The stimulation of regulated exocytosis in vascular endothelial cells (EC) by a variety of naturally occurring agonists contributes to the interrelated processes of inflammation, thrombosis, and fibrinolysis. The Weibel-Palade body (WPB) is a well-described secretory granule in EC that contains both von Willebrand factor (vWF) and P-selectin, but the mechanisms responsible for the targeting of these proteins into this organelle remain poorly understood. Through adenoviral transduction, we have expressed human growth hormone (GH) as a model of regulated secretory protein sorting in EC. Immunofluorescence microscopy of EC infected with GH-containing recombinant adenovirus (GHrAd) demonstrated a granular distribution of GH that colocalized with vWF. In contrast, EC infected with an rAd expressing the IgG1 heavy chain (IG), a constitutively secreted protein, did not demonstrate colocalization of IG and vWF. In response to phorbol ester, GH as well as endogenously synthesized vWF were rapidly released from GHrAd-infected EC. By immunofluorescence microscopy, granular colocalization of GH with endogenous tissue-type plasminogen activator (tPA) was also demonstrated, and most of the tPA colocalized with vWF. These data indicate that EC are capable of selectively targeting heterologous proteins, such as GH, to the regulated secretory pathway, which suggests that EC and neuroendocrine cells share common protein targeting recognition signals or receptors.
REGULATED EXOCYTOSIS provides a means by which endothelial cells (EC) can very rapidly and selectively alter the microenvironment of individual vascular beds and modulate the interrelated processes of coagulation, fibrinolysis, and inflammation. Weibel-Palade bodies (WPB) are secretory granules in EC in which von Willebrand factor (vWF) and P-selectin are stored.1-4 vWF is synthesized exclusively by EC and megakaryocytes, and the vWF stored in WPB consists primarily of very high molecular weight multimers3,5 that, when released, bind avidly to the extracellular matrix and to platelet receptors.6 P-selectin is a type 1 membrane protein that promotes the binding and rolling of monocytes and neutrophils before leukocyte migration into sites of inflammation.7 Plasma vWF levels increase rapidly in response to physiologic stimuli such as exercise and adrenaline in vivo and in response to the clinical administration of DDAVP, a vasopressin analog.8 In cultured EC, exocytosis of WPB results in the release of vWF and surface expression of P-selectin within minutes of exposure to a number of naturally occurring agonists, including thrombin,9 peptido-leukotrienes,10 and histamine.11 EC in vivo also contain large stores of active and releasable tissue-type plasminogen activator (tPA),12the primary initiator of plasma fibrinolysis.13 Plasma levels of tPA increase rapidly after the administration of DDAVP in vivo, and regulated secretion of tPA from EC has also been demonstrated in response to thrombin in vitro.14 15
The mechanisms responsible for the sorting of EC proteins into secretory granules are not well understood. Cultured human EC are difficult to transfect at high efficiency by standard means16,17; therefore, previous transfection studies of regulated EC secretory proteins have been performed in neuroendocrine cell lines.18-21 vWF expressed in AtT-20 (pituitary) cells is found in granular structures that are similar in appearance to WPB, but the vWF cannot be mobilized by agonists that induce secretion of endogenous peptides in this cell type.18 In contrast, P-selectin is targeted to functional secretory granules when expressed in AtT-20 cells,19 and there is evidence that the cytoplasmic domain is responsible for sorting to the regulated pathway in this cell type20 and in EC, but not in platelets.22 It is not known whether the results of these studies can be extended to the targeting of endogenous proteins in EC.
Human growth hormone (GH) is a 191 amino acid protein normally stored in dense granules of the somatotroph cells of the anterior pituitary23 and is secreted in response to GH-releasing hormone. GH has been used as a model of sorting and secretion in AtT-20 cells and PC12 cells.24 25 Through the use of a recombinant adenoviral vector (rAd), we have expressed GH in human umbilical vein EC (HUVEC) as a model for the sorting of proteins into endothelial regulated secretory granules. In this report, we demonstrate that GH expressed in HUVEC colocalized with vWF in granules and that the GH was secreted in a regulated fashion in response to agonists that mobilize WPB. We also provide evidence that tPA resides at least in part in the WPB. These data indicate that EC are capable of targeting a heterologous secretory protein to functional exocytic granules, suggesting that EC and neuroendocrine cells share protein targeting recognition signals or receptors.
MATERIALS AND METHODS
Materials.
