Abstract
Human T cells are transformed in vitro to stable growth after infection with herpesvirus saimiri subgroup C strain C488, and they retain their antigen-specific reactivity and other important functional features of mature activated T lymphocytes. The virus persists as nonintegrating episomes in human T cells under restricted viral gene expression and without production of virus particles. This study analyzes the behavior of herpesvirus-transformed autologous T cells after reinfusion into the donor under close-to-human experimental conditions. T cells of 5 macaque monkeys were transformed to stable interleukin-2 dependent growth and were intravenously infused into the respective donor. The animals remained healthy, without occurrence of lymphoma or leukemia for an observation period of more than 1 year. Over several months virus genomes were detectable in peripheral blood cells and in cultured T cells by polymerase chain reaction. In naive control animals, a high-dose intravenous infection rapidly induced pleomorphic peripheral T-cell lymphoma. In contrast, monkeys were protected from lymphoma after challenge infection if they had previously received autologous T-cell transfusions. High levels of antibodies against virus antigens were detectable after challenge infection only. Taken together, herpesvirus-transformed T cells are well tolerated after autologous reinfusion. This may allow us to develop a novel concept for adoptive T-cell mediated immunotherapy.
Herpesvirus saimiri is a γ2-herpesvirus of the squirrel monkey (Saimiri sciureus), which is the naturally infected host and does not show signs of disease related to this virus. However, when herpesvirus saimiri is experimentally transferred to other New World primate species, such as the cottontop tamarin (Saguinus oedipus) or common marmoset (Callithrix jacchus), the animals develop acute T-cell lymphoma or leukemia within a few weeks after infection. This disease is associated with massive virus replication. Herpesvirus saimiri can easily be recovered by cocultivation of peripheral blood mononuclear cells (PBMCs) from leukemic monkeys with permissive owl-monkey kidney cells.1-3
Herpesvirus saimiri subgroup C strains, especially strain C488, are capable of transforming human T cells to a stable interleukin-2–dependent (IL-2–dependent) growth in culture, which is largely independent of their T-cell subtype.4 The virus genome persists as nonintegrating episomes under restricted virus gene expression. The transformed human T cells are not permissive to the virus, and it has not been possible to induce reactivation of virus replication.4-6 The transformed T cells show the phenotype of mature activated T lymphocytes including expression of CD4 or CD8 and activation markers. Most importantly, the antigen-specific reactivity of parental nontransformed T cells is retained after transformation. After stimulation, herpesvirus saimiri–transformed T cells are cytotoxic and secrete T-helper-1–type cytokines such as IL-2, interferon-γ (IFN-γ), granulocyte macrophage-colony stimulating factor (GM-CSF), and tumor necrosis factor-α (TNF-α).7 Thus, herpesvirus saimiri provides a convenient means to amplify functional human T lymphocytes to large cell numbers in culture.
In theory, this method for in vitro expansion of T cells could be a useful tool for adoptive immunotherapy. However, it has been unclear as to which consequences this type of viral transformation would lead to under in vivo conditions. The observed growth transformation could be a mild phenotype that is only observed in culture. Alternatively, it could be a strong type of oncogenic transformation leading to tumorigenesis in vivo. Therefore, the behavior of transformed autologous T cells after retransfusion into the donor remained to be determined. New World primates, such as S oedipus, are not suitable for this purpose because transformed marmoset T cells express all viral genes tested and produce considerable amounts of virus particles. We addressed this question in macaque monkeys (Macaca mulatta and Macaca fascicularis) because herpesvirus saimiri–transformed T cells from macaques closely resemble their human counterparts.8-10 Moreover, macaques are the closest relatives to humans that are available for animal experiments.
Herpesvirus saimiri–transformed autologous macaque T cells were retransfused into the donor animals. The transfused animals did not develop signs of disease, although virus DNA could be demonstrated in PBMCs by polymerase chain reaction (PCR) over several months. After autologous transfusion, the animals were protected from acute disease caused by a herpesvirus saimiri challenge infection. In summary, transformed autologous T cells did not induce leukemia or lymphoma and were clinically well tolerated in the recipients.
