Oncogenic RAS alleles encode proteins that accumulate in the guanosine triphosphate (GTP)-bound state. Because post-translational processing of Ras by farnesyltransferase is essential for biologic function, inhibitors of this enzyme have been developed as rational cancer therapeutics. We have investigated farnesyltransferase inhibitor (FTI) L-744,832 in an in vivo murine model of myeloid leukemia that is associated with inactivation of the Nf1 tumor suppressor gene.Nf1 encodes a GTPase activating protein for Ras, andNf1-deficient (Nf1−/−) hematopoietic cells show hyperactive Ras signaling through the mitogen-activated protein (MAP) kinase pathway. L-744,832 inhibited H-Ras prenylation in cell lines and in primary hematopoietic cells and abrogated the in vitro growth of myeloid progenitor colonies in response to granulocyte-macrophage colony-stimulating factor (GM-CSF). This FTI also partially blocked GM-CSF–induced MAP kinase activation, but did not reduce constitutively elevated levels of MAP kinase activity in primaryNf1−/− cells. Injection of a single dose of 40 or 80 mg/kg of L-744,832 increased the amount of unprocessed H-Ras in bone marrow cells, but had no detectable effect on N-Ras. Adoptive transfer ofNf1−/− hematopoietic cells into irradiated mice induces a myeloproliferative disorder that did not respond to L-744,832 treatment. We speculate that the lack of efficacy in this model is due to the resistance of N-Ras and K-Ras processing to inhibition by this FTI.
RAS PROTO-ONCOGENES encode 21-kD proteins that regulate cellular growth and differentiation by transducing signals from the plasma membrane to the nucleus through a number of downstream effectors.1-3 The biochemical output of Ras proteins is tightly regulated by their ability to cycle between an active guanosine triphosphate (GTP)-bound state (Ras · GTP) and an inactive guanosine diphosphate (GDP)-bound state (Ras · GDP). RAS mutations are the most common oncogenic alterations in human cancer cells and are frequently detected in carcinomas of the colon and pancreas, as well as in myeloid leukemias.4,5 Mutant RAS alleles encode proteins that accumulate in the GTP-bound conformation because of defective GTP hydrolysis.1-3
Ras proteins undergo post-translational processing at a common C-terminal CAAX sequence in which C is cysteine, A is an aliphatic amino acid, and X is any amino acid.6-9 Ras processing is initiated by farnesyltransferase, which transfers the C15isoprenoid group from farnesyl diphosphate to the cysteine residue of the CAAX box. The 3 terminal amino acids of farnesylated Ras are then cleaved by an endopeptidase; this reaction is followed by methylation of the now-terminal cysteine by a specific methyltransferase. Because farnesyltransferase is essential for biologic activity of mutant and oncogenic Ras proteins, competitive inhibitors of this enzyme have been developed as a new class of rational anticancer therapeutics.6-9
Studies evaluating different farnesyltransferase inhibitors (FTIs) in a number of in vitro and in vivo systems have provided preclinical data supporting selective antitumor effects of these compounds.7,9 FTIs have been shown to block Ras-induced transformation in tissue culture cells, to inhibit the growth of many cancer cell lines, and to halt proliferation of Ras-activated xenografts in nude mice.7,9 FTI L-744,832 also showed efficacy in 2 transgenic mouse models of breast cancer in whichRAS expression is driven from a mammary tumor virus (MMTV) promoter.10,11 Barrington et al12 recently reported that L744-832 induced breast tumor regression was associated with apoptotic cell death that was partially independent of P53function in MMTV-HRAS mice.
Although the data supporting antitumor effects of FTIs are impressive, questions remain with respect to their biochemical mechanism(s) of action, the reason(s) that these compounds selectively inhibit the growth of malignant cells in many models, and the spectrum of antitumor activity. In particular, although data from C elegans andDrosophilia indicate that FTIs can modify phenotypes attributed to hyperactive Ras,13,14 it is unclear if inhibition of mammalian tumor cell growth is mediated through a direct effect on Ras or by targeting other farnesylated proteins such as RhoB.15-17 For example, in a study showing that a FTI inhibited the growth of about 70% of human tumor cell lines, the presence or absence of a RAS mutation did not correlate with responsiveness.18 Understanding the mechanisms of FTI action is complicated because oncogenic Ras proteins perturb signaling in a number of different downstream effector pathways.2 3
GTPase activating proteins (GAPs) negatively regulate Ras output by accelerating the slow intrinsic Ras GTPase activity.19 The neurofibromatosis type 1 tumor suppressor gene (NF1) encodes neurofibromin, which functions as a GAP for Ras.19Individuals with NF1 are predisposed to specific cancers including malignant peripheral nerve sheath tumors (MPNST), pheochromocytomas, and juvenile myelomonocytic leukemia (JMML).20 MPNST cell lines derived from patients with NF1 show elevated levels of Ras · GTP and reduced GAP activity.21,22 Treatment with an FTI blocked growth and reverted the malignant phenotype of one of these cell lines; this result suggested that FTIs might prove useful in treating tumors that are associated with inactivation ofNF1.23
JMML is a myeloproliferative disorder (MPD) of young children that is characterized by overproduction of myeloid cells, a subacute but relentless clinical course, and by the formation of excessive numbers of myeloid progenitor colonies in cultures stimulated with the hematopoietic growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF).24,25 Genetic and biochemical analyses of JMML bone marrow cells from children with NF1 showed inactivation ofNF1, an increase in the percentage of Ras · GTP, a decrease in neurofibromin-associated GAP activity, and evidence of in vivo mitogen-activated protein (MAP) kinase activation.24-28Furthermore, oncogenic RAS mutations are detected in the bone marrows of 20% to 30% of children with JMML and other myeloid leukemias who do not have NF1, but are conspicuously absent in children with NF1.29 Taken together, these data provide compelling evidence that the tumor suppressor function of NF1 in myeloid cells is mediated through the ability of neurofibromin to negatively regulate Ras signaling.
