Abstract
A substantial minority of patients with chronic myelogenous leukemia (CML) achieve a complete response (CR) to treatment with interferon- (IFN), defined as the disappearance of Philadelphia chromosome-positive metaphases. Currently it is unclear how long IFN treatment should be continued for such patients. We used a competitive reverse transcriptase-polymerase chain reaction (RT-PCR) to quantify levels of BCR-ABL transcripts in 297 peripheral blood specimens collected from 54 patients who had achieved CR with IFN. The median duration of observation was 1.9 years (range, 0.3-11.0 years). Total ABL transcripts were quantified as internal control and results were expressed as the ratio BCR-ABL/ABL. All 54 patients had molecular evidence of residual disease, although 3 patients were intermittently PCR negative. The median BCR-ABL/ABL ratio at the time of maximal response for each patient was 0.045% (range, 0%-3.6%). During the period of observation 14 patients relapsed, 11 cytogenetically to chronic phase disease and 3 directly to blastic phase. The median ratio of BCR-ABL/ABL at maximal response was significantly higher in patients who relapsed than in those who remained in CR (0.49% versus 0.021%,P < 0.0001). Our findings show that the level of residual disease falls with time in complete responders to IFN, but molecular evidence of disease is rarely if ever eliminated. The actual level of minimal residual disease correlates with the probability of relapse. We suggest that for patients who reach CR, IFN should be continued at least until relatively low levels of residual leukemia are achieved. (Blood. 2000;95:62-66)
Since the initial description of the polymerase chain reaction (PCR) in 1985,1 numerous applications for amplification of specific DNA sequences have been developed. Because of its remarkable sensitivity, PCR has become a standard technique for the detection of minimal residual disease (MRD) after therapy of various hematologic and infectious diseases.
Chronic myelogenous leukemia (CML) constitutes a clinical model for molecular detection and therapy surveillance because this entity was the first leukemia known to be associated with a specific chromosomal rearrangement, the Philadelphia (Ph) translocation t(9;22)(q34;q11), and the presence of a novel chimeric gene, BCR-ABL. In 1989, the first encouraging results concerning detection of MRD by PCR in patients with CML after allogeneic bone marrow transplantation were reported.2 However, conflicting data from a comparative multicenter study revealed serious problems for the method with a high rate of false-positive results and provoked an open discussion.3,4 Over the past 10 years, PCR has been optimized and developed. Specificity has been considerably increased by the partial standardization of methodology and the introduction of rigorous precautions to avoid contamination.5Sensitivity has been improved by using nested primer pairs and performing 2 consecutive PCR steps. In view of the limited value of qualitative PCR for monitoring CML patients after therapy, quantitative BCR-ABL PCR assays were developed to monitor patients after bone marrow transplantation6,7 or treatment with interferon-α (IFN)8 9 and are now in routine clinical use.
Interferon-α may induce complete hematologic remission in about 70% to 80% of patients with CML in early chronic phase.10Complete response (CR) has been observed by cytogenetic or Southern blot analysis in 6% to 38% of patients.11-16 It is generally agreed that the variability of response rates may be explained by differences in the risk composition of patient groups. Complete responders survive significantly longer than those without cytogenetic remission,15 but some of these patients will ultimately relapse with reappearance of the Ph-positive clone.17 Using the reverse transcriptase-polymerase chain reaction (RT-PCR) assay, persistent disease in CR has been documented in the great majority of cases.9 17-19 The aim of our study was to assess the prognostic significance of sequential quantitative analysis of residual disease in CML patients in CR on IFN.
