Abstract
During recovery from intensive chemotherapy with cyclophosphamide (CTX), mice suffer a severe but transitory impairment in spleen cell proliferation to T-cell mitogens (Con A or anti-CD3 plus IL-2). Although CTX treatment reduced spleen T-cell cellularity, this cannot fully account for T-cell unresponsiveness. The results showed that CTX induces the colonization of spleen by an immature myeloid CD11b+Ly-6G+CD31+ population. Its presence closely correlated with the maximum inhibition of T-cell proliferation. Moreover, this suppressive activity was dependent on nitric oxide (NO) production in cultures since (1) higher amounts of nitric oxide and inducible nitric oxide synthase (iNOS) mRNA were produced in CTX spleen cells (CTX-SC) than in control splenocyte cultures and (2) NOS inhibitors greatly improved the proliferation of T lymphocytes. Nitric oxide production and suppressive activity were also dependent on endogenous interferon-γ (IFN-γ) production since anti–IFN-γ abrogated both effects. Finally, iNOS protein expression was restricted to a heterogeneous population of CD31+cells in which CD11b+Ly-6G+ cells were required to suppress T-cell proliferation. These results indicated that CTX might also cause immunosuppression by a mechanism involving the presence of immature myeloid cells with suppressor activity. This may have implications in clinical praxis since inappropriate immunotherapies in patients treated with intensive chemotherapy could lead to deleterious T-cell responses. (Blood. 2000;95:212-220)
Recovery from immunodeficiency caused by high-dose chemotherapy or radiotherapy remains as a major clinical problem. In medical praxis, restoration of neutrophil and platelet counts is often seen as the hallmark of hematological reconstitution. This view somewhat neglects the important role of cellular interactions in lymphoid organs, which are needed for functional recovery of the immune system. This issue may have important implications in order to design therapeutic approaches to improve the anti-infectious or antitumoral resistance of patients undergoing bone marrow transplantation and/or anti-cancer treatment.1 2
Cyclophosphamide (CTX) is a widely used antineoplasic drug, employed alone or in combination with other products.3 Used as an anticancer drug or in bone marrow transplantation conditioning regimes, treatment with CTX severely injures hematopoietic and lymphoid tissues, thereby leading to a profound leukopenia. However, when CTX is used as a single agent, hematopoietic recovery always occurs, even at the highest doses of the drug. This probably reflects the relative resistance of stem cells to cytotoxic effects of CTX.3,4,5Still, during the repopulation period, a transient unresponsiveness in T and B lymphocytes is observed.1,2 In mice, those anomalies have been correlated with a population of cells in the spleen that are capable of nonspecifically suppressing, in an major histocompatibility complex–unrestricted fashion,9,10 primary and secondary in vitro antibody responses6,7,8 as well as the in vitro proliferative response of normal T lymphocytes. These cells have been poorly defined, and in some cases, they have been characterized as a heterogeneous population of cells that mainly belong to the monocyte/macrophage lineage.9,11 12
On the other hand, the role of NO in immune response has received much attention since its discovery as an activated-macrophage secretory product.13 Nitric oxide is produced by the NO synthase (NOS, EC 1.14.13.39), of which 3 different isoforms have been identified in mammals.14 They are designated as neuronal or type 1 NOS (nNOS), inducible or type 2 NOS (iNOS), and endothelial or type 3 NOS (eNOS). Both nNOS and eNOS isoforms are constitutively expressed in neurons and endothelium as well as in other tissues, and these isoforms produce low amounts of NO upon transient raise in intracellular Ca++ concentration. In contrast, iNOS is induced in macrophages and several other cell types by cytokines and/or products derived from microorganisms. iNOS produces high quantities of NO for extended periods of time,14,15and it has become increasingly evident that iNOS-derived NO is involved in suppression of several immune responses.14 16-20
In this study, we show that immature myeloid cells are the main agents responsible for defective in vitro responses to mitogens in splenic T cells obtained from CTX-treated mice by a NO-dependent mechanism. NO production is dependent on endogenously synthesized interferon-γ (IFN-γ) and is restricted to a heterogeneous population of CD31+ cells.
Materials and methods
Mice
Specific pathogen-free female syngeneic Balb/c mice (8-12 weeks of age) were used throughout the study. The mice (Harlan; Gannat, France) were maintained in the GlaxoWellcome Centro de Investigación Farmacológica (C.I.F.) animal facilities (Tres Cantos, Spain). The animal research described in this paper complied with national and European union legislation and with related codes of practice.
Reagents
We used the following reagents: CTX (Genoxal; Prasfarma, Barcelona, Spain); streptavidin-R-phycoerythrin, lipopolysaccharide fromSalmonella typhi, concanavalin A, L-N6-(1-iminoethyl)lysine (L-NIL), aminoguanidine hemisulfate (AMG), and RPMI-1640 (Sigma, St. Louis, MO); horseradish peroxidase-conjugated streptavidin (Zymed; San Francisco, CA); penicillin/streptomycin mixture, L-glutamine, and fetal calf serum (BioWhittaker, Walkersville, MD); mouse recombinant interleukin-2 (IL-2), IFN-γ, and tumor necrosis factor–α (TNF-α) (Genzyme; Cambridge, MA); and NG-monomethyl-L-arginine (Dr JoséL. Subiza, Madrid, Spain).
Antibodies
We used monoclonal antibodies (mAbs) against the following cell-surface antigens (CSA) (Pharmingen, San Diego, CA): CD3ε (purified and biotin-conjugated hamster IgG, clone 145-2C11); CD16/CD32 (FcBlock, rat IgG2b, clone 2.4G2); CD19 (fluorescein isothyocynate–rat [FITC-rat] IgG2a, clone 1D3); CD45 (FITC-rat IgG2b, clone 30-F11); CD45R/B220 (FITC-rat IgG2a, clone RA3-6B2); Ly-6G (biotin-rat IgG2b, clone RB6-8C5); CD11b (biotin-rat IgG2b, clone M1/70); CD90.2 (Thy-1.2) (FITC-conjugated rat IgG2a, clone 53-2.1); and erythroid lineage-specific TER-119 mAb (phycoerythrin-rat [PE-rat] IgG2b, clone TER-119).
Additional CSA were used: CD31 (biotin-rat IgG2a, ER-MP12; BMA, Augst, Switzerland) and βTcR and CD116 (FITC-hamster IgG, clone H57-597, and FITC-rat IgG2b, clone M1/70.15; Caltag, Burlingame, CA). We also used the following isotype control mAbs (Pharmigen): biotin-, FITC-, and PE-conjugated rat IgG2b (clone R35-38); FITC- and PE-conjugated rat IgG2a (clone R35-95); and anti–TNP biotin-, FITC-, and PE-conjugated hamster IgG (clone G235-2356).
We used anti-iNOS mAb (FITC-mouse IgG2a, clone 6; Transduction Laboratories, Lexington, KY) and mouse IgG2a FITC-conjugated isotypic control (Dr J Rullas, Hospital Clı́nico San Carlos, Madrid, Spain). Neutralizing rat IgG1 antimouse IFN-γ mAb and TNF-α mAb (clones XMG1.2 and XT3; Endogen, Woburn, MA) were used at 10 μg/mL. Their specificity and activity were assayed by inhibiting NO production using resident peritoneal macrophages stimulated with IFN-γ (25 ng/mL) plus TNF-α (50 ng/mL).
The characterization of T lymphocytes was assessed by the expression of CD3ε, βTcR, or CD90.2 (Thy-1.2). The characterization of B lymphocytes was assessed by analyzing the cells expressing CD19 or CD45/B220, both expressed from pro-B lymphocytes to mature B cells. The cells of myeloid/monocytic lineage were identified on the basis of CD11b (Mac-1) or Ly-6G (Gr-1). The expression of leukocyte common antigen CD45 was used to distinguish the cells of hematopoietic origin but of nonerythrocytic lineage. Cells of erythrocytic lineage express an antigen recognized by TER-119 mAb.
