Abstract
Cytotoxic T lymphocytes (CTL) lyse antigen-bearing target cells by two distinct pathways. Whereas granule exocytosis targets any antigen-bearing cell, fas-mediated cytotoxicity kills only fas-expressing cells and does not require antigen expression. Fas pathway activation can potentially lead to lysis of uninfected bystander cells. We examined the relative usage of the two pathways by CTL clones and cell lines directed against four different human immunodeficiency virus (HIV) proteins in lysing primary HIV-infected targets. Although fas was expressed on HIV-infected primary CD4+ T cells, their lysis by antigen-specific CD8+ CTL was only by the granule pathway. Fas ligand (fasL) was not detectable on antigen-specific CD8 clones, T-cell lines, or circulating HIV-specific CD8 T cells from HIV-infected donors, stained with a tetrameric HLA-A2-HIV-peptide complex. FasL expression by HIV-specific CTL clones was not activated by exposure to HIV-presenting cells, but was after unphysiological stimulation with phorbol myristate acetate (PMA). CTL clones did not lyse bystander Jurkat cells, but HIV-infected primary CD4+ T cells lysed uninfected bystander cells by the fas-mediated pathway. These results suggest that HIV-specific CD8+ CTL do not cause HIV immunopathology by lysing bystander cells. On the contrary, fas-mediated lysis of uninfected cells by HIV-infected cells may contribute to CD4 decline.
HUMAN IMMUNODEFICIENCY virus (HIV) infection stimulates a vigorous cytotoxic T-lymphocyte (CTL) response, which may play an important role in host defense against the virus. Evidence for a protective role of antiviral CD8 T lymphocytes comes from a variety of in vitro and clinical studies.1-6However, in most untreated infected individuals, viral replication and CD4 T-cell decrease continue despite a strong CTL response, ultimately with progression to symptomatic acquired immunodeficiency syndrome (AIDS). Because the decline in CD4 numbers in the course of HIV disease is disproportional to the number of circulating infected CD4 T cells, it has been postulated that indirect mechanisms like apoptosis of uninfected T cells may contribute to CD4 T-cell loss.7Histological studies of lymph nodes also suggest that apoptosis occurs predominantly in uninfected cells.8 Tissue damage could result from cytokine release, as well as from lysis of uninfected bystander cells by CTL.9,10 Recent studies in HIV infection have shown that a high level of circulating CD8 T cells expressing the activation markers CD38 and HLA-DR is associated with poor prognosis.11-15 This could reflect an immunopathogenic role for activated CD8 T cells in HIV disease. CTL have been shown to play a central role in both viral clearance and immune-mediated tissue damage in other human and murine viral infections.16-24
CTL recognition of target cells via their T-cell receptor (TCR) activates 2 distinct mechanisms of cell lysis, both of which induce apoptosis.25-28 The calcium-dependent granule exocytosis and the calcium-independent fas-mediated pathways have been shown to account for essentially all T-cell–mediated cytotoxicity.25-27 The granule exocytosis pathway induces the migration of preformed cytolytic granules to the region of CTL apposition to the target cell and release of granule contents into the intercellular space between the CTL and its target. Degranulation releases a pore-forming protein perforin and a group of serine proteases termed granzymes, which induce rapid death of target cells.29-31 Fas-mediated CTL lysis is associated with synthesis of fasL in CTL after TCR engagement. FasL expression on CTL leads to target cell lysis by interaction with fas expressed on a variety of susceptible target cells.32,33 The HIV gp120 and tat proteins have been shown to induce fas expression on CD4+ T cells in vitro and elevated levels of fas have been reported on CD4+ T cells in HIV-infected individuals.34-37
In the present study, we have examined the relative contributions of granule- and fas-mediated pathways to the lysis of HIV antigen-presenting B lymphoblastoid cell lines (BLCL) and HIV-infected primary CD4+ T-cell targets. We also used fas-sensitive Jurkat as indicator cells for bystander lysis during antigen-specific killing by CTL. Our results do not show a major role for fas-based cytotoxicity in CTL lysis of infected targets or in bystander lysis of uninfected cells.
MATERIALS AND METHODS
Isolation of HIV-specific CTL clones.
HIV-specific CTL clones were isolated from Ficoll-Hypaque (Sigma, St Louis, MO)–separated peripheral blood mononuclear cells (PBMC) obtained from HIV-seropositive subjects 307 (stage B2) and 352 (stage A1) with informed consent. PBMC were cultured in 96-well plates at 5 cells per well in the presence of 1 × 105allogeneic irradiated feeder cells and 0.1 μg/mL CD3 monoclonal antibody (MoAb) 12F6 in RPMI 1640 supplemented with 15% heat-inactivated fetal calf serum (FCS) and 600 IU/mL of recombinant interleukin-2 (rIL-2; a kind gift of Chiron Oncology, Emeryville, CA). After 2 to 3 weeks, clones were transferred to 24-well plates and restimulated with anti-CD3 MoAb and allogeneic feeder cells. CTL activity of the clones was tested against autologous B-LCL targets infected with vaccinia virus expressing HIV env, gag, reverse transcriptase (RT), and lacZ control as described.38-40 HIV-specific clones were expanded and maintained in culture by periodic restimulation. The cytolytic activity and specificity of the clones was stable for at least 6 months.
Cell lines.
Epstein-Barr virus (EBV)-transformed BLCL were established from peripheral blood lymphocytes (PBLs) by standard methods. The HIV-expressing T1-nPLAP cell line was derived by transfection of the TxB hybrid cell line T1, with a molecular clone of HIV, HXB-nPLAP, which expresses a placental alkaline phosphatase (PLAP) selection marker in place of the nef gene.41,42 The cell line was monitored regularly for HIV-1 expression by flow cytometry using FACScalibur (Becton Dickinson, San Jose, CA) with fluorescein isothiocyanate (FITC)-conjugated KC57 p24 MoAb (Coulter, Miami, FL). BLCL lines, Jurkat, Molt 1, YT-Indy, T1, and T1-nPLAP cell lines were maintained in RPMI 1640 supplemented with 10% FCS. Bulk T-cell lines were generated from HIV-infected donors by stimulation with 2 μg/mL PHA-P (DIFCO, Detroit, MI) or with 5 μg/mL of an immunodominant gag epitope peptide (VHQAISPRTLNANVKVVEEK)40 43 and were grown in RPMI 1640 supplemented with 600 IU/mL rhuIL-2 and 15% FCS.
Vaccinia vectors.
HIV-infected primary CD4 T cells.
