Abstract
Binding of the adhesive glycoprotein, von Willebrand factor (vWf), to the platelet membrane glycoprotein (GP) Ib-IX-V complex initiates platelet adhesion and aggregation at high shear stress in hemostasis and thrombosis. In this study, the GP Ib-IX-V binding site within the vWf A1 domain was analyzed using a panel of murine monoclonal antibodies raised against a 39/34-kd vWf fragment (Leu-480/Val-481–Gly-718) encompassing the A1 domain. One antibody, 6G1, strongly inhibited ristocetin-dependent vWf binding to platelets, but had no effect on botrocetin- or jaracetin-dependent binding, or asialo-vWf–dependent platelet aggregation. The 6G1 epitope was mapped to Glu-700–Asp-709, confirming the importance of this region for modulation of vWf by ristocetin. Like ristocetin, 6G1 activated the vWf A1 domain, because it enhanced binding of the 39/34-kd fragment to platelets. In contrast, 5D2 and CR1 completely inhibited asialo-vWf–induced platelet aggregation and ristocetin-induced vWf binding to GP Ib-IX-V. However, only 5D2 blocked botrocetin- and jaracetin-induced vWf binding to platelets and binding of vWf to botrocetin- and jaracetin-coated beads. Epitopes for 5D2 and CR1 were conformationally dependent, but not congruent. Other antibodies mapped to epitopes within the A1 domain (CR2 and CR15, Leu-494–Leu-512; CR2, Phe-536–Ala-554; CR3, Arg-578–Glu-596; CR11 and CR15, Ala-564–Ser-582) were not functional, identifying regions of the vWf A1 domain not directly involved in vWf-GP Ib-IX-V interaction. The combined results provide evidence that the proline-rich sequence Glu-700–Asp-709 constitutes a regulatory site for ristocetin, and that ristocetin and botrocetin induce, at least in part, separate receptor-recognition sites on vWf. (Blood. 2000;95:164-172)
Platelet adhesion to the damaged vasculature is not only the initial event in the hemostatic response to injury but is also an important factor in thrombosis underlying cardiovascular disease and stroke. Thrombus formation at high shear stress in hemostasis and thrombosis depends on the adhesive multimeric glycoprotein von Willebrand factor (vWf) binding to its specific platelet membrane receptor, the glycoprotein (GP) Ib-IX-V complex.1-3 This interaction requires activation of either vWf or the receptor or both. Association of vWf with the subendothelial extracellular matrix appears to activate vWf to bind to GP Ib-IX-V constitutively expressed on platelets.1,4 Alternatively, binding of plasma vWf to platelet GP Ib-IX-V is induced by pathologic shear stress by a mechanism that may involve activation of receptor.2
vWf, derived from a complex gene (∼180 kb, 52 exons), is synthesized as pre–pro-vWf, including a 22-residue signal peptide and a 741-residue propeptide, and undergoes extensive posttranslational processing, glycosylation, and assembly in the endoplasmic reticulum, Golgi and post-Golgi.5 vWf consists of 270-kd subunits and forms disulfide-linked multimers of ∼1000 to >10 000 kd. Each vWf subunit contains 2050 amino acids made up of conserved modular domains in the order D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2.6 The GP Ib-IX-V–binding site on vWf is contained within the A1 domain.7,8 A 39/34-kd proteolytic fragment of vWf (Leu-480/Val-481–Gly-718) encompassing the A1 domain binds to GP Ib-IX-V on platelets.8 This fragment contains an intramolecular disulfide bond, Cys-509–Cys-695, defining a predominantly positively charged sequence within the loop, and 2 discontinuous anionic flanking sequences containing sialylated glycosylation sites at residues 468, 485, 492, 493, 500, 705, and 714. The sequence Asp-514–Glu-542 within the disulfide loop has been identified as a potential site involved in binding GP Ib-IX-V.9 Other sequences within the loop have been identified as potential binding sites for sulfatide (Gln-628–Val-646),10 Type III collagen (Glu-542–Met-622),11 and heparin (Tyr-565–Ala-587).12 The vWf-activating compounds, the snake venom proteins botrocetin and jaracetin, and the bacterial glycopeptide ristocetin, also interact with the A1 domain. Botrocetin binds to 1 or more sequences within the disulfide loop,9,13whereas there is evidence that ristocetin binds to proline-rich sequences (Cys-474–Pro-488 and Leu-694–Pro-708) contained in the anionic flanking sequences of the A1 domain.9,14,15 These findings suggested an electrostatic mechanism for activation of the A1 domain.9
The aim of this study was to further investigate structure-activity relationships of the vWf A1 domain. We therefore generated a panel of monoclonal antibodies raised against the 39/34-kd vWf fragment. These antibodies were functionally characterized in terms of their ability to inhibit (1) the interaction of vWf with platelet GP Ib-IX-V in the presence of ristocetin, botrocetin, or jaracetin; (2) asialo-vWf– and bovine vWf-induced platelet aggregation; and (3) binding of vWf directly to the modulators, botrocetin and jaracetin. Specific antibodies discriminated between vWf binding to platelets depending on how the interaction was induced. In addition, epitopes for several functional and nonfunctional antibodies were mapped by immunoblotting the 39/34-kd vWf fragment (Leu-480–Gly-718) and overlapping synthetic peptides, based on the vWf A1 domain sequence and by cross blocking between different antibodies. Overall, the combined results identified sites that are potentially involved in binding the platelet membrane GP Ib-IX-V complex in the presence of ristocetin compared with botrocetin, and support the role of the C-terminal proline-rich sequence Glu-700–Asp-709 in ristocetin-dependent activation of the vWf A1 domain.
Materials and methods
Materials
Bovine serum albumin (BSA), Tween 20, pristane, and Freund's adjuvant were purchased from Sigma, St Louis, MO. Chloramine T was obtained from Riedel-de Häen, Selze, Germany. Neuraminidase was purchased from Hoechst-Behring (Marburg, Germany). DMEM and HAT media were from Trace Scientific (Castle Hill, Australia). Ristocetin sulfate was from Paesel and Lorei (Frankfurt, Germany). Synthetic peptides based on the vWf A1 domain amino acid sequence,6 containing an N-terminal cysteine residue to facilitate coupling, were purified by reverse-phase high-performance liquid chromatography (HPLC) and characterized by mass spectroscopy (Chiron, Clayton, Australia or Auspep, Parkville, Australia). Botrocetin and jaracetin were purified from Bothrops jararaca viper venom (Sigma) as previously described.16 17 Human Factor VIII concentrate was a gift of the Commonwealth Serum Laboratories, Melbourne, Australia.
