Chemokines regulate leukocytes trafficking in normal and inflammation conditions. Thymus-seeding progenitors are made in bone marrow and migrate to the thymus where they undergo their maturation to antigen-specific T cells. Immature T cells are in thymic cortex, while mature thymocytes are in medulla. Chemokines may be important for homing of thymus-seeding progenitors, and/or differential thymocyte localization in thymus. Here we report that GPR-9-6, now called CC chemokine receptor 9 (CCR9), is a receptor for thymus-expressed chemokine, TECK. Among a panel of chemokines tested, TECK specifically induced calcium flux in CCR9-expressing cell lines. We also showed that TECK efficaciously induced chemotaxis of immature CD4+CD8+ double-positive, and mature CD4+ and CD8+ single-positive human thymocytes. Our data suggest that TECK/CCR9 interaction may play a pivotal role in T-cell migration in the thymus.
CHEMOKINES MEDIATE the migration and activation of leukocytes. This primary function results from the binding of chemokines to specific surface receptors, known as the chemokine receptor superfamily.1-3 A growing body of evidence clearly suggests that T cells differentially express a set of chemokine receptors upon activation or differentiation such that specific effector functions of T cells are made possible.4Common lymphoid progenitor cells migrate from bone marrow to the fetal thymus to initiate T-cell development. The T-cell maturation process occurs in specific compartments in thymus.5 This compartmentalizing process may be regulated by interactions between chemokines and chemokine receptors. Several chemokines and/or their cognate receptors have been implicated in T-cell migration in the thymus.6 7 These include SDF-1, which binds the chemokine receptor CXCR4; SLC and CKβ-11, which bind CCR7; and thymic-expressed chemokine (TECK), whose receptor has not yet been identified. SDF-1 preferentially attracts immature murine CD4−CD8− double-negative (DN) and CD4+CD8+ double-positive (DP) subsets, while SLC and CKβ-11 attract mature CD4+, and CD8+single-positive (SP) subsets. Chemotactic specificity of TECK for human or murine thymocyte subsets has not been reported.
Here we report that TECK7 is a specific ligand for an orphan chemokine receptor-like protein known as GPR-9-6 and now designated CCR9. Moreover, we show that TECK induces strong chemotaxis of immature DP thymocytes, and CD4+ and CD8+ SP human thymocytes, suggesting that the CCR9/TECK interaction plays an important role in migration of T-cell progenitors in thymus.
MATERIALS AND METHODS
Chemokines.
Human TECK, MIP-1α, RANTES, MIP-1β, Exodus-2, Eotaxin-2, Fractalkine, MIP-4, LEC, MDC, TARC, Eotaxin, MCP-1 , I-TAC, ENA-78, and MIG were purchased from Peprotech (Rocky Hill, NJ). Human ELC, GRO-γ, interleukin-8 (IL-8), SDF-1α, and mouse TECK were purchased from R&D systems (Minneapolis, MN). Lkn-1 was kindly provided by Dr Doo H. Park (MOGAM Biotechnology Research Institute, Kyunggi-Do, Korea). MPIF-1(CKβ8) was previously described.8 BRAK was kindly provided by Dr Rob Hromas (Indiana University School of Medicine).
Cell culture, fluorescence-activated cell-sorting analysis, and transfection.
HEK 293 cells expressing EBNA were purchased from In Vitrogen (Carlsbad, CA) and cultured in Dulbecco’s modified Eagle medium (DMEM). Immunofluorescent staining was performed, essentially as described in Pharmingen (San Diego, CA) technical protocols. Cells were transfected using Fugene-6 (Roche, Indianapolis, IN) with CCR1, CCR4, CCR5, CCR6, and GPR-9-6. Open reading frames encoding CCR1, CCR4, CCR5, and GPR-9-6 were directly amplified from human genomic DNA with the use of polymerase chain reaction (PCR), whereas CCR6 cDNA was kindly provided by Osamu Yoshie (Shionogi Institute for Medical Science, Osaka, Japan). After 48 hours, 200 μg/mL hygromycin B was added. Pools of hygromycin B-resistant colonies were selected.
Construction of CCR9 expression vector.
