Abstract
Collagen activates platelets through a tyrosine kinase-dependent pathway, involving phospholipase Cγ2. Functional responses such as aggregation and secretion induced by collagen are potentiated by preincubation with thrombopoietin (TPO). In this study, we show that collagen and thrombopoietin activate the phosphatidylinositol 3-kinase (PI 3-kinase) pathway and that this contributes to their respective actions. The structurally distinct inhibitors of PI 3-kinase, wortmannin, and LY294002, completely inhibit formation of phosphatidylinositol 3,4,5-trisphosphate by collagen. This leads to a substantial reduction in the formation of inositol phosphates and phosphatidic acid, 2 indices of PLC activity, and the consequent inhibition of intracellular Ca++[Ca++]i, aggregation and secretion. Potentiation of the collagen response by TPO is prevented in the presence of wortmannin and LY294002. However, when the 2 PI 3-kinase inhibitors are given after the addition of TPO but before the collagen, recovery of potentiation is observed. This suggests that potentiation is mediated through activation of PI 3-kinase. TPO stimulates aggregation of platelets from a low percentage of donors and this is also blocked by wortmannin. These results suggest that the PI 3-kinase pathway plays an important role in signaling by collagen and in the priming action of TPO.
Thrombopoietin (TPO) was described in 1994 as a growth and developmental factor for the platelet precursor cell, the megakaryocyte.1 The cellular receptor for TPO is c-mpl, a transmembrane receptor with close homology to the erythropoietin receptor. Expression of c-mpl has been found restricted to hematopoietic progenitor cells, megakaryocytes, and platelets. However, recent studies suggest c-mpl expression in neutrophils and endothelial cells.2,3 TPO potentiates (or primes) platelet activation by a variety of stimuli, including thrombin, adenosine diphosphate (ADP), collagen, and adrenaline; whereas it has no effect on its own in the majority of donors.4-9 The most thoroughly characterized signaling cascade from the TPO receptor is the JAK/STAT pathway.5,10 In addition, TPO stimulates tyrosine phosphorylation of a number of proteins in platelets, including Cbl, p85 subunit of phosphatidylinositide 3-kinase (PI 3-kinase), Shc, Vav, and cortactin.11-13 The mechanism of platelet priming by TPO is not established.
Over the last few years, we and others14,15 have shown that the major collagen receptor underlying platelet activation is a multimeric assembly consisting of glycoprotein VI (GPVI) and the Fc receptor γ-chain (FcR γ-chain). Activation by collagen leads to tyrosine phosphorylation of the FcR γ-chain on 2 conserved tyrosines in an immunoreceptor tyosine-based activation motif (ITAM), enabling binding of the tyrosine kinase Syk.14 Activation of Syk in turn leads to tyrosine phosphorylation and activation of a number of signaling proteins, including the adapters LAT and SLP-76, and phospholipase Cγ2 (PLCγ2).16-18 The importance of this pathway in platelet activation by collagen is illustrated by the complete inhibition of secretion and aggregation to collagen in platelets from mice deficient in the FcR γ-chain, Syk, LAT, and SLP-76.16-18 The platelet low-affinity immune receptor FcγRIIA is thought to induce activation through a similar pathway.
Recently, a collagen-related peptide (CRP), based on a glycine-proline-hydroxyproline repeat motif, was reported to stimulate formation of 3-phosphorylated inositol lipids in platelets through the PI 3-kinase pathway.19 CRP is a powerful agonist at the collagen receptor GPVI but does not recognize the integrin α2β1 (GPIa-IIa).20 The structurally distinct inhibitors of the PI 3-kinase pathway, wortmannin, and LY294002, partially inhibit activation of phospholipase C (PLC) and aggregation by CRP, suggesting that this pathway has a critical role in signaling by GPVI. This is supported by the observation that chelation of phosphatidylinositol 3,4,5-trisphosphate (PI3,4,5P3) in megakaryocytes by microinjection of the pleckstrin homology (PH) domain to Bruton's tyrosine kinase (Btk), which binds selectively to this lipid species, inhibits CRP-induced elevation of Ca++. The PI 3-kinase pathway also plays a critical role in platelet activation by FcγRIIA.21 PI3,4,5P3 is believed to be the active component in this pathway because it restores full activation in response to cross-linking of the immune receptor in permeabilized platelets.21
In this study, we have investigated the role of PI 3-kinase in the regulation of platelets by TPO and collagen. Our results provide evidence for an important role for PI 3-kinase in the activation of PLCγ2 by collagen and show that priming of the collagen response by TPO is mediated through a PI 3-kinase–dependent pathway.
Materials and methods
Materials
Recombinant human TPO was from Genzyme Diagnostics (West Malling, Kent, UK). The antiphosphotyrosine monoclonal antibody (mAb), 4G10, and the anti–PI 3-kinase p85 serum were from Upstate Biotechnology Inc (TCS Biologicals, Bucks, UK). The mAb IV.3, which is selective for FcγRIIA, was purchased from Medarex Inc (Annandale, NJ). Anti-PLCγ2 polyclonal antibody (Q-20) was from Santa Cruz (Autogen Bioclear, Wiltshire, UK) and anti-PLCγ2 serum was kindly provided by Dr Y. H. Lee (Pohang University of Science and Technology, Kyung-Buk, South Korea). Collagen fibers, as Horm collagen, a suspension of type I fibers from equine tendon, were obtained from Nycomed (Munich, Germany). Bovine thrombin, wortmannin, phosphatidylinositol, and F(ab′)2 antimouse IgG fragments were from Sigma (Poole, Dorset, UK). LY294002 was purchased from Calbiochem-Novabiochem (Nottingham, UK). Nonidet P-40 (NP40) was from BDH (Poole, Dorset, UK). Horseradish peroxidase–conjugated sheep antimouse IgG (NA931),myo-[3H]inositol and [32P]orthophosphate were from Amersham International (Cardiff, UK). Other reagents were from previously described sources.22 23
Fura2-AM, wortmannin, and LY294002 were dissolved in dimethyl sulfoxide (DMSO) with other reagents made up in saline. The concentration of DMSO in the incubation never exceeded 1:1000 final dilution and an equivalent volume of DMSO was present in control samples.