Purification of vWf, asialo-vWf, and the 39/34-kd vWf fragment
vWf was purified from Factor VIII concentrates as described elsewhere.16 Asialo-vWf was prepared from native vWf by neuraminidase treatment as previously described.9 The 39/34-kd dispase fragment of vWf, Leu-480/Val-481–Gly-718, was prepared and isolated by heparin-affinity chromatography as described elsewhere.8 The reduced (2-mercaptoethanol) and alkylated (2-iodoacetamide) 39/34-kd vWf fragment was also prepared as previously described.8 Where appropriate, vWf and the 39/34-kd vWf fragment were radio-iodinated with sodium [125I]iodide (Australian Radioisotopes, Lucas Heights, Australia) using the chloramine T method18 and separated from excess label by gel filtration on a Sephadex G25 PD10 column (Pharmacia, Uppsala, Sweden) equilibrated with TS buffer (0.01 mol/L Tris, 0.15 mol/L sodium chloride, pH 7.4).
Antibodies
Murine monoclonal antibodies, AK2 and WM23, directed against the extracytoplasmic domain of GP Ibα were purified and characterized as described in detail elsewhere.16,18-21 Anti-vWf monoclonal antibodies were produced in BALB/C mice immunized against the 39/34-kd vWf fragment by 3 to 4 intraperitoneal injections (140 μg 39/34-kd fragment/0.2 mL adjuvant/injection) over 8 weeks. A booster injection was given 3 to 4 days before fusion of spleen cells with the NS-1 cell line. Briefly, the spleen was macerated in serum-free DMEM cell culture medium. Both spleen cells and NS-1 cells were washed in serum-free DMEM, equal numbers of both cell types (∼26 × 106cells) were mixed by inversion, centrifuged at 200g for 5 minutes, and resuspended in 1 mL of 40% polyethylene glycol in DMEM. After 5 minutes at 37°C, the solution was diluted to 10 mL with serum-free DMEM, incubated for 1 hour at 37°C with 5% carbon dioxide, and HAT media added to a final volume to 15 mL before plating onto macrophage feeder plates. Positive clones as assessed by immunoblotting vWf (see below) were subcloned to homogeneity by limiting dilution. Ascites was produced from clonal hybridomas by washing cells twice in serum-free DMEM, diluting to 1.5 × 106 cells/mL, and injecting 0.5 mL intraperitoneally into BALB/C mice primed with pristane for 1 to 2 weeks. Ascites was collected after 10 to 30 days from carbon dioxide asphyxiated mice. Anti-vWf monoclonal antibodies were purified on protein-G Sepharose 4B (Pharmacia) and subtyped using a commercial isotyping kit (Pierce, Rockford, IL) according to the manufacturer's instructions. All purified antibodies were radio-iodinated where appropriate using the chloramine T method18 and separated from free label by gel filtration on Sephadex G25.
Immunoblotting
For Western blotting, protein was electrophoresed on SDS-polyacrylamide gels under reducing or nonreducing conditions, electrotransferred to nitrocellulose, and immunoblotted by standard methods.18-21 For dot blotting of synthetic peptides with antibodies, peptides were coupled through an N-terminal Cys residue to BSA (1 mg peptide/10 mg BSA) withm-maleimidobenzoyl-N-hydroxysuccinimide (Pierce) essentially as previously described for coupling peptides to hemocyanin.22Peptide-BSA conjugates (1 μg), native or asialo-vWf (1 μg), the 39/34-kd vWf fragment (0.2 μg), or reduced and alkylated 39/34-kd fragment (0.2 μg), all in TS buffer, were dotted onto nitrocellulose strips (1 μL). After air drying, membranes were blocked with 5% (w/v) skim milk powder in TS buffer for 1 hour and incubated with anti-vWf monoclonal antibodies (1 μg/mL, final concentration) in 0.5% (w/v) skim milk powder in TS buffer for a further 2 hours. The nitrocellulose strips were washed extensively with TS buffer, and incubated with a 1:500 dilution of peroxidase-labeled sheep antimouse IgG (Silenus, Melbourne, Australia) for 90 minutes in 0.5% (w/v) skim milk powder in TS buffer, washed thoroughly with TS buffer, and visualized using the ECL detection system (Amersham, UK).
Binding of 125I-labeled monoclonal antibodies to immobilized vWf or 39/34-kd vWf fragment
Detachable microtiter wells (Immulon I; Dynatech, Chantilly, VA) were coated with either vWf (10 μg/mL) or the 39/34-kd fragment (∼7 μg/mL) in TS buffer for 4 hours at 22°C. Wells were aspirated and replaced with 125I-labeled monoclonal antibody (1 μg/mL) in 0.1% (w/v) BSA in TS buffer. Nonspecific binding was assessed using a 100-fold excess of the unlabeled antibody in a parallel assay. To test the ability of other anti-vWf monoclonal antibodies to cross block, some assays also included a 100-fold excess of these individual antibodies. Alternatively, other assays included synthetic peptides at a final concentration of 100 μM. After 30 minutes at 22°C, the wells were aspirated and washed twice with 100-μL aliquots of 0.1% (w/v) BSA in TS buffer. Radioactivity bound to the vWf-coated wells was measured in a γ counter. Assays were performed in triplicate or quadruplicate, with standard deviation from the mean typically ≤ 5%.
Platelet aggregometry
Platelet aggregation was performed at 37°C in a Lumiaggregometer (Chronolog, Havertown, PA) using citrated platelet-rich plasma stirred at 900 rpm as previously described.9,21 23 The effect of anti-GP Ibα or anti-vWf monoclonal antibodies (final concentration, 20 μg/mL) was determined by preincubating the antibodies with platelets or vWf, respectively, for 3 minutes at 37°C before the addition of ristocetin (final concentration, 1.5 mg/mL), botrocetin (final concentration, 25 μg/mL), asialo-vWf (final concentration, 70 μg/mL), or 10% (v/v) bovine plasma (as a source of bovine vWf).
