Mast cell precursors invade from the peripheral blood into local tissues where they differentiate to their mature phenotypes. However, the mechanism of this migration process has been unclear. We clearly demonstrated here the production and release of matrix metalloproteinase-9 (MMP-9), a matrix-degrading enzyme necessary for leukocyte transmigration, by interleukin-3–dependent mouse mast cell progenitors: bone marrow-derived cultured mast cells and IC-2 mast cells. Because several interleukin-3–independent mast cell lines with active mutations in the c-kit gene did not release MMP-9, the possible involvement of c-kit receptor activation in downregulation of MMP-9 production was predicted. c-kitreceptor activation by stem cell factor led to a significant decrease in MMP-9 production of cultured mast cells and IC-2 mast cells transfected with the c-kit gene. Thus, the present results suggest that mast cell precursors are able to produce MMP-9, which may be essential for mast cell migration into tissues, and that stem cell factor may downregulate the MMP-9 production, resulting in engagement of mast cells to matrix components.
MAST CELL PROGENITORS, which originate from pluripotential hematopoietic stem cells, circulate through the bloodstream and invade into distinct tissues where they differentiate into two well-characterized types of mature mast cells.1-4Proliferation and differentiation of mast cells are regulated by soluble factors locally produced by at least Th2 T cells and fibroblasts in rodents and humans.5,6 Murine bone marrow derived-cultured mast cells (BMCMCs), which develop in the presence of T-cell–derived growth factors, including interleukin-3 (IL-3),7 are able to differentiate into mature mast cells through the peripheral blood at various tissues of mast cell-deficientW/Wv mice.8 On the other hand, stem cell factor (SCF), a specific ligand for the c-kit receptor with tyrosine kinase activity on mast cell precursors,9 is expressed on fibroblasts.10 This factor is not only able to support survival and proliferation of BMCMCs and peritoneal mast cells, but also induces a phenotypic change from BMCMCs to connective tissue-type mast cells.11 12 Much is known regarding how allergic and nonallergic stimuli lead to rapid activation with release of chemical mediators of mast cells, including inflammatory cytokines, resulting in proliferation and differentiation of their precursors at the affected sites. However, it is unclear how the precursors invade from the peripheral blood into local tissues or how the precursors degrade extracellular matrix in the process of their proliferation and differentiation.
Previous studies have demonstrated that the production of matrix-degrading enzymes, such as matrix metalloproteinase-9 (MMP-9), is essential for leukocyte extravasation and recruitment to the affected sites.13,14 MMP-9, which belongs to a large family of Zn2+-requiring enzymes, hydrolyzes basement membrane collagen types IV and V, anchoring collagen type VII, denatured collagens, fibronectin, and elastin.15,16 MMP-9 is secreted as a latent form (proMMP-9) and directly or indirectly activated by MMP-3 released from fibroblasts,17 chymase released from mast cells,18 and a plasminogen activator released from microvascular endothelial cells.19 Therefore, there is a possibility that mast cell precursors produce MMP-9 by themselves to degrade extracellular matrix in the process of their migration in local tissues. In the present study, we demonstrated that BMCMCs and IC-2 mast cell progenitors synthesized and released MMP-9, which was downregulated by SCF.
MATERIALS AND METHODS
Cells and reagents.