Platelet isolation and measurement of aggregation, secretion, and [Ca++]i
Human-washed platelets were prepared from donors who had not taken aspirin in the previous 10 days.23 Platelets were separated from plasma by centrifugation in the presence of prostacyclin (100 ng/mL), resuspended in a modified Tyrodes-HEPES buffer without added Ca++ and recentrifuged in the presence of prostacyclin before final suspension in the Tyrodes-HEPES buffer at a density of 2 to 4 × 108 cells per milliliter. Platelets were left for 30 minutes to allow recovery from exposure to the prostacyclin. Indomethacin (10 μmol/L), EGTA (1 mmol/L), and apyrase (5 U/mL) were included as required. In a limited number of aggregation studies, the platelets were separated from plasma by gel filtration.24
Platelet suspensions (450 μL) were prewarmed to 37°C for 5 minutes before incubation with TPO or inhibitors of PI 3-kinase. Platelets were transferred to a Born-aggregometer (Chronolog; Labmedics, Cheshire, UK) and stirred at 1200 rpm for 60 seconds before addition of agonist. The 5-HT secretion was measured as previously described.22 For measurement of [Ca++]i, the platelets were loaded with Fura2-AM in platelet-rich plasma.25[Ca++]i was measured in a spectofluorimeter at 37°C under continuous agitation, with excitation at 340 and 380 nm and emission at 510 nm.
Measurement of [3H]-inositol phosphates
Washed platelets were labeled with 1.85 MBq/mL (50 μCi/mL) of [3H]myo-inositol for 3 hours at 30°C, washed, and resuspended at 4 × 108 cells per milliliter.26 LiCl (10 mmol/L) was added to inhibit conversion of inositol phosphates into free inositol. Reactions were performed in a final volume of 250 μL and stopped after 5 minutes of stimulation with 0.94 mL chloroform/methanol/HCl (50:100:1, by vol). Water (0.31 mL) and chloroform (0.31 mL) were then added. After the separation of phases, total [3H]inositol phosphates (mono-, bis-, trisphosphates) were eluted by Dowex anion-exchange chromatography as described.26
Measurement of [32P]3-phosphoinositides and [32P] phosphatidic acid
Platelets were labeled with [32P]orthophosphate (22.2 MBq/mL [0.6 mCi/mL]) for 60 minutes, washed, and resuspended at 1 × 109 cells per milliliter. Reactions were stopped at the indicated time by addition of chloroform/methanol (1:1 v/v) containing 0.4 mol/L HCl, and lipids were immediately extracted and analyzed by high-performance liquid chromatography (HPLC) as described.21 Phosphatidic acid was separated by thin-layer chromatography (TLC), detected by autoradiography, and analyzed by densitometry.
Measurement of PI 3-kinase activity
p85 or 4G10 immunoprecipitates (see below) were washed and preincubated in 0.1 mol/L NaCl, 20 mmol/L Tris (pH 7.6), and 10 μg phosphatidylinositol (final volume 50 μL) for 10 minutes. The reaction was initiated by addition of 10 mmol/L MgCl2, 0.02 mmol/L ATP, and 0.37 MBq (10 μCi) [32P]ATP for 30 minutes at 30°C. Labeled phosphoinositides were extracted, separated by TLC, and visualized by autoradiography.27 Silica was scraped from TLC plates and radioactivity was measured in a Beckmann scintillation counter (Beckmann Instruments, High Wycombe, UK).
Immunoblotting and immunoprecipitation studies
For measurement of whole-cell tyrosine phosphorylation, platelet suspensions (4 × 108/mL) were stopped with an equal volume of Laemmli sample buffer, and the mixture was heated for 5 minutes at 100°C.23 For immunoprecipitation studies, platelet suspensions (4 × 108/mL) were stopped by addition of an equal volume of a nondenaturing extraction buffer (2% NP-40, 300 mmol/L NaCl, 20 mmol/L Tris, 1 mml/L PMSF, 10 mmol/L EDTA, 2 mmol/L Na3VO4, 20 μg/mL leupeptin, 20 μg/mL aprotinin, and 5 μg/mL pepstatin; for final concentrations divide by 2). Immunoprecipitations were carried out with 5 μL/mL anti-p85 serum or 5 μL/mL anti-PLCγ2 per sample. Proteins were separated by 10% SDS/PAGE and transferred to polyvinylidene difluoride (PVDF) blotting. Membranes were blotted for tyrosine phosphorylation using 1 μg/mL mAb 4G10, or 1:1000 dilution of the anti–PI 3-kinase p85 or anti-PLCγ2 as described. Horseradish peroxidase–conjugated sheep antimouse IgG (NA931) or sheep antirabbit IgG (NA934) was applied as a secondary antibody and detected by enhanced chemiluminescence (ECL). The membranes were stripped of bound antibody by washing in TBS-T containing 2% SDS for 30 minutes at 80°C. After verifying stripping by the use of the secondary antibody, blots were reprobed with a different primary antibody as appropriate.
Analysis of data
Each experiment was performed at least 3 times. Results are shown as mean ± SE mean of 1 experiment performed in triplicate, or as pooled results where n represents the number of experiments. Statistical testing was by Student t test withP < .05 taken to indicate significance.
Results
Collagen and thrombopoietin stimulate formation of PI 3,4,5 P3
The ability of collagen and TPO to stimulate formation of 3-phosphorylated lipids was measured in platelets labeled with [32P]orthophosphate. Collagen (5 μg/mL) stimulated a significant increase in formation of [32P]PI3,4,5P3 and [32P]PI3,4P2 (phosphatidylinositol 3,4-bisphosphate) by 2 minutes (Table 1), at which time, aggregation and secretion responses were more than 80% complete. The increase in [32P]PI3,4,5P3 was approximately 1 order of magnitude lower than the response to CRP,19 but greater than that to the G protein–coupled receptor agonist ADP.28 TPO stimulated a lower increase in formation of [32P]PI3,4,5P3 and [32P]PI3,4P2 (Table 1). Formation of PI3,4,5P3 and PI3,4P2 by both agonists was completely inhibited by wortmannin (100 nmol/L) (Table 1) and LY294002 (50 μmol/L; not shown). Responses to collagen and TPO were maintained in the presence of cyclooxygenase inhibitor indomethacin, GPIIb-IIIa antagonist, RGDS, and apyrase (Table 1 and not shown).