Binding of 125I-labeled vWf or 39/34-kd vWf fragment to platelets
The effect of monoclonal antibodies on the binding of125I-labeled vWf to platelets in the presence of 1 mg/mL ristocetin or 25 μg/mL botrocetin or jaracetin as vWf modulators was measured using a previously described assay.16,17,19Monoclonal antibodies (final concentration, 50 μg/mL) were incubated with 125I-labeled vWf (1 μg/mL) for 5 minutes at 22°C, before the addition of washed platelets (final concentration, 5 × 108/mL) in TS buffer containing 0.1% (w/v) BSA. After 30 minutes, samples were centrifuged at 8750g for 2 minutes, and label associated with the pellet was measured in a γ-counter after aspiration of the supernatant. Specific binding was calculated from total binding by subtracting nonspecific binding measured in a parallel assay containing a 100-fold excess of unlabeled vWf or 20 μg/mL of the blocking monoclonal antibody, AK2.16,19 Binding of 125I-labeled 39/34-kd vWf fragment to platelets in the presence of 25 μg/mL botrocetin or 10 μg/mL of monoclonal antibody was measured using the same method.8 Nonspecific binding was determined in the absence of botrocetin or antibody in a parallel assay, or by including 20 μg/mL of AK2.
Binding of vWf to botrocetin- or jaracetin-coated beads
Purified botrocetin or jaracetin was covalently coupled to the surface of polyacrylamide Immunobeads (Bio-Rad, Richmond, CA) according to the manufacturer's instructions. Routine assays incorporated 25% (v/v) beads and 0.5 μg/mL 125I-labeled vWf in a final volume of 0.1 mL TS buffer containing 0.1% (w/v) BSA. After 30 minutes at 22°C, samples were centrifuged at 8750g for 2 minutes, and the radioactivity associated with the pellet was measured in a γ counter. Nonspecific binding was determined in a parallel assay by including a 100-fold excess of unlabeled vWf. To assess the effect of monoclonal antibodies on binding of 125I-labeled vWf to the beads, some assays included 50 μg/mL anti-vWf or control IgG. Standard assays were performed in triplicate or quadruplicate, with standard deviation from the mean typically ≤ 5%.
Results
The A1 internal repeat domain of vWf is encompassed by a 39/34-kd dispase fragment of vWf (Leu-480/Val-481–Gly-718) that includes the binding site for the platelet membrane vWf receptor, the GP Ib-IX-V complex.8 Unlike native vWf, this fragment has the capacity to spontaneously bind receptor in the absence of shear stress or vWf modulators. As part of our analysis of structure-activity relationships of the vWf A1 domain, we used the 39/34-kd fragment to generate a panel of monoclonal antibodies to identify receptor-binding and regulatory sites involved in vWf-dependent platelet adhesion and activation. A total of 9 murine monoclonal antibodies were produced from 6 separate fusions using purified 39/34-kd vWf fragment as immunogen. Four of the monoclonal antibodies, 5D2, 6G1, CR1, and CR3, were of the IgG1 subtype, whereas the other 5, CR2, CR4, CR7, CR11, and CR15, were of the IgG2b subtype. The physicochemical and functional characteristics of these antibodies were determined, along with the previously described murine monoclonal antibodies, 2C9, raised against native vWf23 and AK2 and WM23, directed against platelet GP Ibα.19 21
Characterization of anti-vWf monoclonal antibodies by immunoblotting
The anti-vWf monoclonal antibodies were initially characterized by immunoblotting native vWf, asialo-vWf, 39/34-kd vWf fragment and reduced and alkylated 39/34-kd vWf fragment (Table). First, all 9 of the antibodies raised against the 39/34-kd vWf fragment, but not 2C9, Western blotted the 39/34-kd fragment following SDS–polyacrylamide gel electrophoresis under nonreducing or reducing conditions (Table), although CR4 and CR7 reacted only weakly. Both the 39-kd and the 34-kd species have an identical amino acid sequence (Leu-480/Val-481–Gly-718) and differ in apparent molecular mass due to variable glycosylation.8None of the antibodies immunoreacted preferentially with either the 39-kd or 34-kd species under reducing or nonreducing conditions (data not shown).
Second, in immunodot assays, all the antibodies, including 2C9, had similar reactivity toward native and asialo-vWf spotted onto nitrocellulose, with 6G1, CR1, CR3, and CR11 being most reactive (Table). This result confirmed that all the antibodies raised against the proteolytic fragment of vWf had epitopes that were conserved in the native protein. As expected, all 9 antibodies raised against the 39/34-kd vWf fragment reacted with the native, purified 39/34-kd fragment. However, CR2, CR4, CR7, and CR15 were only weakly reactive with the immobilized protein. 2C9 failed to recognize the 39/34-kd vWf fragment suggesting its epitope did not involve the sequence Leu-480–Gly-718. 5D2, CR1, and CR3 showed markedly less reactivity with reduced and alkylated 39/34-kd fragment compared with native fragment suggesting that these epitopes were sensitive to disruption of the Cys-509–Cys-695 disulfide bond. Interestingly, this observation was in contrast to the Western blotting results, where 5D2, CR1, and CR3 were all essentially equally reactive under nonreducing and reducing conditions (Table). This suggests that the epitopes for these 3 antibodies may in part encompass the Cys-509–Cys-695 region and that the effect seen in the immunodot assays may primarily be a consequence of disruption of the epitope by alkylation rather than by reduction.
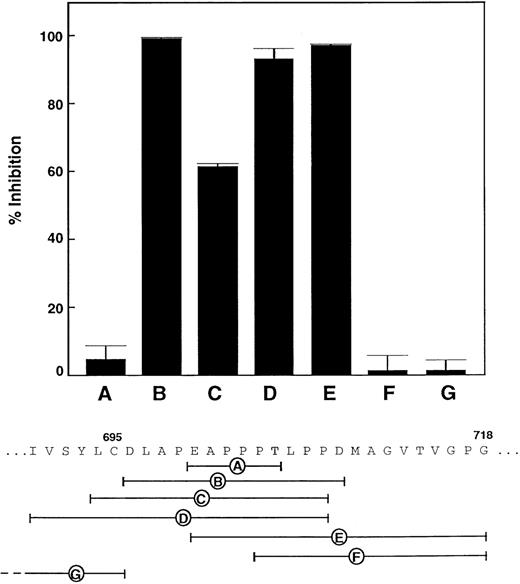
To further characterize the epitopes for these antibodies, overlapping 19-mer peptides together comprising the entire Leu-480–Gly-718 sequence were coupled to bovine serum albumin and their reactivity with the antibodies assessed by immunodot analysis (Table). Four of the antibodies 5D2, CR1, CR4, and CR7 did not react with any of the peptide-albumin conjugates suggesting that these antibodies may be recognizing conformational epitopes. The 6G1 reacted intensely with 2 peptide sequences, Ile-690–Pro-708 and Glu-700–Gly-718, suggesting that the overlap sequence contained the epitope for 6G1. To further map the epitope of 6G1, a series of peptides were evaluated for their ability to inhibit binding of 125I-labeled 6G1 to immobilized vWf (Figure 1). These results showed that the sequence Glu-700–Asp-709 represented a minimal 6G1 epitope. CR2 showed weak binding to 2 noncontiguous sequences, Leu-494–Leu-512 and Phe-536–Ala-554 (Table). CR3 reacted with the sequence Arg-578–Glu-596, whereas CR11 and CR15 both reacted with the peptide Ala-564–Ser-582. CR15 also reacted with Leu-494–Leu-512. The control monoclonal antibodies, AK2 and WM23, were unreactive in all the immunoblotting experiments (data not shown). The location of the epitopes for these antibodies is shown in Figure2. Thus, while CR2 and CR15 reacted with non-contiguous peptide sequences, the sequences recognized by these 2 antibodies were proximal in the 3-dimensional structure.