Cell cultures
SC or immunomagnetically sorted cells (0.4 × 106cells/well) were cultured in 96-well flat-bottom culture plates (Costar, Cambridge, MA) in 250 μL of culture medium (RPMI 1640, 10% FCS, 2 mmol/L L-glutamine, 5 × 10-5 mol/L 2-mercaptoethanol, 100 units/mL penicillin, 0.1 μg/mL streptomycin) in an atmosphere of 5% CO2 at 37°C. Proliferation assays were done in triplicate, culturing the cells for 72 hours. For the last 18 hours of the culture period, proliferative activity was estimated measuring thymidine deoxyribose ([3H]TdR) incorporation after a pulse with 0.5 μCi/well (1450 MicroBeta Liquid Scintillation Counter; Wallac, Turku, Finland).
Immunomagnetic depletion of spleen cells
In some experiments, populations of SC were selectively depleted by immunomagnetic beads (Dynabeads; Dynal, Oslo, Norway). Depletion of CD3 cells or CD31-positive cells was carried out by streptavidin-conjugated beads, following the manufacturer's instructions. On the other hand, CD11b+ (Mac-1) cells were depleted by sheep antirat IgG-coated immunomagnetic beads. Briefly, CTX-SC were incubated for 20 minutes in HBSS 1% FCS 20% normal mouse serum at 4°C. Then the cells (30 × 106 cells/mL) were stained with CD11b–FITC-conjugated (0.5 μg/106)cells for 30 minutes, washed, and incubated at the same cell concentration with antirat IgG-coated immunomagnetic beads (4:1 bead/cell ratio) for 1 hour at 4°C in a rotor. The efficacy of the process was assessed by flow cytometry. In all cases contamination with remaining positive cells was below 5%.
Flow cytometry
A known number of cells (0.2 × 106 cells) were incubated with CD16/CD32 mAbs at 10 μg/mL in 50 μL of staining buffer (HBSS, 1% FCS, 0.05% azide) for 10 minutes at 4°C. Subsequently, appropriate biotin-conjugated mAbs were added at 10 μg/mL and incubated for 30 minutes at 4°C. After washing with staining buffer, samples were stained with FITC-conjugated or PE-conjugated mAbs (10 μg/mL) plus PE- or FITC-streptavidin (20μg/mL), respectively, for 30 minutes at 4°C in the dark. After final washing steps, the cells were fixed with 250 μL of phosphate-buffered saline (PBS) 1% paraformaldehyde. Finally, 5000 viable cells were acquired on the basis of forward/side light scattering and analyzed (Coulter EPICS-XL; Coulter Electronics, Miami, FL).
iNOS protein expression was assessed by flow cytometry. Briefly, after 24-30 hours of stimulation, the cells were recovered from culture wells (about 0.8 × 106 cells per sample) and stained for surface markers as above. After washing with cold HBSS, the samples were fixed with PBS-paraformaldehyde 4% at 4°C for 30 minutes in the dark. Then the cells were washed twice with cold HBSS, twice with permeabilization buffer (PEB, saponin 0.1% in staining buffer), pelleted, and resuspended in 0.05 mL PEB. Subsequently, the cells were stained with mouse IgG–FITC-conjugated antimouse iNOS or isotypic control at 10 μg/mL for 1 hour, after which they were carefully washed 3 times with PEB and twice with staining buffer.
Measurement of cytokines
Cytokine contents (IL-4 and IFN-γ) were measured in 24-hour supernatants of the aforementioned cell cultures using a specific enzyme-linked immunosorbent assay (ELISA) for mice (MiniKit; Endogen), according to manufacturer's instructions.
Reverse transcriptase–polymerase chain reaction
We cultured SC (0.4 × 106 cells/well) in medium alone and in medium with Con A or with anti-CD3 plus IL-2 for 24 hours, as described above. Then total mRNA was isolated (PolyATtract Series 9600 mRNA isolation and cDNA synthesis system; Promega, Madison, WI) as recommended. Subsequently, the cDNA obtained was employed as a template for PCR amplification of mouse glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and mouse iNOS using 5′ and 3′ available primers (Clontech Lab, Palo Alto, CA). PCR reactions and analysis were conducted according to previously described conditions.21
Nitric oxide production
Nitric oxide production was assessed by measuring nitrite accumulation in 72-hour culture supernatants. This was carried out using the Griess reaction.22 Briefly, 100 μL of 0.5% sulfanilamide and 0.05%N-naphtyl-ethylenediamine hydrocloride in 2.5% H3PO4 (Griess reagent) were added to 100 μL of supernatants and incubated for 5 minutes at room temperature in the dark. The absorbance was then measured at 550 nm, and nitrite concentrations were extrapolated from a sodium nitrite standard curve.
Statistical analysis
Experimental differences over the controls were analyzed by the Student's t test. Probability values P > 0.05 were considered nonsignificant. All the experiments described were performed at least twice in order to warrant the reproducibility of the results.
Results
The effect of CTX treatment on spleen T-lymphocyte proliferative activity
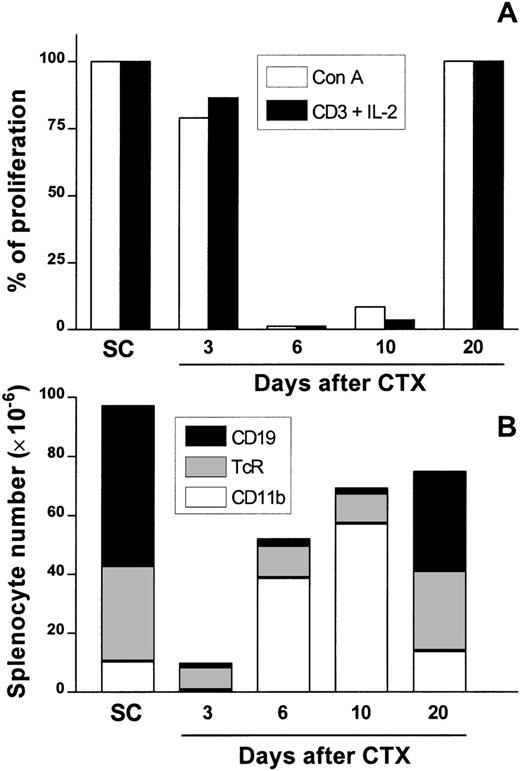
Female BALB/c mice were treated with 3 intraperitoneal (i.p.) injections of CTX at 100 mg/kg of body weight every 72 hours, whereas control mice received PBS. At days 3, 6, 10, and 20 after the last dose of drug was administered, SC were obtained and assayed for T-cell proliferation induced with Con A or anti-CD3 plus IL-2. A strong impairment of T-cell proliferation in response to both stimuli was observed at day 6 (more than 98% suppression) and day 10 (between 50% and 90% suppression, depending on the experiment) after CTX administration (Figure 1A). No relevant suppression was noted at days 3 and 20.
Kinetics of splenic T-cell responsiveness and the major presence of cell populations in the spleen after CTX treatment.
Female Balb/c mice were injected i.p. with CTX (100 mg/kg of body weight) or PBS 3 times every 72 hours. At days 3, 6, 10, and 20 after CTX delivery, SC were aseptically obtained and assayed for T-cell proliferation with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) as previously described. Data are the percentage of proliferation over mitogen-stimulated SC control cultures (A) to account for differences in proliferative response of these cells in different days of the experiment. From the very same spleens, CD19+ B cells, αβTcR+ T cells, and CD11b+ macrophage/myeloid cells were analyzed by flow cytometry as described (B). In this figure, the absolute number of each population is represented by the height of each stacked bar. The data are the means of positive cells of PBS-injected control (2 mice/d) and CTX-injected animals (3 mice/d). SD deviations were <10% in all cases.