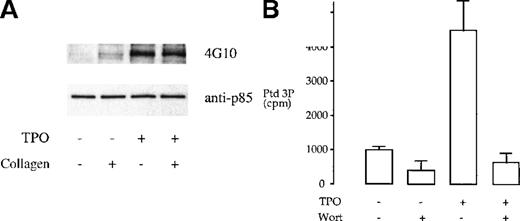
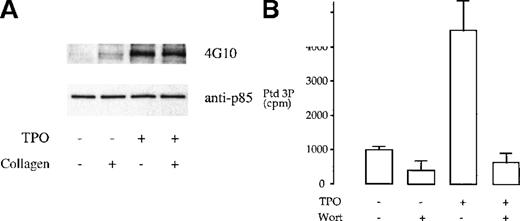
We investigated whether tyrosine phosphorylation plays a role in the regulation of the heterodimeric p85/110 form of PI 3-kinase by TPO and collagen. TPO has been reported to stimulate tyrosine phosphorylation of the p85 subunit of PI 3-kinase in platelets.12 This observation was confirmed in this study (Figure1A). In comparison, collagen (10 μg/mL) induced negligible tyrosine phosphorylation of the p85 subunit of PI 3-kinase and had no effect on phosphorylation induced by TPO (Figure1A). The consequence of phosphorylation of the p85 subunit by TPO was investigated by in vitro measurement of PI 3-kinase activity after immunoprecipitation, using the antiphosphotyrosine mAb 4G10, or the anti-p85 serum, using phosphatidylinositol as the substrate lipid. TPO stimulated an increase in PI 3-kinase activity in both sets of immunoprecipitates that was completely inhibited by 100 nmol/L wortmannin (Figure 1B and not shown). Collagen had no effect on the activity of PI 3-kinase in the p85 or 4G10 immunoprecipitates (not shown).
TPO treatment of platelets results in tyrosine phosphorylation and activation of PI 3-kinase.
(A) Phosphorylation of p85 subunit of PI 3-kinase. Platelet samples were treated with 10 μg/mL collagen (2 minutes), 50 ng/mL TPO (3 minutes), or the combination of both agents (TPO for 3 minutes, followed by collagen for 2 minutes). The platelets were then lysed under nondenaturing conditions in 1% NP-40 and protein immunoprecipitated with p85 antiserum. The immunoprecipitates were submitted to SDS-PAGE and, after transfer, the membrane was blotted with the antiphosphotyrosine monoclonal antibody (mAb) 4G10 and exposed by ECL (upper panel). The same membrane was stripped and reprobed with anti-p85 serum (lower panel). Results are from 1 experiment representative of 5; the extent of phosphorylation varied widely between donors. (B) TPO stimulates an increase in PI 3-kinase in p85 immunoprecipitates. Activity was measured by monitoring formation of phosphatidylinositol 3-monophosphate as described in “Materials and methods.” Results are performed in duplicate and are representative of 2 experiments.
TPO treatment of platelets results in tyrosine phosphorylation and activation of PI 3-kinase.
(A) Phosphorylation of p85 subunit of PI 3-kinase. Platelet samples were treated with 10 μg/mL collagen (2 minutes), 50 ng/mL TPO (3 minutes), or the combination of both agents (TPO for 3 minutes, followed by collagen for 2 minutes). The platelets were then lysed under nondenaturing conditions in 1% NP-40 and protein immunoprecipitated with p85 antiserum. The immunoprecipitates were submitted to SDS-PAGE and, after transfer, the membrane was blotted with the antiphosphotyrosine monoclonal antibody (mAb) 4G10 and exposed by ECL (upper panel). The same membrane was stripped and reprobed with anti-p85 serum (lower panel). Results are from 1 experiment representative of 5; the extent of phosphorylation varied widely between donors. (B) TPO stimulates an increase in PI 3-kinase in p85 immunoprecipitates. Activity was measured by monitoring formation of phosphatidylinositol 3-monophosphate as described in “Materials and methods.” Results are performed in duplicate and are representative of 2 experiments.
These results demonstrate that TPO and collagen stimulate the PI 3-kinase second messenger pathway through distinct mechanisms. Activation of PI 3-kinase by TPO is associated with tyrosine phosphorylation of the p85 subunit of PI 3-kinase. In contrast, collagen does not stimulate tyrosine phosphorylation of p85, but most likely activates the p85p/110 heterodimer through recruitment to the membrane via association with the adapters LAT29 and Cbl.30
The effect of PI 3-kinase inhibitors on PLCγ2 activation
A role for the PI 3-kinase pathway in the activation of PLCγ2 by CRP and FcγRIIA has been proposed.19 21 We were therefore interested to determine whether the PI 3-kinase pathway is also required for activation of PLC by collagen. PLC activity was assessed by measurement of [3H]inositol phosphates and [32P]phosphatidic acid, a metabolite of 1,2-diacylglycerol (Figure 2). LY294002 and wortmannin inhibited the response to collagen (10 μg/mL) over the effective concentration range for inhibition of PI 3-kinase. Maximally effective concentrations of wortmannin (100 nmol/L) and LY294002 (50 μmol/L) reduced the formation of inositol phosphates and phosphatidic acid to collagen by up to 75%. In contrast, they had no significant effect on the formation of inositol phosphate by the G protein–coupled receptor agonist, thrombin (Figure 2A and 2B).
Wortmannin and LY294002 partially inhibit collagen-induced formation of inositol phosphates and phosphatidic acid.
(A) [3H]Inositol labeled platelets resuspended at 4 × 108/mL were incubated with different concentrations of either LY294002 (i) or wortmannin (ii) for 3 to 15 minutes before stimulation with 10 μg/mL collagen (s) or 0.1 U/mL thrombin (l) for 5 minutes. Results are expressed as percentage (%) of the response to collagen or thrombin (= 100% in each case) from 1 experiment made in quadruplicate, which is representative of 4 experiments. The mean ± SEM basal dpm was 101 ± 12.4, and maximum stimulations over basal were 1019 ± 133.2 (collagen) and 954 ± 8.4 (thrombin). (B) [32P]orthophosphate-labeled platelets were stimulated with collagen (10 μg/mL) for 2 minutes and phosphatidic acid extracted as described in “Materials and methods.” LY294002 (LY) or wortmannin (Wt) for 3 to 15 minutes before stimulation with collagen. Results are shown as mean ± SEM percentage of the increase in phosphatidic acid by collagen over basal from 3 experiments.