To obtain uniformly HIV-infected primary CD4+ T-cell targets, autologous PBMC were depleted of CD8 T cells, stimulated with phytohemagglutinin (PHA) (2 μg/mL) for 2 days and infected with viral supernatant from the T1-nPLAP cell line. After overnight incubation, cells were washed and cultured in RPMI 1640 with 10% FCS and 60 IU/mL IL-2. On day 4 after infection, nPLAP-expressing cells were selected immunomagnetically with an IgG2a MoAb against PLAP (DAKO, Carpenteria, CA) and anti-mouse IgG2a Miltenyi beads.42 HIV expression in the selected cells was confirmed by flow cytometry before their use as targets in cytotoxicity assays. To test the nef-specific CTL clone, CD4+ T cells were positively selected with αCD4 Miltenyi beads and activated with PHA. Two days later, cells were infected with HIVIIIB virus at a multiplicity of infection (MOI) of 0.1. Infected cells were selected by the method described by Ferrari et al,44 which is based on the downmodulation of CD4 on HIV-infected cells. After culture for 4 to 7 days, uninfected cells, which continue to express CD4, were removed by negative selection with CD4-Dynal beads as per the manufacturer’s instructions. The negatively enriched population was analyzed for p24-expression and used as targets in cytotoxicity experiments.
Flow cytometry.
For p24 staining, cells were resuspended in 50 μL Hanks’ balanced salt solution (HBSS) and permeabilized using the Caltag Laboratories (Burlingame, CA) Fix and Perm kit according to the manufacturer’s protocol. Fixed cells were incubated for 15 minutes at RT with 2 μL p24 MoAb KC57 conjugated to FITC. After washing with 5 mL of HBSS, cells were resuspended in phosphate-buffered saline (PBS) with 1% formaldehyde for analysis. FasL expression on CTL lines and clones was evaluated after overnight culture in the presence of 10 μmol/L of the metalloprotease inhibitor KB8301 (Pharmingen, San Diego, CA) to prevent release of surface fasL. To assess fas and fasL expression, cells were suspended in 50 μL fluorescence-activated cell sorting (FACS) buffer (2% FCS, 0.02% NaN2 in PBS) to which 2 μL anti-fas MoAb ZB4 (Immunotech, Westbrook, ME) or 5 μL anti-fasL MoAb (Pharmingen) was added. After incubation for 30 minutes at 4°C, cells were washed twice with 1 mL of FACS buffer and stained for 20 minutes with a 1:50 dilution of FITC-conjugated F(ab′)2 goat anti-mouse Ig (Immunotech). Cells were washed with 1 mL FACS buffer and resuspended in FACS buffer with 1% formaldehyde for analysis. For phenotypic analysis, CTL clones were resuspended at 2 × 106 cells/mL in FACS buffer and 50 μL of the suspension was stained with 2 μL CD4-FITC (MoAb 13B8.2), CD8-PE (MoAb B9.11), CD28-PE (MoAb CD28.2), and CD57-FITC (MoAb NC1) or isotype-matched IgG-FITC and IgG-PE antibodies (Immunotech). After incubation for 30 minutes at 4°C, cells were washed and resuspended in FACS buffer with 1% formaldehyde for analysis. Fluorescent staining was analyzed on a FACScalibur with Cell Quest software (Becton Dickinson).
FasL and granzyme A expression in HIV-gag peptide tetramer-stained PBMC.
Bir A modified HLA-A2 heavy chain and β2 microglobulin were synthesized and purified from plasmids (obtained from M. Davis [Stanford University, Palo Alto, CA] and D.C. Wiley [Harvard University, Cambridge, MA], respectively) and refolded with an A2-restricted HIV-gag epitope peptide (SLYNTVATL)45to produce tetramers as described.46,47 To assess fasL expression in circulating tetramer-positive cells, 2 × 106 PBMCs from 2 A2-expressing seropositive subjects, 350 (stage A1) and 606 (stage A2), were resuspended in 500 μL FACS buffer and stained with 0.5 μg/mL of Streptavidin phycoerythrin (PE)-conjugated tetramer for 40 minutes at 4°C. Cells were stained for fasL and CD8-Cy5 as described above. For granzyme A staining, a separate aliquot of tetramer-stained cells was permeabilized using the Caltag Laboratories Fix and Perm kit and stained successively with 3 μL granzyme A-specific MoAb CB948 and FITC-conjugated F(ab′)2 goat anti-mouse Ig (Immunotech). Cells were then washed twice, resuspended in 50 μL FACS buffer, and stained with 2 μL of CD8-Cy5 for 30 minutes at 4°C. After another wash, cells were resuspended in FACS buffer with 1% formaldehyde for flow cytometric analysis.
Cytotoxicity assay.
Log-phase BLCL target cells were infected with 5 pfu/cell recombinant HIV-vaccinia virus or the lacZ control and incubated at 37°C for 16 hours. Cells were then labeled with 100 μCi of 51Cr for an hour, washed 3 times in RPMI 1640 medium with 10% FCS, and resuspended at 105/mL. Labeled targets (104) were added to triplicate wells of U bottom microtiter plates. Effector cells suspended at various E:T ratios in 100 μL were added to target cells and the plates incubated at 37°C over CO2 for 6 hours. Supernatants (35 μL) were counted on a Top Count (Packard, Meriden, CT) microplate reader and percent specific cytotoxicity was calculated from the average cpm as: ([Average cpm − Spontaneous Release]/[Total Release − Spontaneous Release]) × 100. Cytotoxicity assays with HIV-infected cells were performed in the same way except that 60 IU/mL IL-2 was present in the culture medium during the assay.
Inhibition of perforin and fas-mediated pathways of cytolysis.
To evaluate the role of perforin in CTL-mediated cytotoxicity, target cells were pretreated with 2 mmol/L EGTA-MgCl2 for 30 minutes and the cytotoxicity assay was also performed in the presence of EGTA. To evaluate the role of fas in CTL-mediated cytotoxicity, target cells were treated with 5 μg/mL of ZB4 (Coulter), a fas-blocking MoAb for 30 minutes, and assayed for cytotoxicity in a 6-hour assay in the presence of antibody. In some experiments, Brefeldin A (Sigma, St Louis, MO) and Concanamycin A (CMA; Calbiochem, La Jolla, CA) were used as selective inhibitors of fas-mediated and granule-mediated cytotoxicity, respectively. Effector CTL clones were preincubated with 10 μmol/L Brefeldin A or 100 nmol/L CMA for 2 hours as described by Kataoka et al46 and assayed for cytotoxicity in the presence of the drugs.