Kinetics of splenic T-cell responsiveness and the major presence of cell populations in the spleen after CTX treatment.
Female Balb/c mice were injected i.p. with CTX (100 mg/kg of body weight) or PBS 3 times every 72 hours. At days 3, 6, 10, and 20 after CTX delivery, SC were aseptically obtained and assayed for T-cell proliferation with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) as previously described. Data are the percentage of proliferation over mitogen-stimulated SC control cultures (A) to account for differences in proliferative response of these cells in different days of the experiment. From the very same spleens, CD19+ B cells, αβTcR+ T cells, and CD11b+ macrophage/myeloid cells were analyzed by flow cytometry as described (B). In this figure, the absolute number of each population is represented by the height of each stacked bar. The data are the means of positive cells of PBS-injected control (2 mice/d) and CTX-injected animals (3 mice/d). SD deviations were <10% in all cases.
Theoretically, the impaired response to mitogens could be attributed to the cytotoxic effect of CTX on SC. To address this point, we used flow cytometry to analyze the major cell populations present in the spleen. The results obtained are summarized in Figure 1B. CTX treatment induced a sharp decrease in splenic cellularity at day 3, after the last dose (approximately 80% reduction was noted, compared with control mice). Most of the remaining cells were αβTcR+ T lymphocytes (comprising about 70% in CTX-SC versus an average of 35% in control SC). This represented a drop of around 60% in total T-cell numbers with respect to those found in control SC. On the other hand, CD19+ B lymphocytes, which represented 54% in control SC, were <12% in CTX-SC, which is a drop of more than 20 times in total number of B cells. However, the percentage of CD11b+ cells remained unchanged (between 6% and 15% both in SC and CTX-SC).
Overall, the absolute numbers of T and B lymphocytes and myeloid cells markedly decreased in CTX-SC, but T cells seemed to be more resistant to the cytotoxic effects of CTX than B lymphocytes. Conversely, at day 6 after CTX treatment, there was a great increase in CD11b+cells, both in percentage and absolute numbers, that persisted at day 10. At day 20 we found that CD11b+cells were only slightly elevated (about 20%) compared with percentages found in SC. Once more, nearly normal percentages and absolute numbers of T and B lymphocytes were reached at day 20. Therefore, it seems that the decrease in T-cell numbers could not fully explain the strong impairment of T-cell proliferation seen at days 6 or 10. The same number of residual T cells found at day 3 was also found at days 6 or 10, and they could, theoretically, proliferate as well.
Involvement of NO in suppression of proliferative responses of T lymphocytes
The results shown above suggested the existence of an active suppression phenomenon. Thus, we assessed the involvement of NO or other reactive nitrogen intermediates (RNI) in the reduced proliferative activity of spleen T cells from CTX-treated mice at days 6 or 10 after treatment. Unstimulated cultures from both SC and CTX-SC did not release detectable amounts of NO. Interestingly, supernatants of CTX-SC cultures stimulated with ConA or anti-CD3 plus IL-2 produced much larger amounts of NO or other RNI (between 10 and 30 μmol/L), as assessed by nitrite accumulation in 72-hour culture supernatants, than SC from control mice (<3 μmol/L in all cases).
We also analyzed iNOS mRNA expression in cultures of SC or CTX-SC. There was a marked correlation between the increase of iNOS mRNA expression in CTX-SC and the production of high amounts of nitrite in culture supernatants (Figure 2A). These results indicate that there is a strong relationship between NO production and the suppression of proliferation. More interestingly, in Con A- or anti-CD3 plus IL-2–stimulated cultures, the addition of NOS inhibitors markedly improved the proliferation of T lymphocytes (Figure2B). Moreover, there was no significant difference in improving proliferation between the structurally different NOS inhibitors used, such as NG-monomethyl-L-arginine (LMMA), a nonspecific NOS inhibitor, and L-N6-(1-iminoethyl)lysine (LNIL) or AMG, more specific inhibitors of iNOS.23-25 Thus, it seems unlikely that their effect in spleen cultures was unspecific. Taken together, the former results indicate that iNOS-dependent NO production substantially contributes to the inhibition of proliferation of T cells from the spleen of CTX-treated mice.
NO involvement in CTX-induced inhibition of T-cell proliferation.
(A) iNOS mRNA analysis by RT-PCR. 0.4 × 106cells/well were cultured for 24 hours in the presence of Con A (1μg/mL) or anti-CD3 (2μg/mL) plus IL-2 (50 units/mL) in the same conditions used for proliferative assays, and iNOS or control G3PDH mRNA was analyzed by RT-PCR as previously. The figure also shows PCR-positive and -negative controls. (B) The effect of NOS inhibitors on T-cell proliferation. Day 6 or 10 after the last dose of CTX, T-cell proliferative activity induced with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) was assessed in the absence or the presence of LMMA (0.5 mmol/L), L-NIL (0.5 mmol/L), or AMG (0.5 mmol/L) added at culture initiation. The cells were cultured for 72 hours, and proliferation was estimated by [3H]TdR incorporation in the last 18 hours of culture. Data are the mean ±SEM of triplicate cultures from 3 mice injected i.p. with CTX. At the concentrations used, iNOS inhibitors did not have any significant effect over proliferation of control SC cultures that ranged between 100 × 103 and 150 × 103 cpm (not shown). (C) Nitrite accumulation in culture supernatants from SC cultures. Nitrite was measured in the very same cultures used for proliferation assays after 72 hours of culture. As above, data are expressed as the mean ±SEM. Nitrite production by control SC cultures was always <3 μmol/L.
NO involvement in CTX-induced inhibition of T-cell proliferation.
(A) iNOS mRNA analysis by RT-PCR. 0.4 × 106cells/well were cultured for 24 hours in the presence of Con A (1μg/mL) or anti-CD3 (2μg/mL) plus IL-2 (50 units/mL) in the same conditions used for proliferative assays, and iNOS or control G3PDH mRNA was analyzed by RT-PCR as previously. The figure also shows PCR-positive and -negative controls. (B) The effect of NOS inhibitors on T-cell proliferation. Day 6 or 10 after the last dose of CTX, T-cell proliferative activity induced with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) was assessed in the absence or the presence of LMMA (0.5 mmol/L), L-NIL (0.5 mmol/L), or AMG (0.5 mmol/L) added at culture initiation. The cells were cultured for 72 hours, and proliferation was estimated by [3H]TdR incorporation in the last 18 hours of culture. Data are the mean ±SEM of triplicate cultures from 3 mice injected i.p. with CTX. At the concentrations used, iNOS inhibitors did not have any significant effect over proliferation of control SC cultures that ranged between 100 × 103 and 150 × 103 cpm (not shown). (C) Nitrite accumulation in culture supernatants from SC cultures. Nitrite was measured in the very same cultures used for proliferation assays after 72 hours of culture. As above, data are expressed as the mean ±SEM. Nitrite production by control SC cultures was always <3 μmol/L.
Endogenous IFN-γ is required for NO production by CTX-SC
In most cell types, iNOS induction relies on cytokine signaling, particularly by IFN-γ.14 26 Hence, we studied the role of IFN-γ in the production of NO in mitogen-stimulated cultures. First, we assessed IFN-γ production by CTX-SC cultures. As expected, control SC challenged with T-cell mitogens produced large amounts of IFN-γ after 24 hours, being higher in anti-CD3 plus IL-2–stimulated cultures than Con A–stimulated cultures (Table 1). Surprisingly, Con A–stimulated CTX-SC produced as much IFN-γ as control SC, despite the almost complete block in proliferation and the lower number of T cells found in CTX-SC (Figure 1). When stimulated with anti-CD3 plus IL-2, about 50% of IFN-γ production, with respect to SC controls, was detected in CTX-SC cultures.