Wortmannin and LY294002 partially inhibit collagen-induced formation of inositol phosphates and phosphatidic acid.
(A) [3H]Inositol labeled platelets resuspended at 4 × 108/mL were incubated with different concentrations of either LY294002 (i) or wortmannin (ii) for 3 to 15 minutes before stimulation with 10 μg/mL collagen (s) or 0.1 U/mL thrombin (l) for 5 minutes. Results are expressed as percentage (%) of the response to collagen or thrombin (= 100% in each case) from 1 experiment made in quadruplicate, which is representative of 4 experiments. The mean ± SEM basal dpm was 101 ± 12.4, and maximum stimulations over basal were 1019 ± 133.2 (collagen) and 954 ± 8.4 (thrombin). (B) [32P]orthophosphate-labeled platelets were stimulated with collagen (10 μg/mL) for 2 minutes and phosphatidic acid extracted as described in “Materials and methods.” LY294002 (LY) or wortmannin (Wt) for 3 to 15 minutes before stimulation with collagen. Results are shown as mean ± SEM percentage of the increase in phosphatidic acid by collagen over basal from 3 experiments.
The effect of PI 3-kinase inhibitors on aggregation and dense granule secretion
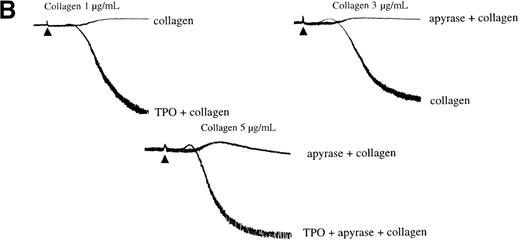
Experiments were designed to investigate whether the inhibitory effect of PI 3-kinase inhibitors on PLC activation by collagen is associated with inhibition of aggregation and dense granule secretion. Aggregation induced by a low concentration of collagen is converted to shape change in the presence of the PI 3-kinase inhibitors LY294002 and wortmannin (Figure 3A). Full inhibition of aggregation by LY294002 and wortmannin occurs at 20 μmol/L and 100 nmol/L, respectively (Figure 3A). These concentrations correspond to those required for full inhibition of PI 3-kinase activity in platelets stimulated with collagen. Aggregation induced by higher concentrations of collagen (more than 3 μg/mL) was slowed and partially inhibited by wortmannin and LY294002 (Figure 3B). The 2 PI 3-kinase inhibitors also inhibited secretion of [3H]5-HT from dense granules, producing a rightward shift in the concentration response curve to collagen (Figure 3C).
Effect of PI 3-kinase inhibitors on platelet aggregation and secretion of 5-hydroxytryptamine (5-HT).
Platelets were isolated as described in “Materials and methods” and resuspended at 2 to 4 × 108/mL in a modified Tyrode's buffer. Platelets were treated with LY294002 (LY, i) or wortmannin (Wt, ii) for 3 minutes at 37°C before stimulation. All experiments were performed in a Born aggregometer. (A) and (B) An increase in optical density (OD) represents shape change that precedes the decrease, due to aggregation. The traces are representative of at least 10 experiments. (C) [3H]5-HT secretion was analyzed as described in “Material and methods,” the concentrations of LY294002 and Wt were 50 μmol/L and 100 nmol/L, respectively. Platelets were incubated with collagen for 2 minutes. Results are shown as the mean ± SEM of 3 experiments.
Effect of PI 3-kinase inhibitors on platelet aggregation and secretion of 5-hydroxytryptamine (5-HT).
Platelets were isolated as described in “Materials and methods” and resuspended at 2 to 4 × 108/mL in a modified Tyrode's buffer. Platelets were treated with LY294002 (LY, i) or wortmannin (Wt, ii) for 3 minutes at 37°C before stimulation. All experiments were performed in a Born aggregometer. (A) and (B) An increase in optical density (OD) represents shape change that precedes the decrease, due to aggregation. The traces are representative of at least 10 experiments. (C) [3H]5-HT secretion was analyzed as described in “Material and methods,” the concentrations of LY294002 and Wt were 50 μmol/L and 100 nmol/L, respectively. Platelets were incubated with collagen for 2 minutes. Results are shown as the mean ± SEM of 3 experiments.
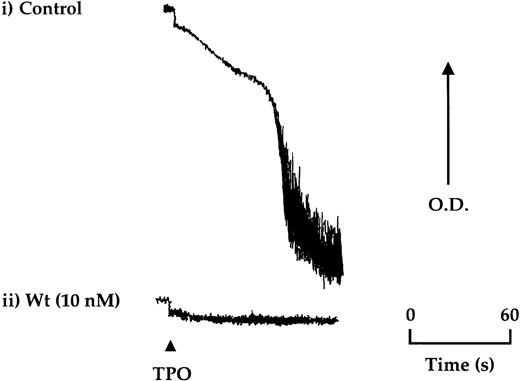
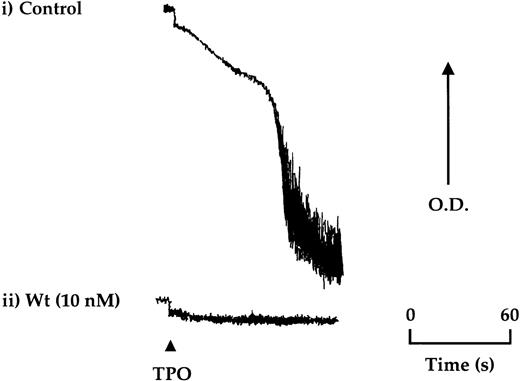
TPO (50 ng/mL) stimulated aggregation in approximately 20% of donors after isolation of platelets by gel filtration, which could be reproduced for the same donor over time (Figure4). A lower frequency of response (less than 5%) was seen in platelets isolated by centrifugation. Aggregation induced by TPO was not preceded by shape change and was inhibited completely by the PI 3-kinase inhibitors, wortmannin, and LY294002, ADP scavengers, and by the protein kinase C antagonist, Ro 31-8220 (Figure4 and not shown).
TPO stimulates aggregation of platelets in a subpopulation of donors.