Bystander lysis.
Bystander cytotoxicity during HIV-specific CTL-mediated cytolysis was assessed using fas-expressing Jurkat cells. Jurkat cells were labeled with 51Cr as above, resuspended at 2 × 105 cells/mL and distributed (50 μL) into microtiter U-bottom wells. Fifty microliters of unlabeled HIV-infected primary CD4+ T cells or uninfected control CD4+ T cells at 2 × 105/mL was added to each well. The effector CTL clone was added to triplicate wells at an E:T Ratio of 5:1. Cells were incubated at 37°C in a humidified CO2 incubator for 4 hours. Supernatants were collected and assessed for cytotoxicity as described above. In some experiments, bystander lysis of fas nonexpressing Molt 1 cells or fas-expressing BLCL targets was also tested.
RT-polymerase chain reaction (PCR).
The CTL clone 352-En52 was cultured for 6 hours in the presence of HIV-vaccinia or lacZ-vaccinia–infected BLCL or stimulated with 10 ng/mL PMA. Total cellular RNA was extracted from 1 × 105 cells using Tri Reagent (Molecular Research Center, Inc, Cincinnati, OH) according to the manufacturer’s instructions. RNA (100 ng) was reverse-transcribed using Moloney murine leukemia virus (M-MuLV) RT (Promega, Madison, WI). cDNAs were amplified for 40 cycles using primers specific for fasL and β-actin and conditions described in Montel et al,49 analyzed by electrophoresis on 10% polyacrylamide gels, and visualized by SyBR Green (Molecular Probes, Eugene, OR) staining. RNA extracted from the fasL-expressing natural killer (NK) cell line YT-Indy (a kind gift of Z. Brahmi, Indiana University School of Medicine, Indianapolis, IN) was used as a positive control for these experiments.
RESULTS
Characterization of HIV-specific CTL clones.
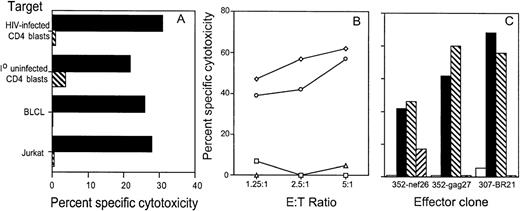
CTL clones were derived from 2 HIV-seropositive subjects by limiting dilution culture of PBMC stimulated with αCD3 in the presence of IL-2 and irradiated allogeneic PBMC feeder cells. CTL activity of expanded clones was tested against autologous BLCL targets infected with recombinant vaccinia virus expressing HIV-gag, env, nef, and RT. Clones from both subjects were directed against multiple proteins of HIV. Clones from subject 352 directed against HIV-envelope (352-env52), gag (352-gag27), and nef (352-nef26) and an RT-specific clone BR21 from subject 307 were used in this study. Clone BR21 recognized a novel A2-restricted RT epitope.42 As shown in Table 1, lysis of primary HIV-infected CD4 T cells by each of the clones was comparable to their lysis of recombinant-HIV–vaccinia-infected BLCL targets. All clones were CD4−CD8+ by flow cytometry (data not shown). The clones were heterogeneous in their expression of activation markers and were representative of expanded populations of activated CD8 T cells in PBMC of HIV-infected donors (Table 1). Two of three clones tested for cell-surface expression of CD28 and CD57 were predominantly CD57+ and CD28−. The third clone was mostly CD57− and had mixed CD28 expression. In HIV infection, CD57 expression may be a marker for end-stage effector CTLs that are unable to undergo clonal expansion in the absence of IL-2.50 51 CD38 expression by each clone was heterogeneous (data not shown). All clones were maintained for over 6 months without loss of specific cytotoxicity.
HIV-specific lysis of BLCL target cells by CTL is granule-mediated.
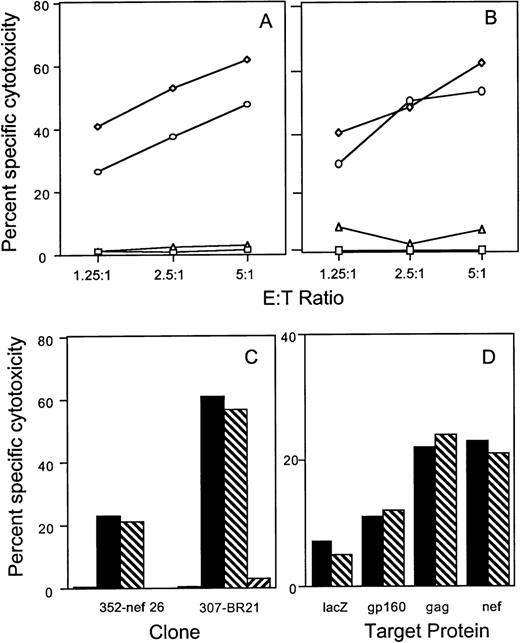
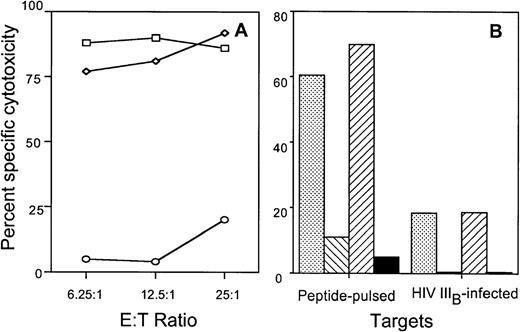
To evaluate the role of the fas-mediated and perforin-mediated pathways in antigen-specific lysis of recombinant HIV vaccinia-infected BLCL targets, cytotoxicity assays were performed in the presence of the fas blocking antibody ZB4 and in the presence of 2 mmol/L MgCl2 plus 2 mmol/L EGTA, a calcium chelating agent known to inhibit the calcium-dependent granule exocytosis pathway. Antigen-specific lysis of BLCL targets by CTL clones directed against envelope, gag, nef, and RT proteins was minimally affected by the fas blocking antibody. Figure 1A and B depict the results obtained with gag-specific clone 352-gag27 and envelope-specific clone 352-env52. Cytotoxicity was inhibited by EGTA at all ratios tested. Similar results were obtained with the RT-specific clone BR21 and the nef-specific clone 352-nef26 (Fig 1C). No fas-dependent cytotoxicity of HIV-vaccinia–infected BLCL targets was present even when a PHA-stimulated bulk T-cell line generated from subject 352 was used as effector cells (Fig 1D).