Patients and methods
Patients
Fifty-four patients who had achieved CR to treatment with IFN were studied prospectively (36 male, 18 female; median age at diagnosis 48 years, range 5-76 years) with informed consent as a cooperative project of the German and UK MRC CML study groups. Thirty-two patients were treated with recombinant IFN-α2a, 5 with recombinant IFN-α2b, 1 with recombinant IFN-α2c, 14 with lymphoblastoid IFN-α1n, 1 with IFN-α2a and 2b, and 1 with IFN-α2a and IFN-αn1 consecutively. The therapeutic goal in all patients was to treat them with the maximum tolerable doses of IFN, starting with 3 million IU/d with increasing dosages to 6, 9, or 12 million IU/d in the UK study11 or starting with 5 million IU/m2/d in the German study.12 The doses were adjusted according to leukocyte and platelet counts to reach a level of 2000 to 4000 white blood cells/μL and > 100 000/μL platelets. The median interval from diagnosis until start of IFN therapy was 0.2 year (range, 0-2.1 years). At diagnosis, 52 patients were Ph positive, 2 patients were Ph negative/BCR-ABL positive. CR was defined as the disappearance of Ph chromosome-positive metaphases or, for Ph-negative/BCR-ABL-positive patients, the disappearance of the rearranged BCR allele as assayed by Southern blot analysis. The median time from start of IFN therapy to first detection of CR was 1.6 years (range, 0.4-6.4 years). The median follow-up period in CR was 1.9 years (range, 0.3-11.0 years). In 44 patients, CR lasted at least 1 year with 10 patients experiencing long-lasting complete remissions of more than 5 years. In 6 patients IFN has been withdrawn after 0.3 to 5.2 years (median, 4.4 years) from CR because of stable CR (n = 3) or because of adverse side effects (n = 3). Additional therapies administered prior to CR were hydroxyurea (n = 30), busulfan (n = 2), hydroxyurea and busulfan (n = 1), and cytosine arabinoside (n = 1). Prognostic risk distribution according to Sokal20 was calculated for 51 patients: low risk, n = 27 (53%); intermediate risk, n = 17 (33%); high risk, n = 7 (14%). For 49 patients a new score established for IFN-treated patients21 was assessed: low risk, n = 27 (55%); intermediate risk, n = 19 (39%); high risk, n = 3 (6%).
Cytogenetics and Southern blot analysis
Cytogenetic and DNA analyses were performed according to standard protocols. For chromosome analysis bone marrow specimens were examined on direct or short-term (24-hour) cultures. Southern blotting for M-bcr rearrangements was performed as reported.22 A minimum number of 20 metaphases was required to assess CR. If fewer than 20 metaphases were available for analysis, CR was only diagnosed if no M-bcr rearrangement was detectable by Southern blot analysis. Relapse was defined as the reappearance of at least 1 Ph-positive metaphase or, alternatively, the reappearance of the rearranged M-bcr band in Southern blot analysis.
Qualitative and quantitative RT-PCR analysis
RNA was extracted from leukocytes obtained from 20 mL peripheral blood. Samples were received either locally or by mail and spent between 1 to 3 days in transit. We have previously shown essentially identical levels of residual disease in peripheral blood and bone marrow.9 Conditions for RNA extraction, reverse transcription, and 2-step (nested) PCR for BCR-ABL have been described.23 An estimate of the number of BCR-ABL transcripts was made applying a competitive PCR titration assay6,9 using a semilogarithmic dilution series of 101 to 106 molecules added to the same volume of patient cDNA. The equivalence point between different competitor concentrations was determined by densitometric analysis. ABL mRNA was quantified in all samples as an internal standard using the same competitor construct but different oligonucleotide primers.9,24 The result was expressed as the percentage ratio between BCR-ABL and ABL. BCR-ABL-negative results obtained from 10 samples with ABL equivalence points < 10.4,5competitor molecules per reaction were regarded as of insufficient quality for sensitive detection of MRD and were therefore discarded. Strict precautions were taken to prevent contamination. All experiments included negative controls from all stages of the reactions.
A total of 297 samples (1-13 per patient, median 5) were analyzed. Seventy samples have been investigated before CR or after relapse, 227 samples were obtained at various time points during CR (median 1.7; range, 0-11.0 years after first CR). Of these 227 samples, 196 were studied during ongoing IFN-α therapy in complete remission, 31 samples of 6 patients after withdrawal of IFN.