The human growth hormone radioisotopic assay (RIA) kit was purchased from Nichols Institute (San Juan Capistrano, CA). Rabbit-anti-GH and anti-vWF polyclonal antibodies were purchased from Dako (Carpinteria, CA), sheep anti-tPA antibody from Enzyme Research Laboratories, Inc (South Bend, IN), and biotinylated sheep anti-tPA and fluorescein isothiocyanate (FITC)-conjugated goat anti-vWF antibody from The Binding Site (Birmingham, UK), anti–lysosomal-associated membrane protein-1 (LAMP-1) monoclonal antibody (H4A3) from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Fluorophore-conjugated avidin and secondary antibodies were purchased from Vector Laboratories (Burlingame, CA), ICN/Cappel (Costa Mesa, CA), or Sigma Chemical Co (St Louis, MO). Sheep antirabbit Ig antibody-coated magnetic beads were obtained from Dynal (Lake Success, NY). Human α-thrombin was purchased from Enzyme Research Laboratories, fibronectin from Collaborative Biomedical Products (Bedford, MA), and endothelial mitogen from Biomedical Technologies, Inc (Stoughton, MA). Other tissue culture reagents were purchased from GIBCO-BRL (Frederick, MD) and Bio-Whittaker (Walkersville, MD). Restriction enzymes were purchased from Pharmacia (Piscataway, NJ), and all other reagents were purchased from Sigma Chemical Co. The polymerase chain reaction (PCR) was performed with reagents from the GeneAmp PCR Reagent kit (Perkin Elmer, Roche Molecular Systems, Inc, Branchburg, NJ).
Construction of the GH and IgG adenoviral vectors.
A human GH-expressing rAd was constructed using methods modified from Becker et al.26 A 2.1-kb BamHI/EcoRI GH genomic DNA fragment was excised from p0GH27 and ligated into Bluescript KS(−) (Stratagene, La Jolla, CA). The insert was excised from Bluescript KS(−) with Xba I andHindIII, gel-purified, and ligated into the Xba I andHindIII sites between the cytomegalovirus (CMV) promoter and SV40 polyadenylation sequences of the rAd transfer vector pACCMV.pLpASR(+).26 This transfer plasmid containing GH was cotransfected with the pJM1728 plasmid into 293 cells29 by use of Lipofectamine (GIBCO-BRL). Single clones were obtained by limiting dilution of viral lysates obtained from the cotransfected 293 cells. One clone, proven to express GH in 293 cells as measured by RIA, was amplified by infection of 293 cells in T175 flasks, and the rAd was purified from crude cell lysates by centrifugation twice through CsCl followed by dialysis in phosphate-buffered saline (PBS)/10% glycerol.26
A control rAd expressing the signal peptide from P-selectin fused to the human IgG1 heavy chain constant region (IGrAd) was constructed in a manner similar to the GHrAd. The signal peptide sequence of P-selectin was amplified from human cDNA by PCR with primers incorporating EcoRI and Xba I sites. The IgG1 heavy chain segment (IG) including the stop codon was amplified with primers containing Xba I and HindIII sites from a plasmid containing the cDNA.30 Digests of the PCR fragments were sequentially cloned into the pACCMV.pLpASR(+) vector so that the IgG sequence was directly 3′ to the P-selectin signal peptide sequence. IgGrAd was then generated in 293 cells as described above.
Cell culture.
EC were isolated from 2 to 4 human umbilical vein segments by collagenase digestion and serially subcultured (2 or 3 passages) in M199 containing 20% heat-inactivated fetal calf serum, 100 μg/mL of porcine heparin, 50 μg/mL of endothelial cell mitogen, and penicillin/streptomycin. Final plating was onto gelatin-coated tissue culture-treated plastic 60-mm or 100-mm dishes or C-24 wells or onto ultrasonically cleaned, fibronectin-coated glass coverslips in C-24 wells. 293 cells were cultured in gelatin-coated T75 or T175 flasks, C-6 wells, or microtiter plates (96 wells) in Dulbecco’s modified Eagle’s medium (DMEM) containing 5% heat-inactivated fetal calf serum and penicillin/streptomycin.
Immunofluorescence staining and microscopy.
HUVEC cultured on glass coverslips in 24-well plates were either mock-infected or infected with approximately 5 to 10 plaque-forming units (pfu) of GHrAd or IGrAd per cell. After approximately 72 hours of incubation at 37°C, the cells were washed in Dulbecco’s phosphate-buffered saline (DPBS), fixed in 3.7% formaldehyde/DPBS for 15 minutes, permeabilized with acetone for 20 minutes, and blocked with 3% bovine serum albumin/1% normal goat serum in DPBS. The cells were then incubated with unconjugated primary antibodies, washed 4 times with DPBS, and then incubated with secondary antibodies or avidin conjugated to fluorophore or with FITC-conjugated anti-vWF antibody, as indicated in the figure legends. The coverslips were then washed, dried, and mounted on glass slides for visualization on an Olympus BH2 fluorescence microscope (Olympus Corp, Lake Success, NY). Some specimens were visualized using an MRC-1024 laser scanning confocal microscope (Bio-Rad, Hercules, CA); images were obtained at 0.36-μm intervals in the Z-axis using a 100× objective.