Lysis of recombinant HIV-vaccinia–infected BLCL by CTL clones and a T-cell line is inhibited by calcium chelation, but not by fas-blocking antibody. Autologous BLCL targets were infected overnight with HIV env (A) or gag (B) expressing-vaccinia or with control lacZ-vaccinia (□) and labeled with 51Cr. HIV-expressing target cells were pretreated with medium (◊), EGTA-MgCl2(▵) or fas blocking antibody (○) before adding either the env-specific clone 352-env52 (A) or the gag-specific clone 352-gag27 (B). (C) The nef-specific clone 352-nef26 and the RT-specific clone 307-BR21 were tested similarly at an E:T ratio of 5:1 against control vaccinia-infected targets (□) or HIV-vaccinia (nef or RT) infected targets in the presence of medium (▪), fas-blocking antibody (▧) or EGTA (▨). (D) Cytotoxicity by a PHA-stimulated bulk T-cell line from subject 352 was tested against autologous BLCL targets infected overnight with HIV-expressing vaccinia or with control lacZ-vaccinia. Target cells were pretreated with medium (▪) or fas-blocking antibody (▧) for use in a 6-hour cytotoxicity assay at an E:T ratio of 25:1.
Lysis of recombinant HIV-vaccinia–infected BLCL by CTL clones and a T-cell line is inhibited by calcium chelation, but not by fas-blocking antibody. Autologous BLCL targets were infected overnight with HIV env (A) or gag (B) expressing-vaccinia or with control lacZ-vaccinia (□) and labeled with 51Cr. HIV-expressing target cells were pretreated with medium (◊), EGTA-MgCl2(▵) or fas blocking antibody (○) before adding either the env-specific clone 352-env52 (A) or the gag-specific clone 352-gag27 (B). (C) The nef-specific clone 352-nef26 and the RT-specific clone 307-BR21 were tested similarly at an E:T ratio of 5:1 against control vaccinia-infected targets (□) or HIV-vaccinia (nef or RT) infected targets in the presence of medium (▪), fas-blocking antibody (▧) or EGTA (▨). (D) Cytotoxicity by a PHA-stimulated bulk T-cell line from subject 352 was tested against autologous BLCL targets infected overnight with HIV-expressing vaccinia or with control lacZ-vaccinia. Target cells were pretreated with medium (▪) or fas-blocking antibody (▧) for use in a 6-hour cytotoxicity assay at an E:T ratio of 25:1.
Fas is expressed on HIV-infected primary CD4 T-cell targets used for CTL assays.
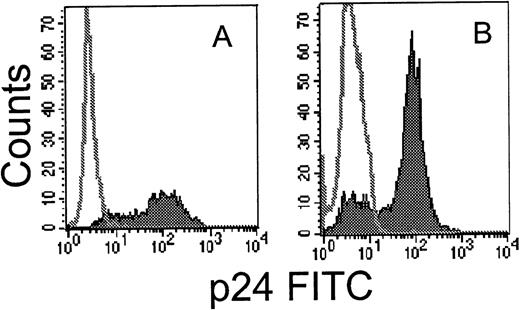
Technical problems associated with obtaining a uniform and viable population of HIV-infected primary target cells have hampered studies of CTL recognition of primary infected cells. We developed a simple method to prepare uniformly HIV-infected primary T cells by immunomagnetic selection after infection with a molecular clone of HIV expressing placental alkaline phosphatase (HXB-nPLAP).41For testing HIV-nef–specific clones, because the nPLAP gene is inserted in place of nef, we used the depletion method described by Ferrari et al.44 HIV-infected T cells, which downmodulate CD4 expression, were enriched by immunomagnetic depletion of CD4-expressing uninfected cells. As shown in Fig 2, a highly enriched population of HIV-infected cells could be obtained by both methods. Cell viability was not a problem when infected cells were used for CTL assays on the day of selection, provided IL-2 was added to the CTL assay medium. Spontaneous release was within the acceptable limit of 15% to 20%.
Flow cytometric analysis of intracellular p24 expression in permeabilized primary CD4 T cells after infection with HIVIIIB (A) or HXB-nPLAP (B) and immunomagnetic selection as described.42 44 The unfilled histogram depicts uninfected control CD4 PHA blasts and the filled histogram, the selected infected cells.
Flow cytometric analysis of intracellular p24 expression in permeabilized primary CD4 T cells after infection with HIVIIIB (A) or HXB-nPLAP (B) and immunomagnetic selection as described.42 44 The unfilled histogram depicts uninfected control CD4 PHA blasts and the filled histogram, the selected infected cells.
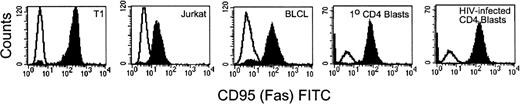
To address the mechanisms involved in lysis of primary infected cells by HIV-specific CTL, we first examined fas expression on HIV-infected primary CD4 T cells and on other targets used in this study. Cell-surface expression of fas was detected on Jurkat cells, BLCL lines, and on autologous uninfected and HIV-infected CD4 PHA blast targets. Figure 3 shows representative flow cytometric analysis of fas expression for each target cell type. Target cells were also exposed to the fas agonistic antibody CH11 to determine their sensitivity to fas-mediated apoptosis. All of the target cell lines examined were lysed by the antibody and the lysis was inhibitable by the fas blocking antibody ZB4 (Fig 4A). Comparable lysis was observed for uninfected CD4 PHA blasts, Jurkat, and BLCL targets. HIVIIIB-infected CD4 T-cell blasts (>80% cells infected) had higher levels of fas expression (mean fluorescence intensity [MFI] uninfected 55 vinfected 111) and were slightly more susceptible to fas-mediated lysis than uninfected targets.
Flow cytometric analysis of fas expression on target cells. T1, Jurkat, 352-BLCL, and uninfected and HIVIIIB-infected CD4 T-cell blasts (negatively selected for CD4 expression) were stained with fas antibody ZB4 and FITC-conjugated anti-mouse IgG. The unfilled histogram depicts staining with isotype-matched control antibody. Fas expression is upregulated on HIV-infected CD4 T-cell blasts compared with uninfected blasts.
Flow cytometric analysis of fas expression on target cells. T1, Jurkat, 352-BLCL, and uninfected and HIVIIIB-infected CD4 T-cell blasts (negatively selected for CD4 expression) were stained with fas antibody ZB4 and FITC-conjugated anti-mouse IgG. The unfilled histogram depicts staining with isotype-matched control antibody. Fas expression is upregulated on HIV-infected CD4 T-cell blasts compared with uninfected blasts.