Materials and methods
Virus and cell culture
The propagation of herpesvirus saimiri strain C488, as well as the transformation and cultivation of transformed T cells, was done according to published protocols.7,10 To enhance the sensitivity of herpesvirus saimiri isolation experiments, potentially existing virus particles were sedimented from large volumes (at least 35 mL) of T-cell culture supernatants. The concentrates were tested on owl-monkey kidney cells for the induction of cytopathic changes. Virus isolation tests from monkey blood were performed on fresh owl-monkey kidney cell layers (25 cm2) with either 0.5 mL plasma or 106 PBMCs.7 To circumvent the problem of foamy virus reactivation,9 the fresh monkey T cells were cloned by limiting dilution in microtiter wells in the presence of 105 irradiated human feeder PBMCs (120 Gy) and 5 μg/mL phytohemagglutinin (PHA) (Murex/Diagnostica, Burguedel, Germany). The primary T-cell lines were further amplified by restimulation with irradiated human feeder cells and mitogen, followed by the addition of 20 U/mL recombinant IL-2 (Roche Biochemicals, Mannheim, Germany) after 24 hours. The cell lines were finally used for the standard transformation procedure in the presence of IL-2.7
Animal experiments
The animal experiments were performed at the German Primate Center, Göttingen, Germany. Based on an approval according to the German animal protection regulations, 5 healthy macaque monkeys (2 M mulatta and 3 M fascicularis) were transfused twice with 80 × 106 washed transformed autologous T cells per kg of body weight (Table 1). Under short-term anesthesia, blood samples were taken at increasing intervals (Figure 1). PBMCs were prepared by Ficoll density gradients (Biochrom, Berlin, Germany) and subjected to DNA-PCR, virus isolation, and long-term T-cell culture. After 1 year of observation, 4 animals and 2 naive control monkeys were intravenously infected by herpesvirus saimiri C488 with 106plaque-forming units in 1 mL. After necropsy, detailed histological analysis was performed. Sections of formalin-fixed, paraffin-embedded tissue were stained with hematoxylin/eosin and subjected to immunohistochemistry by using a streptavidin-biotin–complex peroxidase detection system (StreptABComplex/AP; Dako, Glostrup, Denmark). Immunostaining of deparaffinized sections was performed with a mouse antihuman CD20 monoclonal antibody (mAb) (L26, Dako) or with an affinity-purified rabbit antihuman CD3 mAb (M756, Dako), which proved to react with lymphocytes of rhesus and cynomolgus monkeys.
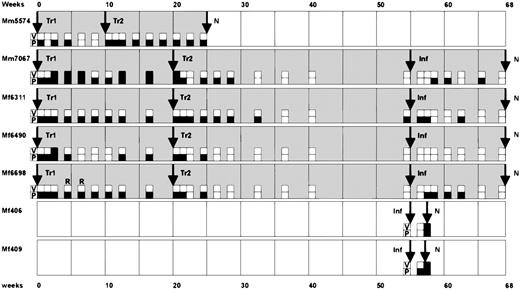
Time course of the animal experiments.
In a pilot experiment, the animal Mm5574 received 2 transfusions (Tr1 and Tr2) of autologous T cells at a 10-week interval. At necropsy (N) in week 25, pathological changes were not observed. Subsequently, the animals Mm7067, Mf6311, Mf6490, and Mf6698 received a first autologous transfusion (Tr1) and then a second transfusion (Tr2) after 20 weeks. At week 55, these animals and 2 naive control monkeys (Mf406 and Mf409) were subjected to intravenous challenge infection (Inf). Whereas the control animals died within 2 weeks from peripheral T-cell lymphoma, the other animals survived without pathological changes at necropsy at week 68. In 2 cases, the transformed T cells were successfully recultivated from peripheral blood, as indicated by (R). The results of virus isolation assays (V) and DNA-PCR (P) from peripheral blood leukocytes are summarized as follows: open squares indicate negative results and filled squares represent positive results for virus isolation (V) or PCR detection (P), respectively. While infectious virus was rarely isolated from peripheral blood, virus DNA was detectable in peripheral blood cells for long periods.
Time course of the animal experiments.
In a pilot experiment, the animal Mm5574 received 2 transfusions (Tr1 and Tr2) of autologous T cells at a 10-week interval. At necropsy (N) in week 25, pathological changes were not observed. Subsequently, the animals Mm7067, Mf6311, Mf6490, and Mf6698 received a first autologous transfusion (Tr1) and then a second transfusion (Tr2) after 20 weeks. At week 55, these animals and 2 naive control monkeys (Mf406 and Mf409) were subjected to intravenous challenge infection (Inf). Whereas the control animals died within 2 weeks from peripheral T-cell lymphoma, the other animals survived without pathological changes at necropsy at week 68. In 2 cases, the transformed T cells were successfully recultivated from peripheral blood, as indicated by (R). The results of virus isolation assays (V) and DNA-PCR (P) from peripheral blood leukocytes are summarized as follows: open squares indicate negative results and filled squares represent positive results for virus isolation (V) or PCR detection (P), respectively. While infectious virus was rarely isolated from peripheral blood, virus DNA was detectable in peripheral blood cells for long periods.