Analysis of GH secretion by RIA.
Confluent HUVEC grown in 24-well plates were infected with approximately 0.5 or 5 pfu/cell GHrAd or were mock-infected. To measure regulated secretion, the cells were washed 3 times with Hanks’ balanced salt solution (HBSS)/HEPES/0.1% gelatin 68 to 72 hours after infection and then treated with agonist for 15 minutes. The supernatants were collected and stored at −20°C for subsequent analysis. RIA was performed in duplicate on the supernatants following the manufacturer’s instructions. To assess constitutive GH secretion, 50-μL aliquots of conditioned growth medium were removed from wells twice a day for 3 days after infection, GH was measured by RIA, and total GH secreted into the media was calculated.
Pulse-chase labeling, immunoprecipitation, and gel electrophoresis.
For radiolabeling experiments, HUVEC were infected with approximately 3 pfu/cell GHrAd or IGrAd or were mock-infected and were then maintained for 3 days in cysteine- and methionine-deficient medium, supplemented with 250 μCi of [35S]-cysteine/methionine (NEN, Boston, MA) per 60-mm plate. Cell supernatants were subjected to immunoprecipitation onto antirabbit Ig-coated magnetic beads (PerSeptive Biosystems, Framingham, MA) with rabbit antihuman GH antibody (Dako) or rabbit antihuman IgG (ICN/Cappel) and electrophoresed through a 5% to 15% gradient sodium dodecyl sulfate (SDS) polyacrylamide gel to confirm the presence of GH or IG at the approximate sizes of 22 and 34 kD, respectively. Regulated secretion experiments were then conducted on GHrAd- or IGrAd-infected, radiolabeled HUVEC that were chased for 16 hours with unlabeled OptiMEM (GIBCO-BRL). The cells were then incubated for 30 minutes at 37°C with control OptimMEM or OptimMEM containing 100 nmol/L phorbol myristate acetate (PMA). Chase and treatment supernatants were collected and analyzed by electrophoresis in 5% to 20% gradient gels. Gels to be imaged by radiographic film were treated with ENHANCE (NEN) and dried. For quantitative analysis, bands were either evaluated by densitometry or by scanning on a phosphorimaging device (Storm 840 PhosphoImager; Molecular Dynamics, Sunnyvale, CA). The relative targeting efficiency of GH compared to vWF within individual experiments was calculated by the following formula: relative efficiency ([GHpma/GHchase]/[vWFpma/vWFchase]). GHpma is the densitometric value assigned to the amount of GH released into the treatment supernatant in response to PMA; vWFpma is the value of vWF present in that same lane. GHchase is the value of GH constitutively released into the chase medium; vWFchase is the value of vWF present in the same lane (see Fig 3C). Because GH and vWF were present in the same samples and comparisons were made from the same gel lanes, no correction is needed for sample volumes.
RESULTS
Colocalization of GH and vWF in GHrAd-infected EC.