Lysis of HIV-infected primary CD4 T cells is mediated by the perforin pathway and is not blocked by fas-blocking antibody. (A) Fas-expressing target cells are sensitive to cytotoxicity induced by fas agonistic antibody CH11. 51Cr labeled Jurkat cells, 352-BLCL, uninfected PHA-stimulated CD4 blasts, and HIVIIIB-infected and selected CD4 blasts were exposed to fas agonistic MoAb CH11 in the absence (▪) or presence (⧄) of fas-blocking antibody ZB4. Cytotoxicity was assayed after 6 hours. (B and C) Autologous HIVIIIB or HXB-nPLAP–infected CD4 T-cell blasts (cultured for 3 days and selected immunomagnetically for HIV expression) and uninfected CD4 T-cell blasts were used as targets for CTL clones directed against the env, gag, nef, and RT proteins of HIV. Target cells were preincubated with EGTA-Mg to block granule-mediated lysis or with fas blocking antibody. Cytotoxicity was assayed at various E:T ratios for clone 352-env52 (B) and at 5:1 for the other clones (C). In (B) the targets are uninfected (□) or infected and incubated with medium (◊), fas blocking antibody (○), or EGTA-MgCl2 (▵). In (C), targets are uninfected (□) or infected and incubated with medium (▪), fas blocking antibody (⧅) or EGTA (⧄).
Lysis of HIV-infected primary CD4 T cells is mediated by the perforin pathway and is not blocked by fas-blocking antibody. (A) Fas-expressing target cells are sensitive to cytotoxicity induced by fas agonistic antibody CH11. 51Cr labeled Jurkat cells, 352-BLCL, uninfected PHA-stimulated CD4 blasts, and HIVIIIB-infected and selected CD4 blasts were exposed to fas agonistic MoAb CH11 in the absence (▪) or presence (⧄) of fas-blocking antibody ZB4. Cytotoxicity was assayed after 6 hours. (B and C) Autologous HIVIIIB or HXB-nPLAP–infected CD4 T-cell blasts (cultured for 3 days and selected immunomagnetically for HIV expression) and uninfected CD4 T-cell blasts were used as targets for CTL clones directed against the env, gag, nef, and RT proteins of HIV. Target cells were preincubated with EGTA-Mg to block granule-mediated lysis or with fas blocking antibody. Cytotoxicity was assayed at various E:T ratios for clone 352-env52 (B) and at 5:1 for the other clones (C). In (B) the targets are uninfected (□) or infected and incubated with medium (◊), fas blocking antibody (○), or EGTA-MgCl2 (▵). In (C), targets are uninfected (□) or infected and incubated with medium (▪), fas blocking antibody (⧅) or EGTA (⧄).
The perforin pathway predominates in the lysis of HIV-infected primary CD4 T cells by HIV-specific CTL clones.
Because fas expression is upregulated on CD4 T cells of HIV-infected subjects,37 we examined the relative importance of the fas-fas-L pathway in the antigen-specific lysis of HIV-infected primary CD4 T-cell targets. Although fas was expressed on infected and uninfected CD4 PHA blasts, the lysis of these targets by CTL directed against 4 different HIV proteins was not affected by the fas blocking antibody (Fig 4B and C). On the other hand, cytotoxicity mediated by these clones was completely abrogated by EGTA.
Lysis of peptide-pulsed and HIV-infected T1 cells by the A2-restricted RT-specific clone BR21 is blocked by CMA but not by Brefeldin A.
Pretreatment of effector CTL with Brefeldin A or CMA selectively blocks the fas or granule-mediated pathways, respectively. Brefeldin A selectively inhibits fas-based cytotoxicity by inhibition of intracellular glycoprotein transport, whereas CMA inhibits granule-mediated lysis by blocking the granule proton pump.46 To confirm further the lack of a role of the fas pathway in HIV-specific CTL lysis, the A2-restricted RT-specific clone BR21 was preincubated with Brefeldin A or CMA and assayed for cytotoxicity against RT peptide-pulsed or HIV-infected A2.1-expressing TxB hybrid T1 cell targets. Although Brefeldin A preincubation did not block the cytotoxicity against HIV-expressing T1 cells, specific cytotoxicity was effectively blocked by pretreatment with CMA or EGTA (Fig 5).
CTL-mediated cytolysis of cognate-peptide pulsed or HIV-infected T1 cells is blocked by EGTA or CMA, but not by Brefeldin A. (A) The RT-specific CTL clone BR21 was tested for cytotoxicity against peptide-pulsed T1 cells that were either untreated (□) or pretreated with EGTA (○). Cytotoxicity was also measured during fas pathway blockade by pretreating the CTL with Brefeldin A and performing the assay in the presence of Brefeldin A (◊). (B) CTL clone BR21 was treated with medium (), CMA (⧅), Brefeldin A (⧄), or EGTA (▪) and assayed in the presence of drugs for cytotoxicity against cognate peptide-pulsed or HIVIIIB-infected T1 cells.
CTL-mediated cytolysis of cognate-peptide pulsed or HIV-infected T1 cells is blocked by EGTA or CMA, but not by Brefeldin A. (A) The RT-specific CTL clone BR21 was tested for cytotoxicity against peptide-pulsed T1 cells that were either untreated (□) or pretreated with EGTA (○). Cytotoxicity was also measured during fas pathway blockade by pretreating the CTL with Brefeldin A and performing the assay in the presence of Brefeldin A (◊). (B) CTL clone BR21 was treated with medium (), CMA (⧅), Brefeldin A (⧄), or EGTA (▪) and assayed in the presence of drugs for cytotoxicity against cognate peptide-pulsed or HIVIIIB-infected T1 cells.
Lack of bystander lysis during antigen-specific lysis by HIV-specific CTL.