DNA and protein methods
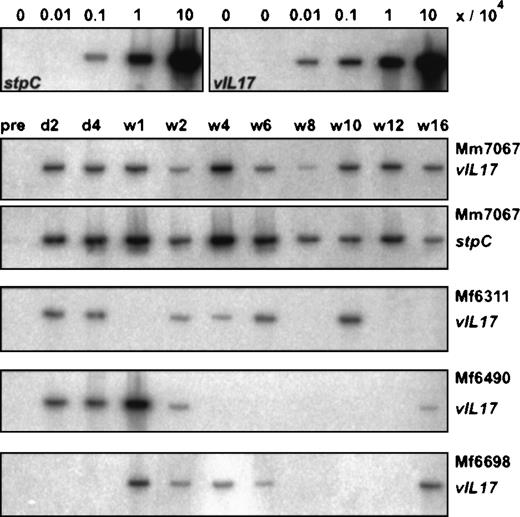
Virus DNA from PBMCs or cultivated T cells was analyzed by PCR. Primer pairs specific for the virus genes stpC andvIL-17 (gene 13) were applied.6 The PCR products were separated on 1.5% agarose gels, transferred onto nylon membranes, and hybridized with phosphorus 32 (32P) radiolabeled probes for stpC or vIL-17 genes. PCR for the cellular β-globin gene served as an internal control. The semiquantitative PCR assays for the virus genes stpC and vIL-17 detected low copy numbers of viral genomes, corresponding to 10 (stpC) or 1 (vIL-17) genome equivalents. This is based on previous estimations that 1 transformed T cell contained up to 100 episomes.4 Increasing numbers of transformed human T cells were mixed with nontransformed T cells as a control for PCR validation. At a mix ratio of 1 in 106 cells, a single virus genome would then be present in the reaction if 1% of the lysate of 106 cells were used for PCR. The signal intensities from fresh PBMCs after autologous transfusion correlated to about 1 transformed T cell in 104 to 106 cells. This semiquantitative estimation was supported by the observation that signals corresponding to about 1 herpesvirus saimiri genome copy per reaction were positive only in a fraction of repetitive tests. In such cases, the PBMC samples were classified positive if at least 50% of the attempts yielded specific PCR products.
Viral nonintegrated episomal DNA was demonstrated by Gardella in situ lysis gel electrophoresis and subsequent Southern blot hybridization with an stpC specific probe.7 The surface phenotype of macaque T cells was analyzed by standard flow cytometry (FACStrak; Becton Dickinson, Heidelberg, Germany). Murine mAbs against the following human antigens were used for flow cytometry (names of hybridoma clones in parentheses): CD2 (clone TS1/18.1.1), CD4 (SK3), CD8 (SK1), CD18/LFA1β (TS1/18.1.2.11.4), CD25 (2A3), CD56 (My31), CD58/LFA3 (TS2/9.1.1.4.3), CD69 (L78), MHC-I (W6/32), and MHC-II/HLA-DR (L243) (Becton Dickinson and American Type Culture Collection, Manassas, Virginia). The rhesus CD3-specific mAb FN1811 was applied together with a secondary antibody against mouse immunoglobulins (Dianova, Hamburg, Germany) in indirect staining reactions. Antibody sets for the human cytokines IFN-γ, TNF-α, and IL-6 (Genzyme, Rüsselsheim, Germany) were applied in enzyme-linked immunosorbent assays (ELISAs). Control tests with supernatants of phorbolester-stimulated transformed T cells from humans and macaques showed that these antibodies were cross-reactive with the respective macaque cytokines (data not shown).
Antiviral immune response
ELISAs were performed with 1:200 diluted plasma samples collected at various time points during the experiment. Gradient-purified virus particles7 (400 ng protein per well) were coated onto 96-well plates (Maxisorp; Nunc, Roskilde, Denmark). Unspecific reactions were blocked with rabbit serum. A peroxidase-conjugated secondary antihuman antibody (Dianova) was applied for the ELISA detection reaction, which was monitored as an optical density at 450 nm. Western blot analyses used antigen derived from cultivated transformed T cells or from gradient-purified virion particles.7 The proteins were separated under reducing conditions on sodium dodecyl sulfate/polyacrylamide (10%) gels, transferred onto nitrocellulose membranes, and tested with 1:200 diluted plasma samples. A peroxidase-labeled secondary antihuman antibody (Dianova) was used for detection.
