the FcαRI-induced respiratory burst response (data not shown). From these data, we conclude that FcαRI induced assembly of a macromolecular complex that contains Shc, SHIP, Grb2, and Sos and that the assembly of the respiratory burst machinery requires the activation of a Src kinase in myeloid cells.
Role for Src kinases in FcRI oxidant signaling in myeloid cells. The Src family kinase inhibitor, PP1, was used to determine the role of Src kinases in phosphorylation of Shc, Shc-adaptor protein interactions, and FcR-induced superoxide response. Shc was immunoprecipitated from resting (lane 2) or FcRI-stimulated U937IF cells (lanes 3 through 6). U937IF cells were preincubated at 37°C with 1 μmol/L PP1 (lane 4), 5 μmol/L PP1 (lane 5), or 10 μmol/L PP1 (lane 6). Lanes 1 through 3 represent immunoprecipitations performed on U937IF cells treated with DMSO (D) at 5 μg/mL. Lane 7 represents a whole cell lysate of U937IF cells. Lane 1 represents a preimmune immunoprecipitation. Membranes were probed with (A) antiphosphotyrosine antibody, (B) anti-Sos immunoblot, (C) anti-SHIP immunoblot, (D) anti-Shc immunoblot, or (E) anti-Grb2 immunoblot. (F) Effects of PP1 on FcRI-induced respiratory burst response. Data shown are the means and the standard deviation of triplicate samples in each experimental group (see legend).
Role for Src kinases in FcRI oxidant signaling in myeloid cells. The Src family kinase inhibitor, PP1, was used to determine the role of Src kinases in phosphorylation of Shc, Shc-adaptor protein interactions, and FcR-induced superoxide response. Shc was immunoprecipitated from resting (lane 2) or FcRI-stimulated U937IF cells (lanes 3 through 6). U937IF cells were preincubated at 37°C with 1 μmol/L PP1 (lane 4), 5 μmol/L PP1 (lane 5), or 10 μmol/L PP1 (lane 6). Lanes 1 through 3 represent immunoprecipitations performed on U937IF cells treated with DMSO (D) at 5 μg/mL. Lane 7 represents a whole cell lysate of U937IF cells. Lane 1 represents a preimmune immunoprecipitation. Membranes were probed with (A) antiphosphotyrosine antibody, (B) anti-Sos immunoblot, (C) anti-SHIP immunoblot, (D) anti-Shc immunoblot, or (E) anti-Grb2 immunoblot. (F) Effects of PP1 on FcRI-induced respiratory burst response. Data shown are the means and the standard deviation of triplicate samples in each experimental group (see legend).
DISCUSSION
IgA is a predominant Ig produced in the human body, yielding up to 66 mg/kg/d.44 IgA plays a major role in host defense either by inhibiting adherence and attachment of environmental pathogens at the sites of mucous membrane or by neutralizing toxins. FcαR, Fc receptor of IgA, is a member of the Ig gene superfamily including TCR, BCR, FcγRs, and FcεRI.2,44,45 FcαR does not have an ITAM in its cytoplasmic tail and, hence, likely signals through an association with the FcεRIγ subunit ITAM.2,30,46 It has been recently reported that the FcεRIγ chain is associated with TCR, FcγRI, and FcαRI.12,38 Our biochemical data shown in Fig 1 support this conclusion. The combined data suggest that a number of signaling molecules for FcαRI may be shared with effectors of FcγR and FcεRI signaling. We questioned whether FcαRI may induce the modulation of adaptor complexes containing Grb2, Shc, Sos, and Cbl as a mechanism to control the Ras/Raf-1/MAP kinase cascade. Our data demonstrate that FcαR is functionally linked to the γ subunit of FcγRIα, a subunit shared with FcγRI and FcεRI (Fig 1). This result is consistent with previous reports of Pfefferkorn and Yeaman12 and Morton et al13 that demonstrate that the γ subunit of FcεRI coprecipitates with FcαR and FcγRI in U937 cells. The γ subunit belongs to the family of multichain immune recognition receptors.12,47 The cytoplasmic tail of the γ subunit contains an ITAM consensus sequence characterized by repeats of YxxL/I.14,18 Tyrosine phosphorylation of the ITAM triggers activation signals originating from these cell surface receptors leading to downstream signaling events. Tyrosine-phosphorylated ITAM provides a docking site for protein tyrosine kinases, Syk and ZAP-70, and for adaptor proteins, Shc and CBL.23,24,26 39 Hence, via these signals, the ITAM can activate small GTPases, MAP kinases, and the PI-3 kinase cascade. Association of γ subunit with FcαR gives a clue for how FcαR transmits signals following the binding of IgA-containing complex. A mobility shift of γ1, tyrosine phosphorylated, upon FcαR stimulation supports the argument that the signal transducing γ subunit of FcαR is shared with FcγRs and FcεRI in myeloid cells.