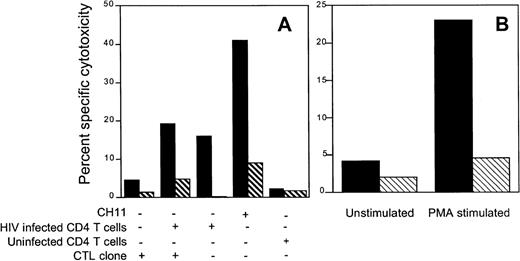
Because Jurkat cells are sensitive to the agonistic anti-fas antibody, we used them as indicator cells in a 51Cr release assay to evaluate the contribution of CTL to bystander lysis during interaction with HIV-infected primary CD4 T cells. The env-specific CTL clone 352-env52 did not lyse Jurkat cells by the fas pathway in the absence of infected targets. However, in the presence of autologous infected targets, a proportion of the labeled Jurkat bystander cells was lysed by the fas-mediated pathway. Surprisingly, comparable lysis was present even when no CTL were added. Thus, HIV-infected CD4 blasts, but not uninfected blasts, lysed the bystander Jurkat cells. This bystander effect was blocked by the fas blocking antibody and occurred within 6 hours, before productive HIV infection of the bystander Jurkat cells could occur. This suggests that HIV-infected primary T cells may lyse uninfected bystander cells by the fas pathway, implying a possible role for infected cells in the lysis of uninfected bystander CD4 cells (Fig 6A). Similar results were found when the gag-specific clone 352-gag27 and the nef-specific clone 352-nef26 were substituted for the env-specific clone (data not shown). In addition, radiolabeled Molt 1 and BLCL were also not lysed as bystanders during CTL lysis by the env-specific clone (data not shown). However, after stimulation with PMA + anti-CD3 antibody, the envelope-specific clone 352-env52 could be induced to lyse Jurkat cells by the fas pathway, as lysis was inhibited by fas-blocking antibody (Fig 6B).
HIV-specific CTL clone 352-env52 does not lyse bystander cells in the presence of specific target cells, but HIV-infected CD4 T-cell blasts do. Fas-mediated cytolysis can be induced by stimulation with anti-CD3 and PMA. (A) Uninfected fas-sensitive Jurkat cells were labeled with 51Cr and used as indicator target cells for bystander lysis by CTL. Combinations of the unlabeled CTL clone, uninfected and magnetically selected HIVIIIB-infected CD4 T-cell blasts were added to the indicator cells. Five times as many CTL were added as CD4 T cells to give an effective E:T ratio of 5:1. The fas agonist antibody CH11 was used as a positive control for fas-mediated Jurkat cell lysis. Assays were performed either without (▪) or with (⧅) fas blocking antibody pretreatment of the radiolabeled indicator cells. (B) PMA activation induces fas-mediated cytotoxicity by clone 352-env52. The clone was treated with medium or was stimulated with PMA and anti-CD3. After 2 hours of culture, the washed CTL were added to 51Cr-labeled Jurkat cells at an E:T ratio of 2:1. Cytotoxicity was assessed against both untreated (▪) or fas blocking antibody-pretreated (⧅) targets.
HIV-specific CTL clone 352-env52 does not lyse bystander cells in the presence of specific target cells, but HIV-infected CD4 T-cell blasts do. Fas-mediated cytolysis can be induced by stimulation with anti-CD3 and PMA. (A) Uninfected fas-sensitive Jurkat cells were labeled with 51Cr and used as indicator target cells for bystander lysis by CTL. Combinations of the unlabeled CTL clone, uninfected and magnetically selected HIVIIIB-infected CD4 T-cell blasts were added to the indicator cells. Five times as many CTL were added as CD4 T cells to give an effective E:T ratio of 5:1. The fas agonist antibody CH11 was used as a positive control for fas-mediated Jurkat cell lysis. Assays were performed either without (▪) or with (⧅) fas blocking antibody pretreatment of the radiolabeled indicator cells. (B) PMA activation induces fas-mediated cytotoxicity by clone 352-env52. The clone was treated with medium or was stimulated with PMA and anti-CD3. After 2 hours of culture, the washed CTL were added to 51Cr-labeled Jurkat cells at an E:T ratio of 2:1. Cytotoxicity was assessed against both untreated (▪) or fas blocking antibody-pretreated (⧅) targets.
FasL is upregulated in HIV-specific CTL clones after PMA exposure, but not after exposure to antigen.
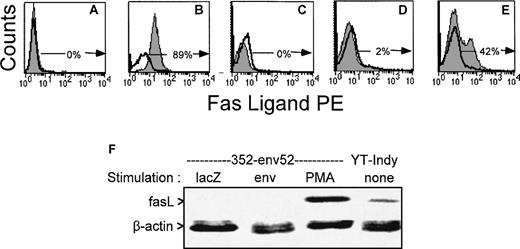
The lack of fas-mediated cytotoxicity and bystander lysis might be due to lack of expression of fasL by the T-cell line and clones used in this study. FasL was not detected above background by flow cytometry on PBMC and PHA-stimulated T-cell lines from HIV-infected donors and the 4 clones used in this study, even when staining was performed in the presence of a metalloproteinase inhibitor to inhibit release of surface fasL and enhance detection sensitivity.52 However, upregulation of fasL expression was observed after nonspecific stimulation with PMA. A representative flow cytometric analysis with a gag-specific CTL line is depicted in Fig 7. We also used a more sensitive RT-PCR amplification to measure fasL mRNA in 1 of the clones (Fig 7F). As fasL expression is upregulated after T-cell activation, we looked for fasL expression 6 hours after stimulation with HIV-vaccinia infected autologous BLCL or PMA. Although PMA activated fasL expression by the env-specific clone that was able to lyse bystander Jurkat cells after PMA induction, exposure to HIV-presenting cells did not. Therefore, HIV-specific CTL can be activated by supraphysiological means to express fasL, but they do not upregulate fasL expression after antigen-specific stimulation. The pattern of fasL expression correlates with what would be predicted based on the results of the CTL and bystander lysis experiments in Figs1 and 4 through 6.
FasL is upregulated in an HIV-specific CTL line after PMA exposure, but not after exposure to antigen. Surface expression of fasL was evaluated in a representative gag-specific CTL line from stage A2 HIV-seropositive subject 606 by flow cytometry after overnight culture and stimulation with unpulsed (C) or cognate peptide pulsed (D) autologous BLCL or 10 ng/mL PMA (E) in the presence of metalloprotease inhibitor KB8301. (A) and (B) depict the negative and positive control cell lines for fasL expression (A:BLCL, B:YT Indy). The unshaded histograms represent control antibody stained cells; the shaded histograms, cells stained for fasL. The percentage of fasL-expressing cells is indicated. (F) RT-PCR analysis of FasL expression by HIV-specific CTL clone 352-env52 after stimulation with antigen or PMA. The CTL clone was incubated for 6 hours with autologous lacZ- or HIV-vaccinia infected BLCL at an E:T ratio of 10:1 or with 10 ng/mL PMA. Total RNA was extracted and reverse transcribed. cDNAs amplified with fasL and β-actin primers were electrophoresed and visualized by SyBer Green staining.