The murine homolog of NF1 has been disrupted by targeted homologous recombination to generate lines of Nf1 knockout mice.30,31 Approximately 10% of heterozygous mice (Nf1+/−) develop a JMML-like MPD during the second year of life that is associated with loss of the wild-type Nf1 allele in bone marrow cells.31 Homozygous Nf1-deficient embryos (Nf1−/−) die in utero between 12 and 14 days of gestation from cardiac defects.30,31 FetalNf1−/− hematopoietic cells show a similar pattern of aberrant in vitro myeloid progenitor colony growth as human JMML cells in response to GM-CSF.27,32 Furthermore, adoptive transfer of these Nf1-deficient fetal liver cells into irradiated recipient mice consistently induces a JMML-like MPD that is characterized by activated Ras-MAP kinase signaling in hematopoietic cells.32 33 The subacute nature of this MPD, the central role of hyperactive Ras in this and other myeloid malignancies, the availability of primary hematopoietic cells for biochemical studies, and the fact the disease phenotype does not depend on overexpressing aRAS transgene from a heterologous promoter makes this in vivo model appealing for preclinical studies of FTIs and other promising anti-Ras therapeutics. Here, we report biochemical and hematologic data from studies examining FTI L-744,832 in this experimental system.
MATERIALS AND METHODS
Compounds.
The L-744,832 used in these studies was provided by the Department of Medicinal Chemistry, Merck Research Laboratories. Compactin was purchased from Sigma (St Louis, MO) and was dissolved in dimethyl sulfoxide as previously described.34
Nf1 mice.
Nf1 knockout mice were produced and characterized as described previously.31 Inbred 129/Sv mice were used as transplant recipients in experiment 1 and both 129/Sv and 129/Sv × C57BL/6 mixed genetic background were used as recipients in the second experiment. The fetal liver cells used as grafts were from a mixed 129/Sv × C57BL/6 genetic background. The experimental procedures were reviewed and approved by the University of California San Francisco Committee for Animal Research.
Transplant procedure.
Nf1+/− mice were mated to produce Nf1−/− embryos. Pregnant Nf1+/− females were killed by CO2inhalation on day E13.5 and the embryos were removed through an abdominal incision. Fetal livers were removed from embryonic tissues and transferred to 1 mL of Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 20% fetal calf serum. Single-cell suspensions were prepared by drawing the livers through progressively smaller needles (22-gauge needle × 2; followed by a 25-gauge needle ×2). A total of 4 to 14 × 106 mononuclear cells were injected into the dorsal tail veins of recipients that have been conditioned with 1,000 cGy of total body irradiation. This protocol induces destruction of recipient hematopoiesis and is associated with more than 80% survival of animals engrafted with donor fetal liver cells (data not shown).
Treatment and observations.
The recipients of wild-type (Nf1+/+), mutant (Nf1−/−) or of a mixture of Nf1+/+, andNf1−/− cells were observed weekly and had complete blood counts (CBCs) performed monthly for at least 2.5 months before being assigned to the study. The genotype of circulating blood cells was confirmed by Southern blot analysis by using a genomic Nf1probe that distinguishes between the wild-type and targeted alleles.31 Recipients transplanted with cells of each genotype were assigned to either receive treatment with L-744,832 or to an untreated control group. L-744,832 was diluted in a citrate-buffered sodium chloride solution and a volume of 0.1 to 0.5 mL was injected subcutaneously once per day. Study mice were weighed weekly and the volume of L-744,832 injected was adjusted as necessary to maintain the dose at 40 mg/kg (in the first experiment) or at 80 mg/kg (in the second experiment). Mice were treated 5 days per week for 8 weeks in the first experiment and 7 days per week for 4 weeks in the second experiment. The animals were observed daily during treatment, and CBCs with differential white blood cell counts were performed every 2 weeks. Mice from the second experiment were killed after their final treatment and their spleens were weighed. Bone marrow and spleen cells were isolated for biochemical studies.
Hematopoietic progenitor colony growth.