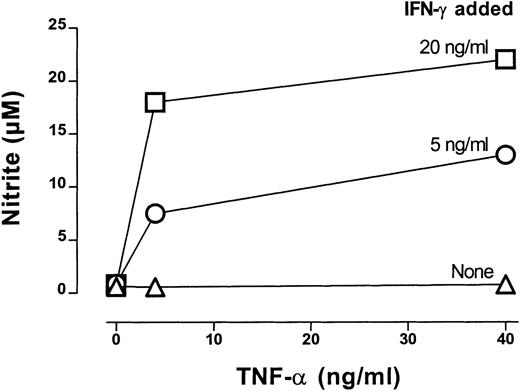
Next we examined the effect of a neutralizing mAb to IFN-γ in CTX-SC or SC cultures treated with Con A or anti-CD3 plus IL-2. The addition of anti–IFN-γ mAb did not affect proliferation by SC (Figure3A). However, it enhanced the proliferation of CTX-SC to Con A or anti-CD3 plus IL-2 in a similar way as NOS inhibitors did. Again, this correlated with a total inhibition of the NO produced in these cultures (Figure 3B). We also determined whether IFN-γ was sufficient to induce high amounts of NO. To assess this, we added increasing concentrations of IFN-γ alone or with other co-stimuli to CTX-SC cultures. Data obtained in a representative experiment are shown in Figure 4. Recombinant IFN-γ (up to 40 ng/mL) was unable to induce the production of large amounts of NO. However, the addition of recombinant TNF-α to IFN-γ–treated cultures triggered a massive NO production. Similar results were obtained with LPS (10 μg/mL) or an agonistic mAb anti-CD40 (1C10)27 employed as costimulus, although this mAb by itself was able to induce NO production in the absence of exogenous IFN-γ in some experiments (data not shown). Therefore, IFN-γ production is necessary, but not sufficient, to achieve high NO levels in cultures of CTX-SC, and IFN-γ neutralization markedly improved the proliferative response of T cells from CTX-SC.
The effect of IFN-γ neutralization in SC cultures.
SC (0.4 × 106 cells/well) were stimulated with Con A (1 μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) and cultured for 72 hours with or without antimurine IFN-γ neutralizing mAb (10 μg/mL) added at culture initiation. [3H]TdR incorporation (A) and nitrite production (B) were assessed at 72 hours after the starting point. Data are the mean ±SD of triplicate cultures of pooled cells from PBS- or CTX-injected mice.
The effect of IFN-γ neutralization in SC cultures.
SC (0.4 × 106 cells/well) were stimulated with Con A (1 μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) and cultured for 72 hours with or without antimurine IFN-γ neutralizing mAb (10 μg/mL) added at culture initiation. [3H]TdR incorporation (A) and nitrite production (B) were assessed at 72 hours after the starting point. Data are the mean ±SD of triplicate cultures of pooled cells from PBS- or CTX-injected mice.
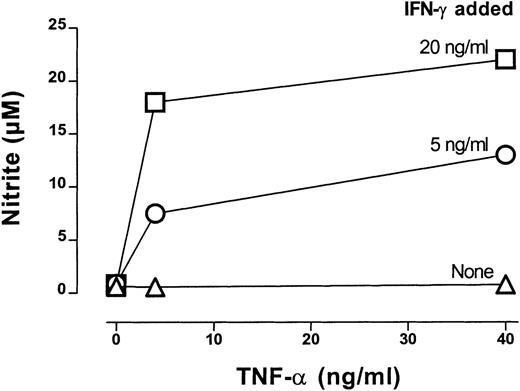
IFN-γ–dependent NO production by CTX-SC.
CTX-SC (0.4 × 106 cells/well) were cultured in the absence or presence of increasing amounts of IFN-γ (5-20 ng/mL) plus TNF-α (4-40 ng/mL). Nitrite accumulation was measured after 72 hours of culture. Data are the mean ±SD of triplicate cultures from pooled spleens.
IFN-γ–dependent NO production by CTX-SC.
CTX-SC (0.4 × 106 cells/well) were cultured in the absence or presence of increasing amounts of IFN-γ (5-20 ng/mL) plus TNF-α (4-40 ng/mL). Nitrite accumulation was measured after 72 hours of culture. Data are the mean ±SD of triplicate cultures from pooled spleens.
Characterization of NO-producing suppressive cells
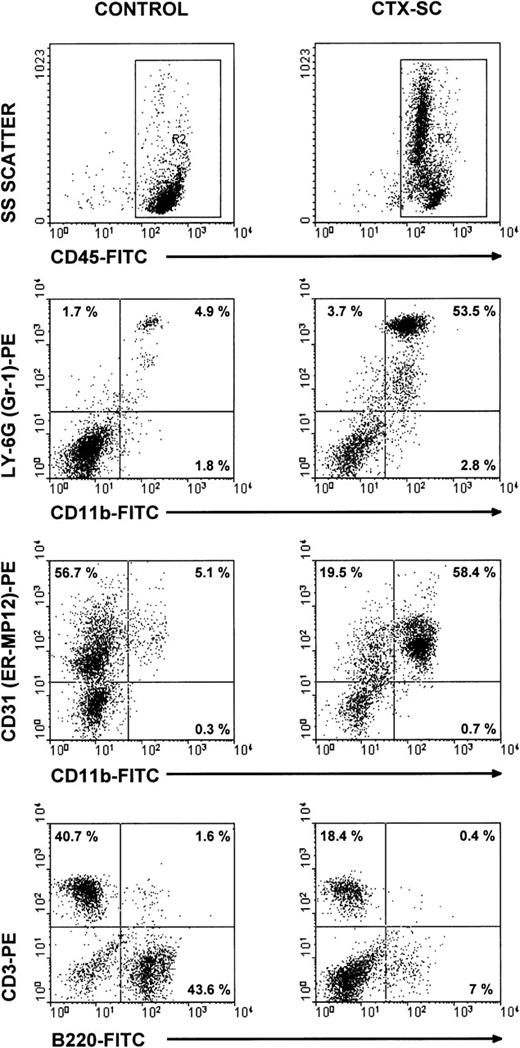
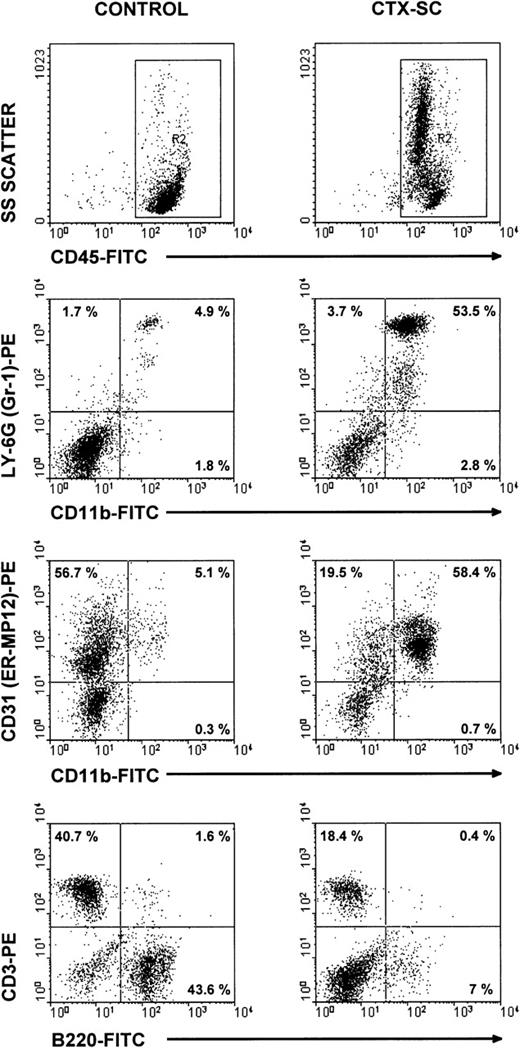
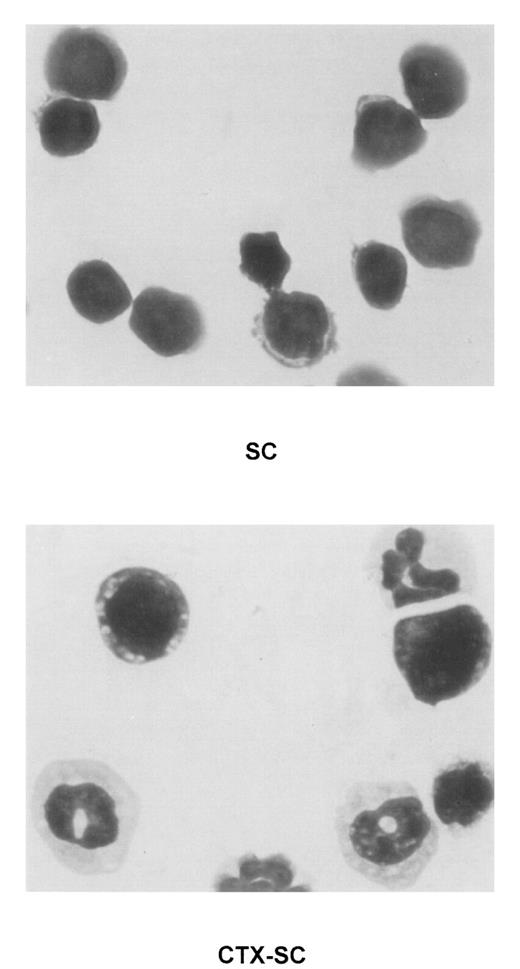