Mapping the epitope for the anti-vWf monoclonal antibody, 6G1.
Inhibition of binding of 125I-labeled monoclonal antibody 6G1 (final concentration, 1 μg/mL) to immobilized vWf by synthetic peptides (final concentration, 100 μM) in 30 minutes at 22°C. Inhibition is expressed relative to maximal specific binding in the absence of peptide. Results are the mean of quadruplicate determinations, with standard deviation from the mean of ≤ 5%.
Mapping the epitope for the anti-vWf monoclonal antibody, 6G1.
Inhibition of binding of 125I-labeled monoclonal antibody 6G1 (final concentration, 1 μg/mL) to immobilized vWf by synthetic peptides (final concentration, 100 μM) in 30 minutes at 22°C. Inhibition is expressed relative to maximal specific binding in the absence of peptide. Results are the mean of quadruplicate determinations, with standard deviation from the mean of ≤ 5%.
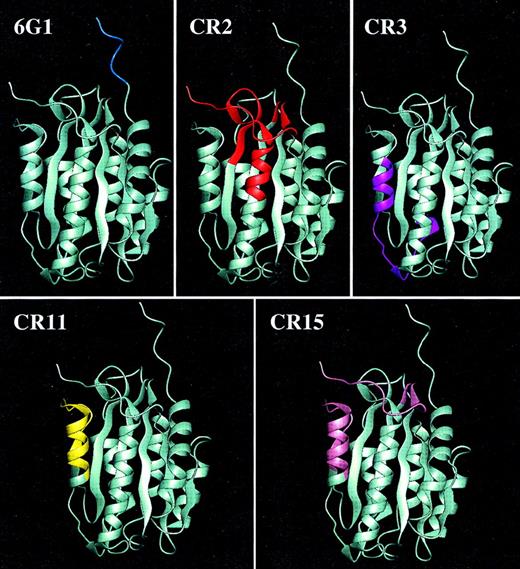
Epitopes for anti-vWf monoclonal antibodies.
Structure of the von Willebrand factor A1 domain based on the x-ray crystal coordinates25 with highlighted sequences representing epitopes for the anti-39/34-kd vWf fragment monoclonal antibodies determined using synthetic peptides.
Epitopes for anti-vWf monoclonal antibodies.
Structure of the von Willebrand factor A1 domain based on the x-ray crystal coordinates25 with highlighted sequences representing epitopes for the anti-39/34-kd vWf fragment monoclonal antibodies determined using synthetic peptides.
Cross blocking of anti-vWf monoclonal antibodies
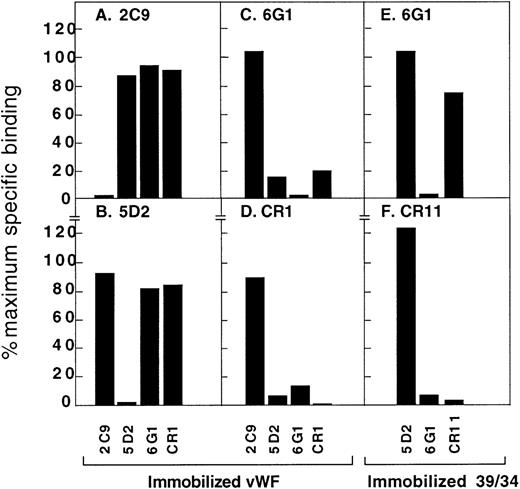
To further characterize the topographic association of the antibodies within the vWf A1 domain and its flanking sequences, cross-blocking studies were performed using immobilized vWf or the 39/34-kd vWf fragment (Figure 3). Although all the antibodies bound to vWf or the 39/34-kd vWf fragment immobilized on nitrocellulose (see above), only 4 antibodies, 2C9, 5D2, 6G1, and CR1, bound to native vWf immobilized on plastic and only 2 antibodies, 6G1 and CR11, recognized plastic-bound 39/34-kd vWf fragment. This suggests that either the immobilization of vWf or fragment is not random (that is, a specific face of the A1 domain binds to plastic), that binding of vWf or fragment to plastic results in conformational changes not recognized by some of the antibodies, or a combination of these possibilities. The simplest interpretation of cross-blocking studies, that is, if antibody A blocks binding of antibody B and the converse is true, then the epitopes for A and B are either identical or sterically proximal, was not the case for the anti-vWf antibodies. This suggested that the assays were affected by conformational effects within the vWf A1 domain. First, binding of radiolabeled 2C9 to immobilized vWf was only inhibited by unlabeled 2C9 (Figure 3A), and 2C9 did not block binding of the other antibodies. However, while binding of 5D2 to vWf was only inhibited by excess cold 5D2 (Figure 3B), unlabeled 5D2 blocked the binding of 6G1 and CR1 (Figures 3C and 3D, respectively). This suggests the epitope for 5D2 was distinct from those of CR1 and 6G1, and that binding of 5D2 induced conformational changes that masked the CR1 and 6G1 epitopes. 6G1 and CR1 cross blocked each other (Figures 3C and 3D), suggesting their epitopes were proximal, even though 6G1 recognized the linear sequence Glu-700–Asp-709; whereas the epitope for CR1 was apparently dependent on conformation and markedly affected by reduction and alkylation of the Cys-509 to Cys-695 disulfide bond (see above). Of the 2 antibodies that bound to the 39/34-kd vWf fragment on plastic, 6G1 binding was only inhibited by itself (Figure 3E), but binding of CR11 was inhibited by both 6G1 and CR11 (Figure 3F), suggesting that 6G1 induces a conformational change that causes the CR11 epitope to become cryptic.
Cross blocking of anti-vWf monoclonal antibodies.