Statistical methods
Relapse-free survival analysis was based on Kaplan-Meier estimation and groups were compared by log-rank test. Relapse-free survival was dated from the first PCR analysis in complete remission to avoid selection bias for the time interval between first detection of CR and first PCR analysis. The impact of the minimum individual MRD level on the stability of CR was proven by the Mann-Whitney test. The influence of the level of residual disease and the relationship to prognostic risk groups at diagnosis on the risk of relapse were determined by Fisher's exact test.
Results
All 54 patients had evidence of residual BCR-ABL transcripts in complete remission. Twenty-six patients (48%) expressed b3a2, 18 (33%) b2a2, 9 (17%) b2a2 and b3a2, and 1 (2%) b3a3 BCR-ABL transcripts. For 3 patients, nested PCR was intermittently negative (1 of 4, 2 of 7, and 1 of 11 samples, respectively). The median BCR-ABL/ABL ratio of all 227 samples investigated during complete remission was 0.07% (range, 0%-7.0%) and the median ratio of the 196 samples investigated during ongoing IFN therapy was 0.1% (range, 0%-7.0%). The lowest level of residual disease on sequential analysis (maximal response) for individual patients in CR ranged between 0% and 3.6% (median, 0.045%). Levels of residual disease in 153 samples of 40 non-relapsing patients declined over time after first CR (Table), indicating an ongoing effect of IFN in reducing the levels of MRD. Five of the 6 patients who had ceased IFN therapy were in stable remission at the date of analysis, 2.2 to 9.5 years (median, 5.6 years) after IFN withdrawal. Levels of MRD in these 5 patients ranged from 0% to 3.0% (median, 0.07%) BCR-ABL/ABL. The sixth patient relapsed 2.5 years after IFN withdrawal and had rising levels of MRD on sequential analysis.
Fourteen patients (26%) relapsed at 0.3 to 5.1 years (median, 1.3 years) after first diagnosis of CR and 0.2 to 3.9 years (median, 1.1 years) after first molecular analysis. Eleven patients relapsed into chronic phase CML with the reappearance of Ph-positive metaphases or M-bcr rearrangement. Three patients relapsed directly into blast crisis (1 myeloid, 2 lymphoid). Four patients died, 3 of blast crisis and 1 of liver failure in chronic phase CML (Figure1). The relapsing patients have been treated with IFN-α2a (n = 9), IFN-α2b (n = 1), IFN-αn1 (n = 3), and IFN-α2c (n = 1). Additional therapies in relapsing patients were hydroxyurea (n = 10), busulfan (n = 1), and none (n = 3). The relapsing patients were recruited from the German studies (n = 7), from the UK studies (n = 6), or neither trial (n = 1). There was no specific pattern of the type of IFN, additional therapies, or allocation to a specific study in relapsing patients.
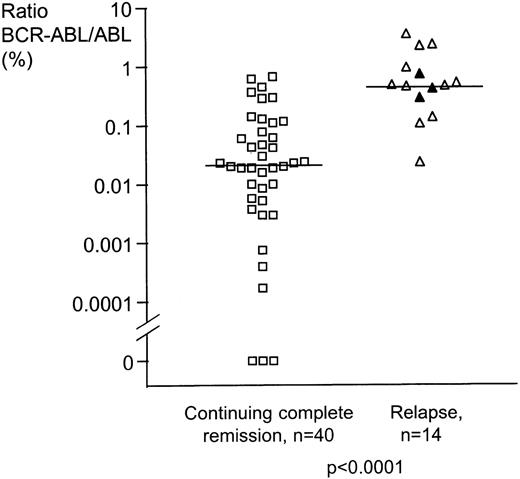
Maximum response on sequential analysis for the 54 CML patients in CR on IFN.
For the 14 patients who relapsed, the minimum BCR-ABL/ABL ratio achieved was significantly higher (P < 0.0001, 2-sided Mann-Whitney test) than that seen in patients who remained in CR. ▵ = relapse in chronic phase CML, n = 11; ▴ = relapse in blast crisis, n = 3; □ = continuing complete remission, n = 40.