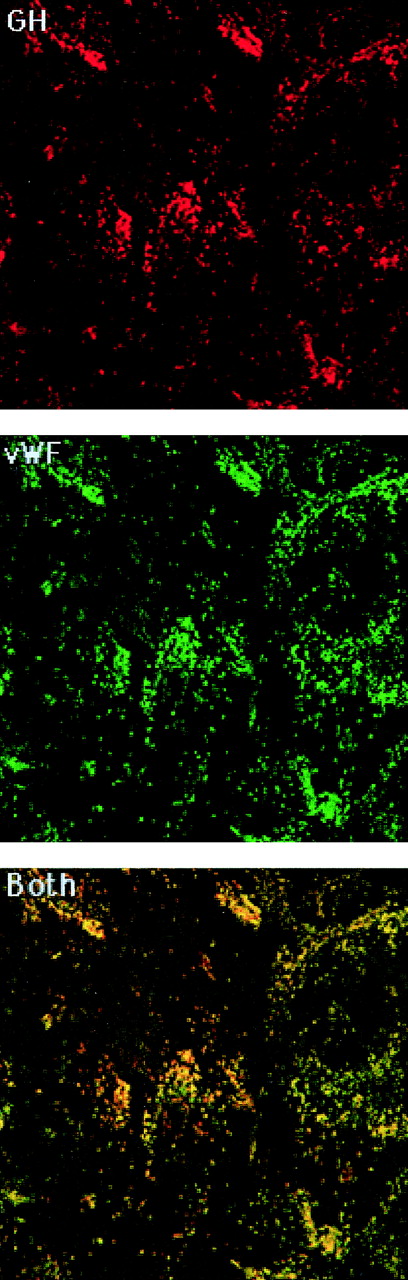
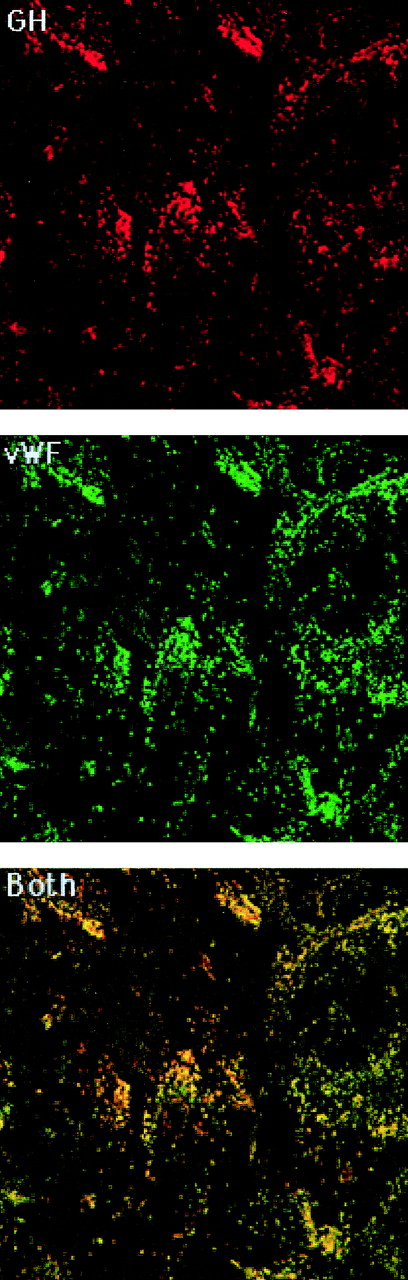
Mock- and GHrAd-infected HUVEC were examined for vWF and GH distribution by conventional and confocal fluorescence microscopy. Staining with FITC-conjugated anti-vWF antibody demonstrated punctate granules corresponding to WPB, similar to those described previously.3 18 In GHrAd-infected cells stained with anti-GH antibody and rhodamine-conjugated secondary antibody, we observed punctate granules similar to those containing vWF. Most of the granular immunoreactive GH colocalized with vWF, consistent with targeting of GH to WPB (Fig 1). There was also occasional staining for GH in smaller granules that did not colocalize with vWF, suggesting that a small proportion of GH was targeted to EC granules that were distinct from WPB. As anticipated, there were numerous granules staining for vWF, but not GH. GH was not detected in uninfected cells, and GHrAd-infected cells did not stain with the rhodamine-conjugated antibody in the absence of primary anti-GH antibody (data not shown). Unpermeabilized GHrAd-infected HUVEC did not stain to any significant degree for either GH or vWF (data not shown).
Colocalization of GH with vWF. GHrAd-infected HUVEC were fixed, permeabilized, and stained with rabbit anti-GH antibody followed by rhodamine-conjugated sheep antirabbit antibody and then FITC-conjugated sheep anti-vWF antibody. Images were obtained by scanning confocal microscopy at 0.36-μm intervals in the Z-axis using a 100× objective. Shown is a representative scan from the approximate midsection of the Z-axis. The red and green images were merged to demonstrate colocalization, which appears yellow.
Colocalization of GH with vWF. GHrAd-infected HUVEC were fixed, permeabilized, and stained with rabbit anti-GH antibody followed by rhodamine-conjugated sheep antirabbit antibody and then FITC-conjugated sheep anti-vWF antibody. Images were obtained by scanning confocal microscopy at 0.36-μm intervals in the Z-axis using a 100× objective. Shown is a representative scan from the approximate midsection of the Z-axis. The red and green images were merged to demonstrate colocalization, which appears yellow.
The patterns of lysosomal and GH staining were also compared in GHrAd-infected HUVEC. Although some perinuclear staining for GH corresponding with LAMP-1 was observed, staining with this lysosomal marker did not colocalize with the distinct peripheral granules of GH (Fig 2A). HUVEC infected with the control IGrAd demonstrated a diffuse, fine granular pattern of cytoplasmic staining that did not colocalize with vWF (Fig 2B). HUVEC cotransduced with GHrAd and IGrAd did not demonstrate any significant colocalization of these 2 proteins by dual-label immunofluorescence microscopy (Fig 2C). These results argue against the possibility of heterologous proteins expressed in EC being nonspecifically targeted to the WPB.