The open reading frame previously known as GPR-9-6 (GenBank accession no. U45982) was amplified with the following set of primers: forward primer (5′-CCGGCTCGAGCCTATTCCTAACATGGCTGATGAC-3′) and reverse primer (5′-CGCGGGATCCAAGACCCCTCAGAGGGAGAGTGC-3′) using Pfupolymerase (Stratagene, La Jolla, CA). The amplified fragment was digested with XhoI/BamHI and cloned into pCEP4 (Invitrogen, Carlsbad, CA).
Northern blot.
The multiple-tissue Northern blots were purchased from Clontech (Palo Alto, CA) and hybridized to GPR-9-6 or human β-actin probe according to the manual provided.
Calcium flux and chemotaxis assay.
RESULTS AND DISCUSSION
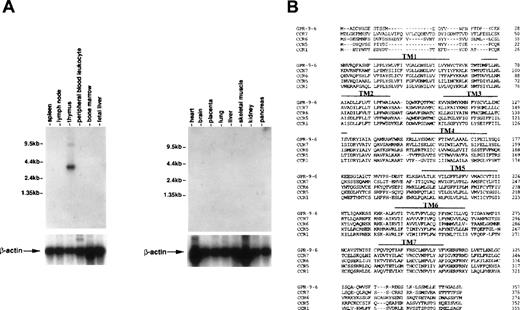
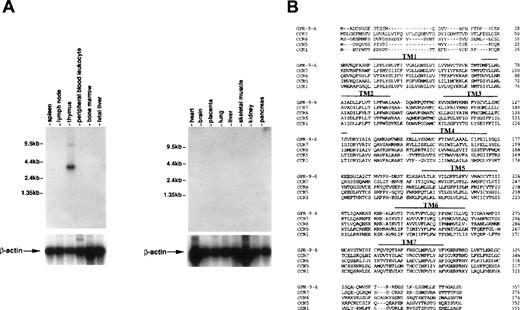
To see whether thymus-specific chemokine receptors exist, we screened expression patterns of known chemokine receptors and orphan receptors using Northern blots as well as expression sequence tags (EST) through GenBank. This screening led to identification of a cDNA previously known as GPR-9-6 (GenBank accession no. U45982), whose expression was highly restricted to thymus (Fig 1A). No other immune tissues expressed the 3.5-kb GPR-9-6 mRNA, suggesting that GPR-9-6 could be a good candidate receptor involved in thymocyte migration. GPR-9-6 contains 7 putative transmembranes (TM) denoted by overlines in Fig 1B. There is a potential N-glycosylation site (Asn20-Phe21-Thr22) in the N-terminal segment. One notable feature is that the length of the N-terminal segment is shortest among known chemokine receptors (Fig1B). Considering the general role of the N-terminal segment of chemokine receptors in ligand binding, the N-terminal segment of GPR-9-6 may be involved in chemokine selectivity. The rank order of amino acid identity of several known chemokine receptors to GPR-9-6 is CCR7 (39%) > CCR6 (35%) = CCR5 (35%) > CCR1 (33%).
Thymus-specific expression of GPR-9-6 and comparison of the primary structure of GPR-9-6 with other chemokine receptors. (A) Human immune system multiple-tissue Northern blot II and human multiple-tissue Northern blot were sequentially hybridized to GPR-9-6 cDNA and human β-actin probes at 68°C for 1 hour, washed with 0.1X standard saline citrate (SSC) at 50°C for 40 minutes, and exposed to x-ray films for 12 hours. (B) An alignment of GPR-9-6 with CCR7, CCR6, CCR5, and CCR1 with the use of GeneWork 2.5.1. Putative transmembranes are indicated by overlines.
Thymus-specific expression of GPR-9-6 and comparison of the primary structure of GPR-9-6 with other chemokine receptors. (A) Human immune system multiple-tissue Northern blot II and human multiple-tissue Northern blot were sequentially hybridized to GPR-9-6 cDNA and human β-actin probes at 68°C for 1 hour, washed with 0.1X standard saline citrate (SSC) at 50°C for 40 minutes, and exposed to x-ray films for 12 hours. (B) An alignment of GPR-9-6 with CCR7, CCR6, CCR5, and CCR1 with the use of GeneWork 2.5.1. Putative transmembranes are indicated by overlines.