T-cell proliferation tests were carried out with fresh PBMCs during the time course experiment after the autologous transfusions. For this purpose, 105 PBMCs per well were cultivated in 100 μL complete culture medium with 1% human AB serum and without exogenous IL-2. The cultures were either left untreated or supplemented with 10 μg/mL PHA or inactivated purified virion particles at 1 μg/mL final concentration. On day 6, 0.0185 MBq (0.5 μCi) tritium-labeled thymidine (Amersham Pharmacia Biotech) in a volume of 10 μL was added to each well. On day 7, the microcultures were harvested onto glass-fiber filters and measured for tritium activity in a β-counter. Control PBMCs were reactive to mitogen treatment.
Results
T-cell transformation and autologous reinfusion in macaque monkeys
Herpesvirus saimiri–transformed T-cell lines were generated from 5 macaques (Table 1): 2 rhesus monkeys (M mulatta; Mm5574 and Mm7067), and 3 cynomolgus monkeys (M fascicularis; Mf6311, Mf6490, and Mf6698). Direct transformation of freshly isolated peripheral blood T cells from macaques was rarely successful (eg, animal Mm5574) because T-cell experiments in macaques are hampered by the highly prevalent foamy virus infection at primate centers. In most cases, T-cell cloning by limiting dilution using mitogenic activation in the presence of irradiated human feeder cells was required to generate foamy virus-free T-cell lines. The transformed T cells carried high copy numbers of viral nonintegrated episomes, whereas linear DNA molecules typical for virion particles were not detectable by Southern blot analyses of Gardella gels. In contrast to their human counterparts, most of the transformed macaque T-cell lines from this study did release small amounts of virus particles (Table 1). The surface phenotype of these T cells was very similar to that of transformed human T cells including expression of CD2, CD3, CD18/LFA1β, CD25, CD56, CD58/LFA3, CD69, MHC-I, and MHC-II. Frequently, CD4 and CD8 were coexpressed on cultivated parental and transformed macaque T cells (Table 1).
When stable IL-2–dependent T-cell lines had been established for several months, autologous transformed T cells (80 × 106/kg body weight) were washed in saline and intravenously infused into the donor animal (Table 1). After 10-20 weeks, a second infusion with identical cell numbers was performed. Animal Mm5574 was treated prior to the others in a shorter time course protocol in order to obtain first information from a pilot experiment. Six months after the first transfusion, animal Mm5574 was subjected to necropsy without pathological findings. The other animals were frequently monitored for more than 1 year (Figure 1). There were neither signs of pathological disorders nor hints of tumor development.
Behavior of transformed T cells after autologous transfusion
During several months, blood samples were taken at weekly, biweekly, or monthly intervals (Figure 1). Sensitive virus isolation experiments were positive only in rare cases (PBMCs) but never in plasma samples (Figure 1). The persistence of viral genomes was demonstrated by semiquantitative DNA-PCR. Viral genomes were detected over months in PBMCs and in cultivated T cells (Figure 1, Figure 2). The signal intensities from fresh PBMCs correlated to about 1 transformed T cell in 104to 106 cells. The PCR results from PBMCs were confirmed by testing T-cell cultures originating from the same blood samples. Using long-term T-cell culture, herpesvirus saimiri–transformed T cells were recultivated from the peripheral blood of animal Mf6698 in some cases (Figure 1).
Virus DNA in PBMCs after autologous transfusion.
Semiquantitative DNA-PCR was performed from fresh PBMCs. DNA from cell mixtures was used as control. For this purpose, herpesvirus saimiri–transformed human T cells were mixed with PHA/IL-2–stimulated human T cells at ratios of 0/106, 1/106, 10/106, 102/106, and 103/106. The time points of the PBMC preparations after the first autologous transfusion are marked for day 2 (d2), day 4 (d4), and week 1 (w1) to week 16 (w16). PCR for 2 different virus genes, stpC and vIL-17, is shown for monkey Mm7067.
Virus DNA in PBMCs after autologous transfusion.