P-815 mouse mastocytoma, RBL-2H3 rat mast cell leukemia, and HMC-1 human mast cell leukemia cell lines were cultured in α-minimum essential medium (α-MEM; GIBCO RBL, Grand Island, NY) containing 10% fetal bovine serum (FBS; Filtron, Brooklyn, Australia), 100 U/mL penicillin, and 100 μg/mL streptomycin. An IC-2 mouse mast cell progenitor line was maintained in α-MEM containing 10% FBS and 100 U/mL of recombinant mouse IL-3 (Kirin Brewery, Tokyo, Japan). The wild-type c-kit gene or V814 mutant gene was introduced into IC-2 cells by the retroviral vector, and the infected cells were selected in G418-containing growth medium as described previously.20 Control IC-2 cells were transfected with the vector alone and selected in the same way. BMCMCs were obtained from bone marrow cells of WBB6F1-+/+ mice in α-MEM containing 10% FBS and 10% pokeweed mitogen-stimulated spleen cell-conditioned medium according to the method described previously.21Recombinant rat SCF (rSCF) was a gift from Amgen Inc (Thousand Oaks, CA). Rabbit anti–c-kit polyclonal antibody and goat anti–MMP-9 polyclonal antibody were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Peroxidase-conjugated mouse antiphosphotyrosine monoclonal antibody (MoAb; clone 4G10) and peroxidase-conjugated mouse antigoat IgG antibody were from Upstate Biotechnology Inc (Lake Placid, NY) and Jackson ImmunoResearch Laboratories Inc (West Grove, PA), respectively. Metal ion chelators, EDTA, and 1,10-phenanthroline were purchased from Kanto Chemical Co, Inc (Tokyo, Japan). Serine protease inhibitors, 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (ABSF) and aprotinin, were from Wako Pure Chemicals Industries (Osaka, Japan) and Boehringer Mannheim (Mannheim, Germany), respectively.
Gelatin zymography.
Cells (5 × 106/mL) were incubated in serum-free medium for 72 hours and culture supernatants were harvested. The supernatants were electrophoresed using 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel with copolymerized 1 mg/mL gelatin (TEFCO, Tokyo, Japan) under nonreducing conditions. After washing gels in 2.5% Triton X-100 for 30 minutes twice to remove SDS, gelatinolytic enzymes were activated by incubating gels in 50 mmol/L Tris-HCl buffer containing 200 mmol/L NaCl and 10 mmol/L CaCl2 (pH 7.5) for 24 hours at 37°C. Gels were stained with 0.2% Coomassie Brilliant blue for 60 minutes and destained in 10% acetic acid/25% methanol. Gelatinolytic activity was identified as a clear band on a blue background. Images were analyzed by Gel Print 2000i/VGA and Basic Quantifier (Genomic Solutions, Tokyo, Japan).
To define that gelatinolytic enzymes belong to MMPs, gels were incubated in buffer containing metal chelators (20 mmol/L EDTA or 2 mmol/L 1,10-phenanthroline) after electrophoresis and after SDS removal. For further determination of MMPs, collected cultured media were mixed with either of the serine protease inhibitors, 1 mmol/L ABSF or 30 nmol/L aprotinin, before application onto gelatin zymography. In each experiment, gels were stained and destained as described above.
Northern blot analysis.
BMCMCs and IC-2 transfectants were washed twice and incubated with or without 50 ng/mL 10-O-tetradecanoylphorbol-13-acetate (TPA; Sigma, St Louis, MO) or various concentrations of rSCF in α-MEM containing 1% bovine serum albumin (BSA; Sigma). Six hours later, mRNA was isolated from 5 × 106 cells per each condition using a QuickPrep mRNA purification kit (Pharmacia, Uppsala, Sweden). mRNA obtained from each sample was electrophoresed on formaldehyde-agarose (1.2%) gels, transferred onto nylon membrane (Boehringer Mannheim), and UV cross-linked. After prehybridization for 60 minutes at 42°C, the samples were hybridized for 16 hours at 60°C with digoxigenin-labeled single-strand cDNA probes specific for mouse MMP-9 and β-actin (Nippon Flower Milles, Tokyo, Japan), 5′-GGACACATAGTGGGAGGTGCTGTCGGCTGT-3′ (988 through 1018) and 5′-GGCTGGGGTGTTGAAGGTCTCAAACATGATCTGGGTCATC-3′ (394 through 434), respectively. Hybridized filters were washed, blocked for 60 minutes in blocking buffer (100 mmol/L maleic acid, 3 mol/L NaCl, 0.3% Tween 20, and 0.5% blocking reagent, pH 8.0), and incubated with alkaline phosphatase-conjugated polyclonal sheep antidigoxigenin Fab fragments (Boehringer Mannheim) diluted to 75 mU/mL (1:15,000) in blocking buffer for 30 minutes. After washing 3 times, the membrane was immersed in detection buffer (100 mmol/L Tris-HCl, 100 mmol/L NaCl, 50 mmol/L MgCl2, and 0.25 mmol/L CSPD [Boehringer Mannheim], pH 9.5) for 30 minutes, repacked in a hybridization bag without buffer, and incubated for 30 minutes at 37°C to enhance the luminescent reaction. The signals were exposed to x-ray film at room temperature, and the images were digitalized as described.