Aggregation of gel-filtered platelets was monitored by light transmission where a decrease in optical density (OD) reflects aggregation. (i) Platelets from approximately 1 in 5 donors were found to undergo aggregation in response to TPO (50 ng/mL). (ii) Wortmannin (Wt) preincubated 15 minutes before the addition of TPO was found to completely block aggregation. The aggregation trace is representative of 4 experiments.
TPO stimulates aggregation of platelets in a subpopulation of donors.
Aggregation of gel-filtered platelets was monitored by light transmission where a decrease in optical density (OD) reflects aggregation. (i) Platelets from approximately 1 in 5 donors were found to undergo aggregation in response to TPO (50 ng/mL). (ii) Wortmannin (Wt) preincubated 15 minutes before the addition of TPO was found to completely block aggregation. The aggregation trace is representative of 4 experiments.
Potentiation of collagen-induced platelet activation by thrombopoietin abrogated by inhibitors of PI 3-kinase
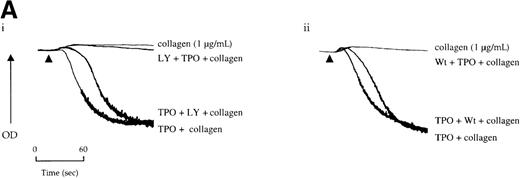
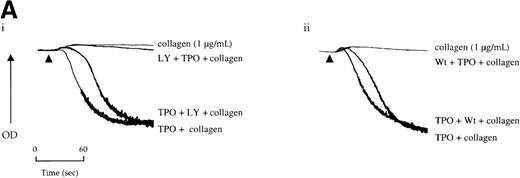
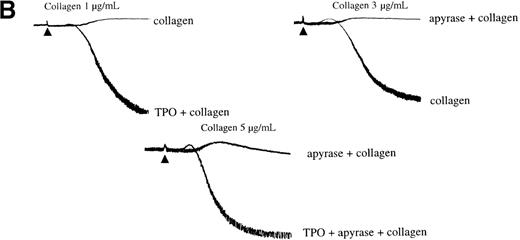
Potentiation of the collagen response by TPO is illustrated in Figure 5, in which the response to a threshold concentration of the matrix protein is converted to maximal aggregation after treatment with the cytokine. We sought to determine whether activation of the PI 3-kinase pathway by TPO underlies this potentiation. Wortmannin and LY294002 inhibited aggregation induced by the combination of TPO and the threshold concentration of collagen (Figure 5A). However, it is unclear whether this inhibitory action is due solely to an effect against collagen or because of an additional action against TPO. To address this, the platelets were treated with TPO before the addition of wortmannin or LY294002 and then challenged with collagen. Recovery of potentiation was observed in both cases (Figure 5A).
Priming of platelet responses to collagen by TPO is mediated through PI 3-kinase.
(A) Platelets were preincubated with (i) 50 μmol/L LY294002 (LY) or (ii) 100 nmol/L wortmannin (Wt) given either 3 minutes before or 2 minutes after TPO (50 ng/mL). In both sets of experimental paradigms, platelets were left for 5 minutes after the addition of TPO before stimulation by collagen (1 μg/mL). (B) Platelets were preincubated 3 minutes with TPO (100 ng/mL) and/or apyrase (1 U/mL) as indicated and then stimulated by collagen. Results are shown from 1 experiment that is representative of at least 5.
Priming of platelet responses to collagen by TPO is mediated through PI 3-kinase.
(A) Platelets were preincubated with (i) 50 μmol/L LY294002 (LY) or (ii) 100 nmol/L wortmannin (Wt) given either 3 minutes before or 2 minutes after TPO (50 ng/mL). In both sets of experimental paradigms, platelets were left for 5 minutes after the addition of TPO before stimulation by collagen (1 μg/mL). (B) Platelets were preincubated 3 minutes with TPO (100 ng/mL) and/or apyrase (1 U/mL) as indicated and then stimulated by collagen. Results are shown from 1 experiment that is representative of at least 5.
Secretion of ADP serves as a positive feedback signal in the activation of platelets by collagen. It was therefore important to establish whether the ability of TPO to induce potentiation was maintained in the presence of the ADP scavenger, apyrase. Apyrase causes a shift to the right in the collagen concentration response curve to collagen for aggregation, as illustrated in Figure 5B. Potentiation by TPO was retained in the presence of apyrase, demonstrating that it is not dependent on release of ADP (Figure 5B).
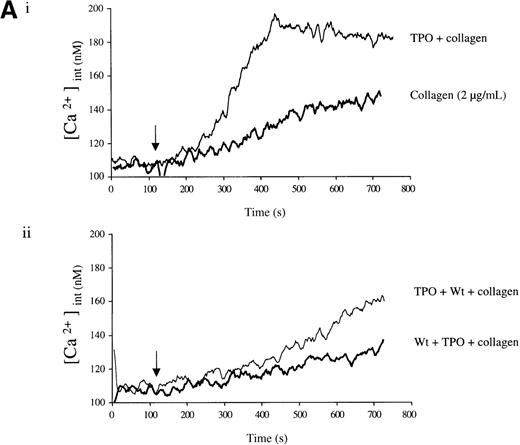
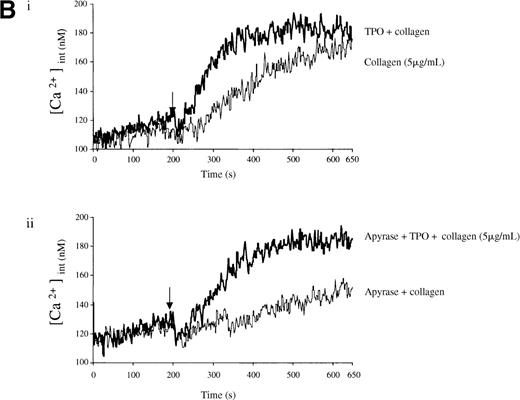
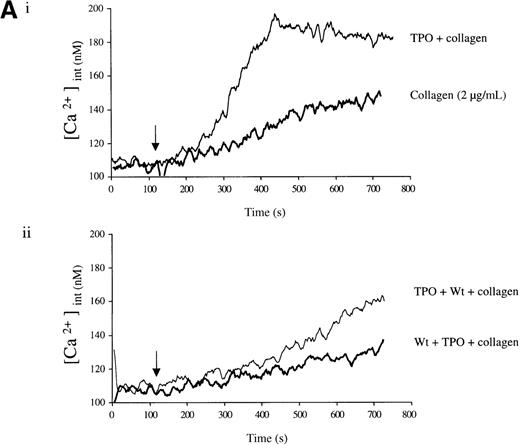
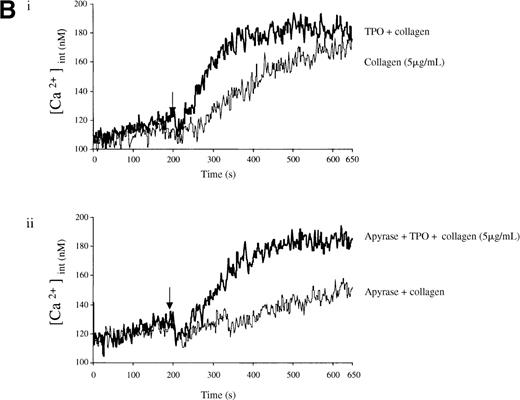
Experiments were designed to investigate whether the priming action of TPO on collagen is associated with elevation of [Ca++]i. TPO potentiated the rate and magnitude of the Ca++ response to collagen (Figure6A, i). When wortmannin was added before TPO and collagen, the increase in Ca++ was reduced to a lower level than for collagen alone (Figure 6A, ii). In contrast, when wortmannin was added after TPO but before collagen, potentiation of the Ca++ response was restored (Figure 6A, ii). Potentiation of the collagen Ca++ response by TPO was also observed in the presence of apyrase, confirming that it is not dependent on the release of ADP (Figure 6B).