To characterize the phenotype of NO-producing cells, we used flow cytometry to analyze the cell populations found in CTX-SC. In all cases, at least 2 markers per cell population were studied in order to control for any effect of CTX on the expression of critical surface molecules.28 B lymphocytes, assessed by CD19 (already shown in Figure 1B) or B220 expression, were severely depleted at day 6 and particularly day 10, after the last CTX dose, which in most cases was <7% CTX-SC (Figure 5). Moreover, the percentage of CD3+ (Figure 5), CD90.2 (Thy-1.2+), or βTcR+ T cells (not shown) was reduced from 50% to 35% of positive cells found in control SC, depending on the experiment (10%-20% of total cells in CTX-SC versus 35%-42% of total cells in control SC). However, a prominent population of large and granular cells CD45+CD11b+Ly-6G+CD31 (ER-MP12)+ accounted for about 50%-75% of splenocytes from CTX-treated mice, whereas PBS-treated control SC contained <7% of that population. Accordingly, Giemsa-stained cytospin preparations of CTX-SC showed the predominance of blasts, immature granulocytic, and monocytic cells (Figure 6).
Major cell populations in CTX-SC.
Fresh SC from PBS- and CTX-injected mice were obtained 6 days after the last injection of PBS/CTX and stained with anti-CD45, CD11b, or B220 FITC-labeled mAbs or biotinylated CD31, CD3, or anti–Ly-6G followed by streptavidin R-phycoerythrin conjugate (AvPE). Isotypic controls showed <0.5% in selected regions.
Major cell populations in CTX-SC.
Fresh SC from PBS- and CTX-injected mice were obtained 6 days after the last injection of PBS/CTX and stained with anti-CD45, CD11b, or B220 FITC-labeled mAbs or biotinylated CD31, CD3, or anti–Ly-6G followed by streptavidin R-phycoerythrin conjugate (AvPE). Isotypic controls showed <0.5% in selected regions.
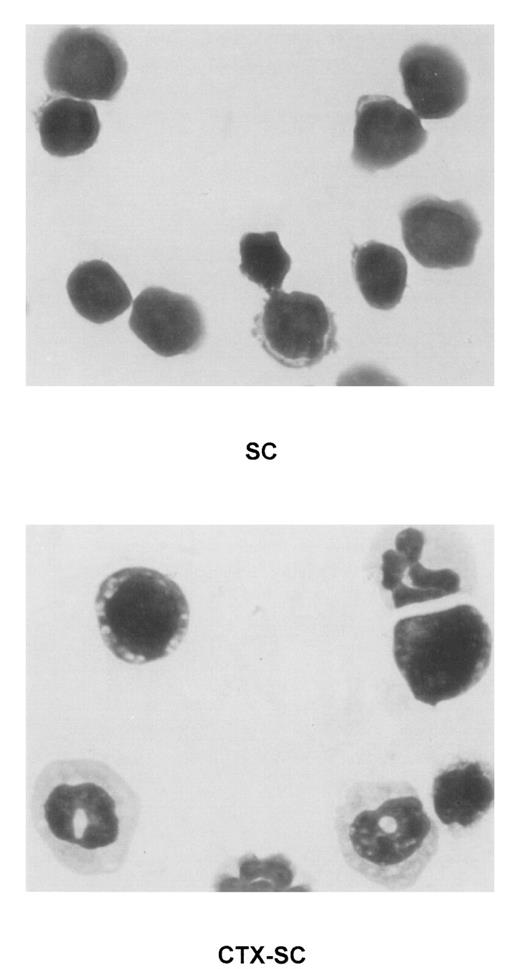
Presence of immature myeloid cell in CTX-SC.
Giemsa staining was performed in cytospin preparations of concentrated fresh SC of PBS-injected (top, SC) or CTX-injected (bottom, CTX-SC) mice 6 days after the last dose of CTX. Samples were analyzed using an Olympus Vanox AHBT3 microscope, original magnification ×1000.
Presence of immature myeloid cell in CTX-SC.
Giemsa staining was performed in cytospin preparations of concentrated fresh SC of PBS-injected (top, SC) or CTX-injected (bottom, CTX-SC) mice 6 days after the last dose of CTX. Samples were analyzed using an Olympus Vanox AHBT3 microscope, original magnification ×1000.
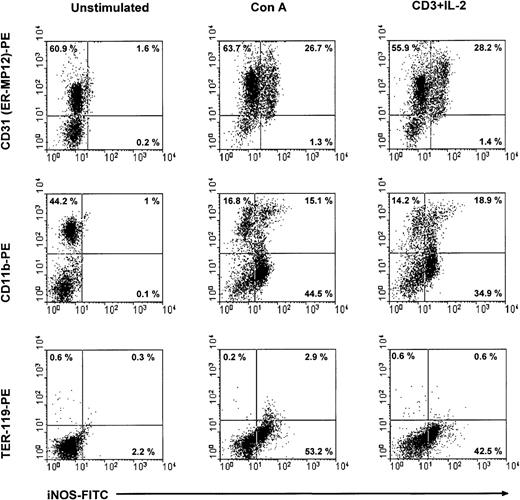
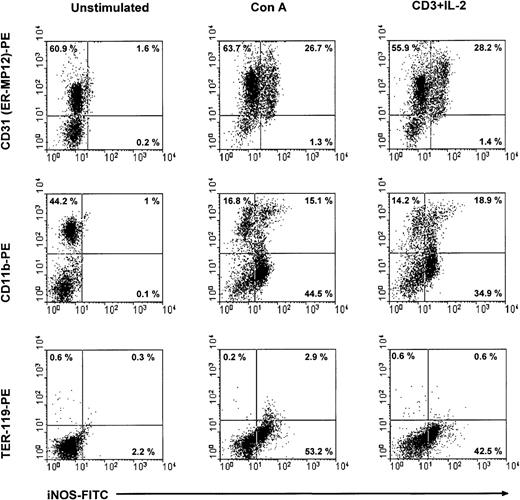
Subsequently, we used flow cytometry to verify the expression of iNOS in CD11b+ CD31+ cells of CTX-SC after stimulation with Con A or anti-CD3 plus IL-2. CTX-SC were stimulated with mitogens for 24 or 30 hours. The cells were then recovered, stained for suitable surface markers, and assayed for intracellular expression of iNOS. The results depicted in Figure7 show that intracellular expression of iNOS protein is restricted to CD31 (ER-MP12)+ cells or between 30%-60% of cells in mitogen-treated cultures. In addition, it was clearly seen that iNOS protein was induced in CD11b+CD31 (ER-MP12)+ cells, although a substantial percentage of iNOS+ cells did not express CD11b. We concluded that the NO-producing ability was induced by mitogens in a heterogeneous population, such as CTX-SC, and in particular in CD11b+Ly-6G+CD31+cells.
iNOS expression in CTX-SC.