Immunoprecipitation and Western blotting.
Protein A-Sepharose CL-4B (Pharmacia) was equilibrated in lysis buffer (20 mmol/L Tris-HCl, 137 mmol/L NaCl, 10% glycerol, 1% NP-40, and protease and phosphatase inhibitors, pH 8.0) and adjusted to 50% suspension. To make beads-antibody complexes, 0.5 μg of anti–c-kit antibody and 100 μL of 50% Protein A-Sepharose suspension were mixed in 500 μL of lysis buffer for 4 hours at 4°C with gentle rotation. The complexes were washed twice with lysis buffer and kept on ice until use. BMCMCs and IC-2WTcells (1 × 107 cells per each condition) were incubated in serum-free α-MEM containing 1% BSA for 4 hours at 37°C to reduce basic levels of phosphorylation. After the treatment with or without 100 ng/mL rSCF for 15 minutes, cells were collected and lysed in 800 μL of lysis buffer, frozen and thawed 3 times, and centrifuged for 10 minutes at 15,000 rpm. Supernatants were harvested, applied onto the beads-antibody complexes, and incubated at 4°C overnight with gentle rotation. Immunoprecipitates were washed twice with lysis buffer, suspended in 50 μL of 2× SDS sample buffer containing 12% urea, and boiled for 7 minutes. Proteins were separated by 6% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membrane (Millipore, Bedford, MA), and immunoblotted with peroxidase-conjugated antiphosphotyrosine MoAb (Upstate Biotechnology). Immune complexes were visualized using an ECL system (Amersham, Arlington Heights, IL).
Immunocytochemistry.
Cytospin preparations of mast cell progenitors were fixed in 4% paraformaldehyde for 15 minutes at 4°C and then washed in phosphate-buffered saline (PBS). Endogenous peroxidase was invalidated by immersing samples in 3% H2O2 containing PBS for 5 minutes at room temperature. Nonspecific reaction was blocked by incubating slides with 10% filtrated mouse serum in PBS for 30 minutes at room temperature. Goat anti–MMP-9 polyclonal antibody diluted in 0.05% Tween 20 containing PBS was applied onto the samples and incubated at 4°C overnight. Filtrated normal goat serum was used as a control. After washing, the preparations were incubated with peroxidase-conjugated mouse antigoat IgG antibody in 0.05% Tween 20 containing PBS for 60 minutes. The reaction products were visualized with diaminobenzidine as a substrate, and then the cells were counterstained with Mayer’s hematoxylin.
RESULTS
MMP-9 production of mast cell progenitors.