FasL is upregulated in an HIV-specific CTL line after PMA exposure, but not after exposure to antigen. Surface expression of fasL was evaluated in a representative gag-specific CTL line from stage A2 HIV-seropositive subject 606 by flow cytometry after overnight culture and stimulation with unpulsed (C) or cognate peptide pulsed (D) autologous BLCL or 10 ng/mL PMA (E) in the presence of metalloprotease inhibitor KB8301. (A) and (B) depict the negative and positive control cell lines for fasL expression (A:BLCL, B:YT Indy). The unshaded histograms represent control antibody stained cells; the shaded histograms, cells stained for fasL. The percentage of fasL-expressing cells is indicated. (F) RT-PCR analysis of FasL expression by HIV-specific CTL clone 352-env52 after stimulation with antigen or PMA. The CTL clone was incubated for 6 hours with autologous lacZ- or HIV-vaccinia infected BLCL at an E:T ratio of 10:1 or with 10 ng/mL PMA. Total RNA was extracted and reverse transcribed. cDNAs amplified with fasL and β-actin primers were electrophoresed and visualized by SyBer Green staining.
FasL is not expressed by freshly isolated HLA-A2 HIV-tetramer positive T cells in the blood.
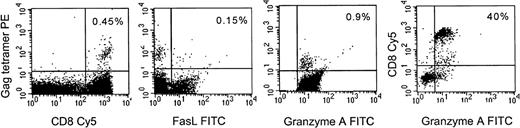
To assess whether the results observed with CTL lines and clones cultured in vitro also hold for circulating HIV-specific CTL, gag-specific CTL in PBMCs of 2 A2-expressing HIV-seropositive subjects were stained with a major histocompatibility complex (MHC)-gag tetramer for a frequently recognized A2-restricted gag epitope SLYNTVATL.45 Uncultured tetramer positive CD8+ T cells expressed granzyme A, an effector molecule for granule-mediated lysis, but did not express fasL. Representative data from 1 subject is shown in Fig 8. Tetramer positive cells failed to express fasL even after brief culture in the presence of metalloprotease inhibitor (data not shown). This suggests that in vivo HIV-specific CTL are also likely to use the granule-mediated pathway, but not the fas pathway, to lyse virus-infected cells.
Fas-L is not expressed in HIV-gag-tetramer positive circulating CD8+ T cells, but granzyme A is. Representative results from an A2-expressing seropositive subject are shown. PBMC were costained with Streptavidin PE-conjugated HLA-A2-gag tetramer, CD8-Cy5 and fasL or granzyme A MoAbs. The first 3 panels depict staining of gated CD8 bright lymphocytes; the last panel depicts all cells in the lymphocyte gate to show that most CD8−lymphocytes do not stain for granzyme A. The percent of dually positive cells is shown.
Fas-L is not expressed in HIV-gag-tetramer positive circulating CD8+ T cells, but granzyme A is. Representative results from an A2-expressing seropositive subject are shown. PBMC were costained with Streptavidin PE-conjugated HLA-A2-gag tetramer, CD8-Cy5 and fasL or granzyme A MoAbs. The first 3 panels depict staining of gated CD8 bright lymphocytes; the last panel depicts all cells in the lymphocyte gate to show that most CD8−lymphocytes do not stain for granzyme A. The percent of dually positive cells is shown.
DISCUSSION
The role of the granule and fas-based pathways in CTL-mediated lysis of virus-infected targets has been a subject of debate.25-28Naı̈ve CD8 T cells cannot kill by either pathway. After TCR engagement, the molecules required for CTL lysis by the granule pathway (granzymes and perforin) and for fas-mediated lysis (fasL) are expressed. Although antigen-specific CD8 CTL can upregulate fasL expression after activation and lyse fas-expressing compliant targets by the fas-mediated pathway, the granule exocytosis pathway has been shown to be the major pathway used by CD8+ CTL for elimination of lymphocytic choriomeningitis virus (LCMV)-infected cells and melanoma cells.53-56 On the other hand CD4+ cytotoxic T cells have been thought to lack perforin expression and lyse target cells by the fas-fasL–mediated pathway.27 The importance of granule-mediated killing in clearing virally infected targets was shown in perforin knockout mice, which succumbed to LCMV infection.53-55 A recent study of human mycobacteria-specific CTL clones showed that lysis of infected target cells by class I restricted CD8+ CTL was exclusively perforin-mediated.57 In another study, tumor killing by human melanoma reactive CD8+, as well as CD4+, CTL clones isolated from tumor lesions was also exclusively by the granule-dependent, fas-fasL–independent pathway.56 We have found similar results for lysis of HIV-infected CD4+ T cells by CD8 T cells. Lysis of both HIV-vaccinia–infected BLCL targets and primary HIV-infected CD4 T-cell targets by cytotoxic T-cell clones directed against 4 different HIV proteins is granule-mediated, despite the expression of fas on target cells.