Binding of 125I-labeled monoclonal antibodies (final concentration, 1 μg/mL) to immobilized native vWf (A-D) or 39/34-kd vWf fragment (E-F) in 30 minutes at 22°C. Unlabeled antibodies were at a final concentration of 100 μg/mL. Specific binding in the presence of blocking antibodies was expressed as a percentage of maximal specific binding measured in the absence of blocking antibody. Panel, monoclonal antibody: A, 2C9; B, 5D2; C, 6G1; D, CR1; E, 6G1; F, CR11. Results are the mean of triplicate determinations, with standard deviation from the mean of ≤ 5%.
Cross blocking of anti-vWf monoclonal antibodies.
Binding of 125I-labeled monoclonal antibodies (final concentration, 1 μg/mL) to immobilized native vWf (A-D) or 39/34-kd vWf fragment (E-F) in 30 minutes at 22°C. Unlabeled antibodies were at a final concentration of 100 μg/mL. Specific binding in the presence of blocking antibodies was expressed as a percentage of maximal specific binding measured in the absence of blocking antibody. Panel, monoclonal antibody: A, 2C9; B, 5D2; C, 6G1; D, CR1; E, 6G1; F, CR11. Results are the mean of triplicate determinations, with standard deviation from the mean of ≤ 5%.
Effect of anti-vWf antibodies on vWf binding to GP Ib-IX-V on platelets
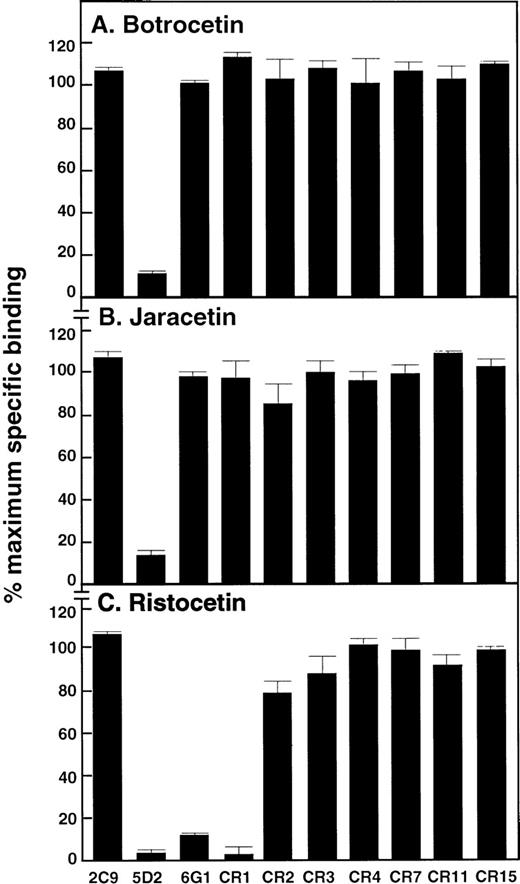
In initial studies, the anti-vWf monoclonal antibodies at 50 μg/mL (final concentration) were tested for the ability to inhibit aggregation of platelet-rich plasma induced by either asialo-vWf or bovine-vWf. Of the 10 antibodies tested, 5D2 and CR1 inhibited asialo-vWf–induced platelet aggregation, whereas 2C9 and the other anti-vWf antibodies were without effect (data not shown). CR1 was the only monoclonal antibody to inhibit bovine vWf–induced platelet agglutination (not shown), although whether the other antibodies recognized bovine vWf was not determined. Anti-vWf antibodies were then tested for their ability to inhibit botrocetin-, jaracetin-, and ristocetin-induced vWf binding to washed platelets. 5D2 was the only antibody that significantly inhibited botrocetin- and jaracetin-induced vWf binding to washed platelets (Figures 4A and 4B, respectively). In both cases, binding of vWf to platelets was inhibited by ∼90%. Three antibodies, 5D2, CR1, and 6G1, blocked ristocetin-induced vWf binding to platelets (Figure 4C). Consistent with previously reported results,16,19 21 the anti-GP Ibα antibody, AK2, completely blocked vWf binding to platelets in all the assay systems used, whereas WM23 against GP Ibα (data not shown) and 2C9 against vWf (Figure 4) were without effect in any of the assays.
Effect of anti-vWf monoclonal antibodies on vWf binding to platelets.
Antibodies (final concentration, 50 μg/mL) were included in assays measuring binding of 125I-labeled vWf (1 μg/mL) to GP Ib-IX-V on washed platelets induced by (A) botrocetin (25 μg/mL), (B) jaracetin (25 μg/mL), or (C) ristocetin (1 mg/mL). Antibodies were preincubated with vWf for 5 minutes at 22°C before the addition of washed platelets (final concentration, 5 × 108/mL). Specific binding was expressed as a percentage of maximal specific binding measured in the absence of antibody. Nonspecific binding was assessed in the presence of 50 μg/mL of the inhibitory anti-GP Ibα monoclonal antibody, AK2. Results are the mean of quadruplicate determinations, with standard deviation from the mean of ≤ 5%.
Effect of anti-vWf monoclonal antibodies on vWf binding to platelets.
Antibodies (final concentration, 50 μg/mL) were included in assays measuring binding of 125I-labeled vWf (1 μg/mL) to GP Ib-IX-V on washed platelets induced by (A) botrocetin (25 μg/mL), (B) jaracetin (25 μg/mL), or (C) ristocetin (1 mg/mL). Antibodies were preincubated with vWf for 5 minutes at 22°C before the addition of washed platelets (final concentration, 5 × 108/mL). Specific binding was expressed as a percentage of maximal specific binding measured in the absence of antibody. Nonspecific binding was assessed in the presence of 50 μg/mL of the inhibitory anti-GP Ibα monoclonal antibody, AK2. Results are the mean of quadruplicate determinations, with standard deviation from the mean of ≤ 5%.