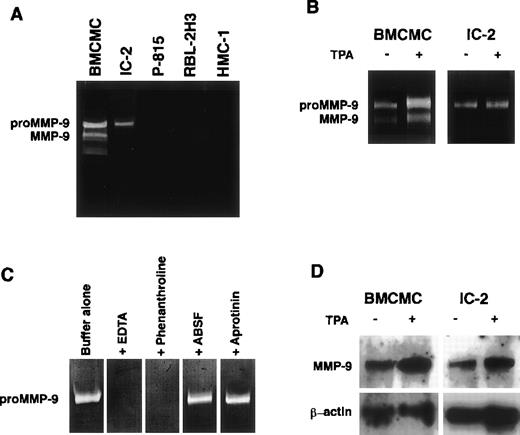
BMCMCs and several mast cell lines were incubated in serum-free α-MEM for 72 hours, and gelatinolytic activities in collected culture media were examined by gelatin zymography. The culture media of IL-3–dependent mast cell progenitors, BMCMCs, and IC-2 mouse mast cells possessed gelatin-degrading activities corresponding to proMMP-9 and its active form and to proMMP-9 alone, respectively, whereas little or no gelatinolytic activity was detected in the culture media of P-815 mouse mastocytoma cells, RBL-2H3 rat mast cell leukemia cells, and HMC-1 human mast cell leukemia cells (Fig1A). Because the synthesis of MMP-9 is stimulated with phorbolesters,22 BMCMCs and IC-2 cells were incubated with 50 ng/mL TPA for 72 hours; the more potent gelatinolytic activities corresponding to proMMP-9 and its active form were noted (Fig 1B). The proteolytic bands disappeared when the gels were incubated in buffer containing EDTA or 1,10-phenanthroline (chelators of Ca2+and Zn2+, respectively) after loading the samples. Because serine proteases possess a weak gelatinolytic activity, serine protease inhibitors, ABSF and aprotinin, were added to samples before electrophoresis. The treatment with the inhibitors showed no effect on the positive activity, indicating that this gelatinolytic activity was an MMP23 (Fig 1C). Northern blot analysis using digoxigenin-labeled oligonucleotide cDNA probes specific for mouse MMP-9 clearly demonstrated the expression of MMP-9 mRNA (2.4 kb), which was increased in both of the cells stimulated with TPA (Fig 1D).
MMP-9 production from mast cell progenitors. Gelatinolytic activities comparable to proMMP-9 and MMP-9 are observed in IL-3–dependent BMCMCs or IC-2 cells, but not in P-815, RBL-2H3, and HMC-1 cells (A). The addition of 50 ng/mL TPA enhances the gelatinolytic activities released from BMCMCs and IC-2 cells (B). The gelatinolytic activity released from IC-2 cells disappears in gels incubated in buffer containing 20 mmol/L EDTA or 2 mmol/L 1,10-phenanthroline. Enzymatic activity is not affected by incubation with serine protease inhibitors, 1 mmol/L ABSF, or 30 nmol/L aprotinin, respectively (C). Expression of MMP-9 mRNA is observed in BMCMCs and IC-2 cells; and treatment with 50 ng/mL TPA for 6 hours significantly increases the expression of MMP-9 mRNA of both the cells (D).
MMP-9 production from mast cell progenitors. Gelatinolytic activities comparable to proMMP-9 and MMP-9 are observed in IL-3–dependent BMCMCs or IC-2 cells, but not in P-815, RBL-2H3, and HMC-1 cells (A). The addition of 50 ng/mL TPA enhances the gelatinolytic activities released from BMCMCs and IC-2 cells (B). The gelatinolytic activity released from IC-2 cells disappears in gels incubated in buffer containing 20 mmol/L EDTA or 2 mmol/L 1,10-phenanthroline. Enzymatic activity is not affected by incubation with serine protease inhibitors, 1 mmol/L ABSF, or 30 nmol/L aprotinin, respectively (C). Expression of MMP-9 mRNA is observed in BMCMCs and IC-2 cells; and treatment with 50 ng/mL TPA for 6 hours significantly increases the expression of MMP-9 mRNA of both the cells (D).
Deficiency in MMP-9 production of mast cell lines with active c-kit tyrosine kinase.
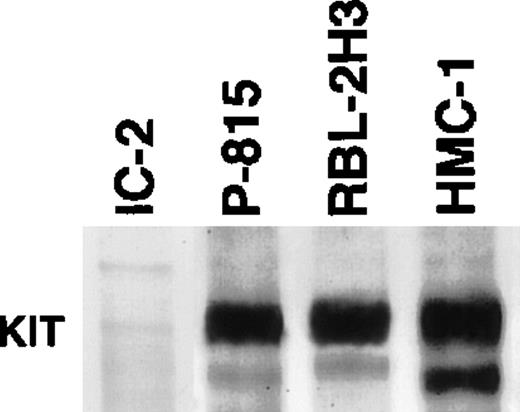
SCF, which is one of fibroblast-derived growth factors, regulates survival, differentiation, and functions of mast cells through the c-kit receptor.12,24 P-815, RBL-2H3, and HMC-1 cells have point mutations in the c-kit proto-oncogene, leading to constitutive activation of the c-kit tyrosine kinase even without ligation of SCF to the receptor.25-27 Because no MMP-9 secretion was detected in culture media obtained from these cell lines (Fig 1A), there is a possibility that c-kitautophosphorylation may lead to suppression in MMP-9 production of mast cell progenitors. To confirm active c-kit receptors in these cell lines, Western blot analysis with antiphosphotyrosine MoAb was performed on immunoprecipitated products with anti–c-kitreceptor antibody. As shown in Fig 2, a marked positive response showing autophosphorylation of the c-kit receptor was detected in P-815, RBL-2H3, and HMC-1 cells, but not in IC-2 cells.