Specificity of GH targeting to WPB. (A) GH (red) versus LAMP-1 (green). GHrAd-infected HUVEC were fixed, permeabilized, and stained with rabbit anti-GH and mouse anti-LAMP-1 (H4A3) antibodies, followed by rhodamine-conjugated antirabbit and FITC-conjugated antimouse antibodies. Several images were obtained by scanning confocal microscopy at 0.36-μm intervals in the Z-axis and then combined into a single image. (B) IG (red) versus vWF (green). IGrAd-infected HUVEC were fixed, permeabilized, and stained with rhodamine-conjugated antihuman IgG and FITC-conjugated anti-vWF antibodies. Shown is a representative confocal scan from the approximate midportion of the Z-axis. (C) GH (red) versus IG (green). HUVEC transduced with both GHrAd and IGrAd were fixed, permeabilized, and stained with rabbit anti-GH antibody and biotinylated goat antihuman IgG antibody, followed sequentially with rhodamine-conjugated goat antirabbit antibody and FITC-conjugated avidin. Shown is a representative confocal scan from the approximate midsection of the Z-axis. In each of these images, there are few yellow granules, indicating minimal colocalization of proteins in each case.
Specificity of GH targeting to WPB. (A) GH (red) versus LAMP-1 (green). GHrAd-infected HUVEC were fixed, permeabilized, and stained with rabbit anti-GH and mouse anti-LAMP-1 (H4A3) antibodies, followed by rhodamine-conjugated antirabbit and FITC-conjugated antimouse antibodies. Several images were obtained by scanning confocal microscopy at 0.36-μm intervals in the Z-axis and then combined into a single image. (B) IG (red) versus vWF (green). IGrAd-infected HUVEC were fixed, permeabilized, and stained with rhodamine-conjugated antihuman IgG and FITC-conjugated anti-vWF antibodies. Shown is a representative confocal scan from the approximate midportion of the Z-axis. (C) GH (red) versus IG (green). HUVEC transduced with both GHrAd and IGrAd were fixed, permeabilized, and stained with rabbit anti-GH antibody and biotinylated goat antihuman IgG antibody, followed sequentially with rhodamine-conjugated goat antirabbit antibody and FITC-conjugated avidin. Shown is a representative confocal scan from the approximate midsection of the Z-axis. In each of these images, there are few yellow granules, indicating minimal colocalization of proteins in each case.
Constitutive and regulated secretion of GH from GHrAd-infected HUVEC.
Confluent HUVEC mock-infected or infected with 0.5 to 5 pfu/cell of GHrAd demonstrated constitutive secretion of GH into the medium, the rate of which increased gradually over 3 days, but varied somewhat from experiment to experiment (data not shown). When GHrAd-infected cells were treated for 30 minutes with PMA, secretion was modestly increased compared with controls (fold increase 1.52 ± 0.16 [mean ± SEM] for 0.5 pfu/cell and 1.37 ± 0.11 for 5 pfu/cell [n = 4, each experiment performed in triplicate]), suggesting release of GH from an agonist-sensitive pool.
To further demonstrate that a stored pool of GH was being mobilized, pulse-chase experiments were performed. GH and IG were immunoprecipitated from both [35S]-cysteine/methionine labeling medium and chase supernatants of infected HUVEC. Immunoprecipitates were then analyzed by SDS polyacrylamide gel electrophoresis (PAGE) to confirm their approximate molecular weights (Fig 3A). Pulse-chase experiments of mock-infected or GHrAd-infected HUVEC showed that GH secretion increased by 3- to 4-fold in response to PMA, in conjunction with a 4- to 5-fold increase in vWF secretion (Fig 3B). These data support the view that a portion of the GH heterologously expressed in HUVEC was sorted into functional secretory granules. In contrast, similar experiments with HUVEC infected with IGrAd demonstrated only constitutive secretion of radiolabeled IG, which was not increased further in response to PMA (Fig 3C).
Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.
Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.
To assess the relative efficiency of targeting, further pulse-chase and secretion experiments of GHrAd or IgGrAd-infected HUVEC were performed (Fig 3C). Densitometric analysis of SDS-PAGE gels indicated that the relative efficiency of GH compared with vWF targeting was 0.2 ± 0.14 (mean ± SD, n = 3). Essentially none of the IG was targeted to the WPB as measured by this method. These data indicate that the targeting efficiency of GH is low compared with vWF, although greater than the targeting efficiency of IG.