To find a chemokine ligand for GPR-9-6, we searched the literature for expression patterns of known chemokines. Among these chemokines, TECK is produced by thymic dendritic cells,7 indicating that TECK might be a ligand for GPR-9-6. We stimulated a panel of chemokine receptor–expressing cell lines (CCR1, CCR4, CCR5, CCR6, GPR-9-6) with human (h) TECK (hereafter termed TECK) and measured calcium flux. TECK specifically induced a calcium flux in GPR-9-6 expressing cells in a dose-dependent manner (Fig 2A and B). Primary stimulation of GPR-9-6 expressing cells with TECK completely desensitized the cells to TECK. We also tested 14 CC chemokines (MIP-1α, MIP-1β, RANTES, Lkn-1, MCP-1, Eotaxin, Eotaxin-2, Exodus-2, ELC, MDC, TARC, MIP-4, LEC, and MPIF-1), 9 CXC chemokines (IL-8, Gro-γ, ENA78, I-TAC, GCP-2, MIG, IP10, SDF-1α, and BRAK), and a CX3C chemokine (Fractalkine) in calcium flux assays. These chemokines did not induce transient calcium mobilization in GPR-9-6–expressing cells or influence the subsequent calcium flux induced by TECK (data not shown), suggesting that TECK may be a specific ligand for GPR-9-6. Mouse TECK (mTECK) also induced robust calcium flux. Primary stimulation with mTECK partially desensitized the cells to TECK. These data suggest that TECK is a specific ligand for GPR-9-6. There are currently 9 human CC chemokine receptors (CCR). Of these receptors, CCR9, previously also known as D6 or CCR10, does not appear to be a signaling receptor.9 10 Therefore, according to the established nomenclature system, we propose GPR-9-6 to be designated as human CCR9.
Functional expression of GPR-9-6 and chemotactic behavior of human thymocytes in response to TECK. (A) HEK293 cells were stably transfected with CCR1, CCR4, CCR5, CCR6, and GPR-9-6. Transfected cells were loaded with Fura-2AM and sequentially stimulated with the chemokines (100 nmol/L) indicated. Transient calcium mobilization was monitored by measuring relative fluorescence of Fura-2AM. (B) Concentration dependence of calcium flux. Fura-2AM–loaded GPR-9-6-expressing cells were stimulated with the indicated concentrations of TECK (1 to 100 nmol/L), and fluorescence was monitored. The peak amplitude of the calcium response was plotted. (C) TECK is a broad-spectrum chemoattractant for immature and mature human thymocyte subsets. (D) Comparison of chemotactic specificity of TECK and SDF-1 for human thymocyte subsets. Input thymocytes before chemotaxis and thymocytes migrated to control medium, TECK (3 μg/mL), and SDF-1 (200 ng/mL) are shown with the percent composition of each thymocyte subset shown in each panel. (E) TECK-induced chemotaxis of human thymocytes is sensitive to pertussis toxin. Thymocytes were pretreated with pertussis toxin (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK (3 μg/mL). Percent inhibition was obtained from a formula [% Inhibition = 100 − (% Cell Migration With Pertussis Toxin Treatment − Background Migration)/(% Cell Migration Without Pertussis Toxin Treatment − Background Migration) ×100].
Functional expression of GPR-9-6 and chemotactic behavior of human thymocytes in response to TECK. (A) HEK293 cells were stably transfected with CCR1, CCR4, CCR5, CCR6, and GPR-9-6. Transfected cells were loaded with Fura-2AM and sequentially stimulated with the chemokines (100 nmol/L) indicated. Transient calcium mobilization was monitored by measuring relative fluorescence of Fura-2AM. (B) Concentration dependence of calcium flux. Fura-2AM–loaded GPR-9-6-expressing cells were stimulated with the indicated concentrations of TECK (1 to 100 nmol/L), and fluorescence was monitored. The peak amplitude of the calcium response was plotted. (C) TECK is a broad-spectrum chemoattractant for immature and mature human thymocyte subsets. (D) Comparison of chemotactic specificity of TECK and SDF-1 for human thymocyte subsets. Input thymocytes before chemotaxis and thymocytes migrated to control medium, TECK (3 μg/mL), and SDF-1 (200 ng/mL) are shown with the percent composition of each thymocyte subset shown in each panel. (E) TECK-induced chemotaxis of human thymocytes is sensitive to pertussis toxin. Thymocytes were pretreated with pertussis toxin (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK (3 μg/mL). Percent inhibition was obtained from a formula [% Inhibition = 100 − (% Cell Migration With Pertussis Toxin Treatment − Background Migration)/(% Cell Migration Without Pertussis Toxin Treatment − Background Migration) ×100].