Immunoblot analysis of tyrosine phosphorylation of the c-kit receptor. Marked positive reaction is observed in P-815, RBL-2H3, and HMC-1 cells, but not in the original IC-2 cells.
Immunoblot analysis of tyrosine phosphorylation of the c-kit receptor. Marked positive reaction is observed in P-815, RBL-2H3, and HMC-1 cells, but not in the original IC-2 cells.
MMP-9 release of IC-2 cells denuded by transfection with mutant c-kit gene.
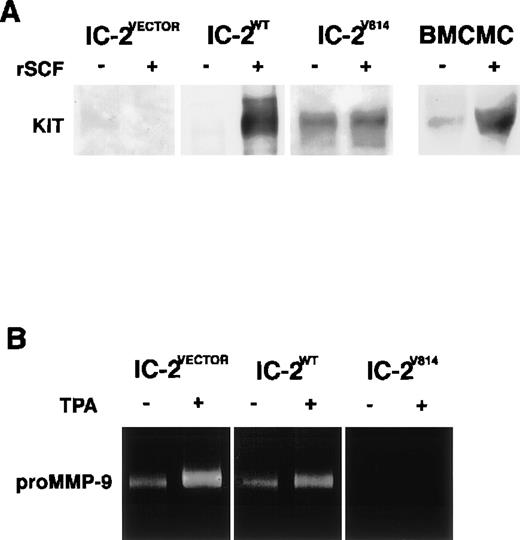
To clarify whether activation of the c-kit receptor downregulates MMP-9 production of mast cells, we transferred a wild-type of the c-kit gene or c-kit gene with one point mutation to IC-2 cells that express no c-kit receptors on the surface28 and assessed MMP-9 activity in culture media of these transfectants by gelatin zymography. In HMC-1 cells, the c-kit gene was found to include a mutant allele with point mutations, resulting in amino acid substitutions of Gly-560 for Val and Asp-816 for Val.20 Because the activating mutation corresponding to Asp codon of HMC-1 cells was also found in both P-815 cells (Asp-814 to Tyr) and RBL-2H3 cells (Asp-817 to Tyr),25,26 we introduced c-kit V814 mutant (Asp-814 to Val) into IC-2 cells using the retroviral vector as previously described.20 Wild-type c-kit gene transfectants (IC-2WT) exhibited activation of c-kit receptor tyrosine kinase dependent on rSCF as well as BMCMCs, whereas mutant gene transfected cells (IC-2V814) expressed active c-kit receptors even without an addition of rSCF (Fig 3A). Although IC-2WT cells were capable of releasing proMMP-9 comparable to original IC-2 cells, such a gelatinolytic activity was undetectable in the culture medium of IC-2V814 cells (Fig 3B). These results clearly demonstrated the negative correlation between c-kit receptor activation and MMP-9 secretion of IC-2 cells.
Detection of c-kit receptor activation and MMP-9 secretion of IC-2 transfectants and BMCMCs. SCF-dependent activation of the c-kit receptor was observed in BMCMCs and IC-2WT cells, whereas SCF-independent phosphorylation was detected in a constitutively active c-kit transfectant (IC-2V814; A). ProMMP-9 activity is marked in control IC-2VECTOR cells and IC-2WT cells as well as the original IC-2 cells, but not in IC-2V814 cells even when stimulated with TPA (B).