Colocalization of tPA with vWF and GH.
Uninfected and GHrAd-infected HUVEC were examined for the intracellular location of tPA, which, similar to vWF, is stored in EC and secreted in a regulated fashion.15,31 In contrast to the findings of Emeis et al,31 we found numerous granules in which tPA and vWF colocalized, as assessed by conventional and confocal immunofluorescence microscopy (Fig 4A). There were also occasional smaller tPA-containing granules in which vWF was not detected. We confirmed the colocalization of tPA and vWF by repeating the immunostaining successively with sheep anti-tPA, followed by Texas Red-conjugated rabbit antisheep, followed by FITC-conjugated goat anti-vWF or with biotinylated sheep anti-tPA plus mouse anti-vWF, followed by rhodamine-conjugated avidin and then FITC-conjugated goat antimouse antibody (data not shown). There was considerable heterogeneity among EC both within individual cultures and among different EC platings with respect to the number of tPA-containing granules present. In GHrAd-infected cells, many of the granules staining for GH also stained for tPA (Fig 4B). The total number of GH and tPA granules and the degree of colocalization varied among cells.
Colocalization of tPA with vWF and GH. (A) tPA versus vWF. Uninfected HUVEC were fixed, permeabilized, and stained with biotinylated sheep anti-tPA antibody followed by rhodamine-conjugated avidin and then FITC-conjugated goat anti-vWF. (B) tPA versus GH. GHrAd-infected HUVEC were fixed, permeabilized, and stained with biotinylated sheep anti-tPA and rabbit anti-GH antibodies, followed by rhodamine-conjugated avidin and then FITC-conjugated antirabbit antibody. Scanning confocal microscopy images were obtained at 0.36-μm intervals in the Z-axis using a 100× objective. Shown are representative scans from the approximate midsection of the Z-axis. The red and green images were merged to demonstrate colocalization, which appears yellow.
Colocalization of tPA with vWF and GH. (A) tPA versus vWF. Uninfected HUVEC were fixed, permeabilized, and stained with biotinylated sheep anti-tPA antibody followed by rhodamine-conjugated avidin and then FITC-conjugated goat anti-vWF. (B) tPA versus GH. GHrAd-infected HUVEC were fixed, permeabilized, and stained with biotinylated sheep anti-tPA and rabbit anti-GH antibodies, followed by rhodamine-conjugated avidin and then FITC-conjugated antirabbit antibody. Scanning confocal microscopy images were obtained at 0.36-μm intervals in the Z-axis using a 100× objective. Shown are representative scans from the approximate midsection of the Z-axis. The red and green images were merged to demonstrate colocalization, which appears yellow.
DISCUSSION
The targeting of secretory proteins into regulated exocytotic granules has not been extensively studied in EC. Results obtained primarily in cell lines of neuroendocrine origin have supported several different theories regarding the sorting of proteins between the constitutive and regulated secretory pathways. These include selective condensation and concentration of the secretory product within the Golgi cisternae, selective protein targeting through binding to specific granule receptors, carrier vesicle-mediated targeting to the secretory granule, and temporal regulation of granule protein biosynthesis.24,32-34 These mechanisms may not be mutually exclusive within a given cell type. Selective protein aggregation in the trans-Golgi network is thought to play an important role in the development of immature secretory granules and may depend in part on an acidic milieu and high calcium concentration.35,36 In EC, both vWF multimer and WPB formation is abolished by the presence of a weak base in the culture medium.37 However, selective condensation is unlikely to be an exclusive sorting mechanism, because constitutively secreted proteins have also been found in immature secretory granules in some cell types.38
The primary amino acid sequences of numerous regulated secretory proteins have been analyzed in search of a transport sorting signal similar to those described for sorting between other intracellular compartments,39 but to date no such consensus sequence has been found. It is likely that secondary or higher protein structures determine sorting into secretory granules. Analysis of the predicted secondary structure of a variety of secretory proteins has shown the presence of an N-terminal hydrophobic domain that may be both necessary and sufficient for their sorting to the regulated secretory pathway.40 Also, chromogranin B36 and pro-opiomelanocortin (POMC)41 contain N-terminal amphipathic loop conformational motifs that are necessary for sorting. The structure responsible for the sorting of GH is unknown.