Priming of platelet responses to collagen by TPO is accompanied by a PI 3-kinase–dependent Ca++ increase.
Fura-2–loaded platelets (108/mL) were stimulated in a plastic cuvette with stirring at 37°C in the presence of 1 mmol/L extracellular Ca++. Fluorescence emission was recorded at 510 nm for an excitation ratio 340/380 nm. In (A) experiments were performed as follows: (i) collagen (2 μg/mL) or TPO (100 ng/mL) for 3 minutes and then collagen (2 μg/mL); (ii) wortmannin (Wt) (100 nmol/L) for 3 minutes, followed by TPO for 3 minutes and then collagen (2 μg/mL), or TPO (100 ng/mL) for 3 minutes, followed by Wt (100 nmol/L) for 3 minutes and then collagen (2 μg/mL). In (B) experiments were performed as follows: (i) collagen (5 μg/mL), or TPO (100 ng/mL) for 3 minutes, followed by collagen (5 μg/mL); (ii) same as (i) but in the presence of apyrase (1 U/mL). The addition of collagen is indicated by an arrow. Results are from 1 experiment made in triplicate representative of 4.
Priming of platelet responses to collagen by TPO is accompanied by a PI 3-kinase–dependent Ca++ increase.
Fura-2–loaded platelets (108/mL) were stimulated in a plastic cuvette with stirring at 37°C in the presence of 1 mmol/L extracellular Ca++. Fluorescence emission was recorded at 510 nm for an excitation ratio 340/380 nm. In (A) experiments were performed as follows: (i) collagen (2 μg/mL) or TPO (100 ng/mL) for 3 minutes and then collagen (2 μg/mL); (ii) wortmannin (Wt) (100 nmol/L) for 3 minutes, followed by TPO for 3 minutes and then collagen (2 μg/mL), or TPO (100 ng/mL) for 3 minutes, followed by Wt (100 nmol/L) for 3 minutes and then collagen (2 μg/mL). In (B) experiments were performed as follows: (i) collagen (5 μg/mL), or TPO (100 ng/mL) for 3 minutes, followed by collagen (5 μg/mL); (ii) same as (i) but in the presence of apyrase (1 U/mL). The addition of collagen is indicated by an arrow. Results are from 1 experiment made in triplicate representative of 4.
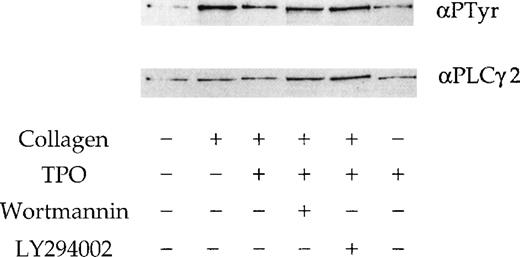
We investigated whether the effect of the 2 PI 3-kinase inhibitors and TPO on the response to collagen is mediated by alteration of phosphorylation of PLCγ2. A submaximal concentration of collagen (10 μg/mL) stimulated tyrosine phosphorylation of PLCγ2 (Figure7), whereas TPO (50 ng/mL) had only a negligible effect (not shown). Neither wortmannin nor LY294002 had a significant effect on the collagen-stimulated tyrosinephosphorylation of PLCγ2 (Figure 7), although a small reduction in phosphorylation was observed in some experiments (not shown). A similar observation was reported in platelets stimulated by CRP.18 There was no significant change in the tyrosine phosphorylation of PLCγ2 in response to collagen in the presence of TPO (Figure 7).
Phosphorylation of PLCγ2 by collagen in the presence of TPO.
PLCγ2 tyrosine phosphorylation induced by TPO and collagen. Platelets were stimulated with stirring by collagen (10 μg/mL) for 90 seconds; TPO (50 ng/mL) for 300 seconds; TPO (50 ng/mL) for 300 seconds, followed by collagen for 90 seconds. Wortmannin (100 nmol/L) and LY294002 (50 μmol/L) are preincubated 3 minutes before the addition of TPO or collagen. PLCγ2 was immunoprecipitated and probed for tyrosine phosphorylation using the monoclonal antibody 4G10 (upper panel). Membranes are stripped and reprobed for PLCγ2 (lower panel). Results are from 1 experiment representative of 3.
Phosphorylation of PLCγ2 by collagen in the presence of TPO.