Semiquantitative DNA-PCR was performed from fresh PBMCs. DNA from cell mixtures was used as control. For this purpose, herpesvirus saimiri–transformed human T cells were mixed with PHA/IL-2–stimulated human T cells at ratios of 0/106, 1/106, 10/106, 102/106, and 103/106. The time points of the PBMC preparations after the first autologous transfusion are marked for day 2 (d2), day 4 (d4), and week 1 (w1) to week 16 (w16). PCR for 2 different virus genes, stpC and vIL-17, is shown for monkey Mm7067.
Herpesvirus saimiri challenge infection
One year after the first autologous reinfusion, 4 of these animals (Mm7067, Mf6311, Mf6490, and Mf6698) and 2 naive control cynomolgus monkeys (Mf406 and Mf409) were subjected in parallel to a high-dose intravenous challenge infection with herpesvirus saimiri C488 (106 tissue culture infectious doses in 1 mL). The 4 animals that had previously received autologous infusions did not develop the disease. It was not possible to reisolate the virus from PBMCs. At necropsy 2 months later, no pathological findings were made. Using PCR with DNA from necropsy materials, only low amounts of virus DNA were detected, similar to the levels observed in PBMCs during the time course experiment after autologous transfusion.
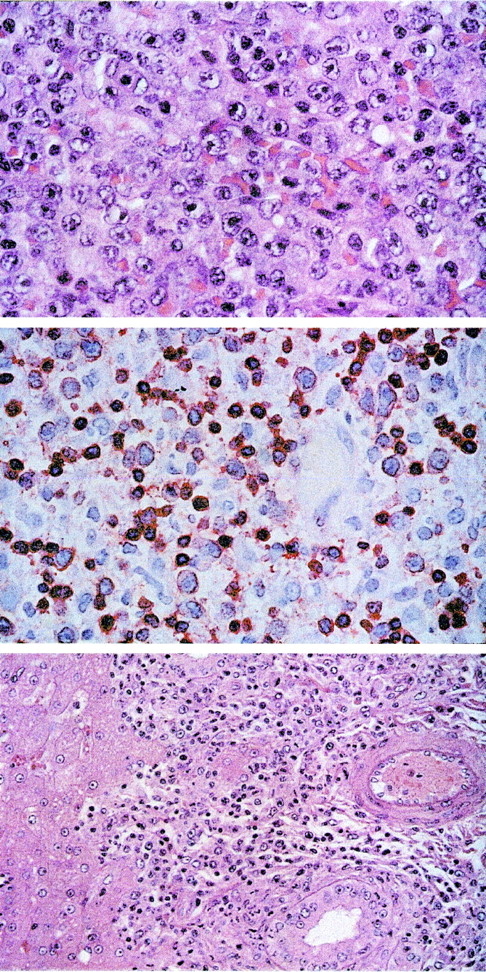
In contrast, the naive control animals died from lymphoma at day 13 or 14 after high-dose infection. Lymphoma cell lines were established ex vivo from both animals, and the virus was easily reisolated from PBMCs. Foamy virus reactivation terminated the cultures from 1 animal after several weeks. T cell lines in the other animal were phenotypically indistinguishable from in vitro transformed T cells. Necropsy samples of lymph nodes, thymus, spleen, bone marrow, kidney, and brain of these animals contained large amounts of virus DNA (data not shown). Whereas IFN-γ, IL-6, and TNF-α were not detectable in plasma samples after autologous transfusions, IFN-γ (up to 225.1 pg/mL) and IL-6 (up to 10.2 pg/mL) were increased in plasma samples from the terminally ill animals Mf406 and Mf409. Histopathological and immunohistochemical analysis showed widespread infiltrates of a pleomorphic peripheral T-cell lymphoma consisting of medium-sized and large blasts with vesicular nuclei and prominent nucleoli (Figure3, upper panel). The blasts were positive for the T-cell marker CD3 (Figure 3, central panel) but negative for CD20. The infiltrates of blasts involved the lymph nodes, spleen, Waldeyer's ring, intestine, and pancreas of animal Mf406, and they were detected in lymph nodes, spleen, intestine, kidney, salivary glands, lung, and liver (Figure 3, lower panel) of animal Mf409. The lymph nodes were enlarged in both animals and showed a widening of sinuses and T zones by the infiltrating blasts as well as a loss of follicular structure.
T-cell lymphoma after intravenous infection with herpesvirus saimiri C488 of naive cynomolgus monkeys.
The tissue sections were stained with hematoxilin/eosin. Upper panel: Lymph node showing peripheral pleomorphic T-cell lymphoma with medium-sized and large blasts containing vesicular nuclei and prominent nucleoli (original magnification ×100). Central panel: Immunostaining of the medium-sized and large blasts and some accompanying small lymphocytes for CD3 (original magnification ×63). Lower panel: Infiltration of the portal tracts of the liver by the blast cells (original magnification ×40).