We next questioned whether adaptor proteins are involved in FcαRI signaling. We observed that Grb2 inducibly interacts with tyrosine-phosphorylated Shc and CrkL and also binds the nucleotide exchange protein, Sos, which is mobility shifted upon FcαRI cross-linking (Fig 2B). Consistent with these results, our previous results demonstrate that Grb2 binds Shc in a tyrosine phosphorylation-dependent manner upon FcγRI stimulation in U937IF cells.25 There is some evidence that the interaction of Grb2 with Shc (on Tyr317 residue of tyrosine-phosphorylated Shc) through the Grb2 SH2-domain is essential for binding of guanidine nucleotide exchange factor Sos to Grb2 and regulation of Ras activity.48 Our data support the notion that Grb2 recruits tyrosine-phosphorylated Shc, and the Grb2/Shc complex may lead to localization of Sos to the cytoplasmic surface of the cell membrane. Sos exchanges GDP-Ras to GTP-Ras, which sequentially activates Raf-1/MEK/MAP kinase and PI-3 kinase pathways. We recently demonstrated that cross-linking of FcγRI markedly increases GTP-bound Ras in U937IF cells (unpublished observation). Stimulation of FcγRI also induces the tyrosine phosphorylation of Raf-1 and mobility shift of Erk1 in U937IF cells.21 25
p120cbl is the cellular homologue of the oncogene contained within the Cas NS-1 retrovirus.49 Sequence analysis of Cbl cDNA shows that the protein contains a PTB binding motif and a ring finger motif in the N-terminus, 11 proline-rich (PXXP) sequences in the C-terminus, and 22 potential tyrosine phosphorylation sites. Although Cbl contains a nuclear localization signal and putative DNA-binding motif, there is no evidence of Cbl localization in the nucleus.50 Recent studies demonstrate that Cbl is a major substrate of protein tyrosine kinases after stimulation of various receptors, including TCR, BCR, FcγRs, and growth factors.26,30,32,51,52 Cbl, through its proline-rich region, has already been shown to bind to the SH3 domains of a number of proteins, including Fyn, Grb2, Lck, Fgr, Nck, PLCγ1, and p85 of PI-3 kinase.30,31 Cbl also interacts with the SH2 domains of Fyn, Lck, and Blk after tyrosine phosphorylation.30,32Our data show that Cbl is heavily tyrosine phosphorylated and, in turn, recruits more SLP-76 upon FcαR stimulation of U937IF cells (Fig4B).53 Interestingly, in resting cells the Cbl associated with SLP-76 is not tyrosine phosphorylated but becomes tyrosine phosphorylated upon FcαRI cross-linking. The SLP-76 bound to Cbl is not tyrosine phosphorylated and is further induced to bind Cbl by FcαRI aggregation (Fig 4B). Reciprocal anti-Cbl blots of SLP-76 immunoprecipitates show that Cbl is constitutively bound with SLP-76 and undergoes tyrosine phosphorylation upon FcαR stimulation. The augmented SLP-76-Cbl interaction in Fig 4B (compare lanes 2 and 3) is not observed in SLP-76 immunoprecipitates (Fig 4B, lanes 4 and 5). In other experiments, we observed an increase in Grb2 binding to SLP-76 in vivo but no increase in Grb2 binding to Cbl.26,53 Previous work from our laboratory has mapped the region of Cbl C-terminus that binds Grb2.26 The antisera used in these experiments was directed against the Cbl C-terminus and hence may effect the capacity to detect certain C-terminal Cbl interactions, ie, the binding of Cbl-Grb2 and/or Cbl-Shc. Our preliminary data support the argument that the enhanced binding of SLP-76 to Cbl in Cbl IP is the result of augmented binding of tyrosine-phosphorylated Shc to Cbl, which, in turn, binds more Grb2 via the Grb2-SH2 domain. Grb2-SH2, which is bound to the Shc-Cbl complex, leaves the Grb2-SH3 domain free to bind more SLP-76 via its PXXP motif in the C-terminus. We observe that the proportion and kinetics of increased SLP-76 binding to Cbl follows the same time course as the tyrosine phosphorylation of Shc (data not shown). We would argue that receptor induced alterations in SLP-76-Cbl-Grb2-Shc binding may eventually prove to have some physiologic significance in regulation of nucleotide exchange protein recruitment after ITAM stimulation in vivo.
In summary, our results indicate that signaling through the FcαRI receptor occurs through the γ subunit of FcεRI by forming a multimolecular adaptor complex containing tyrosine-phosphorylated Shc, SHIP, Cbl, SLP-76, and CrkL/Grb2/Sos in U937IF cells. This adaptor complex likely results in the recruitment of Sos to exchange GDP-Ras to GTP-Ras and, in turn, activates Raf-1/MEK/MAP serine/threonine kinases and PI-3 kinase upon cross-linking of FcαRI. Preliminary data from our laboratory have demonstrated that the FcαRI-induced activation of superoxide response is sensitive to the PI-3 kinase inhibitors, wortmannin and LY294022, thus supporting a role for the above-described Src-dependent adaptor protein interactions in the downstream activation of PI-3 kinase and AKT kinase. Our data provide the first evidence that the Src family kinases are required for FcαRI signaling and that Src kinases induce the phosphorylation of multiple adaptor proteins, resulting in the formation of important protein-protein interactions in myeloid cells. Our results provide the first evidence that Src kinases are involved in upstream tyrosine phosphorylation of Shc, which is required for the interaction of Shc with Grb2, Sos, and SHIP in vivo. These pathways are likely involved in the regulation of important biological responses induced by FcαR aggregation, including the control of Ras, the calcium flux, and the respiratory burst response in myeloid cells.
ACKNOWLEDGMENT
The authors thank Drs Gary A. Koretzky and K. Mark Coggeshall for providing antisera for performance of these experiments. We gratefully acknowledge Dr Anat-Erdreich Epstein for helpful discussions during this work and the other members of Durden laboratory for intellectual support during this project.
Supported by National Cancer Institute Grant No. RO1 CA75637 and American Cancer Society Grant No. RPG-98-244-01-LBC. The work was performed in the Wells Center for Pediatric Research. R.-K.P. was supported by Wonkwang University during 1998.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donald L. Durden, MD, PhD, Department of Pediatrics, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46204; e-mail:ddurden@iupui.edu.