We examined recombinant TECK for its ability to attract T-cell subsets in human thymus. TECK efficaciously attracted CD4+CD8+ DP, CD4+, and CD8+ SP thymocytes (Fig 2C). Chemotactic activity of TECK for CD4−CD8− (DN) thymocytes was weaker than that for other subsets. TECK began to attract thymocytes at concentrations around 1 μg/mL. At 3 μg/mL, a significant percentage of each thymocyte subset (50% SP, 20% DP) was attracted to TECK in 3 hours. Chemotactic specificity of TECK on human thymocytes was similar to that of SDF-1 (Fig 2D), attracting all 4 immature and mature subsets of thymocytes. SDF-1 has been reported as a chemoattractant for immature T-cell progenitors in mouse thymus.6 Chemotaxis of human thymocytes to TECK was completely abolished by pretreatment of thymocytes with pertussis toxin (Fig 2E), suggesting that the TECK receptor, now called CCR9, signals through pertussis toxin-sensitive Gi protein family proteins for chemotaxis.
TECK is expressed mainly in thymus and small intestine. In this regard, TECK receptor appears to be specifically expressed in thymus, suggesting a specific role of TECK in movement and production of T cells. It is possible that this TECK receptor may be expressed in other tissues, but below the detection limit of our present assessment. In thymus, TECK is expressed from MHC II+ N418+dendritic cells in thymic medullary stroma.11 Thymic medulla is the place where SP thymocytes are localized and emigrate to the blood system.5 Thus, the expression pattern suggests that TECK attracts DP thymocytes from thymic cortex to medulla. Our results that TECK efficaciously induces chemotaxis of DP thymocytes support this potential role of TECK in the thymus. Because TECK induces chemotaxis of mature SP thymocytes as well, TECK appears to regulate localization in medulla and emigration of SP thymocytes out of thymus. In the mouse system, TECK has been shown to attract thymus seeding of early fetal T-cell progenitors.11 However, because pertussis toxin, but not neutralizing antibody to TECK, inhibits seeding of thymus by progenitors, TECK may not be the only chemokine that attracts these early T-cell progenitors. In this regard, SDF-1 may be a potential chemokine that can perform this function, because it induces chemotaxis of bone marrow CD34+ cells and very early triple-negative thymocytes.
In summary, we have identified a thymus-specific chemokine receptor, now called CCR9, which is a specific receptor for TECK. During the review of this article, a publication by Zaballos et al12reported that GPR-9-6 was a specific receptor for TECK and suggested that the receptor be termed CCR9. TECK induces chemotaxis of immature and mature thymocytes in a pertussis toxin–dependent manner, suggesting that TECK/CCR9 interactions may be important for the regulation of T-cell development. Obviously, elucidation of the physiological roles of TECK/CCR9 system will be greatly facilitated by generation of mutant mice lacking these respective genes.
ACKNOWLEDGMENT
We thank Dr John W. Brown (Indiana University) for help in obtaining human thymic tissue.
Supported by U.S. Public Health Service Grants No. R01 HL56416 and RO1 DK53674 from the National Institutes of Health to H.E.B.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Byung-S. Youn, PhD, Department of Microbiology/Immunology and the Walther Oncology Center, Indiana University School of Medicine, Bldg R4, Room 302, 1044 W Walnut St, Indianapolis, IN 46202.