CTX-SC (0.4 × 106 cells/well) were cultured for 24-36 hours in the absence of or with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL). The cells were then recovered and stained with biotinylated anti-CD11b, anti-CD31, or PE-conjugated control TER-119 mAbs followed by AvPE, if required, for surface markers and FITC-conjugated antimouse iNOS. Isotypic controls showed <0.8% background in the selected regions. The results shown were obtained in 3 separate experiments.
iNOS expression in CTX-SC.
CTX-SC (0.4 × 106 cells/well) were cultured for 24-36 hours in the absence of or with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL). The cells were then recovered and stained with biotinylated anti-CD11b, anti-CD31, or PE-conjugated control TER-119 mAbs followed by AvPE, if required, for surface markers and FITC-conjugated antimouse iNOS. Isotypic controls showed <0.8% background in the selected regions. The results shown were obtained in 3 separate experiments.
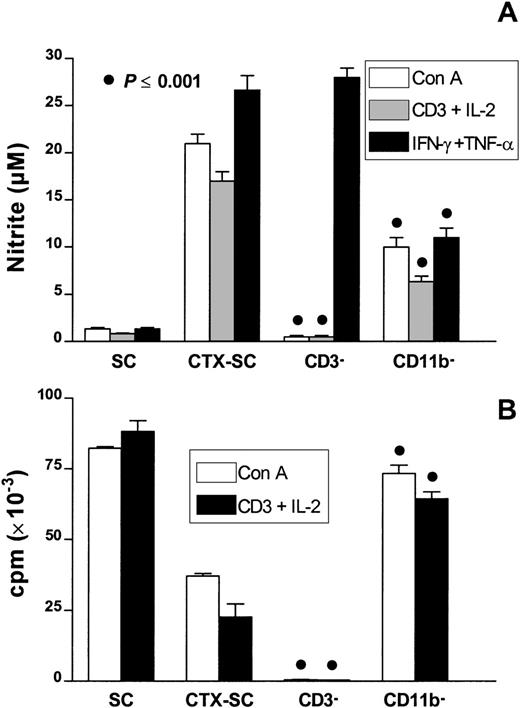
To confirm the involvement of CD11b+Ly-6G+CD31+ cells in the suppression of T-cell proliferation, we performed a series of experiments by selective cell depletion. Thus, we depleted CD3+ or CD11b+ cells from CTX-SC. Data depicted in Figure 8A show that depletion of CD3+ cells abolished the potential of CTX-SC to release NO promoted by T-cell mitogens (Con A or anti-CD3 plus IL-2) but not that promoted by the exogenous addition of IFN-γ plus TNF-α. As expected, there was a lack of proliferation in CD3− cells to both mitogens (Figure 8B). Interestingly, depletion of CD11b+ cells reduced NO production by about 50% but fully restored T-cell responsiveness. This suggests that CD11b+Ly-6G+CD31 (ER-MP12)+ cells are the main NO producers in mitogen- or cytokine-stimulated CTX-SC and are the cells responsible for suppressing the proliferation of splenic CTX-SC T lymphocytes.
The effect of selective cell depletion in T-cell proliferation of CTX-SC.
SC and CTX-SC were obtained at day 6 (CD11b+ cell depletion) or day 10 (CD3+ cell depletion) after the last dose of PBS/CTX; 0.4 × 106 cells/well were stimulated with Con A (1μg/mL), anti-CD3 (2μg/mL) plus IL-2 (50 U/mL), or IFN-γ (5 ng/mL) plus TNF-α (40 ng/mL) as indicated. The cells were assayed for (A) NO production after 72 hours of culture and (B) T-cell proliferation. Data are the mean ±SD of triplicate cultures.
The effect of selective cell depletion in T-cell proliferation of CTX-SC.
SC and CTX-SC were obtained at day 6 (CD11b+ cell depletion) or day 10 (CD3+ cell depletion) after the last dose of PBS/CTX; 0.4 × 106 cells/well were stimulated with Con A (1μg/mL), anti-CD3 (2μg/mL) plus IL-2 (50 U/mL), or IFN-γ (5 ng/mL) plus TNF-α (40 ng/mL) as indicated. The cells were assayed for (A) NO production after 72 hours of culture and (B) T-cell proliferation. Data are the mean ±SD of triplicate cultures.
Discussion
In this work, we have investigated the mechanisms responsible for T-cell deficiency observed after a course of intensive chemotherapy with CTX. Our results show that, besides its well-known cytotoxic effect, the immunosuppressive activity of CTX also results from the presence of a heterogeneous splenic cell population induced by CTX treatment. Those cells have been characterized as mostly immature myeloid/monocytic cells, CD11b+Ly-6G+CD31+, and their inhibitory activity is dependent on iNOS-derived NO production. They can account for the profound impairment in the proliferative activity displayed by splenic T cells during recovery from chemotherapy. Nitric oxide–producing suppressor cells are CD11b+, although there is also a population of CD11b− cells that express iNOS. In this regard, preliminary data from our laboratory indicate that immature CD11b−CD117 (c-kit)+ cells are also able to express iNOS.
Our results are compatible with a mechanism in which activated T lymphocytes release cytokines (especially IFN-γ), which together with other yet undefined signals, induce iNOS expression by immature myeloid cells. Nitric oxide or other RNI generated in this way, acting alone or together with other suppressive signals, can suppress further T-cell activation and proliferation. Similar mechanisms involving IFN-γ–induced NO-mediated suppression have been proposed in order to account for the suppression observed in a number of experimental systems including hematopoietic tissues,21,29,30 infectious diseases,14,17,18,31 graft-versus-host disease (GVHD),19,32 tumor bearers,33,34 and exposure to toxic compounds.35 The following evidence supports the aforementioned model.