Maximum response on sequential analysis for the 54 CML patients in CR on IFN.
For the 14 patients who relapsed, the minimum BCR-ABL/ABL ratio achieved was significantly higher (P < 0.0001, 2-sided Mann-Whitney test) than that seen in patients who remained in CR. ▵ = relapse in chronic phase CML, n = 11; ▴ = relapse in blast crisis, n = 3; □ = continuing complete remission, n = 40.
Of the 14 patients who relapsed, 13 had persistently high levels of MRD (> 0.045% BCR-ABL/ABL, the median lowest level of MRD for patients in CR) on sequential analysis while still in CR. Of the 27 patients who failed to achieve BCR-ABL/ABL ratios of < 0.045%, 13 (48.1%) subsequently relapsed. In contrast, of the 27 patients who did achieve BCR-ABL/ABL ratios of < 0.045%, only 1 patient subsequently relapsed (3.7%; P =.0003). This patient, referred to above, ceased IFN therapy 2.5 years before relapse.
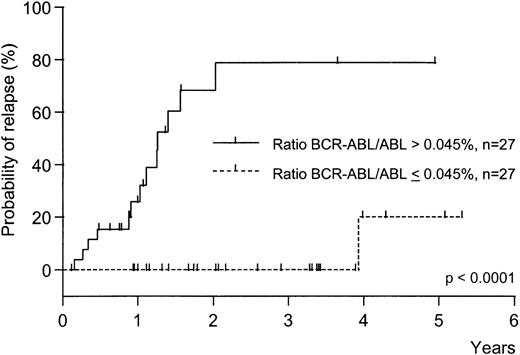
Relapse-free survival was significantly different between patients with residual BCR-ABL/ABL ratios ≤ 0.045% as compared to patients with ratios > 0.045% (P < .0001 2-sided log-rank test, Figure2). We found no association between the rate of relapse and the prognostic risk of individual patients at diagnosis: for the Sokal score, 5 of 27 low-risk versus 8 of 24 intermediate/high-risk patients relapsed (P = .34; not significant, Fisher's exact test); for the IFN-score, 6 of 27 low-risk versus 7 of 22 intermediate/high-risk patients relapsed (P = .52; not significant, Fisher's exact test).
Relapse-free survival from first PCR analysis of patients in CR compared to maximum response to IFN therapy.
One of 27 patients with low levels of residual disease (≤ 0.045% BCR-ABL/ABL) relapsed compared to13 of 27 patients with relatively high levels (> 0.045%, P < .0001, 2-sided logrank test).
Relapse-free survival from first PCR analysis of patients in CR compared to maximum response to IFN therapy.
One of 27 patients with low levels of residual disease (≤ 0.045% BCR-ABL/ABL) relapsed compared to13 of 27 patients with relatively high levels (> 0.045%, P < .0001, 2-sided logrank test).