Mononuclear cells from fetal livers were plated on the day they were harvested in duplicate 35-mm plates at a final concentration of 5 × 104 cells per milliliter in culture medium consisting of 0.8% methyl cellulose supplemented with 30% fetal calf serum, L-glutamine, and fully supplemented IMDM (Stem Cell Technologies, Vancouver, British Columbia). Cells, recombinant murine GM-CSF, and L-744,832 were added directly to the methylcellulose culture medium and the solution was mixed thoroughly before plating. The cells were grown at 37°C in a humidified 21% O2, 5% CO2incubator. Colonies derived from granulocyte-macrophage colony-forming units (CFU-GM) were counted by indirect microscopy.
MAP kinase assay.
To measure endogenous MAP kinase activity, the ERK2 isoform was selectively immunopurified by using an antibody directed to the carboxy-terminal amino acids 345-358 (Santa Cruz Biotechnology Inc, Santa Cruz, CA; Catalog No. sc-154). Cells were lysed in 20 mmol/L Tris, pH 8, 137 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 50 mmol/L NaF, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 1 mmol/L vanadate, 1 mmol/L Pefabloc, 20 μg/mL leupeptin, and 10 μg/mL aprotinin, and cell debris was removed by centrifugation. Lysates were rocked with 20 μL Protein A Sepharose FF (PAS) (Pharmacia, Piscataway, NJ) beads for 30 minutes at 4°C. Sepharose resin was pelleted and cleared lysate was added to 10 μL ERK2 antibody and 90 μL PAS beads and incubated overnight at 4°C with constant rotation. The beads were pelleted and washed 2 times with lysis buffer and then once with kinase buffer (20 mmol/L 3-[N-Morpholino] propanesulfonic acid [MOPS], pH 7.2, 30 mmol/L β glycerol phosphate, 5 mmol/L EGTA, 20 mmol/L MgCl2, 1 mmol/L dithiothreitol [DTT], 1 mmol/L vanadate, 1 mmol/L Pefabloc, 20 μg/mL leupeptin, 10 μg/mL aprotinin). Kinase activity was then measured by adding 30 μL kinase buffer containing 13 μmol/L of cold adenosine diphosphate (ATP) and 2.3 μCi of33Pγ-ATP (2,500 Ci/mmol) and 10 μg Elk-1 fusion protein (New England Biolabs, Beverly, MA) and incubated for 30 minutes at 30°C. Assays were stopped by addition of sodium dodecyl sulfate (SDS) sample buffer and incubation at 95°C for 5 minutes and resolved on 15% SDS-polyacrylamide gels. Incorporation of 33P into the Elk-1 fusion protein was assessed by autoradiography and quantitated on a phosphoimager.
Ras processing.
Lysates prepared as described above were rocked with 20 μL PAS FF beads (Pharmacia) for 30 minutes at 4°C. Sepharose resin was pelleted and the cleared lysate was incubated with Y13-259 beads at 4°C for 1 hour with constant rotation. Beads were then washed 3 times with phosphate-buffered saline (PBS) and 2 mmol/L MgCl2, resuspended in SDS sample buffer, and heated 95°C for 5 minutes. Ras proteins were resolved on a 15% SDS-polyacrylamide gel and electrotransferred to a PVDF membrane (Millipore). The membrane was stained with Coomassie brilliant blue R, blocked for 1 hour in PBS, 0.1% Tween, 1 mmol/L EDTA (PBST), 3% bovine serum albumin (BSA), and then incubated for 1 hour with a monoclonal antibody directed to either H-Ras residues 157-181 (LAO69, Quality Biotech., diluted 1:10,000 in PBST, 1% BSA) or N-Ras (F155, Santa Cruz Biotechnology, Inc.; diluted 1:400). The membrane was washed 3 times with PBST, 1% BSA and incubated for 1 hour with goat anti-mouse IgG-peroxidase conjugate (diluted 1:10,000 in PBST, 1% BSA). Proteins were visualized with an enhanced chemiluminescence detection system (ECL; Amersham, Arlington Heights, IL).
RESULTS
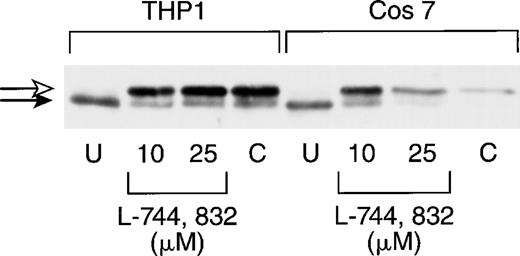
We first tested the ability of L-744,832 to inhibit Ras processing in COS 7 cells and in the THP-1 human myeloid leukemia cell line, which contains an oncogenic NRAS mutation.35 In these and subsequent experiments, cell lysates were first immunoprecipitated with a pan-Ras antibody (Y13-259), followed by electrophoresis, transfer, and blotting with a second anti-Ras antibody (see Materials and Methods). Compactin (100 μmol/L), which blocks Ras farnesylation by depleting farnesyl diphosphate levels, was included as a positive control.34 L-744,832 concentrations of 10 μmol/L and 25 μmol/L were tested on the basis of previous data showing that the growth of 70% of human tumor cell lines was inhibited by 2 to 20 μmol/L of this compound.18 Figure1 shows that all of the H-Ras detected in untreated COS 7 and THP-1 cells has been processed, and that either L-744,832 or compactin induced the appearance of a higher molecular weight band, which corresponds to the unprenylated protein.