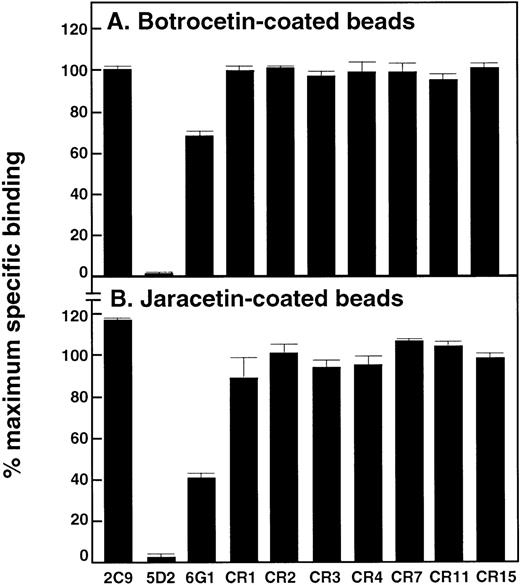
Effect of anti-vWf antibodies on binding of vWf to botrocetin- and jaracetin-coated beads
Previously established assays measuring the interaction of vWf with the modulators botrocetin or jaracetin immobilized on beads16,17 24 were used to determine whether any of the anti-vWf monoclonal antibodies blocked access to regulatory sites within the A1 domain. Binding of 125I-labeled vWf to botrocetin- or jaracetin-coated beads was only completely inhibited by 5D2 (Figures 5A and 5B, respectively). 6G1 partially inhibited binding of vWf to botrocetin and jaracetin under these conditions, by ∼30% and ∼60%, respectively.
Effect of anti-vWf monoclonal antibodies on vWf binding to botrocetin or jaracetin.
Antibodies (final concentration, 50 μg/mL) were included in assays measuring binding of 125I-labeled vWf (1 μg/mL) to (A) botrocetin-coated beads or (B) jaracetin-coated beads for 30 minutes at 22°C. Specific binding was expressed as a percentage of maximal specific binding measured in the absence of antibody. Nonspecific binding was assessed in the presence of a 100-fold excess of unlabeled vWf. Results are the mean of triplicate determinations, with standard deviation from the mean of ≤ 5%.
Effect of anti-vWf monoclonal antibodies on vWf binding to botrocetin or jaracetin.
Antibodies (final concentration, 50 μg/mL) were included in assays measuring binding of 125I-labeled vWf (1 μg/mL) to (A) botrocetin-coated beads or (B) jaracetin-coated beads for 30 minutes at 22°C. Specific binding was expressed as a percentage of maximal specific binding measured in the absence of antibody. Nonspecific binding was assessed in the presence of a 100-fold excess of unlabeled vWf. Results are the mean of triplicate determinations, with standard deviation from the mean of ≤ 5%.
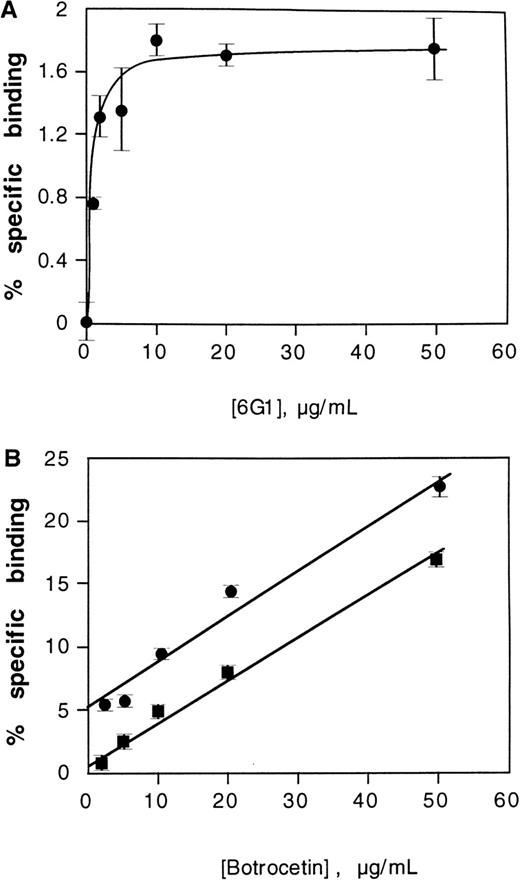
Effect of 6G1 on binding of the 39/34-kd vWf fragment to washed platelets
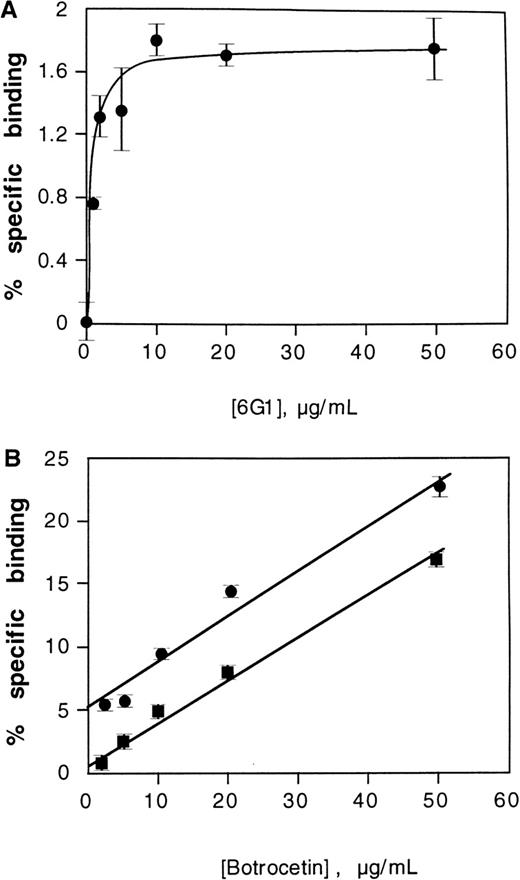
Because the anti-vWf monoclonal antibody 6G1 mapped into a proline-rich sequence at the C-terminal flank of the vWf A1 domain (Glu-700–Asp-709) previously identified as a binding site for the modulator, ristocetin,9,14,15 and because it induced conformational changes in the vWf A1 domain as suggested by cross-blocking studies with CR11, we tested whether 6G1 was itself able to activate vWf to bind GP Ib-IX-V on platelets. In preliminary experiments, 6G1 did not induce binding of 125I-labeled vWf to washed platelets (data not shown). However, saturable binding of125I-labeled 39/34-kd vWf fragment to platelets was induced by increasing concentrations of 6G1, with maximal binding at concentrations above 10 μg/mL (Figure6A). Binding induced by 6G1 was specifically inhibited by the blocking anti-GP Ibα monoclonal antibody, AK2, by ∼80% (data not shown), indicating that 6G1 induced a specific interaction between the 39/34-kd vWf fragment and GP Ib-IX-V. To examine the relationship between binding of the 39/34-kd vWf fragment to platelets as induced by 6G1 and botrocetin, botrocetin-dependent binding of the 39/34-kd vWf fragment to platelets was evaluated over the botrocetin concentration range, 1 to 50 μg/mL, in the absence and presence of 50 μg/mL of 6G1. The modulatory effects of botrocetin and 6G1 on vWf binding to platelets were additive consistent with these modulators activating vWf by distinct mechanisms (Figure 6B). In previous studies, it was not technically feasible to directly demonstrate modulator-independent binding of the 39/34-kd vWf fragment to the GP Ib-IX-V complex. Direct binding was inferred by its capacity to inhibit asialo-vWf–induced platelet aggregation and by cross-linking analysis.8 Consistent with this, there was no measurable specific binding of 125I-labeled 39/34-kd vWf fragment to platelets in the absence of 6G1 or other modulator (Figure6B), and the level of nonspecific binding was unaffected by the presence of AK2 (not shown).