Discussion
Cytogenetic analysis is the standard method to ascertain the quality of remission in patients with CML treated with IFN, but this technique has a maximum sensitivity of only 10−2. Because patients with leukemia at presentation usually have a total burden of more than 1012 malignant cells,25 individuals in CR may harbor as few as zero or as many as 1010 leukemia cells.26 Conventional RT-PCR for BCR-ABL is routinely able to detect residual disease down to a level of a single cell in a background of 105 to106 normal cells and therefore is up to 4 orders of magnitude more sensitive than cytogenetic analysis. Although rare BCR-ABL-positive cells (1/107-108) have been detected in a significant proportion of normal healthy adults,27,28 the finding of BCR-ABL-positive myeloid progenitors in CML patients in CR strongly supports the conclusion that the routine assay is detecting cells derived from the leukemic clone.29
The finding of long-lasting cytogenetic remission in some CML patients treated with IFN raises the question of whether this agent actually cures the disease. Using standardized methods for blood processing, RNA extraction, cDNA synthesis, and nested RT-PCR, we found evidence of residual disease in all of 54 patients. Discordant findings in the literature concerning the ability of qualitative PCR to detect residual disease in CML patients in CR on IFN are probably attributable to the sensitivity with which BCR-ABL transcripts have been detected in different studies and samples. Quantification of BCR-ABL transcripts by competitive PCR revealed levels of residual disease in CR that spanned more than 4 orders of magnitude. Analyzing all samples investigated in non-relapsing patients continuing IFN therapy after CR revealed that BCR-ABL levels declined over time, consistent with the findings of Kurzrock et al.18 This suggests an ongoing process of quantitative disease depletion by IFN treatment. However, in our study, all 10 patients with follow-up of 5 years or more in CR were still BCR-ABL positive. It is possible therefore that the level of residual disease declines slowly but eventually reaches a plateau. This is consistent with the hypothesis that in some cases residual leukemia cells have no capacity to regenerate clinically significant leukemia, a situation tantamount to “operational” cure.
In some patients, however, cytogenetic or hematologic relapse occurs after CR. We have found a significant difference in the risk of relapse in patients with relatively high BCR-ABL transcript levels as compared to patients with low levels. In particular, relapse was rare in patients with BCR-ABL/ABL ratios of ≤ 0.045%. As compared to the total population in randomized studies,21 complete responders represent a relatively high proportion of low-risk patients according to the Sokal score20 (53% versus 35%) or the interferon score21 (55% versus 41%). However, we have found that the risk of relapse after reaching CR does not depend on the prognostic risk at diagnosis, but on the residual BCR-ABL levels in CR. Furthermore, we found that only a minority of patients (1 of 6) who have achieved low levels of MRD subsequently relapsed following withdrawal of treatment (patient B, Figure3). These findings suggest that it is advisable to continue IFN treatment until at least relatively low levels of BCR-ABL are achieved. If, for whatever reason, IFN is discontinued, rising BCR-ABL levels in CR could be used as an indicator to reinitiate treatment.
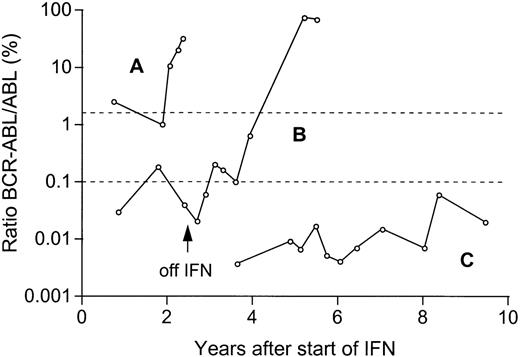
Molecular monitoring in 3 representative patients in CR on IFN therapy.
In patient A, a transient complete cytogenetic response was followed by a hematologic, cytogenetic, and molecular relapse. In patient B, relapse occurred after withdrawal of IFN because of adverse effects. Patient C is in stable complete cytogenetic remission with very low ratios BCR-ABL/ABL 10.4 years after initiation of IFN therapy. The upper dotted line indicates the levels of 2% BCR-ABL/ABL at which cytogenetic relapse is expected,9 and the lower dotted line (0.1% BCR-ABL/ABL) the median ratio of all samples of patients investigated in CR.
Molecular monitoring in 3 representative patients in CR on IFN therapy.
In patient A, a transient complete cytogenetic response was followed by a hematologic, cytogenetic, and molecular relapse. In patient B, relapse occurred after withdrawal of IFN because of adverse effects. Patient C is in stable complete cytogenetic remission with very low ratios BCR-ABL/ABL 10.4 years after initiation of IFN therapy. The upper dotted line indicates the levels of 2% BCR-ABL/ABL at which cytogenetic relapse is expected,9 and the lower dotted line (0.1% BCR-ABL/ABL) the median ratio of all samples of patients investigated in CR.