L-744,832 inhibits H-Ras farnesylation in cultured cell lines. COS 7 or THP-1 cells were cultured for 48 hours, lysed, immunoprecipitated with pan-Ras antibody Y13-259, blotted, and probed with an antibody specific for H-Ras. Cells were either untreated (U) or were cultured with either 10 μmol/L or 25 μM of L-744,832 or with 100 μmol/L of compactin. Unprocessed H-Ras is indicated by the white arrow and processed Ras is designated with a black arrow.
L-744,832 inhibits H-Ras farnesylation in cultured cell lines. COS 7 or THP-1 cells were cultured for 48 hours, lysed, immunoprecipitated with pan-Ras antibody Y13-259, blotted, and probed with an antibody specific for H-Ras. Cells were either untreated (U) or were cultured with either 10 μmol/L or 25 μM of L-744,832 or with 100 μmol/L of compactin. Unprocessed H-Ras is indicated by the white arrow and processed Ras is designated with a black arrow.
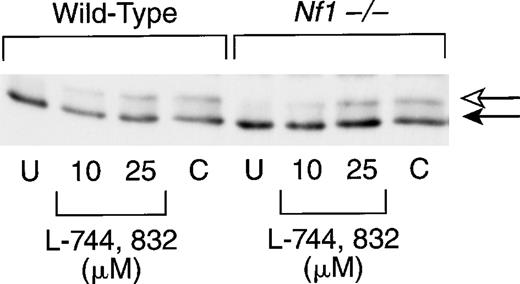
We next investigated bone marrow cells from recipient mice that had been reconstituted with hematopoietic cells from either wild-type (Nf1+/+) or knockout (Nf1−/−) embryos. Because these primary cells did not appear as robust as the cell lines (this was particularly true of the compactin-treated cells), the time in culture was reduced from 48 to 20 hours. As in the cultured cell lines, exposure to L-744,832 or compactin was associated with the appearance of a slower-migrating band that corresponds to unprenylated H-Ras (Fig2). The relative amount of unprocessed Ras was lower in primary cells than in either cell line and appeared to be concentration dependent in cells treated with L-744,832 (Fig 2). Reprobing these blots with an antibody specific for N-Ras revealed no inhibition of Ras processing (data not shown).
L-744,832 inhibits H-Ras farnesylation in murine hematopoietic cells. Mononuclear cells harvested from the bone marrows of mice transplanted with wild-type or Nf1−/− cells were cultured for 20 hours. Cells were either untreated (U) or were cultured with either 10 μmol/L or 25 μmol/L of L-744,832 or with 100 μmol/L of compactin. The cell lysates were prepared and H-Ras processing was assessed as described in Fig 1. Unprocessed H-Ras is indicated by the white arrow and processed Ras is designated with a black arrow.
L-744,832 inhibits H-Ras farnesylation in murine hematopoietic cells. Mononuclear cells harvested from the bone marrows of mice transplanted with wild-type or Nf1−/− cells were cultured for 20 hours. Cells were either untreated (U) or were cultured with either 10 μmol/L or 25 μmol/L of L-744,832 or with 100 μmol/L of compactin. The cell lysates were prepared and H-Ras processing was assessed as described in Fig 1. Unprocessed H-Ras is indicated by the white arrow and processed Ras is designated with a black arrow.
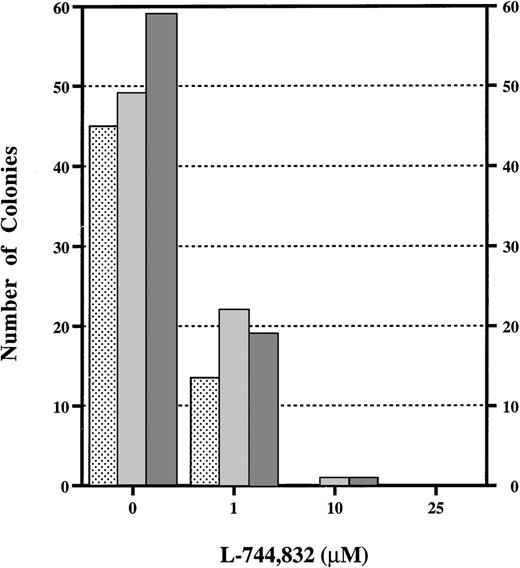
Although these data indicate that L-744,832 can partially inhibit H-Ras prenylation in cultured cell lines and in primary hematopoietic cells, they provide no insights regarding the functional consequences of treatment. To approach this question, we assayed the effects of L-744,832 on the growth of the CFU-GM myeloid progenitor colonies from fetal hematopoietic cells in response to GM-CSF. We were specifically interested in GM-CSF because this growth factor is known to signal through Ras and is implicated in the abnormal growth of human JMML cells and of murine Nf1−/− fetal hematopoietic cells.24,25,27,32 36 In the CFU-GM assay, 10 μmol/L of L744,732 abrogated colony growth from Nf1−/− or controlNf1+/+ fetal hematopoietic cells in response to saturating concentrations of GM-CSF (Fig 3). In addition to a marked reduction in the number of colonies that formed in methylcellulose cultures containing 1 μmol/L of L-744,832, the individual CFU-GM were smaller and had many fewer cells than those that developed in control dishes (data not shown).