In this study, we expressed GH to determine whether EC possess the cellular apparatus necessary to target heterologous secretory proteins to functional exocytotic granules. GH has served as a model of sorting and secretion in the PC12 (pheochromocytoma) and AtT-20 (pituitary) cell lines. In these cell types, heterologously expressed GH was found to reside in secretory granules that contain the endogenous secretory proteins and was released in a regulated fashion together with the endogenous proteins.24 25 In this report, we demonstrate that GH expressed in HUVEC was targeted to functional secretory granules that contain endogenous vWF and that GH was secreted rapidly in response to PMA, along with vWF. Pulse-chase experiments clearly demonstrated that a portion of the GH was secreted in a regulated manner, along with secretion of mature vWF and the cleaved vWF propeptide (Fig 3). HUVEC infected with the control IGrAd did not demonstrate colocalization of IG with vWF, and HUVEC infected with both GHrAd and IGrAd did not demonstrate colocalization of GH with IG. These data support the notion that the heterologously expressed GH was specifically targeted to the WPB; however, the efficiency of this targeting was low.
The mechanism by which GH is sorted to regulated exocytotic granules has not been fully elucidated, although carboxypeptidase E appears to play a role in the anterior pituitary. Membrane-associated carboxypeptidase E has been shown to act as a sorting receptor for POMC in mouse neuroendocrine cells.42 Mice deficient in carboxypeptidase E secrete GH constitutively, and secretion is not increased in response to specific agonists.43Carboxypeptidase E is present in HUVEC44 and may therefore be responsible for the sorting of GH to the WPB when expressed in HUVEC. Alternatively, GH expressed in EC may be targeted to the WPB through coaggregation or specific interaction with vWF or other endogenous protein constituents of this granule. There is in vitro evidence that GH forms aggregates with other pituitary secretory proteins under mildly acidic conditions, but undergoes only minimal self-aggregation under similar conditions.45 Procoagulant factor VIII (FVIII) is sorted to vWF-containing granules when expressed in bovine aortic endothelial cells or in AtT-20 cells stably transfected with vWF.46 This sorting appears to be due to specific coaggregation, because mutation of the FVIII binding site in vWF abolishes the sorting of FVIII to granules.
The results of the immunofluorescence microscopy experiments suggest that GH was located in more than 1 granule compartment within transduced EC. Dual-label immunofluorescence staining of GH and LAMP-1 indicated that some of the non-WPB staining may be due to the processing of a small amount of GH into lysosomes, and staining of GH in transit through the endoplasmic reticulum and Golgi would also be expected. We also found that some GH-containing granules stained positively for tPA. In contrast to the reports by Emeis et al31 47 suggesting that tPA in EC resides in granules that are wholly distinct from WPB, our data indicate that tPA is located in the WPB. Different culture conditions may possibly account for this discrepancy. Our data are most consistent with the notion that GH expressed in HUVEC is targeted primarily to the WPB pool, a portion of which may contain both tPA and vWF.
Knowledge of the basic mechanisms of protein sorting and regulated secretion in EC may aid in understanding the pathophysiology of certain thrombotic and inflammatory conditions and in developing potential therapies. To date, our knowledge of protein sorting to the regulated pathway in EC has been largely inferred from studies conducted in neuroendocrine cell lines. We have now shown the feasibility of using adenoviral vectors to express heterologous secretory proteins in human EC with the intention of selective targeting to functional secretory granules. Significantly, there was no discernible interference of the targeting of endogenous proteins in rAd-infected EC. Our studies demonstrate that EC have the cellular apparatus necessary to sort a model secretory protein of neuroendocrine origin to the regulated pathway of secretion. However, the use of GH to direct heterologous proteins of potential therapeutic benefit to the WPB may not be desirable, due to the low sorting efficiency observed. Sequences from endogenous EC proteins are likey to prove to be of more value in this strategy.
ACKNOWLEDGMENT
The authors thank Andrew Ritchie for technical assistance and Andrew McShea and Christopher Wrighton for advice regarding rAd propagation.
Supported by National Institutes of Health Grants No. K08 HL03499-01A1 and HL-15157.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bruce M. Ewenstein, PhD, Hematology Division, Brigham and Women’s Hospital, Longwood Medical Research Center, Room 617, 221 Longwood Ave, Boston, MA 02115; e-mail:bmewenstei@bics.bwh.harvard.edu.