Acknowledgments
We wish to thank all hematologists and cytogeneticists in Germany, Great Britain, Switzerland, and Austria who provided samples and data. Cooperating institutions include the following:
Germany: Hämatologische Gemeinschaftspraxis Aachen, U. Essers; Hämatologische Praxis Aachen, L. Habets; Gemeinschaftspraxis Bad Oeynhausen, P. Harms; Humboldt-Universität-Charité, Berlin, K. Possinger; Zentralkrankenhaus St. Jürgen-Strasse, Bremen, C. R. Meier; Medizinische Klinik und Poliklinik, Universität Düsseldorf, C. Aul; St. Johannes-Hospital, Duisburg, M. Westerhausen; Nordwest-Krankenhaus Frankfurt, A. Knuth; Allg. Krankenhaus St. Georg, Hamburg, R. Kuse; Zytogenetisches Labor, Med. Klinik, Universität Hamburg, D. K. Hossfeld; Medizinische Klinik und Poliklinik V, Universität Heidelberg, M. Bentz; Kreiskrankenhaus Herford, U. Schmitz-Huebner; Städt. Klinik Kassel, W. D. Hirschmann; Caritas-Krankenhaus Lebach, D. Hufnagl; III. Medizinische Klinik Mannheim, Universität Heidelberg, R. Hehlmann; Gemeinschaftspraxis München, M. Fromm; Städt. Klinikum Nürnberg, C. Falge, W. Brockhaus; Diakonie-Krankenhaus, Schwäbisch-Hall, H. H. Heißmeyer; Praxis Tübingen, M. Haen; Bundeswehrkrankenhaus Ulm, A. P. Schoengen; Zytogenetisches Labor, Universität Ulm, B. Heinze; Kreiskrankenhaus Waldbröl, L. Labedzki; Med. Poliklinik, Universität Würzburg, M. Wilhelm.
United Kingdom: Aberdeen Royal Infirmary, A. A. Dawson; Gwynedd Hospitals, Bangor, R. Williams; St. Helier Hospital, Carshalton, K. Wilson; Western General Hospital, Edinburgh, P. C. A. Shepherd, N. C. Allan; Epsom General Hospital, L. Jones; Farnborough Hospital, A. K. Lakhani; Frimley Park Hospital, J. van de Pette; St. Luke's Hospital, Guildford, G. Robbins; Huddersfield Royal Infirmary, C. Carter; Walton Hospital, Liverpool, P. A. Stevenson; St. John's Hospital Howden, Livingston, M. K. Cook; Hammersmith Hospital, London, J. M. Goldman, A. Chase, S. Coulthard; Royal Marsden Hospital, London, D. Catovsky; University College London, A. H. Goldstone; St. Bartholomews Hospital, London, J. S. Lilleyman; Norfolk & Norwich Hospital, G. E. Turner; Queen's Medical Centre, Nottingham, J. Davies; Poole General Hospital, A. Worsley; Northwick Park Hospital, Watford, C. D. L. Reid.
Switzerland: Kantonsspital Basel, A. Tichelli; Inselspital, Bern, A. Tobler; Hämatologische Praxis, Breitenbach, J. Haberthür.
Austria: Universität Graz, Abt. Hämatologie, H. Sill.
We are grateful to Prof Dr J. Hasford and Dipl-Stat M Pfirrmann, Institut für Medizinische Informationsverarbeitung, Biometrie und Epidemiologie der Universität München, Germany, for their valuable statistical advice.
Supported by the Leukaemia Research Fund of Great Britain, the Dr. Mildred Scheel Stiftung, the Deutsche José-Carreras-Leukämiestiftung e.V., and the Forschungsfonds der Fakultät für Klinische Medizin Mannheim der Universität Heidelberg, Germany.
Reprints:Andreas Hochhaus, III. Medizinische Universitätsklinik, Fakultät für Klinische Medizin, Mannheim der Universität Heidelberg, Wiesbadener Strasse 7-11, 68305 Mannheim, Germany; e-mail:hochhaus@rumms.unimannheim.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.