PLCγ2 tyrosine phosphorylation induced by TPO and collagen. Platelets were stimulated with stirring by collagen (10 μg/mL) for 90 seconds; TPO (50 ng/mL) for 300 seconds; TPO (50 ng/mL) for 300 seconds, followed by collagen for 90 seconds. Wortmannin (100 nmol/L) and LY294002 (50 μmol/L) are preincubated 3 minutes before the addition of TPO or collagen. PLCγ2 was immunoprecipitated and probed for tyrosine phosphorylation using the monoclonal antibody 4G10 (upper panel). Membranes are stripped and reprobed for PLCγ2 (lower panel). Results are from 1 experiment representative of 3.
Discussion
We have previously reported that collagen stimulates tyrosine phosphorylation and activation of PLCγ2 in platelets. In this study, we show that collagen also activates the PI 3-kinase pathway in platelets, leading to the formation of PI3,4,5P3 and PI3,4P2. The PI 3-kinase pathway is required for full activation of PLC activity because 2 structurally distinct inhibitors of PI 3-kinase, LY294002 and wortmannin, partially inhibit formation of inositol phosphates and phosphatidic acid by collagen at concentrations that result in complete inhibition of PI 3-kinase activity. This is associated with inhibition of aggregation, secretion, and Ca++ elevation. In contrast, neither inhibitor has a major effect on the activation of PLC by the G protein–coupled receptor agonist thrombin. These observations confirm our previous study using CRP,19 which provided evidence for a partial role for the PI 3-kinase pathway in the regulation of PLCγ2 by the GPVI-selective peptide, CRP. A similar conclusion has also been made for the regulation of PLCγ2 by FcγRIIA.21 It is notable that collagen induces a much lower increase in the formation of PI3,4,5P3, compared with CRP, but that wortmannin and LY294002 cause a similar reduction in the formation of inositol phosphates by both stimuli. This suggests that the level of the newly generated 3-phosphorylated lipids is not rate limiting in the regulation of PLCγ2. In support of this, we have shown that the elevation of PI3,4,5P3 in murine platelets deficient in the 5′ lipid phosphatase, SHIP, in response to GPVI cross-linking, does not lead to increased activation of phospholipase C (J. Pasquet and S. Watson, unpublished data).
Our study also provides evidence that the cytokine TPO enhances platelet secretion and aggregation by collagen through a PI 3-kinase–dependent pathway. TPO stimulates formation of PI3,4,5P3 and its metabolite PI3,4P2. The treatment of platelets with either LY294002 or wortmannin before the addition of TPO prevents potentiation of the response to collagen, although this could be due solely to inhibition of the response to collagen. However, when either inhibitor is given after TPO but before the addition of collagen, potentiation is restored. This potentiation is maintained for several minutes in the presence of wortmannin and LY 294002, suggesting that the liberated 3-phosphorylated lipids are protected against metabolism, presumably as a consequence of interaction with the PH domains.
There are several mechanisms whereby elevation in PI3,4,5P3and/or PI3,4P2 in response to TPO could lead to potentiation of the response to collagen. These include enhanced recruitment and activation of the tyrosine kinase Btk and/or PLCγ2 to the plasma membrane, and potentiation of Ca++ entry. PI3,4,5P3 has been shown to support the translocation of Btk to the plasma membrane, where it is able to phosphorylate PLCγ2.31 PI3,4,5P3 has also been shown to be required for translocation of PLCγ1 to the membrane through interaction with its src homology 2 (SH2) or PH domains.32,33 PI 3-kinase is also required for the recruitment of PLCγ2 to the membrane by GPVI in megakaryocytes because translocation is inhibited by the pretreatment with wortmannin (R. Bobe and S. Watson, unpublished data). Additionally, elevation of PI3,4,5P3 in murine mast cells deficient in the 5′ lipid phosphatase, SHIP, leads to enhanced Ca++ entry34; a similar result has been observed in platelets (J. Pasquet and S. Watson, unpublished data).
Platelets from a limited number of donors aggregate in response to TPO through a pathway that is exquisitely sensitive to wortmannin, and which may therefore be related to the mechanism that underlies potentiation of the response to collagen. Additionally, we show that the activation of protein kinase C and the release of ADP are required for aggregation by TPO in these donors. Although the relationship between these events is not established, it is notable that 3-phosphorylated lipids support activation of atypical forms of protein kinase C, and that this pathway contributes to platelet aggregation.35 36 Further, the requirement for ADP in aggregation suggests that this might be the result of potentiation of the ADP response by TPO, possibly through the same pathway as described in this study.
In summary, we have shown that PI 3-kinase has an important role in platelet activation by collagen through the regulation of PLCγ2, and that the priming effect of TPO on collagen is mediated through a PI 3-kinase–dependent pathway, leading to potentiation of PLC activity and increased intracellular Ca++ concentration.
Supported by funding from The Wellcome Trust and The British Heart Foundation. S.P.W. is a British Heart Foundation Research Fellow. B.S.G. is a Wellcome Trust Prize Student. L.Q. holds a BHF Studentship. G.V.W. is a Fellow of the Catharijne Foundation. This project was initiated by J.C.M., who laid much of the foundation for the work.
J.P., B.S.G., and M.-P.G. contributed equally to this work.