T-cell lymphoma after intravenous infection with herpesvirus saimiri C488 of naive cynomolgus monkeys.
The tissue sections were stained with hematoxilin/eosin. Upper panel: Lymph node showing peripheral pleomorphic T-cell lymphoma with medium-sized and large blasts containing vesicular nuclei and prominent nucleoli (original magnification ×100). Central panel: Immunostaining of the medium-sized and large blasts and some accompanying small lymphocytes for CD3 (original magnification ×63). Lower panel: Infiltration of the portal tracts of the liver by the blast cells (original magnification ×40).
Antiviral immune response
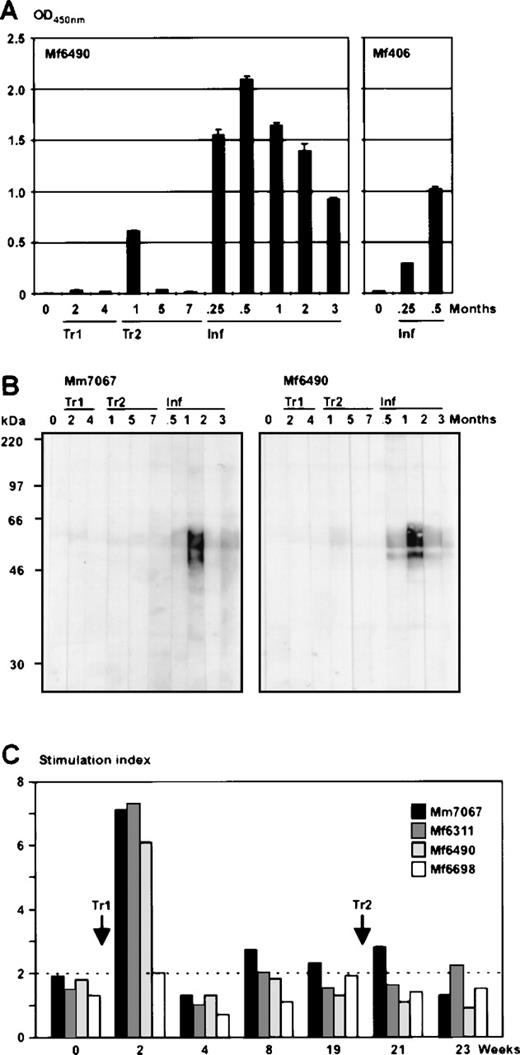
To analyze the humoral immune response, plasma samples were taken following autologous transfusions and challenge infections. The samples were tested using both ELISA with virus particles as antigen and Western blot strips carrying proteins from purified virions or transformed T cells. Transformed T cells as antigen did not reveal signals on Western blots. In contrast, gradient-purified virus particles as antigen caused strong signals in ELISA and Western blots, but this only occurred after the challenge infection (Figure4A, B). The cellular immune response was analyzed in T-cell proliferation assays. Shortly after the first transfusion (2 weeks), in 3 of the 4 animals tested, an increased proliferation was observed when inactivated purified virion particles were added as antigen (Figure 4C). During the further time course of the transfusions, this reactivity was no longer observed, which may indicate that only small amounts of virus antigens had been exposed for a short period after the transfusion.
Antiviral immune response after autologous transfusions and challenge infection.
(A) Seroreactivity of the animals was analyzed by ELISA using purified virus particles as antigen and plasma dilutions of 1:200. Preimmune sera (indicated at time point 0) did not show relevant background reactivity. As examples, the time courses for the animals Mf6490 and Mf406 are shown. The sera after autologous transfusion (Tr1 and Tr2) were either negative or produced weak signals. In animal Mf6490, an unstable antibody response was observed shortly after the second transfusion. After challenge infection (Inf), the protected animals rapidly developed strong seroreactivity. (B) To confirm the ELISA results, Western blot strips analyses carrying virion proteins were stained with 1:200 diluted plasma samples from monkeys Mm7067 and Mf6490 after autologous T-cell transfusion (Tr1 and Tr2) or challenge infection (Inf), respectively. The time points of plasma preparations are given in months after transfusion or infection. The animals Mf6311 and Mf6698 had similar levels of reactive antibodies at equivalent time points. (C) T-cell proliferation tests were performed during the time course of the autologous transfusions. Two weeks after the first transfusion (Tr1), T cells from the blood of 3 out of 4 animals showed proliferative reactivity against inactivated virus particles as an antigen, as demonstrated by the stimulation index of tritium thymidine incorporation assays.