L-744,832 inhibits CFU-GM colony growth from fetal liver cells. Fetal hematopoietic cells from Nf1+/+ (),Nf1+/− ( ), or Nf1−/− (▩) embryos were cultured in methylcellulose medium supplemented with 1 unit/mL of recombinant murine GM-CSF with either 0, 1, 10, or 25 μmol/L of L-744,832. CFU-GM colonies were counted after 7 days in culture.
), or Nf1−/− (▩) embryos were cultured in methylcellulose medium supplemented with 1 unit/mL of recombinant murine GM-CSF with either 0, 1, 10, or 25 μmol/L of L-744,832. CFU-GM colonies were counted after 7 days in culture.
L-744,832 inhibits CFU-GM colony growth from fetal liver cells. Fetal hematopoietic cells from Nf1+/+ (),Nf1+/− ( ), or Nf1−/− (▩) embryos were cultured in methylcellulose medium supplemented with 1 unit/mL of recombinant murine GM-CSF with either 0, 1, 10, or 25 μmol/L of L-744,832. CFU-GM colonies were counted after 7 days in culture.
), or Nf1−/− (▩) embryos were cultured in methylcellulose medium supplemented with 1 unit/mL of recombinant murine GM-CSF with either 0, 1, 10, or 25 μmol/L of L-744,832. CFU-GM colonies were counted after 7 days in culture.
We also measured MAP kinase activities in Nf1+/+ andNf1−/− hematopoietic cells isolated from recipient mice after adoptive transfer. As recently reported for purified c-kit positive cells,33 basal MAP kinase activities were generally higher in the unfractionated Nf1−/− hematopoietic cells than in Nf1+/+ cells that we studied, with some variation noted between individual experiments. Treatment with 25 μmol/L L-744,832 had no effect on the baseline MAP kinase activity of eitherNf1−/− or Nf1+/+ cells; however, the FTI blunted activation in response to either GM-CSF or interleukin-3 (IL-3) (Fig4). In contrast, treatment with compactin both reduced baseline MAP kinase activities and eliminated the response to growth factor stimulation (Fig 4).
L-744,832 blunts MAP kinase activation in response to hematopoietic growth factors. Mononuclear cells collected from recipients engrafted with either Nf1+/+ (left 6 lanes) orNf1−/− (right 9 lanes) cells were incubated at 37°C overnight with medium alone (−), medium plus 25 μmol/L L-744,832 (L) or medium and 100 μmol/L compactin (C). The cultures were then split and either not stimulated with any growth factors (−) or stimulated with 10 ng/mL of either GM-CSF (G) or IL-3 (I). The cells were lysed and MAP kinase activity was measured. The top graph shows a phosphoimager plot (counts per minute [CPM]-background) for each condition over the raw data from the autoradiograph. Loss ofNf1 is associated with constitutive activation of MAP kinase (compare lane 1 with 7) that is unaffected by L-744,832 (compare lane 7 with 10) but is inhibited by compactin (compare lane 7 with 13). In this experiment, L-744,832 blunted MAP kinase activation induced by GM-CSF by 31% in Nf1+/+ cells (compare lane 2 with 4) and by 30% in Nf1−/− cells (compare lane 8 with 11). Similarly, FTI treatment decreased the kinase activity measured inNf1−/− cells stimulated with IL-3 by 37% (compare lane 9 with 12). In contrast, compactin completely abrogated this induction of MAP kinase activity (compare lane 2 with 6, lane 8 with 15, and lane 9 with 16).
L-744,832 blunts MAP kinase activation in response to hematopoietic growth factors. Mononuclear cells collected from recipients engrafted with either Nf1+/+ (left 6 lanes) orNf1−/− (right 9 lanes) cells were incubated at 37°C overnight with medium alone (−), medium plus 25 μmol/L L-744,832 (L) or medium and 100 μmol/L compactin (C). The cultures were then split and either not stimulated with any growth factors (−) or stimulated with 10 ng/mL of either GM-CSF (G) or IL-3 (I). The cells were lysed and MAP kinase activity was measured. The top graph shows a phosphoimager plot (counts per minute [CPM]-background) for each condition over the raw data from the autoradiograph. Loss ofNf1 is associated with constitutive activation of MAP kinase (compare lane 1 with 7) that is unaffected by L-744,832 (compare lane 7 with 10) but is inhibited by compactin (compare lane 7 with 13). In this experiment, L-744,832 blunted MAP kinase activation induced by GM-CSF by 31% in Nf1+/+ cells (compare lane 2 with 4) and by 30% in Nf1−/− cells (compare lane 8 with 11). Similarly, FTI treatment decreased the kinase activity measured inNf1−/− cells stimulated with IL-3 by 37% (compare lane 9 with 12). In contrast, compactin completely abrogated this induction of MAP kinase activity (compare lane 2 with 6, lane 8 with 15, and lane 9 with 16).