Fas or fasL deficiency in mice and in humans result in lymphoproliferation and autoimmunity, but not increased susceptibility to viral infections.58-62 Because of these results, fas-based apoptosis has been thought to be important in regulating lymphocyte homeostasis.27,53 Although both granule and fas-based pathways are activated after TCR stimulation, it is not clear if these two pathways are mutually exclusive in clonal populations of CTL or modulated differentially for lysis of intracellular pathogens versus homeostatic regulation of lymphocytes. We found that after stimulation with PMA, CTL clones could be induced to express high levels of fasL and lyse Jurkat cells by the fas pathway (Fig 6B). Thus, fas-based cytotoxicity can be selectively induced in CTL under certain conditions. Signaling pathways for fasL induction differ after TCR engagement and PMA stimulation.63 The former pathway requires PI3 kinase to induce fasL expression, whereas induction by PMA is dependent on PMA-sensitive protein kinase C (PKC) isoforms.63 A recent study found that a viral epitope activated the perforin mechanism of killing in a CTL clone, whereas exposure to a homologous self-epitope peptide induced only fas-mediated lysis in the same clone.64 This suggests that selective TCR signaling induced by different affinity ligands might modulate the immune response. Differences in accessory molecule requirement have also been suggested to play a role in studies with CD8 CTL clones. Clones that kill only by the perforin pathway require CD8 engagement, while clones that kill by the fas pathway appear to require a leukocyte function associated molecule-1 (LFA-1)/intercellular adhesion molecule (ICAM)-1 interaction.65
Although cytotoxic T lymphocytes provide a major host defense against viral infection, they have also been implicated in immunopathogenesis of several viral and autoimmune diseases.16-24 Despite being highly directional, CTL can mediate tissue damage during antigen-specific killing by lysis of bystander cells or by the release of soluble mediators like tumor necrosis factor-α (TNF-α).9,10 FasL-based mechanisms are more likely to result in bystander lysis than granule-mediated mechanisms, because in the effector phase, fasL-expressing CTL can potentially lyse any fas-expressing targets, including uninfected cells.10 In murine LCMV infection, an infectious model with significant parallels to HIV disease, CTL have been shown to play a central role in both viral clearance and immune-mediated tissue damage.25,27,53-55,66 CTL present among the liver infiltrating lymphocytes in chronic hepatitis due to hepatitis B and C infections are thought to actively contribute to liver damage.18,19 A potential pathogenic role for hepatitis B virus (HBV)-specific CTL in liver injury is supported by the observation that adoptive transfer of murine CD8+HBV-specific CTL mediate liver cell necrosis in a transgenic mouse model of HBV infection.20 In chronic hepatitis C infection, fas expression is upregulated on hepatocytes, and fasL-expressing CTL present in the liver can lyse bystander hepatocytes by the fas-mediated pathway.10 21-23
It has been speculated that CTL may play a similar role in HIV infection because the degree of CD4 loss in HIV infection is disproportional to the number of circulating infected CD4 T cells66-69 and apoptosis is considered to contribute significantly to CD4 loss.7,36 Fas expression on CD4+ T cells is upregulated by CD4 cross-linking by HIV-gp120 in vitro.34,35,37 In a recent study, a nef-specific CTL line was able to lyse uninfected Jurkat cells in a calcium-independent manner, suggesting lysis by a granule-independent pathway.68 However, we did not find significant bystander lysis of fas-sensitive bystander Jurkat cells with any of the CTL clones or lines that we tested. Moreover, these results were found even with an env-specific clone. Uninfected CD4+ Jurkat cells, which might have internalized soluble gp120, might have been recognized by the env-specific clone, but were not. Although all of the antigen-expressing target cell lines, as well as uninfected primary CD4+ T-cell blasts, expressed fas and could be readily lysed by the fas agonist antibody CH11, none of these targets were killed by CTL in a fas-dependent manner.
These results were obtained with a bulk CTL line and 4 CTL clones of different specificities that expressed a range of cell-surface activation phenotypes. Therefore, it is reasonable to suspect that similar results would be true of CD8 cells in vivo. In support of that conclusion, we were unable to detect fasL expression on freshly isolated HIV-gag tetramer staining CD8 T cells from HIV-infected PBMC, even when cells were incubated with a metalloproteinase inhibitor to block fasL release.52 Moreover, fasL expression was undetectable or barely detected even with 40 cycles of RT-PCR amplification when a representative clone was specifically activated by HIV-presenting BLCL stimulator cells infected with recombinant vaccinia virus. The observed fasL independence of antigen-specific lysis by HIV-specific CTL was not due to a general inability of these cells to upregulate functional fasL, as the molecule could be upregulated with resultant lysis of fas-sensitive Jurkat cells by unphysiological stimulation with PMA.
Because fasL expression is transient after T-cell activation, our finding that the tetramer+ CD8 T cells contained in the peripheral blood of HIV-infected donors are fasL−cannot completely rule out the possibility that these cells express fasL after T-cell activation. However, we were unable to assess fasL expression on circulating tetramer+ CD8 T cells after in vitro stimulation because tetramer staining disappeared after stimulation with antigen presenting cells (APCs) pulsed with the cognate peptide (data not shown). This may have been due to TCR downmodulation after antigenic stimulation, a well-described phenomenon. However, a large proportion of circulating CD8 T cells and, in particular, tetramer+ CD8 T cells express activation markers in vivo and are likely to have been recently activated.47
Most of the clones we have generated from seropositive subjects are CD28−CD57+ and show a mixed pattern of CD38 expression (Table 1 and data not shown). They were representative of the phenotypes of expanded populations of CD8 T cells in the blood of HIV-infected donors. Our results suggest that fas-mediated bystander cell lysis and fasL expression are not properties of the CD38+ subset, adequately represented in our experiments. This suggests that expansion of activated CD8+ CTL expressing CD38 and HLA DR that has been associated with high viral load and poor prognosis in studies of the Multicenter AIDS Cohort Study (MACS) may not be due to indiscriminate lysis of fas-expressing uninfected cells by CTL.14 Although CD8+ CTL do not cause CD4 depletion by lysing uninfected bystander cells, they might contribute to HIV immunopathology by interfering with immune function via their secreted products, including interferon-γ (IFN-γ), TNF-α, and chemokines. Alternatively, the view we favor is that antiviral CTL are not pathogenic and that a high level of activated CTL is an indicator of ongoing viral production. In fact, there may be qualitative defects in the ability of circulating CTL to lyse HIV-infected cells in vivo, despite efficient lysis of recombinant-vaccinia and HIV-infected target cells in vitro.48,70 71
On the other hand, we found that HIV-infected primary CD4+T cells contributed significantly to bystander lysis of Jurkat cells via the fas-mediated pathway. Viral nef induces fasL upregulation on simian immunodeficiency virus (SIV)-infected CD4 T cells.72HIV-infected macrophages have also been shown to participate actively in inducing apoptosis of CD4+ T cells by fasL- and TNF-α–dependent mechanisms.73 Thus, it is likely that the dominant fasL-expressing effector cells for lysis of fas-bearing cells in HIV infection are infected CD4+ T cells and macrophages rather than CTL.
In summary, our study of the lytic mechanism used by CTL against HIV-infected targets suggests that granule-mediated lysis is the major CTL lytic pathway in the CD8 T-cell response against the virus. Our studies do not suggest a role for fas-mediated cytotoxicity as an important effector mechanism in HIV-specific lysis by CD8+T cells. However, HIV-infected CD4 T cells themselves may contribute significantly to immunodepletion of CD4 cells and other bystander cells, as they can lyse uninfected bystander cells by the fas-mediated pathway.
ACKNOWLEDGMENT
We thank B. Chen and D. Baltimore for HXB-nPLAP, Z. Brahmi for YT-Indy, M. Davis and D. Wiley for plasmids used to synthesize the tetramers, and Chiron Oncology for rIL-2.
Supported by Grants No. AI-38819 (to P.S.), AI-36611 (to J.L.), and AI-45406 (to J.L.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Judy Lieberman, MD, PhD, The Center for Blood Research, 800 Huntington Ave, Boston, MA 02115; e-mail: lieberman@cbr.med.harvard.edu.