First, NO production is required to suppress the proliferation of T lymphocytes. The fact that all 3 NOS inhibitors tested (LMMA, AMG, L-NIL) greatly improved the proliferative activity of T lymphocytes from CTX-SC in response to Con A or anti-CD3 plus IL-2 suggests that the suppressive activity relies on iNOS induction. Moreover, given their different structures and mechanism of inhibition,23-25 it is highly improbable that their effects were due to unspecific activities and not to iNOS inhibition. In addition, much higher levels of nitrite and iNOS mRNA expression were found in CTX-SC cultures stimulated with mitogens than in SC control cultures. This evidence indicates that NO or other RNI contribute substantially to the profound inhibition of T-cell proliferation in CTX-SC cultures. However, although it has been shown that NO itself is able to inhibit proliferation of T lymphocytes36 and other cell types,37 we cannot discard the fact that other signals could also be involved in the mechanism of suppression, as it has been described in the Fas-mediated destruction of pancreatic β-cells in insulin-dependent diabetes mellitus.38 In this work we have not addressed the molecular mechanism of NO-mediated immunosuppression. Preliminary results suggest that most of the suppressive activity is due to NO-dependent apoptosis of T cells in mitogen–stimulated CTX-SC cultures. T-cell apoptosis is clearly reduced by iNOS inhibitors (data not shown). In this regard, it is worth mentioning that NO has been shown to induce apoptosis in several other experimental systems.39 40
Second, T lymphocytes are absolutely required for mitogen-induced NO production as deduced from cell-depletion experiments. This points to signals derived from activated T lymphocytes as the main inducers of NO production. Nevertheless, depletion of T cells did not impair the NO-releasing ability of CTX-SC promoted by exogenous IFN-γ plus TNF-α. In this regard, IFN-γ has been shown to be an essential signal for NO induction in most experimental systems.14,16-19,21,26,32 Upon suitable activation, IFN-γ is produced in large amounts by T lymphocytes and NK cells26 and some macrophages.41,42 In our experimental system, IFN-γ also seems to be a key molecule, both in NO production and in the inhibition of T-cell proliferation. However, our results suggest that additional signals are also required to achieve a significant NO production, in accordance with previous reports from a variety of cell systems.16,18-21,26,32Specifically, TNF-α could contribute to NO production.14Nevertheless, although a neutralizing anti–TNF-α mAb strongly inhibited NO production in our cultures, it did not recover T-cell proliferation (data not shown). This effect appeared related to the fact that this mAb markedly diminished the normal mitogen-induced proliferation of control SC (data not shown). So, it is likely that the dominant activity of anti–TNF-α in our cultures was the impairment of early T-cell activation events rather than the signaling interference of NO-producing cells. Similar inhibition of cell proliferation by neutralizing anti–TNF-α mAb has been previously reported in human T cells.43 Although other cytokines and colony stimulating factors are putative candidates to cooperate with IFN-γ, it seems likely that cognate cell interactions can play a decisive role both in NO production and in T lymphocyte suppression. In support of this possibility we have found that the agonist CD40 mAb (1C10)27 acted synergistically with IFN-γ in inducing high amounts of NO by CTX-SC. This is not surprising because CD40-CD40L(CD154) interactions have been shown to play a role in NO production in allograft rejection44 and in macrophages,45 dendritic cells,46 and bone marrow–derived immature myeloid cells with natural suppressor (NS) activity (Angulo et al, submitted manuscript).
Third, the dramatic drop in proliferative activity of T lymphocytes from CTX-SC is associated with the colonization of spleen by large and granular immature myelo-monocytic CD45+CD11b+Ly-6G+CD31+cells and not with depletion of T cells. Kinetic analysis showed that the absolute number of T lymphocytes remained relatively unchanged between days 3 and 10 after the last dose of CTX. The recovery of cellularity seen at days 6 and 10 was due to the colonization of the spleen mainly by immature cells expressing myeloid markers47-49 and not due to T or B lymphocytes. This fact agrees with previous data showing that after chemotherapy, progenitor cells are mobilized from the bone marrow to the periphery, and the spleen becomes a major site of extramedullary hematopoiesis.50,51Moreover, NO production by hematopoietic progenitors has been demonstrated by a number of authors.52 Indeed, CD11b+CD31+ cells are able to express iNOS in cultures stimulated with T-cell mitogens. Thus, those cells have a high potential to produce NO in CTX-SC cultures upon induction by T-cell–derived signals. This was further supported by depletion of selected cell populations. Depletion of CD11b+ cells, which included almost all Ly-6G+ and expressed CD31 cells, reduced NO production and restored T-cell responsiveness, indicating that this population is mainly responsible for inhibition of T-lymphocyte proliferation.
Consistent with these results were those obtained by depletion of CD31+ cells from CTX-SC. Unfortunately, interpretation of data obtained in these latter experiments has been hampered by 2 facts: (1) CD31 cells seemed to be directly or indirectly up-regulated by Con A or anti-CD3 plus IL-2, and (2) depletion of CD31+ cells abolished proliferation of T lymphocytes from CTX-SC despite the high percentage of remaining TcR+ cells. Thus, although CD31+ depletion nearly eliminated NO production in response to mitogens, it also caused a severe impairment of T-cell activation, possibly due to depletion of accessory cells. This view is further supported by the strong inhibition of NO production observed when CTX-SC cells are stimulated with IFN-γ plus TNF-α. Overall, the evidence points to CD11b+Ly-6G+CD31+ immature myeloid cells as the main actors responsible for T-cell inhibition in CTX-SC cultures.
It is also noteworthy that, as given in previous reports, the generation of suppressor cells in the spleen of mice treated with CTX is able to inhibit in vitro responses of T and B lymphocytes obtained from normal lymphoid organs.6-12 In some cases, this inhibition occurs in an IFN-γ–dependent fashion.10,12 In the best characterized system, the suppressor activity was assayed in mixed lymphocyte reaction (MLR), and the suppressive cell population was heterogeneous, comprising null cells or B220+, TcR+, or Mac-1+ subpopulations, this latter being the most potent in suppressing lymphocyte proliferation.12 Although these results are basically in agreement with ours, the authors discarded NO involvement in the mechanism of suppression. Still, suppression appeared to be dependent on IFN-γ production and was attributed in part to prostaglandin production and/or blocking of IL-2/IL-4 utilization.53 The discrepancy with our results could be explained by differences in the experimental models; Brooks et al12 used a single injection of 200 mg/kg CTX and assayed suppressive activity by MLR. Importantly, Krenger et al32 found that inhibition of proliferative responses of spleen cells from mice undergoing acute GVHD was due to NO production if the cells were stimulated by Con A. This did not occur in MRL assays. Therefore, the nature of the mitogenic stimulus and the assay system used could have decisive importance in the mechanisms of suppression. In fact, in our experimental system, which lacks allogeneic cells, nitrite levels were quite similar in Con A–stimulated and anti–CD3 plus IL-2–stimulated CTX-SC cultures. Microscopic examination, however, showed an extensive cellular death in the latter, whereas in Con A–stimulated cultures, this phenomenon was not so evident. Therefore, it seems likely that different signals are involved in the mechanism of suppression, as a function of the T-cell mitogen employed.38 54
Among the problems associated with intensive chemotherapy, restoration of immunocompetence is a critical issue in patient management.1,2 Here we have shown that CTX treatment could induce an state of immunodepression by at least 2 mechanisms: (1) direct cytotoxic effects that severely reduce the number of T lymphocytes and (2) the presence in the spleen of a heterogeneous population of immature myeloid cells able to suppress T-cell proliferation upon activation. So, it is conceivable that activation of splenic T cells in an altered context could lead to their NO-dependent deletion or functional inactivation effected by those immature CD11b+Ly-6G+CD31+ cells. This hypothesis is in close agreement with some recent data from other authors. Thus, it has been described that vaccination-induced splenic immunosuppressive Mac-1+/Gr-1+, comprising monocytes and a population of immature myeloid cells, can suppress specific CTL responses in vivo and in vitro in mice immunized with powerful immunogens.55 Moreover, from our point of view, this phenomenon is likely related to the NO-dependent suppressive effects caused by IL-12 in different systems of vaccination.56-58 Accordingly, in both Trypanosoma cruzi and Histoplasma capsulatum infectious models, it has been suggested that splenic T cells can be deleted by NO-dependent apoptosis.59 60
In conclusion, our results may have clinical importance in implementing immunotherapies in immunocompromised patients due to the risk of undesired activation of T lymphocytes in the presence of suppressive cells able to delete or inactivate T lymphocytes in lymphoid tissues.
Acknowledgments
We are grateful to Drs J. L. Subiza and J. Rullas (Hospital Clı́nico, Madrid, Spain) for their help and expertise in flow cytometry experiments. We are also indebted to Dr F. J. Gamo (Glaxo Wellcome, Tres Cantos, Spain) for his help in PCR analysis, Dr Andrew W. Heath (University of Sheffield, Sheffield, England) for the generous gift of purified 1C10, and Lucı́a Horrillo for secretarial assistance.