Antiviral immune response after autologous transfusions and challenge infection.
(A) Seroreactivity of the animals was analyzed by ELISA using purified virus particles as antigen and plasma dilutions of 1:200. Preimmune sera (indicated at time point 0) did not show relevant background reactivity. As examples, the time courses for the animals Mf6490 and Mf406 are shown. The sera after autologous transfusion (Tr1 and Tr2) were either negative or produced weak signals. In animal Mf6490, an unstable antibody response was observed shortly after the second transfusion. After challenge infection (Inf), the protected animals rapidly developed strong seroreactivity. (B) To confirm the ELISA results, Western blot strips analyses carrying virion proteins were stained with 1:200 diluted plasma samples from monkeys Mm7067 and Mf6490 after autologous T-cell transfusion (Tr1 and Tr2) or challenge infection (Inf), respectively. The time points of plasma preparations are given in months after transfusion or infection. The animals Mf6311 and Mf6698 had similar levels of reactive antibodies at equivalent time points. (C) T-cell proliferation tests were performed during the time course of the autologous transfusions. Two weeks after the first transfusion (Tr1), T cells from the blood of 3 out of 4 animals showed proliferative reactivity against inactivated virus particles as an antigen, as demonstrated by the stimulation index of tritium thymidine incorporation assays.
Discussion
The adoptive transfer of human T lymphocytes is limited by the laborious procedures to grow large numbers of T cells. Herpesvirus saimiri offers a convenient way to amplify human T cells. Easily amplifiable antigen-specific T cells, eg, directed against tumor-specific epitopes, would form an interesting tool for adoptive cancer immunotherapy. Alternatively, the antigen specificity of the transformed T cells could be modified by using the transforming virus simultaneously as a gene expression vector. However, the biological safety of such transformed T cells has been unclear. In this report we show that transfusions with autologous herpesvirus saimiri–transformed T cells are well accepted and even provide protection against acute lethal leukemogenesis after experimental intravenous infection.
In this study, 80 × 106 autologous T cells per kg of body weight were applied during transfusion, which resulted in total cell numbers of 130-600 × 106 (Table 1). This is approximately the same order of magnitude as that published previously for retrovirally transduced rhesus monkey T cells, where 54-140 × 106 cells per kg were transferred.12 In human studies, there is little experience in the autologous situation. Early gene marking experiments with autologous melanoma-infiltrating T cells used absolute cell numbers between 0.2 and 43 × 106.10,13,14 Under allogeneic conditions, 0.5-38.6 × 106 cells per kg of retrovirally transduced T cells from bone marrow donors were transfused.15 In other studies, dose increments of antigen-specific donor T cells, ranging from 33 × 106 to 3.3 × 109 cells per m2, were used.12,16-18Retrovirally transduced human or macaque T cells were detectable for 64 days, 12 months, or even up to 727 days, depending on the study.12,14 15 Thus, our observations of cell numbers and survival of the grafted cells are compatible with published transfusion results of nontransformed T cells.
T cells of different primate species have distinct properties concerning the replication and persistence of herpesvirus saimiri. In contrast to the lack of virion production by herpesvirus saimiri–transformed human T cells, transformed T lymphocytes from marmoset monkeys release easily detectable amounts of virus particles. Moreover, linear virion DNA and a broad transcription pattern of virus genes have been demonstrated in herpesvirus saimiri–transformed marmoset T cells.5 10 Unexpectedly, most transformed macaque T-cell lines tested in this study released small amounts of virus particles in culture when a virus isolation procedure with enhanced sensitivity was applied. Such a release of virions from transformed human T cells has never been demonstrated in a large test series. It is noteworthy that the productive activity of the transformed macaque T cells was very low because lytical linear DNA forms had not been detected. In addition, Western blots with virion proteins remained negative after T-cell transfusion, whereas a strong antibody response developed only after challenge infection.
Obviously, there are differences between human and macaque monkeys regarding the control of virus replication in transformed T cells. However, this low-level replication does not seem to be biologically critical, as neither disease nor humoral reactivity developed after autologous transfusion. The observed minimal virus replication in macaque cells is relevant for the safety discussion of potential applications in human therapy: Even if the low-level replication is not critical for pathogenicity and even if this has not been observed for human T cells, any unwanted side effects due to virus replication have to be excluded for potential applications in human patients. This could be achieved by packaging cell-dependent, replication-deficient virus variants. Although rhesus or cynomolgus monkeys show differences to humans with the respect of the virus persistence and replication, these animals provide the only available and valuable close-to-human experimental possibility to test the basic applicability of herpesvirus saimiri–transformed T cells for therapeutic purpose.