Effect of the anti-vWf monoclonal antibody 6G1 on binding of the 39/34-kd vWf fragment to platelets.
(A) Specific binding of 125I-labeled 39/34-kd vWf fragment (final concentration, 1 μg/mL) to washed platelets (final concentration, 5 × 108/mL) in the presence of 6G1 in 30 minutes at 22°C. Nonspecific binding was determined in the absence of 6G1 (see Materials and Methods). (B) Specific binding of125I-labeled 39/34-kd vWf fragment (final concentration, 1 μg/mL) to platelets in the presence of botrocetin and either the absence (squares) or presence (circles) of 6G1 (final concentration, 50 μg/mL).
Effect of the anti-vWf monoclonal antibody 6G1 on binding of the 39/34-kd vWf fragment to platelets.
(A) Specific binding of 125I-labeled 39/34-kd vWf fragment (final concentration, 1 μg/mL) to washed platelets (final concentration, 5 × 108/mL) in the presence of 6G1 in 30 minutes at 22°C. Nonspecific binding was determined in the absence of 6G1 (see Materials and Methods). (B) Specific binding of125I-labeled 39/34-kd vWf fragment (final concentration, 1 μg/mL) to platelets in the presence of botrocetin and either the absence (squares) or presence (circles) of 6G1 (final concentration, 50 μg/mL).
Discussion
Binding of von Willebrand factor (vWf) to its adhesive receptor on the platelet surface, the glycoprotein (GP) Ib-IX-V complex, initiates platelet adhesion and activation at high shear stress. In the normal circulation, plasma vWf does not bind to platelets, but becomes platelet adhesive when bound to the subendothelial matrix, or when platelets and vWf are exposed to pathologic hydrodynamic shear stress.1-4 The interaction of vWf and GP Ib-IX-V is induced by activation of the ligand, the receptor or both. The GP Ib-IX-V–binding domain on vWf has been localized to the internal A1 domain,7 encompassed by a 39/34-kd proteolytic fragment of vWf (Leu-480/Val-481–Gly-718), including the disulfide bond between Cys-509 and Cys-695.8 The crystal structure of the vWf A1 domain has recently been solved at 0.23 nm,25 revealing a central core of 5 parallel and 1 short antiparallel β-sheets surrounded by 7 α-helices and loop regions (Figure 2). A sequence within the A1 domain (Asp-514–Glu-542) identified as a GP Ib-IX-V binding site9overlaps a β1 sheet-loop–α1 helix structure. NMR analysis of a synthetic peptide based on the Asp-514–Glu-542 sequence shows it adopts a solution structure consistent with the crystal structure.26 In this study, characterization of a panel of monoclonal antibodies directed against the vWf A1 domain provides further evidence on functionally important regions of the domain, and how binding to the GP Ib-IX-V complex may be regulated.
First, a monoclonal antibody, 6G1, was identified which blocked ristocetin-dependent binding of vWf to platelets but had no effect on botrocetin-dependent binding or on asialo-vWf–induced platelet agglutination. These results were consistent with 6G1 competing for a ristocetin-binding site rather than a site that recognized receptor. 6G1 mapped into 2 linear overlapping peptides corresponding to residues Ile-690–Pro-708 and Glu-700–Gly-718 (Table), and further analysis using synthetic peptides based on these sequences localized the 6G1 epitope to Glu-700–Asp-709. Previous evidence has suggested that proline-rich sequences Cys-474–Pro-488 and Leu-694–Pro-708 flanking the vWf A1 domain constituted binding sites for ristocetin. Synthetic peptides, based on these sequences, specifically inhibited ristocetin-dependent vWf binding and directly bound ristocetin,9 while mutagenesis of a triple proline sequence, 702-704, in native vWf specifically abolished ristocetin modulation of vWf binding to platelets.15 6G1 also enhanced binding of the 39/34-kd vWf fragment to platelets, consistent with the sequence Glu-700–Asp-709 acting as a regulatory site, binding to which can trigger conformational activation of the vWf A1 domain. Interestingly, 6G1 did not induce binding to receptor of intact vWf. This difference may reflect that the 39/34-kd vWf fragment, as opposed to intact vWf, has the capacity to weakly bind receptor in the absence of modulators8 and therefore may be more susceptible to a trigger of conformational change.
The functional effects of 6G1 combined with the mapping of its epitope to the C-terminal proline-rich sequence Glu-700–Asp-709 strongly support this region being a ristocetin recognition site, and suggest it is more important than the N-terminal flank. Consistent with this supposition, CR2 and CR15 that map into Leu-494–Leu-512 on the N-terminal side of the disulfide bond (Table; Figure 2) did not inhibit ristocetin-dependent vWf binding. In the 3-dimensional structure of the domain (Figure 2), Glu-700–Asp-709 is proximal to the putative GP Ib-IX-V–binding sequence Asp-514–Glu-542. The finding that 6G1 induced binding of the 39/34-kd vWf fragment to platelets additive to that induced by botrocetin also suggests that 6G1 activates the vWf A1 domain by a different mechanism to that of botrocetin. This is consistent with previous evidence that ristocetin (that uses the 6G1 binding site) and botrocetin modulate vWf by distinct mechanisms. Three noncontiguous peptide sequences within the vWf A1 domain, Asp-539– Val-553, Lys-569–Gln-583, and Arg-629–Lys-64313, in addition to Asp-514–Glu-5429 have been proposed as potential botrocetin binding sites mediating vWf binding to the GP Ib-IX-V complex. In the present study, 4 monoclonal antibodies, CR2, CR3, CR11, and CR15, were identified whose epitopes overlapped the 539-553 or 569-583 sequences (Table; Figure 2). None of these antibodies affected botrocetin-dependent binding of vWf to platelets or vWf binding to immobilized botrocetin or jaracetin. This is consistent with the observation that although the 539-553 and 569-583 sequences block botrocetin-dependent vWf binding to platelets, they do not inhibit binding of vWf to botrocetin,13 suggesting their effects on receptor-ligand interaction are either nonspecific or occur by a different mechanism than interference with modulator. In this study, binding of vWf to botrocetin and jaracetin was only blocked by the monoclonal antibody, 5D2, and partially by 6G1, suggesting that the binding site for botrocetin may be proximal to the Glu-700–Asp-709 sequence constituting the 6G1 epitope. The epitope for 5D2 has not been determined and appears dependent on conformation.