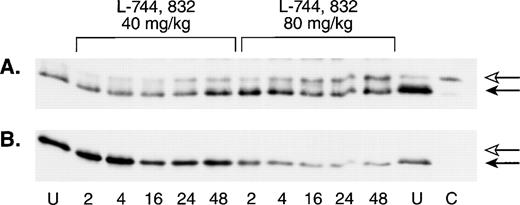
To assess the in vivo effects of FTI treatment on Ras processing in hematopoietic cells, a single L-744,832 dose of either 40 mg/kg or 80 mg/kg was administered to a group of untransplanted mice, and the animals were then sacrificed at defined time intervals. L-744,832-treated animals showed a progressive increase in the proportion of unprenylated H-Ras that was dose and time dependent (Fig5A). However, when this blot was stripped and reprobed with an antibody specific for N-Ras, a single band corresponding to processed Ras was detected (Fig 5B).
L-744,832 inhibits H-Ras, but not N-Ras, farnesylation in vivo. Wild-type mice were injected with a single dose of either 40 mg/kg of 80 mg/kg of L-744,832, then killed 2, 4, 16, 24, or 48 hours after treatment. Mononuclear cells were isolated from hematopoietic tissues, lysed, immunoprecipitated, subject to gel electrophoresis, and blotted as described in Materials and Methods. Bands corresponding to unprocessed and processed Ras are labeled with open and closed arrows, respectively. Cells from untreated mice are labeled “U” and a lysate prepared from a compactin-treated cell line is labeled “C.” (A) Western blot probed with an H-Ras antibody. (B) The same Western blot was stripped and reprobed with an N-Ras antibody.
L-744,832 inhibits H-Ras, but not N-Ras, farnesylation in vivo. Wild-type mice were injected with a single dose of either 40 mg/kg of 80 mg/kg of L-744,832, then killed 2, 4, 16, 24, or 48 hours after treatment. Mononuclear cells were isolated from hematopoietic tissues, lysed, immunoprecipitated, subject to gel electrophoresis, and blotted as described in Materials and Methods. Bands corresponding to unprocessed and processed Ras are labeled with open and closed arrows, respectively. Cells from untreated mice are labeled “U” and a lysate prepared from a compactin-treated cell line is labeled “C.” (A) Western blot probed with an H-Ras antibody. (B) The same Western blot was stripped and reprobed with an N-Ras antibody.
In a breast cancer model in which expression of an HRASoncogene is driven by an MMTV promoter, treatment with L-744,832 at a daily dose of 40 mg/kg was associated with objective tumor responses that were superior to doxorubicin with no evidence of toxicity.10 Therefore, we assigned mice that had been engrafted with either Nf1−/− or with Nf1+/+ fetal liver cells to either receive treatment with L-744,832 (at a dose of 40 mg/kg/d 5 days each week for 8 weeks) or vehicle for 8 weeks. Two mice of each transplant genotype were assigned to each arm (total: n = 8). All of the Nf1−/− recipients were entered at least 3 months after adoptive transfer and had markedly elevated white blood cell counts (Table 1). Although recipient mice tolerated FTI administration with no apparent adverse effects, there was no improvement in white blood cell counts (Table1).
Based on the dose-response effect of L-744,832 on H-Ras prenylation (Fig 5) and on the absence of toxicity in the initial cohort, a second experiment was performed to evaluate an intensive treatment protocol in which the FTI was administered daily at a dose of 80 mg/kg/d for 4 weeks. In addition to 10 mice that had been transplanted withNf1−/− or Nf1+/+ cells alone, we studied 4 animals that had been transplanted with a mixture of Nf1−/− andNf1+/+ cells to determine if treatment with L-744,832 might selectively inhibit the growth of Nf1−/− cells in vivo.Nf1−/− cells have a proliferative advantage overNf1+/+ cells, and mice that receive these transplants develop a myeloid disorder that is similar to what is seen in mice that are givenNf1−/− cells only (data not shown). As in the first experiment, there were no differences in white blood cell counts between FTI-treated and control mice (Table 2). At sacrifice, the spleens of both FTI-treated and control Nf1−/− recipients were enlarged and were infiltrated with myeloid cells (Table 2). Immunoprecipitation and Western blotting experiments showed partial inhibition of H-Ras processing in bone marrow cells from mice that received L-744,832 with no effect on N-Ras (data not shown). MAP kinase activities varied widely in unstimulated bone marrow cells from treated and untreated mice; however, bone marrow cells from mice that had been given the FTI showed a blunted MAP kinase response to GM-CSF (1.7-fold above baseline kinase activity v 3.3-fold for cells from untreated mice). This higher dose of L-744,832 was associated with significant clinical toxicity that included decreased activity, weight loss, abscess formation, and crusted skin lesions at the sites of injections and blood draws.