Supported by grants from Glaxo Wellcome S.A., Dirección General de Investigación Cientı́fico y Técnica, and the Fundación Ramón Areces.
Reprints:Manuel Fresno, Centro de Biologı́a Molecular Severo Ochoa, C.S.I.C.-U.A.M., Cantoblanco, 28049 Madrid, Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. NO involvement in CTX-induced inhibition of T-cell proliferation. / (A) iNOS mRNA analysis by RT-PCR. 0.4 × 106cells/well were cultured for 24 hours in the presence of Con A (1μg/mL) or anti-CD3 (2μg/mL) plus IL-2 (50 units/mL) in the same conditions used for proliferative assays, and iNOS or control G3PDH mRNA was analyzed by RT-PCR as previously. The figure also shows PCR-positive and -negative controls. (B) The effect of NOS inhibitors on T-cell proliferation. Day 6 or 10 after the last dose of CTX, T-cell proliferative activity induced with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) was assessed in the absence or the presence of LMMA (0.5 mmol/L), L-NIL (0.5 mmol/L), or AMG (0.5 mmol/L) added at culture initiation. The cells were cultured for 72 hours, and proliferation was estimated by [3H]TdR incorporation in the last 18 hours of culture. Data are the mean ±SEM of triplicate cultures from 3 mice injected i.p. with CTX. At the concentrations used, iNOS inhibitors did not have any significant effect over proliferation of control SC cultures that ranged between 100 × 103 and 150 × 103 cpm (not shown). (C) Nitrite accumulation in culture supernatants from SC cultures. Nitrite was measured in the very same cultures used for proliferation assays after 72 hours of culture. As above, data are expressed as the mean ±SEM. Nitrite production by control SC cultures was always <3 μmol/L.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.212/5/m_bloo00127002w.jpeg?Expires=1769324626&Signature=ve2ts958xzZpFvjKQkZ8qgyHmCu1WKr8Wu8lurUKpDcHBt55W7fuc6f3hi1jWxbBx4P0Rbpzi-nx3w6mDY-8Gs9XwQbfz4WCf8vr9~oSvVTncj1xyz59z0E~ZdFs5R2g-Wu9QpoLwUSlJFlnW0STz2RZPcXANal16HhNCL0AltfnWql2PWVfVfoD8~SYmU-ebsmSJuGSlFuoZtArI4VVA9GWOj9A4GTh17BqGHFrZCnCw18M7mzZxPnPvxEzDIJxk7XgB4Y-GLzZKSIzlNZicsaXRMdrn0JHkWwi9NnGA5fOsYAi1RfYeRZZNwxM1AMA2-K8uXoMuT0YxGsVZp9h8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. The effect of IFN-γ neutralization in SC cultures. / SC (0.4 × 106 cells/well) were stimulated with Con A (1 μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) and cultured for 72 hours with or without antimurine IFN-γ neutralizing mAb (10 μg/mL) added at culture initiation. [3H]TdR incorporation (A) and nitrite production (B) were assessed at 72 hours after the starting point. Data are the mean ±SD of triplicate cultures of pooled cells from PBS- or CTX-injected mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.212/5/m_bloo00127003x.jpeg?Expires=1769324626&Signature=a6XBDf3gcGxzM3nLwGesEaKKgTNG6xACMNvMeLO6rWkVMJTCMY3mnbzHMXHO8ZqF1s-m2C4pZP-7e-sEdwwmBeUkha3K9zS~8ZsLSviGHYqI7o1PBZdfjBj4DvY~Za8wQDaIfngoLsTEg~HE981ic-JQO3guv56oHjLc7EdqrR0rRQbaLij1iimqeoJ~1nuTs-5gblbdBsAqDpnnLUks~N-Q8uLfK9pd4NjQa~GpGFKcT~bc5AsAd0XM~bpDLvoBwiQMaB0VlcKIMjTgf6C5sRH3aqbyub4SSMgw1TqYcxv~h4tyHtkWD7aNgq0z5NqVRtugFOmUcyaJqoOKxY6SDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. NO involvement in CTX-induced inhibition of T-cell proliferation. / (A) iNOS mRNA analysis by RT-PCR. 0.4 × 106cells/well were cultured for 24 hours in the presence of Con A (1μg/mL) or anti-CD3 (2μg/mL) plus IL-2 (50 units/mL) in the same conditions used for proliferative assays, and iNOS or control G3PDH mRNA was analyzed by RT-PCR as previously. The figure also shows PCR-positive and -negative controls. (B) The effect of NOS inhibitors on T-cell proliferation. Day 6 or 10 after the last dose of CTX, T-cell proliferative activity induced with Con A (1μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) was assessed in the absence or the presence of LMMA (0.5 mmol/L), L-NIL (0.5 mmol/L), or AMG (0.5 mmol/L) added at culture initiation. The cells were cultured for 72 hours, and proliferation was estimated by [3H]TdR incorporation in the last 18 hours of culture. Data are the mean ±SEM of triplicate cultures from 3 mice injected i.p. with CTX. At the concentrations used, iNOS inhibitors did not have any significant effect over proliferation of control SC cultures that ranged between 100 × 103 and 150 × 103 cpm (not shown). (C) Nitrite accumulation in culture supernatants from SC cultures. Nitrite was measured in the very same cultures used for proliferation assays after 72 hours of culture. As above, data are expressed as the mean ±SEM. Nitrite production by control SC cultures was always <3 μmol/L.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.212/5/m_bloo00127002w.jpeg?Expires=1769640702&Signature=pOSDzRh7bq-JyVeV2AEu0lKGDdwIj~TzD4uQnpqynVmk0O2lvI6~jVHryqrdqHBio9JtqPHIcHRfetsHl9QJrMkwUUn5qSs91yUcb2SpDfSyjmJrtKMP0q1XBABTnvORhjdCJXbQzkhZFHxo1P3GdAXrFHKkWHkxAasrYaNMQOYTgkIA7TNsN1ZjqGaT0wZYn0dCZXu9MeXWHFFU96O8mkhd3gbEEOq2LjxWGWip2ZyW-m5u0ihuUh51jQQTQLvJXMsh81VrRz82tWHPHcP-lls-jFZSzkvFZQVVIoqHgt7sd9JfKXsyxlMV2OkzMOct7IFhJXUsjoP0K8XoYCaZOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. The effect of IFN-γ neutralization in SC cultures. / SC (0.4 × 106 cells/well) were stimulated with Con A (1 μg/mL) or anti-CD3 (2 μg/mL) plus IL-2 (50 units/mL) and cultured for 72 hours with or without antimurine IFN-γ neutralizing mAb (10 μg/mL) added at culture initiation. [3H]TdR incorporation (A) and nitrite production (B) were assessed at 72 hours after the starting point. Data are the mean ±SD of triplicate cultures of pooled cells from PBS- or CTX-injected mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.212/5/m_bloo00127003x.jpeg?Expires=1769640702&Signature=fEM0-ze7Hl~MFSZNbY7yyOPB8OlhMLmMhaCZf7-YtzBhu068uTMGc2BdVsRCv7vWhuyNifqubDDEf6KZD7WMr0IozxZeum~BNBE7m14dp-82Isg5n1rqg9KPxWPCoYUUvrFV0ZF2x-IH63JV~u2y9BRFY-DszV40fy6oV8G-w7Te6YWRTNNIAdXEgNRXyk4FkBJdJ~GMxdVu4TtPD~s0S8EAlie~dUwNy3QHAtn7oCHHbK2A7R3Qdh0IP8xf-K-f7mFt10M1iysoQyGhutQR~bzpSZpBWUkNe6EVYqduDErfZTOzicZU0TKY4Vsl4NEkJsi0yMCGnHw6n4pTcOgDDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)