Binding of ristocetin to the negatively charged Glu-700–Asp-709 sequence (this study9,14,15) or botrocetin to the predominantly positively charged Asp-514–Glu-542/Arg-629–Lys-643 sequences9,13 conceivably exposes a GP Ib-IX-V binding site within Asp-514–Glu-542 or elsewhere by altering electrostatic interactions within this region. Previous evidence supports an electrostatic mechanism for vWf A1 domain activation. First, vWf from type 2B von Willebrand's disease patients, where vWf spontaneously binds GP Ib-IX-V, contains point mutations within the A1 domain.29 A number of these gain-of-function mutations are clustered in the region immediately C-terminal to the GP Ib-IX-V–binding sequence (Asp-514–Glu-542). Three major loci are Arg-543/Trp or Gln, Arg-545/Cys or Pro, and Arg-578/Gln or Leu, mutations that involve loss of a positively charged arginine residue. Second, mutagenesis of charged residues within or proximal to the sulfatide-binding region (Gln-628–Val-646) inhibits binding of vWf to platelets.27 Third, a number of sulfated glycans and polyanionic compounds block ristocetin- and botrocetin-dependent binding of vWf to platelets,10,24,30-32 implying that positive charge in the vWf A1 domain is important in mediating receptor binding. The heparin-binding sequence in the A1 domain, Tyr-565–Ala-587, is unlikely to be directly involved in binding GP Ib-IX-V, because antibodies that mapped into this region (CR3, CR11, and CR15) had no effect on vWf–GP Ib-IX-V interaction. However, heparin and some analogous compounds that inhibit both ristocetin- and botrocetin-dependent vWf binding to platelets do not inhibit vWf binding to sulfatides.10,24 30-32 A possible explanation for these observations is that heparin binding to Tyr-565–Ala-587 prevents formation of an active GP Ib-IX-V–binding state, heparin sterically inhibits vWf binding, or heparin interacts with other positively charged residues within the A1 domain.
Unlike 6G1 that specifically blocked ristocetin-dependent binding, 2 monoclonal antibodies, 5D2 and CR1, had profound effects on the vWf–GP Ib-IX-V complex interaction. Cross-blocking studies, while complicated by apparent comformational effects, indicated that the 2 antibodies recognized distinct epitopes, neither of which could be identified by immunoblotting linear peptides. CR1 inhibited ristocetin-dependent binding of vWf to platelets as well as asialo-vWf– and bovine-vWf–induced platelet agglutination, but had no effect on botrocetin-dependent binding of vWf to platelets. In this regard, CR1 appears similar to the previously described anti-vWf monoclonal antibody, 3F8.16,19 (The clone for 3F8 has been lost, precluding further characterization.) In contrast, 5D2 inhibited both ristocetin- and botrocetin-dependent binding of vWf to platelets as well as asialo-vWf–induced platelet aggregation. This difference therefore cannot be explained on the basis that ristocetin and botrocetin bind to different modulation sites, because both CR1 and 5D2 inhibited asialo-vWf–dependent aggregation. The data for these antibodies strongly suggest that different regions of vWf, possibly overlapping, interact with receptor depending on whether vWf is activated by ristocetin or botrocetin. This proposition is consistent with analysis of recombinant vWf fragments by scanning mutagenesis within the A1 domain. Specific amino acid substitutions at Glu-626 or within Asp-520–Lys-534 preferentially inhibited ristocetin-dependent vWf binding as opposed to botrocetin-dependent binding.27These studies, however, do not exclude the possibility that mutations are inducing conformational changes in the A1 domain that preclude activation by ristocetin, rather than directly affecting a receptor-binding sequence.
A model in which different forms of vWf have the capacity to bind receptor depending on the mechanism of vWf activation is supported by complementary structure-activity studies on the GP Ib-IX-V complex. vWf binds to the N-terminal domain of the GP Ibα chain (His-1–Glu-282), consisting of 7 leucine-rich repeats, their conserved N- and C-terminal flanking sequences, and an anionic sequence, Tyr-276–Glu-282, containing 3 sulfated tyrosines.1-3 The sulfated tyrosine sequence is more important for binding vWf activated by botrocetin compared with ristocetin, as demonstrated using proteolytic fragments of native vWf lacking the sulfated tyrosine domain21 or recombinant GP Ibα where sulfation is deficient.33,34 In addition, 2 anti-GP Ibα monoclonal antibodies that map to this region, SZ2 and ES85, blocked botrocetin-induced binding of vWf to platelets and asialo-vWf–dependent platelet aggregation, but not ristocetin-dependent binding.21 In contrast, several antibodies against the N-terminal region of GP Ibα (His-1–Leu-275) blocked ristocetin-dependent, botrocetin-dependent, and asialo-vWf binding.16,19,21 Finally, consistent with separate ristocetin- and botrocetin-dependent receptor-binding sites on vWf, an anti-GP Ibα monoclonal antibody, OP-F1, has been described that abolishes ristocetin-induced, but not botrocetin-induced vWf binding to platelets.35
In conclusion, characterization of a panel of anti-39/34-kd vWf fragment monoclonal antibodies, and epitope analysis of inhibitory and noninhibitory antibodies, has provided further insight into the structure and function of the vWf A1 domain. In particular, this study provides strong additional support for the involvement of the proline-rich A domain C-terminal flanking sequence in binding the modulator, ristocetin, and supports a model in which separate sites within the A1 domain are critical for binding GP Ib-IX-V, depending on how vWf is activated to recognize receptor.
Acknowledgments
We are grateful to Ms Carmen Llerena for outstanding technical assistance. We also thank the National Health and Medical Research Council of Australia for financial support.
M.D.L. and D.A.F. are co–first authors.
Reprints:Michael C. Berndt, Baker Medical Research Institute, PO Box 6492, St Kilda Road Central, Melbourne, VIC, Australia, 8008; Robert K. Andrews, Baker Medical Research Institute, PO Box 6492, St Kilda Road Central, Melbourne, VIC, Australia, 8008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.