The intravenous infection of naive macaques with herpesvirus saimiri C488 at high doses induced peripheral pleomorphic T-cell lymphomas. Based on the histopathology, distribution, and acute onset, the infiltrates could also be designated as a pleomorphic T lymphoproliferative disorder. In fact, the disease shows similarities to human Epstein Barr virus–induced (EBV-induced) posttransplantation B-lymphoproliferative disorders, which can be polyclonal, oligoclonal, or monoclonal. Clonality was not investigated in our cases, but the acute onset of disease favors a polyclonal proliferation. The observed T-cell lymphoma is compatible with a previous brief report in which the infection of 1 rhesus monkey with herpesvirus saimiri C488 caused an unspecified acute lymphoproliferative disease and allowed the establishment of a transformed ex vivo T-cell line.19
In our study, a previous transfusion with autologous transformed T cells protected the animals from leukemic disease caused by high-dose challenge infection. Whereas the naive animals died within 2 weeks from lymphoma, the monkeys with previous autologous transfusions remained healthy and controlled the systemic infection. Two months after infection, virus DNA was not anymore detectable in PBMCs, and histopathological changes could not be found at necropsy. Either latently or lytically expressed virus antigens could mediate this protection. Herpesvirus saimiri–transformed T cells of both humans and macaques show a restricted range of virus gene expression. Only few latently expressed viral proteins would be likely candidates for targets of cellular and humoral immune reactivity: StpC, Tip, and IE14/vSag.5 6 By testing monkey sera against antigens of transformed T cells on Western blots, these proteins were largely ruled out as either lytically or persistently expressed antigens that induce humoral immune response.
After intravenous infection, Western blots with virion antigen revealed signals corresponding to a protein of approximately 60 kd (Figure 4B). This protein appears to be glycosylated, as the diffuse band shape may suggest. It is a virion protein that is expressed upon lytic virus replication. Most likely, the observed protection from leukemogenesis can be explained by the generation of T cells reactive against antigens which are produced during lytic replication. T-cell proliferation tests with inactivated virion particles as antigen revealed a weak reactivity early after autologous transfusions (Figure 4C). This form of immune control may be comparable to the persistence of EBV in humans, in which cells with virus replication are efficiently eliminated by cytotoxic T cells. The observed protective effect may even lead to an enhanced biological safety of herpesvirus saimiri–transformed autologous T cells upon potential therapeutic applications. Because we did not detect humoral or cellular reactivity to transformed T cells, we speculate that nonpermissive transformed autologous T cells are subjected to the normal immunoregulatory mechanisms and not to antiviral immune response.
Autologous herpesvirus saimiri–transformed T cells were tolerated after transfusion without signs of disease. Virus DNA was detected in freshly isolated PBMCs and cultivated T cells for several months after autologous transfusion. In contrast, virus isolations from PBMCs were positive in rare exceptions only. It is most probable that the DNA-PCR signals correspond to the transfused cells or their descendents. However, it cannot be ruled out that a low-level release of virus particles occurs in vivo. This question could be addressed in the future by using retroviral vectors to gene mark the autologous transformed T cells before transfusion. In the case of potential clinical applications, any lytic replication of herpesvirus saimiri vectors must be excluded. Establishing replication-deficient variants and the use of prodrug activation genes for targeted elimination20 will form the next steps for developing herpesvirus saimiri to a T-cell vector for gene therapy. The fact that transformed autologous T cells were well tolerated may allow us to develop a novel concept for adoptive T-cell mediated immunotherapy.
Acknowledgments
The authors thank Dirk Lorenzen and Christiane Stahl-Hennig for stimulating discussions and Gerhard Hunsmann, Franz-Josef Kaup, and Bernhard Fleckenstein for continuous support.
Supported by grants to H.F from the Bayerische Forschungsstiftung, Munich, Germany, and from the Wilhelm Sander-Stiftung, Neustadt/Donau, Germany.
Reprints:Dr Helmut Fickenscher, Institut für Klinische und Molekulare Virologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Schlossgarten 4, D-91054 Erlangen, Germany; e-mail: fickenscher@viro.med.uni-erlangen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.