Reprints:Steve P. Watson, Department of Pharmacology, University of Oxford, Mansfield Rd, Oxford Ox1 3QT, UK; e-mail: stevewatson@pharm.ox.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Wortmannin and LY294002 partially inhibit collagen-induced formation of inositol phosphates and phosphatidic acid. / (A) [3H]Inositol labeled platelets resuspended at 4 × 108/mL were incubated with different concentrations of either LY294002 (i) or wortmannin (ii) for 3 to 15 minutes before stimulation with 10 μg/mL collagen (s) or 0.1 U/mL thrombin (l) for 5 minutes. Results are expressed as percentage (%) of the response to collagen or thrombin (= 100% in each case) from 1 experiment made in quadruplicate, which is representative of 4 experiments. The mean ± SEM basal dpm was 101 ± 12.4, and maximum stimulations over basal were 1019 ± 133.2 (collagen) and 954 ± 8.4 (thrombin). (B) [32P]orthophosphate-labeled platelets were stimulated with collagen (10 μg/mL) for 2 minutes and phosphatidic acid extracted as described in “Materials and methods.” LY294002 (LY) or wortmannin (Wt) for 3 to 15 minutes before stimulation with collagen. Results are shown as mean ± SEM percentage of the increase in phosphatidic acid by collagen over basal from 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3429/6/m_bloo01122002x.jpeg?Expires=1765904190&Signature=30424Xmuz6T9CEexA5MjTqeDMUVw39hxgfxd5of2EW4pg6aqjMiuoRWSf3y250ydgqYc2tKNC0ahPWDsuuolBOMqHTbkTL2qeP895LlyJ0HY~ZZzss51~lj~13NBr-GK6kNOzFIaXeR-j7L7pKBqlOBEwvlKC1hQNPSU1VFpJLs9rWcqwW1fNo9fmgecwLkJ1Yil-VGsP7qVEJreHxccbnVAzd2wN0QWd1RTGUFVZZe7QAlfJN-4~j34R~A6VNtejpi2fqx4Pwn6z-FPf31SgioRzoJ7EB9IXzAec-4Rv~SOfznxJfuMHHeJ3v5NLNGVn-fWkDaj7ViBc1RKKpMkxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of PI 3-kinase inhibitors on platelet aggregation and secretion of 5-hydroxytryptamine (5-HT). / Platelets were isolated as described in “Materials and methods” and resuspended at 2 to 4 × 108/mL in a modified Tyrode's buffer. Platelets were treated with LY294002 (LY, i) or wortmannin (Wt, ii) for 3 minutes at 37°C before stimulation. All experiments were performed in a Born aggregometer. (A) and (B) An increase in optical density (OD) represents shape change that precedes the decrease, due to aggregation. The traces are representative of at least 10 experiments. (C) [3H]5-HT secretion was analyzed as described in “Material and methods,” the concentrations of LY294002 and Wt were 50 μmol/L and 100 nmol/L, respectively. Platelets were incubated with collagen for 2 minutes. Results are shown as the mean ± SEM of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3429/6/m_bloo01122003x.jpeg?Expires=1765904190&Signature=aNizr4WIrQgUa22O7GHRqyLsXgyh35in87BIg1k1KANTxjCu~usbdySZmgTdh8AzJvuA2LXZa2PUIyLG19kGRnlKdOidw72fC7pGAONzdZH1PttVGHnBNsxX3v~VQOAz1EwTo5sx7yEbGYspaMFuMths~UwQVppFeGm3bX8NsXtiF~69epMiCfROAy0PncIqrc3OKvdx5LdO9KKH-1ucdaXa8XMIr3vw5-kqfMUfj-hCG4YfhLvxND9KQYZLUG0sIT1Mkb06SU6dEn5DE1OiFnu-GJ919arKohuBn5lNaiHj86-79a6CtlbGIP6ap8WyxR7Me4yQ00RQEXdjUVKGCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Wortmannin and LY294002 partially inhibit collagen-induced formation of inositol phosphates and phosphatidic acid. / (A) [3H]Inositol labeled platelets resuspended at 4 × 108/mL were incubated with different concentrations of either LY294002 (i) or wortmannin (ii) for 3 to 15 minutes before stimulation with 10 μg/mL collagen (s) or 0.1 U/mL thrombin (l) for 5 minutes. Results are expressed as percentage (%) of the response to collagen or thrombin (= 100% in each case) from 1 experiment made in quadruplicate, which is representative of 4 experiments. The mean ± SEM basal dpm was 101 ± 12.4, and maximum stimulations over basal were 1019 ± 133.2 (collagen) and 954 ± 8.4 (thrombin). (B) [32P]orthophosphate-labeled platelets were stimulated with collagen (10 μg/mL) for 2 minutes and phosphatidic acid extracted as described in “Materials and methods.” LY294002 (LY) or wortmannin (Wt) for 3 to 15 minutes before stimulation with collagen. Results are shown as mean ± SEM percentage of the increase in phosphatidic acid by collagen over basal from 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3429/6/m_bloo01122002x.jpeg?Expires=1765994372&Signature=XOZg2NHx~B0V02SBDTfUw3mqgxj3y6A4P2585yDd6oZaLZOV4yadyzy~j7fRRmk~RihZDAzR8f4gx-1YxyWutgL~nve37kAsRHwNAfmaZG~bL7S8m9VOkxlRNFBrQkLEyUnnllBh5y9GgkuvGnTTYm8TtxKrpc8K692biPirVTn3tFvW5B6~dqScjQkGU1EVJ4drU3NYdI413e0iin7Wx8qqf-jQ4DReUc0Z4ATAyKstnHSsi-3mNor2td2IXQYhPkE0Ap1f5qWgQUJzp-k5tZiumqH6wALCRK1t80vf1rvtYx3dhWl9RvSXKvAFvPd2hf-JBFmeXjDZNde4XdyXNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of PI 3-kinase inhibitors on platelet aggregation and secretion of 5-hydroxytryptamine (5-HT). / Platelets were isolated as described in “Materials and methods” and resuspended at 2 to 4 × 108/mL in a modified Tyrode's buffer. Platelets were treated with LY294002 (LY, i) or wortmannin (Wt, ii) for 3 minutes at 37°C before stimulation. All experiments were performed in a Born aggregometer. (A) and (B) An increase in optical density (OD) represents shape change that precedes the decrease, due to aggregation. The traces are representative of at least 10 experiments. (C) [3H]5-HT secretion was analyzed as described in “Material and methods,” the concentrations of LY294002 and Wt were 50 μmol/L and 100 nmol/L, respectively. Platelets were incubated with collagen for 2 minutes. Results are shown as the mean ± SEM of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3429/6/m_bloo01122003x.jpeg?Expires=1765994372&Signature=bbnuPuIhUUrLgNuQ2p5wCsFe7FrZjw3WxGjrCVbdeanR5qIZheoLlDhuTv3gkgxFTyRv4lD1iHfb3BSOoh10epa8RoGZIoGvtXPBdPjHOarHtsIX0YTlYrnLd~2uGiWEGxKqJu7Y4mbNdK85wngs4smMVDS~iUhVc4z9A4ErjTtqTpyNEKGj-wX7dq5Ym2CLHz1J6W5OK7gFYowxV~2hWh20-6s-HZ6m91f1Vyp1ygKee~VhGRP0W5J6lSRyrim9kluiulgZzGq3oZsMj4R9oz-0iCMBFMCWEMIe2jM3AtF1D42e4u5FcO-raTbr7Dzb0jccwVeB9Q3IoQZxJbQ8Vg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)