DISCUSSION
A number of observations have suggested that Ras is not the primary biochemical target of FTIs in mammalian cells.6-9 In a comprehensive survey in which L-744,832 inhibited the growth of over 70% of 42 human tumor cell lines, in vitro efficacy did not correlate with the presence of a RAS mutation.18Farnesylation of lamin B was inhibited to a similar degree by FTI treatment in 1 resistant cell line and 1 sensitive line; however, MAP kinase activation in response to epidermal growth factor was only blocked in sensitive cells.18 Studies of K-Ras processing have also cast doubt on the notion that FTIs act as Ras inhibitors in mammalian cells. In contrast to H-Ras, the carboxy termini of K-Ras and N-Ras proteins are good substrates for processing by geranylgeranyl transferase type 1 (GGTase-1),7,9,37 and geranylgeranylation of K-Ras has recently been demonstrated in vivo.38 Consistent with this, treatment with both an FTI and a GGTase inhibitor were required to block K-Ras prenylation in 5 human tumor cell lines.39 Furthermore, in both tissue culture and in nude mouse xenograft assays, FTI treatment inhibited the growth of 2 cancer cell lines with oncogenic KRAS mutations (A-549 and Calu-1), but had no effect on K-Ras processing.40
If FTIs do not function as Ras inhibitors, what other farnesylated proteins might account for the dramatic antiproliferative effects of these compounds in many assays? Studies performed by Prendergast et al15 and Lebowitz et al16,17 implicate RhoB, which is essential for Ras-induced transformation, as a potential in vivo target of FTIs. Their results are consistent with a recent study showing that RhoB function is required for oncogenic Ras to stimulate DNA synthesis in fibroblasts.41 In the absence of RhoB function, activated Ras induces p21Waf1 and, in turn, blocks proliferation.41 These data further suggest that cells expressing oncogenic Ras might be highly sensitive to FTI-induced growth arrest because they efficiently induce p21Waf1 in response to inhibition of RhoB.
These questions regarding the mechanisms of FTI action underscore the need for studies in relevant immunocompetent animal models. A major advantage of the JMML-like MPD that follows adoptive transfer ofNf1-deficient fetal cells for testing therapeutics is that clinical responses can be correlated with biochemical effects by measuring Ras prenylation and MAP kinase activity in primary hematopoietic cells. Furthermore, compelling genetic and biochemical data implicate deregulated Ras signaling as playing a central role in these and other myeloid leukemias.5,42,43 We found that L-744,832 partially inhibited H-Ras processing in hematopoietic cells but had no effect on N-Ras. Although we did not have a reliable antibody that is specific for K-Ras, Western blotting with pan-Ras antibodies indicated that most of the Ras was fully processed in vivo and, therefore, suggested that K-Ras was also resistant to L-744,832 (data not shown). This is consistent with other data showing that K-Ras is relatively insensitive to inhibition by FTIs.37,39 40 At the doses tested in this study, L-744,832 had no effect on MAP kinase activity in unstimulated hematopoietic cells, but blunted the normal induction of this kinase in response to GM-CSF. Given these biochemical data and the observation that almost all of the RAS mutations in myeloid leukemia involve KRAS or NRAS, it is not surprising that FTI treatment was clinically ineffective in a model in which hyperactive Ras underlies abnormal myeloid cell growth.
In 2 previous studies, FTIs inhibited the in vitro growth of THP-1 cells and of all 6 other human leukemia cell lines tested.18,44 Similarly, we found that 10 μmol/L 744,832 abrogated CFU-GM colony formation in methylcellulose cultures of wild-type and Nf1-deficient fetal hematopoietic cells. In this system, colony formation from individual myeloid progenitor cells requires extensive proliferation in response to high concentrations of cytokine growth factors. It is likely that the profound inhibition of colony formation that we observed was because of the ability of L-744,832 to partially block MAP kinase activation in response to GM-CSF. This interpretation is supported by our finding of relatively small colonies containing few cells in cultures containing 1 μmol/L L-744,832. Our results also differ from a previous report in which FTI treatment induced growth inhibition and morphologic reversion in anNF1-deficient MPNST cell line.23 Potential explanations include: (1) FTIs might differentially affect the growth of neural crest and hematopoietic cells, (2) genetic variations between immortal tumor-derived cell lines and primary cells, and (3) higher concentrations of drug may be achievable in tissue culture than in whole animals. Whatever the reason, the discordant results seen in vitro and in this relevant animal model emphasize the importance of testing experimental therapeutics in an in vivo preclinical setting.