Detection of c-kit receptor activation and MMP-9 secretion of IC-2 transfectants and BMCMCs. SCF-dependent activation of the c-kit receptor was observed in BMCMCs and IC-2WT cells, whereas SCF-independent phosphorylation was detected in a constitutively active c-kit transfectant (IC-2V814; A). ProMMP-9 activity is marked in control IC-2VECTOR cells and IC-2WT cells as well as the original IC-2 cells, but not in IC-2V814 cells even when stimulated with TPA (B).
MMP-9 production of mast cell progenitors suppressed by rSCF.
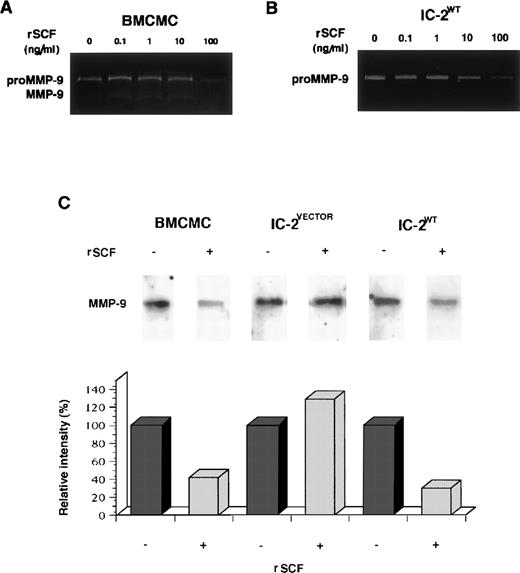
To further investigate the suppressive effect of SCF on MMP-9 production from mast cell progenitors, BMCMCs and IC-2WTcells were incubated with various concentrations of rSCF in α-MEM containing 1% BSA for 72 hours. The addition of rSCF to BMCMCs resulted in a significant decrease of the proMMP-9 activity in a dose-dependent manner (Fig 4A); the inhibitory effect of rSCF on release of proMMP-9 was observed in IC-2WT cells as well (Fig 4B). Northern blot analysis showed that the addition of rSCF led to the diminished expression of MMP-9 mRNA in BMCMCs and IC-2WT cells, but not in IC-2VECTOR cells (Fig 4C).
SCF-induced downregulation of MMP-9 production of mast cell progenitors. BMCMCs and IC-2WT cells were incubated with or without various concentrations of rSCF for 72 hours at 37°C. Dose-dependent inhibitory effect on release of proMMP-9 and MMP-9 is obvious (A and B). mRNA was purified from mast cell progenitors stimulated with or without 100 ng/mL rSCF for 6 hours. MMP-9 mRNA expression of IC-2VECTOR cells is stable despite the addition of rSCF, but MMP-9 mRNA expression of both BMCMCs and IC-2WT cells is markedly reduced by the treatment with rSCF (C).
SCF-induced downregulation of MMP-9 production of mast cell progenitors. BMCMCs and IC-2WT cells were incubated with or without various concentrations of rSCF for 72 hours at 37°C. Dose-dependent inhibitory effect on release of proMMP-9 and MMP-9 is obvious (A and B). mRNA was purified from mast cell progenitors stimulated with or without 100 ng/mL rSCF for 6 hours. MMP-9 mRNA expression of IC-2VECTOR cells is stable despite the addition of rSCF, but MMP-9 mRNA expression of both BMCMCs and IC-2WT cells is markedly reduced by the treatment with rSCF (C).
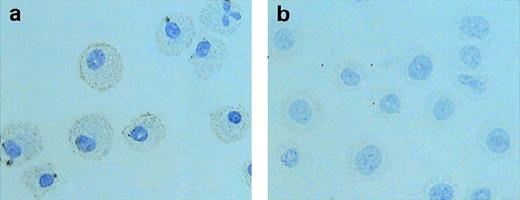
Next, we conducted immunocytochemical experiments to examine whether SCF downregulated MMP-9 synthesis of mast cell progenitors. BMCMCs were incubated with 100 ng/mL rSCF for 72 hours. Every 24 hours, cells were collected and treated with anti–MMP-9 antibody. BMCMCs incubated with rIL-3 in the absence of rSCF were strongly stained with anti–MMP-9 antibody in their cytoplasm (Fig 5a). On the other hand, BMCMCs exposed with rSCF alone showed little or no reaction for MMP-9 (Fig 5b). The specificity of anti–MMP-9 antibody against mouse MMP-9 was confirmed by Western blotting (data not shown). To examine an intracellular gelatinolytic activity, BMCMCs were collected and lysed; MMP-9 activities were diminished depending on the incubation period with rSCF (data not shown). Thus, we concluded that SCF was able to suppress MMP-9 production of mast cell progenitors.