![Fig. 3. Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2696.420k29_2696_2703/7/m_blod42029003aw.jpeg?Expires=1765931714&Signature=DMkghoIiLE3xMHepVBhnDZ7RznWhW4x~M2bGKeMElQtbIfYS2mD-fguO6Nhw0b8qwEXwU5prdSJOPvKnUC1aFlSqrbtkUSslFj7n00gJYImDZug--H4ZZFpbh0EGutgdrqL1J5nsFUAD9lN5BcChbgjHhmTsuceproIubY5vt3b7AU0KUvA0Q0GmzVKej5Ox4gIf6~arC3xjriaIcW5~YcZepus3WWBnHHhzit-uH20ZXUeTWJCdNFWWUuzvE1r-UsfrBQ2d6PrTRLt410UHt4FjvXc~MZbIVLQwo8sZ8IhX0VNP22uYSPeCaWPD7dBhF0JZrpnKpI9aBdU9cI82sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2696.420k29_2696_2703/7/m_blod42029003cw.jpeg?Expires=1765931714&Signature=pG5LjSRBiD7h6fnp8Cuaz4KRnJxXKoiiYjmDyg~RsDXa7Sqq-4WPYohxjYpBivuK810wFmKpW-SkcuNLf2mg~Ljm83wcCzMewOje41LgWr3RFpV75Yj6LXBZK0Pz7e1gRjltfXlAV9ZuukT6sIQ5BHpqc3Gfv5eHcaEHUR-N9w9fvnvm1uQCMc-vpAKm-kEQdmedOQa4zEXEf80ue~JW4pMeY-gjVct-HtU23Iwlf2f8GB1qUXxMw19VwtFhNNTkzgKqN97AVQJKeeSy1hFIF3OiSjvhQpnxvQf-2rAowRipKD5GsPylXFx91MVkbHLZ7zb-gEgUWPe5ESGFW3CfPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2696.420k29_2696_2703/7/m_blod42029003aw.jpeg?Expires=1766009072&Signature=V78lrHj0dvnHXI3NtaFUnoeWwKedXX-iInSgAKFYqKHBPVgzHOsg~YMbrkbADIpnsOJDG0Jy6dLbwfN8vUlk1VsFzGkDZ8~AGhADhgaW73-uI6vdsp9W9mWKlqE72gnwjMmOB7kn0nC59o8E9ZIAVstnbP8HX9T76-5ZIOL8dvS~lhrUvHPjqH3~AZVytDz5XdB7DdTenE8~Zri0S8FFN2SwOzdxRREzl4ftYlQSSqbD7Q86AS~FqOr50uNcnT~kUKd8RUdvym~FmowJa16-ExTjDOB-T318iNRNdRiH7XmaWAXEpW9zbW1k~lhbg8gc5G4vJWFPT5Ty39BAOII83g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Regulated secretion of GH and vWF from HUVEC. (A) HUVEC were infected with IGrAd (1 and 2) or GHrAd (3 and 4) and labeled with [35S]-methionine/cysteine for 3 days. Labeling medium (1 and 3) and chase supernatants (2 and 4) were then subjected to immunoprecipitation and SDS-PAGE as described in Materials and Methods, indicating sizes of approximately 22 and 34 kD, respectively. (B and C) HUVEC were infected and labeled with [35S]-methionine/cysteine, and after 16 hours of chase with unlabeled medium, the cells were treated for 30 minutes with control buffer or 100 nmol/L PMA. Supernatants were not subjected to immunoprecipitation before loading onto gels. (B) Treatment supernatants were analyzed by SDS-PAGE and phosphorimaging. Shown is a gel representative of 3 similar experiments, with mock-infected HUVEC treated with control buffer (1) or PMA (2) and GHrAd-infected HUVEC treated with control buffer (3) or PMA (4) (m-vWF, mature vWF; propeptide, the cleaved vWF propeptide). (C) Shown are chase and treatment supernatants analyzed by SDS-PAGE and densitometry, representative of 3 different experiments; chase supernatants from IGrAd (1 and 2) and GHrAd (3 and 4), control treatment supernatants from IGrAd (5) and GHrAd (7), and PMA treatment supernatants from IGrAd (6) and GHrAd (8). The relative sorting efficiency or GH compared with vWF was calculated from densitometric analysis of pro-vWF and GH in lane 4 and m-vWF and GH in lane 8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2696.420k29_2696_2703/7/m_blod42029003cw.jpeg?Expires=1766009072&Signature=a4DMV8en8JfU8Qi5qYsB9H7FpfXybVNVpMyUzqS7xpmzcOA173K98BWZKCTEfSmfexf-Uucvyo7dj61~0tMmIO8tnzMGmVFaLdVR6XGxaA4V2NYvRF6aJ0nc3Ckf539wZZKuZMe6QokXb7CJZHV~n5PWETqLGli1rK-OCxNt41tlMdaMLWWxbVcagl9RFLIvUI52XV3YomUjyP8blEa9YbLSZJ3-HelLW1Ewhgk~HegmAma6tHlJMazq~Ouhe36J3ykW~J9n0iuh9TTSaMzluTQjnzzmJuu27~rI~doT5TDQpJaPe61-HMAuDjKg5janWwtCeuQL3HA7GR7KkHQ-OQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