The efficacy of L-744,832 has been evaluated previously in 2 murine cancer models in which tumors arise spontaneously in immunocompetent mice.10,11 In a line of transgenic mice that overexpress oncogenic HRAS from an MMTV promoter, Kohl et al10found that treatment with 40 mg/kg/d of L-744,832 induced tumor regression in 100% of the animals. The FTI was much more effective than the conventional chemotherapeutic agent doxorubicin in this model, and a subsequent study showed an increased rate of apoptosis in tumors from L-744,832–treated animals.12 In a recent study, the MMTV promoter was also used to drive overexpression of a wild-typeNRAS gene.11 The response was not as dramatic as in the previous study; however, tumor growth was reduced in mice that received L-744,832 and this was associated with an increase in the percentages of apoptotic cells within the tumors. Importantly, almost all of the N-Ras was processed normally in the tumor cells of these FTI-treated mice.11
We conclude that treatment with FTI L-744,832 partially inhibits H-Ras processing and blunts GM-CSF–induced MAP kinase activation in a murine model of Ras-activated myeloid leukemia. However, N-Ras and K-Ras proteins were processed normally even at an FTI dose of 80 mg per kg, and there was no clinical efficacy. These data provide direct evidence that L-744,832 (and perhaps other FTIs presently in preclinical and clinical trials) does not efficiently inhibit N-Ras or K-Ras processing at clinically tolerable doses in mice. Our finding that H-Ras prenylation is partially inhibited in vivo is consistent with the impressive antitumor effects of L-744,832 seen in an MMTV-HRAS model of breast cancer.10 However, L-744,832 reduced the growth of MMTV-NRAS–driven breast tumors without inhibiting N-Ras processing.11 It remains to be determined if the discrepant therapeutic results are explained by differences between how breast cancer and myeloid cells respond to FTI-induced inhibition of RhoB or other non-Ras proteins, by the presence or absence of specific cooperating mutations in tumor cell clones, and/or by genetic differences in the way hyperactive Ras is induced in these murine models. The growing evidence that the antitumor effects of FTIs are not caused by inhibition of K-Ras or N-Ras and the markedly different results obtained in murine breast cancer and myeloid leukemia models, emphasize both the importance of identifying the authentic in vivo targets of this promising class of anticancer agents as well as the need to test the clinical efficacy of these compounds against a broad spectrum of human tumors.
ACKNOWLEDGMENT
We are grateful to Allen Oliff and George Hartman for support and helpful discussions, to Gideon Bollag for the gift of antibody Y13-259 and for advice on performing the kinase assays, and to Connie Gebbia and Jennifer Alkire for administrative assistance.
Supported by the Leukemia Society of America Translational Research Grant Award No. 6306-97 and by the National Institutes of Health (NIH) Grant No. R01 CA72614. N.M. was supported by NIH Training Grant No. DK07636.
N.M. and B.R.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kevin M. Shannon, MD, Room HSE-302, Box 0519, University of California, San Francisco, CA 94143; e-mail:kevins@itsa.ucsf.edu.

![Fig. 4. L-744,832 blunts MAP kinase activation in response to hematopoietic growth factors. Mononuclear cells collected from recipients engrafted with either Nf1+/+ (left 6 lanes) orNf1−/− (right 9 lanes) cells were incubated at 37°C overnight with medium alone (−), medium plus 25 μmol/L L-744,832 (L) or medium and 100 μmol/L compactin (C). The cultures were then split and either not stimulated with any growth factors (−) or stimulated with 10 ng/mL of either GM-CSF (G) or IL-3 (I). The cells were lysed and MAP kinase activity was measured. The top graph shows a phosphoimager plot (counts per minute [CPM]-background) for each condition over the raw data from the autoradiograph. Loss ofNf1 is associated with constitutive activation of MAP kinase (compare lane 1 with 7) that is unaffected by L-744,832 (compare lane 7 with 10) but is inhibited by compactin (compare lane 7 with 13). In this experiment, L-744,832 blunted MAP kinase activation induced by GM-CSF by 31% in Nf1+/+ cells (compare lane 2 with 4) and by 30% in Nf1−/− cells (compare lane 8 with 11). Similarly, FTI treatment decreased the kinase activity measured inNf1−/− cells stimulated with IL-3 by 37% (compare lane 9 with 12). In contrast, compactin completely abrogated this induction of MAP kinase activity (compare lane 2 with 6, lane 8 with 15, and lane 9 with 16).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2469.419a01_2469_2476/6/m_blod41901004w.jpeg?Expires=1769310558&Signature=vXIQUi2WbygEysgxdzJ9J4578qWvizwuM0D8S1XiYZvwnwgx02iPX0pcxEseAVxKxxt3TBjdnaOpHCzIpKJxDoV7~6oCpj3pzDdzzcN53qTAKEV5d1NkqmLRdYlXl~LXwUf6aUArCvWZxc-ic-NfQEVd3i4LVi~ZhxnBTYR8Qo1zzw4k5Sx3xrZHtXCkf3lUP00mekSOJvCAArCK6WD0h3RtNcFs5QpAmwMGR8GyG6zcFNRHgPTw7NBui39OOSiWOcKTKI2MvGunmRw4mR9grSxIuwWe4xTbaPjp1e2FDmGk937ZyLGctBhXwwoaS1a34Qw9loGa16FNkUtdIHjoDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)