![Fig. 2. Functional expression of GPR-9-6 and chemotactic behavior of human thymocytes in response to TECK. (A) HEK293 cells were stably transfected with CCR1, CCR4, CCR5, CCR6, and GPR-9-6. Transfected cells were loaded with Fura-2AM and sequentially stimulated with the chemokines (100 nmol/L) indicated. Transient calcium mobilization was monitored by measuring relative fluorescence of Fura-2AM. (B) Concentration dependence of calcium flux. Fura-2AM–loaded GPR-9-6-expressing cells were stimulated with the indicated concentrations of TECK (1 to 100 nmol/L), and fluorescence was monitored. The peak amplitude of the calcium response was plotted. (C) TECK is a broad-spectrum chemoattractant for immature and mature human thymocyte subsets. (D) Comparison of chemotactic specificity of TECK and SDF-1 for human thymocyte subsets. Input thymocytes before chemotaxis and thymocytes migrated to control medium, TECK (3 μg/mL), and SDF-1 (200 ng/mL) are shown with the percent composition of each thymocyte subset shown in each panel. (E) TECK-induced chemotaxis of human thymocytes is sensitive to pertussis toxin. Thymocytes were pretreated with pertussis toxin (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK (3 μg/mL). Percent inhibition was obtained from a formula [% Inhibition = 100 − (% Cell Migration With Pertussis Toxin Treatment − Background Migration)/(% Cell Migration Without Pertussis Toxin Treatment − Background Migration) ×100].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2533.419k37_2533_2536/6/m_blod41937002x.jpeg?Expires=1768155977&Signature=JDcq7nZFu50ul3fAo5jOgiH6PWP4UGlTw7pjpxBhsf0-CtLK~lB8lsHa5t9Fn-8hGv3KpirriP3fvTuJY3ehhW~XquBlQqZf06882jHv~aOkHAgtT408PURUzFSNIvd4Z4cgU3~LSqy4pMRmnUEw-Bm-NM56CrCUb5s-gOwugD9W4B-VgzeKOq-Tdu81slbAIf1RH0FQvKhaTAUshCmO8Km~9b9uEJC3mQJog1NbVo-Sf1PKQpn5zdBw~Sst5-0IYbW-GrdE95KWoc24yWXLqE5QaLwHygKnzjmpMHYsTcqOSpDp6bV4qrW0Eh68GI5nQuJnZdW2CjHeAokfztnjhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Functional expression of GPR-9-6 and chemotactic behavior of human thymocytes in response to TECK. (A) HEK293 cells were stably transfected with CCR1, CCR4, CCR5, CCR6, and GPR-9-6. Transfected cells were loaded with Fura-2AM and sequentially stimulated with the chemokines (100 nmol/L) indicated. Transient calcium mobilization was monitored by measuring relative fluorescence of Fura-2AM. (B) Concentration dependence of calcium flux. Fura-2AM–loaded GPR-9-6-expressing cells were stimulated with the indicated concentrations of TECK (1 to 100 nmol/L), and fluorescence was monitored. The peak amplitude of the calcium response was plotted. (C) TECK is a broad-spectrum chemoattractant for immature and mature human thymocyte subsets. (D) Comparison of chemotactic specificity of TECK and SDF-1 for human thymocyte subsets. Input thymocytes before chemotaxis and thymocytes migrated to control medium, TECK (3 μg/mL), and SDF-1 (200 ng/mL) are shown with the percent composition of each thymocyte subset shown in each panel. (E) TECK-induced chemotaxis of human thymocytes is sensitive to pertussis toxin. Thymocytes were pretreated with pertussis toxin (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK (3 μg/mL). Percent inhibition was obtained from a formula [% Inhibition = 100 − (% Cell Migration With Pertussis Toxin Treatment − Background Migration)/(% Cell Migration Without Pertussis Toxin Treatment − Background Migration) ×100].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/7/10.1182_blood.v94.7.2533.419k37_2533_2536/6/m_blod41937002x.jpeg?Expires=1768155978&Signature=r~C4jEzx-MyqHS0lLHti~dM3JoQT5uzDGco3mxScs5oSXZU5cXIr3gDtk917KgBbC2jDytgxZ8gUcc0l09V3UYXa-dkVxfr1l3ErwgbJrvf4DkDVe9x98OJy2I90p5yoTBg2EKAUfIwMT3jMeSzdx9OHlfHZisiLd-0MurWdmQ6Se7nqChbylGsvVrIcWSF-Wxut61PsQjt8T5gKLl2dNOaTdsrq4xfTISPhxCS6a9Bkqo~q~Fb40Q4tZL1ILHADuN6~FKHJ1ZsInhuTaUl1E5IIDpIIDqyLNSAndOnVCWIkLcN~uTyZbo7MVe0fP1j21BqH4YIhdDeW~cJAz0raTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)