Immunocytochemical staining for MMP-9. BMCMCs incubated with rIL-3 (a) or with 100 ng/mL rSCF (b) for 72 hours were fixed in 4% paraformaldehyde and treated with anti–MMP-9 antibody. BMCMCs incubated with rSCF show a weak reaction or no reaction for MMP-9.
Immunocytochemical staining for MMP-9. BMCMCs incubated with rIL-3 (a) or with 100 ng/mL rSCF (b) for 72 hours were fixed in 4% paraformaldehyde and treated with anti–MMP-9 antibody. BMCMCs incubated with rSCF show a weak reaction or no reaction for MMP-9.
DISCUSSION
Much is known regarding how MMP-9 produced by inflammatory cells is crucially involved in their migration from peripheral blood vessels into affected tissues.13,14 For mast cell progenitors, MMP-9, which is able to degenerate basement membrane collagen types IV and V, anchoring collagen type VII, and other matrix components, may be essential to invade from blood vessels into local connective tissues where they complete their differentiation. In the present study, we clearly demonstrated that the IL-3–dependent mouse mast cell progenitors produced and released proMMP-9 spontaneously. Because a plasminogen activator released from vascular endothelial cells is able to indirectly activate proMMP-9,19 proMMP-9 produced by mast cell precursors might be transformed to its active form under the influence of the activator, crossing the endothelial wall.
In the process of maturation, mast cells are considered to migrate toward SCF expressed on fibroblasts and engaged to matrix components of local tissues eventually. In fact, SCF is able not only to function as a chemoattractant for BMCMCs and IL-3–dependent mouse mast cell lines,29 but also to induce their adhesion to fibronectin30 through α5β1 integrin.31Because no MMP-9 activity was detectable in the culture media obtained from the cell lines with active mutations in the c-kit gene, we attempted to determine the possible involvement of the c-kitreceptor activation on MMP-9 production of mast cell progenitors. c-kit receptor tyrosine kinase of P-815, RBL-2H3, and HMC-1 cells was demonstrated to be activated spontaneously as described.25-27 Moreover, transfection of c-kitV814 mutant gene to IC-2 cells resulted in significant suppression of MMP-9 production, indicating that c-kit receptor activation and MMP-9 production may be reciprocally regulated in mast cell progenitors.
Allergic and nonallergic stimuli lead to rapid activation with release of chemical mediators, including inflammatory cytokines, resulting in proliferation and differentiation of their fixed precursors at the affected site.32 Recently, the presence of committed precursors of mast cells in the peripheral blood has been reported,3 4 but it is unknown how the precursors invade from the peripheral blood into tissues or how the precursors fixed in local tissues degrade extracellular matrix in the process of their proliferation and differentiation. The present study strongly suggests the novel role of MMP-9 produced by mast cell precursors in their tissue invasion and the downregulation of mast cell motility mediated by SCF due to decreasing MMP-9 production. Further investigation must take place to elucidate the mechanisms of these responses in detail, but this discovery may bring a new insight into the mechanism of mast cell differentiation and distribution in local tissues.
ACKNOWLEDGMENT
The authors thank Dr Stephen J. Galli (Department of Pathology, Harvard Medical School, Boston, MA) for valuable discussions. We also thank Amgen Inc for providing rat rSCF and Kirin Brewery Co for supplying mouse rIL-3.
Supported by grants from the Ministry of Education, Science, Sports, and Culture, Japan; from the Pioneering Research Project in Biotechnology, the Ministry of Agriculture, Forestry, and Fisheries, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hiroshi Matsuda, DVM, PhD, Department of Veterinary Clinic, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan.