Abstract
Mechanisms underlying fetal hemoglobin (HbF) reactivation in adult life have not been elucidated; particularly, the role of growth factors (GFs) is controversial. Interestingly, histone deacetylase (HD) inhibitors (sodium butyrate, NaB, trichostatin A, TSA) reactivate HbF. We developed a novel model system to investigate HbF reactivation: (1) single hematopoietic progenitor cells (HPCs) were seeded in serum-free unilineage erythroid culture; (2) the 4 daughter cells (erythroid burst-forming units, [BFU-Es]), endowed with equivalent proliferation/differentiation and HbF synthesis potential, were seeded in 4 unicellular erythroid cultures differentially treated with graded dosages of GFs and/or HD inhibitors; and (3) HbF levels were evaluated in terminal erythroblasts by assay of F cells and γ-globin content (control levels, 2.4% and 1.8%, respectively, were close to physiologic values). HbF was moderately enhanced by interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor treatment (up to 5%-8% γ-globin content), while sharply reactivated in a dose-dependent fashion by c-kit ligand (KL) and NaB (20%-23%). The stimulatory effects of KL on HbF production and erythroid cell proliferation were strictly correlated. A striking increase of HbF was induced by combined addition of KL and NaB or TSA (40%-43%). This positive interaction is seemingly mediated via different mechanisms: NaB and TSA may modify the chromatin structure of the β-globin gene cluster; KL may activate the γ-globin promoter via up-modulation of tal-1 and possibly FLKF transcription factors. These studies indicate that KL plays a key role in HbF reactivation in adult life. Furthermore, combined KL and NaB administration may be considered for sickle cell anemia and β-thalassemia therapy.
In humans, the embryonic, fetal, and adult β-globin cluster genes are developmentally regulated. In the perinatal switch, fetal hemoglobin (HbF) is almost completely replaced by adult Hb (HbA).1 The HbF present in adult life (less than 1% of total Hb) is restricted to F cells, which represent less than 5% (mean values, 2.7% ± 1.4%) of the red blood cells (RBCs).2 3
In diverse postnatal conditions, particularly rapid marrow regeneration (stress erythropoiesis), HbF synthesis may be reactivated up to 10% to 20% relative γ-globin content.4,5 HbF reactivation provides an interesting model of partial reverse of the HbF → HbA perinatal switch. Furthermore, pharmacologic reactivation of γ-globin expression may provide an effective treatment for patients affected by β-globin disorders, as sickle cell anemia or β-thalassemia. In sickle cell anemia, HbF interferes with sickling by inhibiting the polymerization of sickle hemoglobin; subjects with elevated HbF levels appear to have a milder disease.6,7 In thalassemia, a significant increase in γ-globin production could partially replace the defective β-globin, thus preventing precipitation of unpaired α-globin chains.8
In humans and primates, HbF reactivation has been induced by diverse agents. Cytostatic drugs such as 5-azacytidine, bromo-deoxyuridine or hydroxyurea (HU) are effective in vitro.9-11 HU has been successfully used for treatment of sickle cell anemia12,13; however, myelotoxicity, potential long-term carcinogenicity, and only moderate therapeutic effects limited this clinical use. Histone deacetylase (HD) inhibitors (ie, trapoxin [TPX], trichostatin A [TSA], arginine butyrate [ArgB], sodium butyrate, and sodium phenylbutyrate [NaB and NaPB]) increase HbF levels in human cultures.14 NaB and NaPB are currently evaluated in clinical trials15,16; intermittent therapy with ArgB sustainedly induced HbF in sickle cell anemia.17
The role of hematopoietic growth factors (HGF) in HbF reactivation is controversial. Large dosages of erythropoietin (Epo), while stimulating HbF production in primates,18,19 induced modest and variable reactivation of HbF in clinical trials.20-22 In the serum-free culture of adult hematopoietic progenitor cells (HPCs), granulocyte-macrophage colony-stimulating factor (GM-CSF)23and interleukin-3 (IL-3)24 induced a significant rise of HbF synthesis, but these results were not confirmed25; furthermore, the c-kit ligand (KL) markedly stimulated HbF production in sickle cell anemia26 and normal27 HPC culture. However, these bulk culture studies involved methodology limitations (see “Discussion”), which hampered elucidation of the role of HGFs in HbF reactivation.
We have investigated HbF reactivation by a novel in vitro approach. Purified adult HPCs were grown in a single-cell unilineage erythroid culture. The generated sibling erythroid burst- forming units (BFU-Es) were then reseeded in unicellular erythroid cultures differentially treated with diverse HGFs (IL-3, GM-CSF, KL), and/or HD inhibitors (TSA, NaB).
Materials and methods
Hematopoietic growth factors and chemical inducers
Recombinant human IL-3 and GM-CSF were supplied by Behring (Behringwerke AG, Marburg, Germany) and Sandoz (Basel, Switzerland), respectively. Recombinant human KL was provided by R & D Systems (Minneapolis, MN), whereas recombinant human Epo was supplied by Amgen (Thousand Oaks, CA). TSA and NaB were provided by Sigma Chemical, (St Louis, MO).
Peripheral blood
Adult peripheral blood (PB) was obtained from healthy adult male donors after informed consent. Blood (450 mL) was collected in preservative-free citrate/phosphate/dextrose/adenine (CPDA-1) anticoagulant. A buffy coat was obtained by centrifugation (Beckman J6M/E, 1400 rpm per 20 minutes at room temperature; Beckman Instruments, Fullerton, CA).
Hematopoietic progenitor cell purification
Adult PB HPCs were purified according to a modification28 of a method previously reported,29 which consists of 3 steps. These purified cells are more than 90% CD34+ and comprise 90% to 95% of early hemopoietic progenitor cells, HPCs (including colony-forming units mixed, CFU-Mix, BFU-E, CFU-GM, and CFU-MK), 5% to 10% of primitive HPCs (high proliferative potential colony-forming cells, HPP-CFCs and colony-forming units-blast, CFU-B), and 0.5% to 2% of putative hematopoietic stem cells (HSCs) (8-week long-term culture initiating cells, LTC-ICs).30
Unilineage erythroid differentiation cultures
Unicellular cultures.
Unicellular cultures were performed in flat-bottomed 96-microwell plates in 0.1 mL of 5% fetal calf serum (FCS)-medium supplemented with all the ingredients used in FCS− culture as previously reported.31 32
In the sibling cell assay, to avoid 2 cells in the same well, the cells were plated at 0.5 cell per well.
To selectively stimulate erythroid differentiation, low dosages of IL-3 and GM-CSF (0.01 units and 0.001 ng/mL, respectively) and saturating levels of Ep (3 U/mL) were added to the cultures (this culture condition was defined in the text as erythroid medium).
The sibling cells were picked up by a micromanipulator from 4 cell clones scored at day 3 and day 4 and seeded in 4 different wells containing 0.1 mL of the same medium supplemented or not with graded amounts of IL-3 (1, 10, or 100 U/mL) or GM-CSF (1, 10, or 100 ng/mL) or KL (1, 10, or 100 ng/mL) or KL + TSA (100 ng and 10 ng/mL, respectively). Cultures were incubated in a fully humidified atmosphere of 5% CO2/5% O2/90% N2 and colonies were analyzed on day 14 to day 18.
Mini-bulk cultures.
Purified HPCs grown in FCS− liquid culture (5.104 cells per milliliter per well in IMDM, supplemented as reported by Valtieri et al32) were induced to specific erythroid differentiation by an appropriate HGF cocktail (Epo and IL-3/GM-CSF as indicated above) supplemented or not with KL (100 ng/mL), TSA (10 ng/mL), NaB (0.5 mmol/L), or their combination, in a fully humidified atmosphere as indicated above.
Morphologic analysis.
Cells were harvested from day 14 to day 21, smeared on glass slides by cytospin centrifugation and stained with standard May-Grünwald-Giemsa. For single sibling colony analysis, specific polylysinated glass slides were used.
F cells
The percentage of erythroblasts containing HbF was evaluated by indirect fluorescence as previously described.3
Briefly, cells from pooled or single bursts33 were cytocentrifuged on a glass slide, fixed for 5 minutes at room temperature in acetone-methanol (9:1, vol/vol), washed 3 times with phosphate-buffered saline (PBS), once with PBS containing 2 mg/mL bovine serum albumin (BSA), and incubated for 40 minutes at 37°C with a 1:40 dilution of an antihuman HbF MoAb (Caltag Laboratories, Burlingame, CA). The slides were washed twice with PBS, once with PBS/BSA, incubated for 30 minutes at room temperature with a 1:20 dilution of fluorescein isothiocyanate (FITC)-conjugated F(ab1)2 antimouse IgGs (Dakopatts, Copenhagen, Denmark), and extensively washed in PBS. The slides were then mounted in PBS/glycerol (50:50, vol/vol) and observed under an Axiophot Zeiss microscope (Zeiss, Jena, Germany) equipped for fluorescence. As a negative control, cells were incubated with normal mouse IgG instead of anti-HbF and processed as above.
γ-Chain content
High-performance liquid chromatographic (HPLC) separation of globin chains was performed according to Leone et al.34 Briefly, cell lysates from bulk culture and/or pooled sibling erythroid colonies were separated on chromatographic columns (Merck LiChrospher 100 CH8/2,5 μm; E. Merck, Darmstadt, Germany) using as eluents a linear gradient of acetonitrile/methanol/0.155 mol/L sodium chloride (pH 2.7, 68:4:28 vol/vol/vol) (eluent A), and acetonitrile/methanol/0.077 mol/L sodium chloride (pH2.7, 26:33:41 vol/vol/vol) (eluent B). Gradient was from 20% to 60% eluent A in 60 minutes at a flow rate of 0.8 mL/min. The optimal absorbance of the different globin was evaluated at 214 nm, because the absorbance coefficients of the different chains are identical at this wavelength.34
Tal-1 and FKLF expression
Tal-1 expression was investigated at the messenger RNA (mRNA) level by reverse transcriptase–polymerase chain reaction (RT-PCR) analysis and at the protein level by Western blotting on erythroid bulk cultures, according to experimental procedures previously described.35 In the same cultures, FKLF mRNA expression was investigated by RT-PCR using primers and amplification conditions as recently reported.36
Results
We have analyzed reactivation of human HbF synthesis in erythroid colonies generated by sibling adult BFU-Es in unicellular culture. Several studies also involved minibulk cultures of purified BFU-Es.
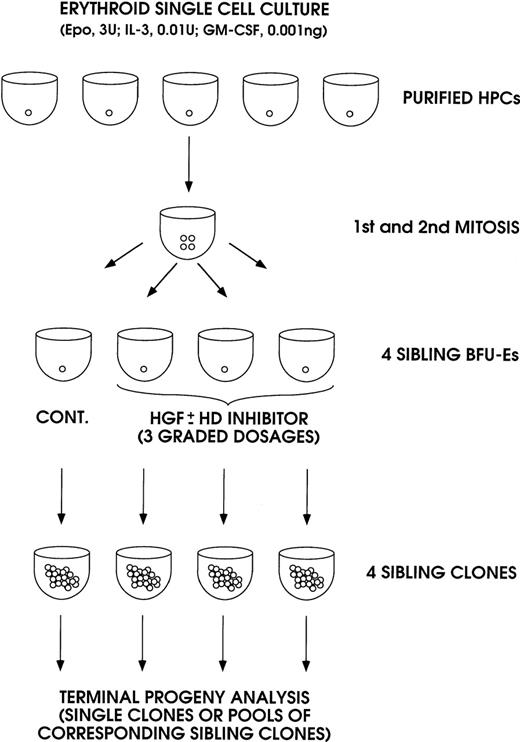
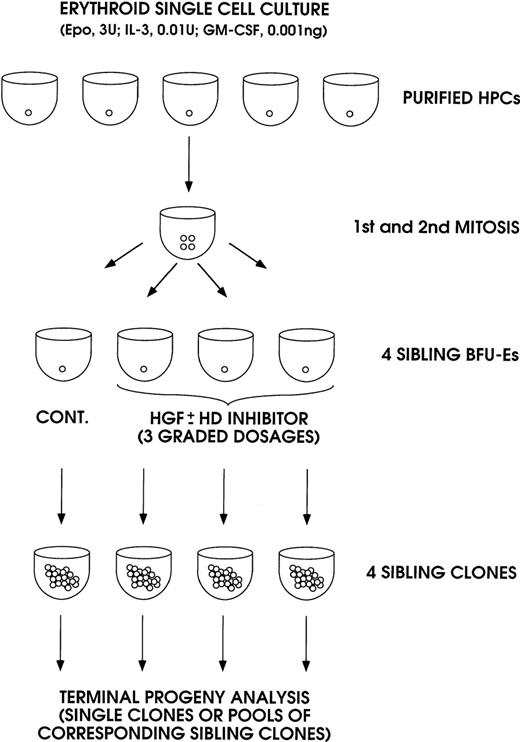
In the first series of experiments, the effect of IL-3, GM-CSF, and KL was analyzed in sibling BFU-Es (Figure 1). The purified HPCs were grown in unicellular erythroid liquid suspension culture (ie, in medium supplemented with saturating amounts of Epo, combined with very low concentrations of IL-3 and GM-CSF to allow erythroid differentiation and to stimulate cell proliferation up to terminal maturation, Sposi et al37). After 2 cell divisions, the 4 generated BFU-Es were subdivided by micromanipulation and individually reseeded in secondary erythroid culture. Three siblings were grown in erythroid culture supplemented with graded concentrations of IL-3, GM-CSF, or KL, whereas the fourth control sibling was grown in standard erythroid culture. Finally, we evaluated HbF levels in the mature erythroblasts of terminal erythroid colonies; it is noteworthy that we analyzed single colonies and/or pools of corresponding sibling colonies.
A model system for analysis of sibling BFU-Es, generated in unicellular erythroid culture (0.5 cell per well) supplemented with low dosages of IL-3 and GM-CSF and saturating levels of Epo.
The 4 sibling BFU-Es were subdivided and individually reseeded in unicellular erythroid culture: 3 siblings were treated with graded amounts of IL-3, GM-CSF, or KL ± HD inhibitor (NaB or TSA); the fourth control sibling was untreated. The sibling clones were analyzed on day 14 through day 18 as single or pooled mature erythroblast colonies.
A model system for analysis of sibling BFU-Es, generated in unicellular erythroid culture (0.5 cell per well) supplemented with low dosages of IL-3 and GM-CSF and saturating levels of Epo.
The 4 sibling BFU-Es were subdivided and individually reseeded in unicellular erythroid culture: 3 siblings were treated with graded amounts of IL-3, GM-CSF, or KL ± HD inhibitor (NaB or TSA); the fourth control sibling was untreated. The sibling clones were analyzed on day 14 through day 18 as single or pooled mature erythroblast colonies.
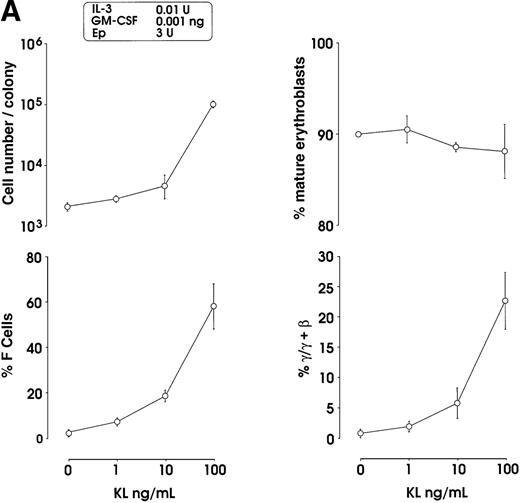
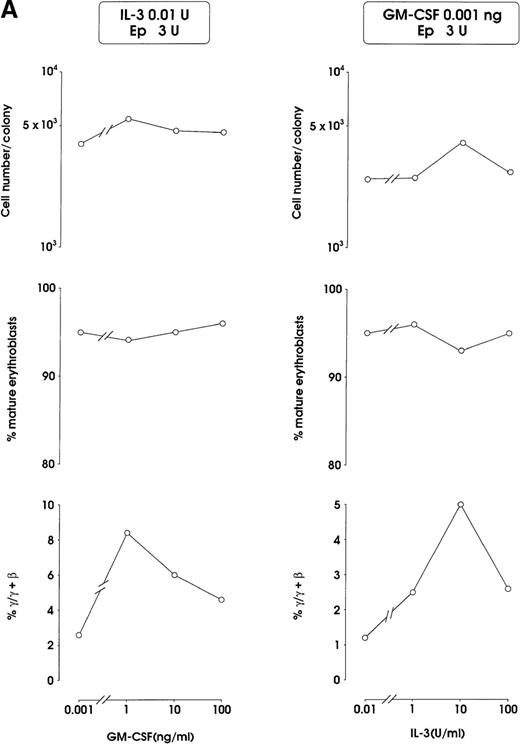
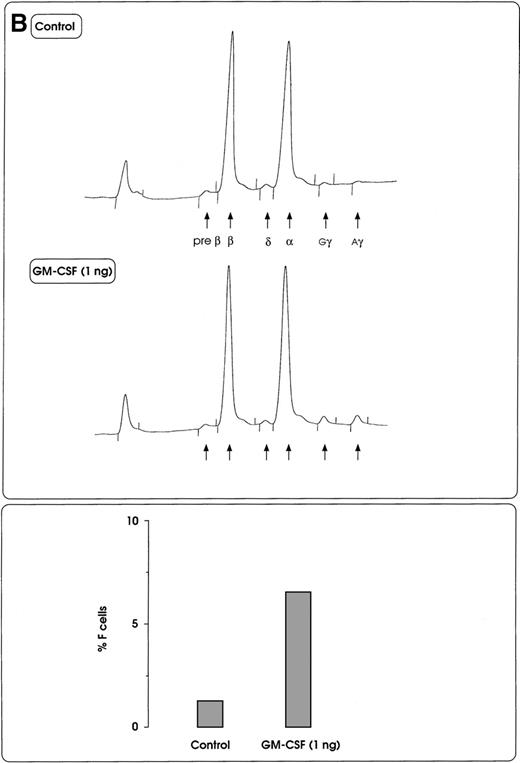
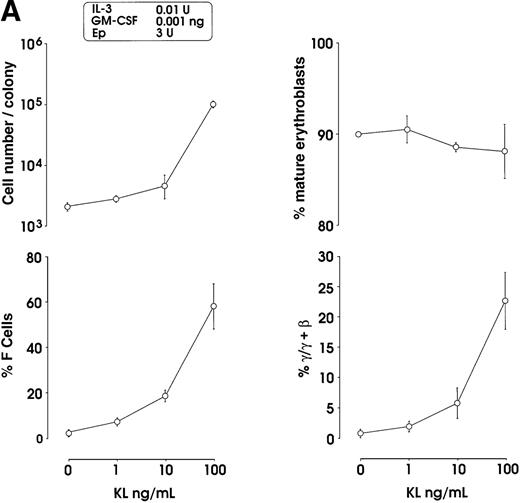
Both IL-3 and GM-CSF reactivated HbF synthesis, as evaluated in terms of γ-chain content (up to 5% and 8% at 10 units and 1 ng, respectively, mean values) and F cells (Figure2A and 2B, and data not shown). Comparison with control erythroid cultures indicates that the HbF reactivation was unrelated to maturation stage and cell proliferation, ie, percentage of late erythroblasts was unmodified and cell number per colony showed only a borderline increase in cultures treated with graded GM-CSF or IL-3 dosages (Figure 2A).
HbF reactivation in sibling BFU-E colonies.
(A) HbF reactivation in sibling BFU-Es grown in the presence of graded concentrations of GM-CSF or IL-3. HbF synthesis was evaluated in terms of γ-chain content in pooled sibling erythroid colonies. A representative experiment is shown. (B) Globin chain HPLC scans and percentage of F cells from pooled sibling BFU-E colonies grown in unilineage erythroid culture in the presence or absence of GM-CSF (1 ng/mL) (representative results).
HbF reactivation in sibling BFU-E colonies.
(A) HbF reactivation in sibling BFU-Es grown in the presence of graded concentrations of GM-CSF or IL-3. HbF synthesis was evaluated in terms of γ-chain content in pooled sibling erythroid colonies. A representative experiment is shown. (B) Globin chain HPLC scans and percentage of F cells from pooled sibling BFU-E colonies grown in unilineage erythroid culture in the presence or absence of GM-CSF (1 ng/mL) (representative results).
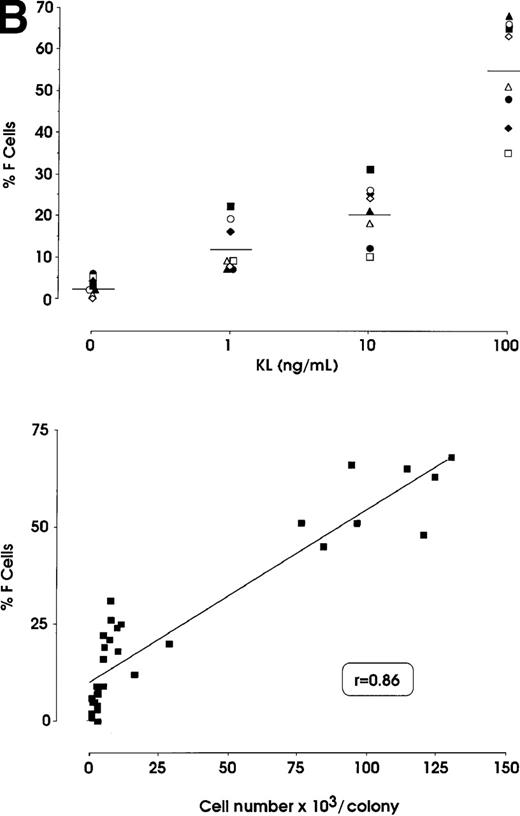
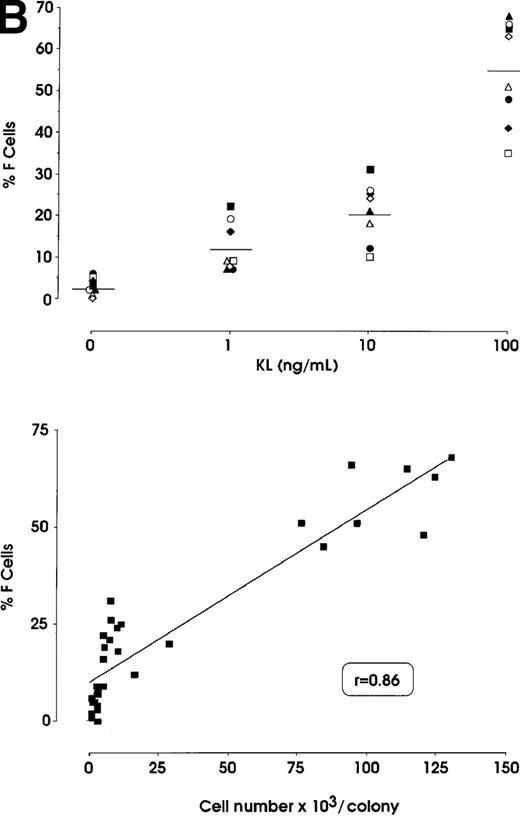
The effects of KL were evaluated in either pooled or single erythroid colonies generated by sibling BFU-Es (Figure3). At pooled colony level (Figure 3A), KL induced a dose-dependent increase in percentage of F cells (from 2.8% up to 58%) and γ-chain content (from 0.8% up to 22.5%). HbF reactivation was paralleled by an increase of colony size (from 2 × 103 up to 105 cells per colony). The correlation between cell numbers per colony and percentage values of F cells or HbF content was highly significant (P < .001, legend of Figure 3A). Although erythroid maturation was delayed in KL-treated culture, we consistently analyzed erythroid clones composed of more than 90% mature orthochromatic erythroblasts. At the single colony level (Figure 3B), KL induced a comparable dose-related HbF reactivation (from 2.5% up to 55% F cells) (top); percentage of F cell values and cell numbers per colonies were strictly correlated (P < .001) (bottom).
HbF reactivation in sibling BFU-E colonies.
(A) HbF reactivation in pooled sibling BFU-E colonies grown in erythroid culture in presence of graded amounts of KL. Clonogenetic parameters are shown in top panels. In the bottom part, HbF synthesis is monitored in terms of percentage of F cells and γ-chain content. Mean ± SEM values from 3 separate experiments. The correlation between cell numbers per colony and percentage values of F cells (r = 0.8, P = .0002) or HbF content (r = 0.91, P < .0001) is highly significant. (B) HbF reactivation in single sibling BFU-E colonies treated with graded amounts of KL, as evaluated in terms of F cells (% values) in each colony. Top panel, results from 8 groups of sibling colonies (mean values are shown). Bottom, direct correlation between F cell number (% values) and cell number × 103 per colony in the 32 sibling clones (see top panel).
HbF reactivation in sibling BFU-E colonies.
(A) HbF reactivation in pooled sibling BFU-E colonies grown in erythroid culture in presence of graded amounts of KL. Clonogenetic parameters are shown in top panels. In the bottom part, HbF synthesis is monitored in terms of percentage of F cells and γ-chain content. Mean ± SEM values from 3 separate experiments. The correlation between cell numbers per colony and percentage values of F cells (r = 0.8, P = .0002) or HbF content (r = 0.91, P < .0001) is highly significant. (B) HbF reactivation in single sibling BFU-E colonies treated with graded amounts of KL, as evaluated in terms of F cells (% values) in each colony. Top panel, results from 8 groups of sibling colonies (mean values are shown). Bottom, direct correlation between F cell number (% values) and cell number × 103 per colony in the 32 sibling clones (see top panel).
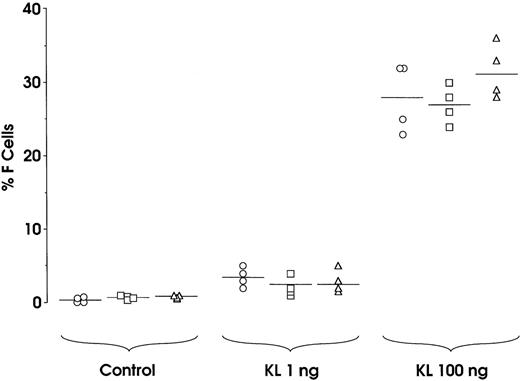
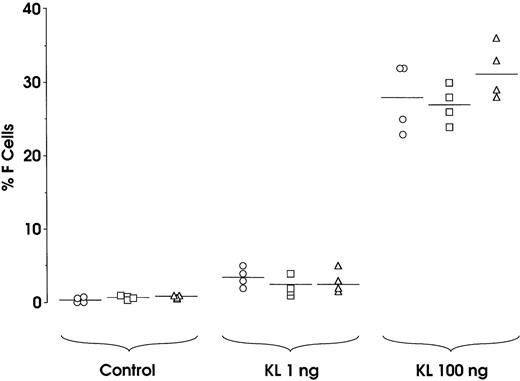
To comparatively evaluate the HbF synthesis potential of sibling BFU-Es, we analyzed 9 experimental groups, each composed of 4 daughter progenitors reseeded in a unicellular erythroid culture. At variance with the standard protocol (Figure 1), all sibling BFU-Es within each group were treated with 0, 1, or 100 ng KL; specifically, 3 groups were treated with each KL concentration. In all groups, the 4 sibling colonies consistently showed equivalent size, maturation stage, and percentage of F cells (Figure 4 and results not shown). This indicates that sibling BFU-Es have a similar potential, in terms of proliferation and differentiation,38as well as HbF synthesis.
Variation of HbF synthesis among sibling BFU-E clones in 9 experimental groups.
At variance with the standard protocol (Figure 1), the 4 sibling colonies within each group were identically treated, ie, the 4 sibling wells were supplemented with 0, 1, or 100 ng KL. Correlation between percentage values of F cells and cell number × 103per colony: r = 0.8603, P < .0001.
Variation of HbF synthesis among sibling BFU-E clones in 9 experimental groups.
At variance with the standard protocol (Figure 1), the 4 sibling colonies within each group were identically treated, ie, the 4 sibling wells were supplemented with 0, 1, or 100 ng KL. Correlation between percentage values of F cells and cell number × 103per colony: r = 0.8603, P < .0001.
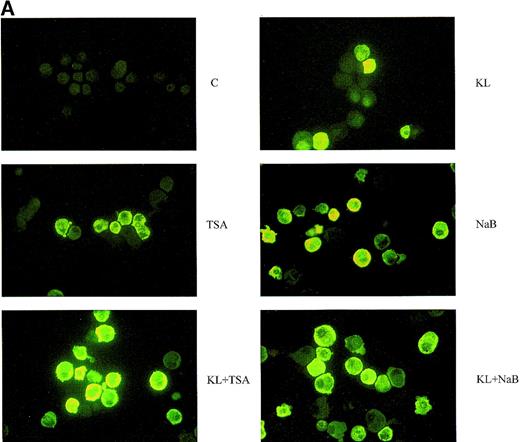
In a second series of experiments, we have analyzed the effects of the HD inhibitors NaB and TSA in minibulk erythroid culture. Treatment with TSA alone blocks erythroid differentiation at an early stage, thus rendering a difficult analysis of HbF content in mature erythroblasts. Nevertheless, the analysis of F cells in minibulk cultures revealed an increase of F erythroblasts (Figure 5A). In a dose-response experiment, NaB markedly reactivated HbF with a peak effect at 0.5 mmol/L, as evaluated in terms of F cells (up to 26%) and γ-globin content (up to 24%) (Table 1and Figure 5A and 5B).
Minibulk HPC erythroid cultures.
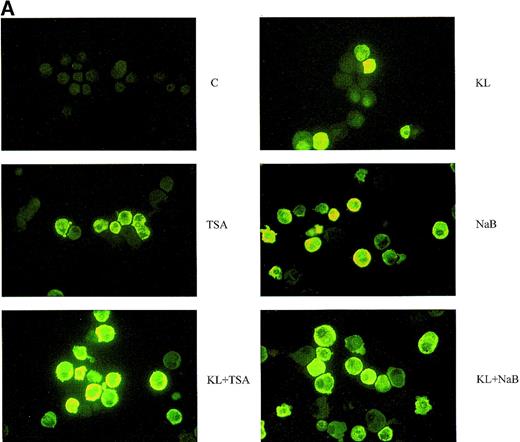
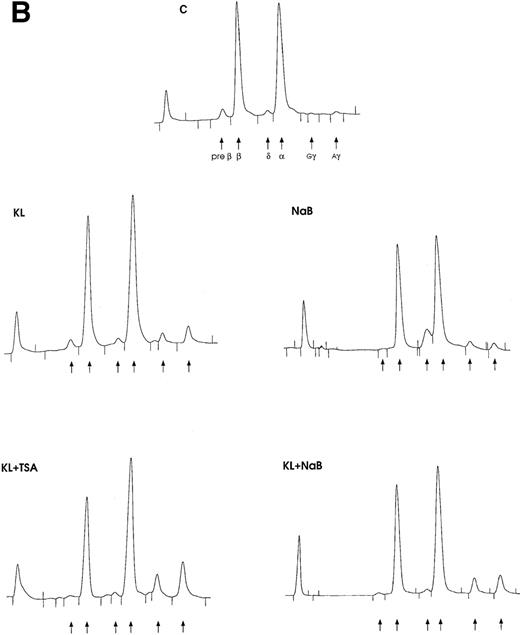
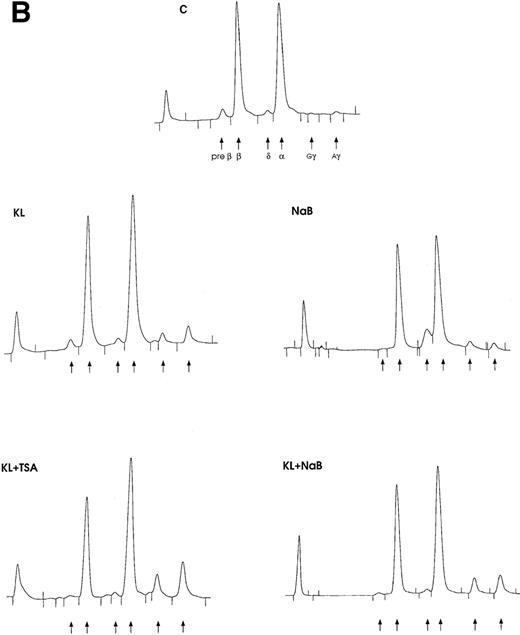
(A) F cells from minibulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± TSA (10 ng/mL) ± NaB (0.5 mmol/L). F cells were evaluated by indirect immunofluorescence using an antihuman HbF mAb as a primary antibody and an FITC-conjugated goat antimouse IgG as a secondary antibody. Original magnification × 630. Representative results are shown. (B) Globin chain HPLC scans from mature erythroblasts obtained in minibulk HPC erythroid cultures supplemented or not with KL, TSA, NaB, or combinations thereof (representative results).
Minibulk HPC erythroid cultures.
(A) F cells from minibulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± TSA (10 ng/mL) ± NaB (0.5 mmol/L). F cells were evaluated by indirect immunofluorescence using an antihuman HbF mAb as a primary antibody and an FITC-conjugated goat antimouse IgG as a secondary antibody. Original magnification × 630. Representative results are shown. (B) Globin chain HPLC scans from mature erythroblasts obtained in minibulk HPC erythroid cultures supplemented or not with KL, TSA, NaB, or combinations thereof (representative results).
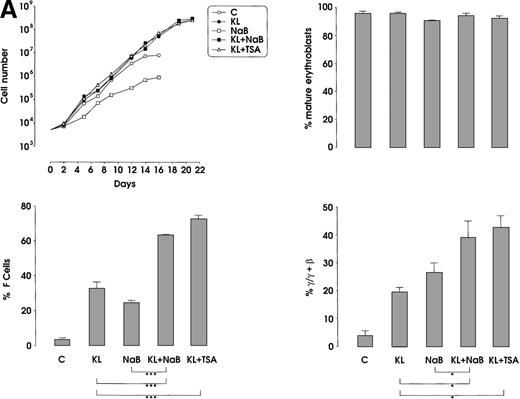
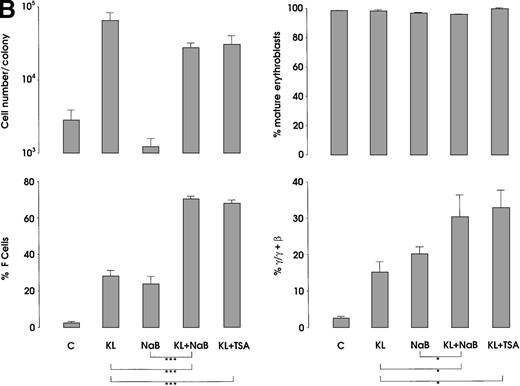
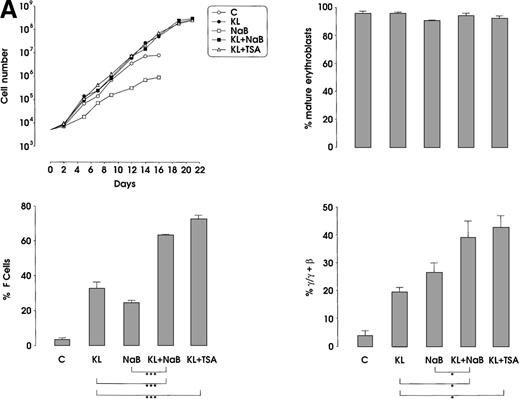
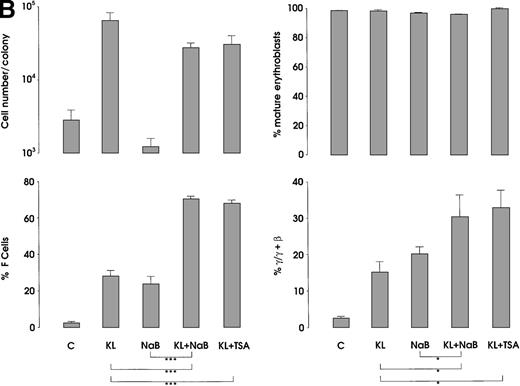
The third series of experiments was performed by combined addition of plateau levels of KL (100 ng/mL) and NaB or TSA (0.5 mmol/L and 10 ng/mL, respectively) in both minibulk and sibling BFU-E erythroid cultures (Figure 6A and 6B). Interestingly, the combined addition of KL and NaB or TSA induced a striking increase of HbF levels in terms of F cells and γ-globin content, up to 71% or 73% and 40% or 43%, respectively (Figure 6A). Although colonies treated with NaB alone were markedly small, KL restored a virtually normal proliferation in NaB-treated cultures, as well as in TSA-supplemented ones (Figure 6B). Furthermore, both NaB and TSA delayed differentiation/maturation along the erythroid pathway (more than 90% of mature erythroblasts were observed at day 20 through day 25 in KL + NaB or TSA culture, ie, 5 days later than in the KL-treated group) (Figure 6A and 6B).
HbF reactivation and synthesis.
(A) HbF reactivation in minibulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± NaB (0.5 mmol/L) or ± TSA (10 ng/mL). Clonogenetic parameters are shown in top panels. On the bottom, Hb synthesis is shown as F cells and γ-chain content percentage. Mean ± SEM values from 3 separate experiments. Variance analysis was performed to evaluate the difference between groups and expressed as Bonferroni P values. *P < .05 and ***P < .001 when compared with the indicated group. (B) HbF synthesis, evaluated in terms of F cells and γ-chain content, in pooled sibling BFU-E colonies grown in unilineage erythroid culture supplemented or not with KL ± NaB or ± TSA. Mean ± SEM values from 3 separate experiments. *P < .05 and ***P < .001 when compared with the indicated group.
HbF reactivation and synthesis.
(A) HbF reactivation in minibulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± NaB (0.5 mmol/L) or ± TSA (10 ng/mL). Clonogenetic parameters are shown in top panels. On the bottom, Hb synthesis is shown as F cells and γ-chain content percentage. Mean ± SEM values from 3 separate experiments. Variance analysis was performed to evaluate the difference between groups and expressed as Bonferroni P values. *P < .05 and ***P < .001 when compared with the indicated group. (B) HbF synthesis, evaluated in terms of F cells and γ-chain content, in pooled sibling BFU-E colonies grown in unilineage erythroid culture supplemented or not with KL ± NaB or ± TSA. Mean ± SEM values from 3 separate experiments. *P < .05 and ***P < .001 when compared with the indicated group.
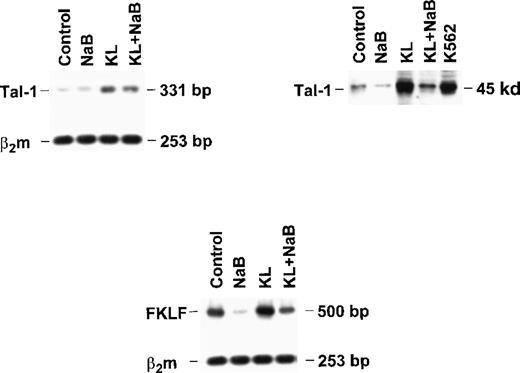
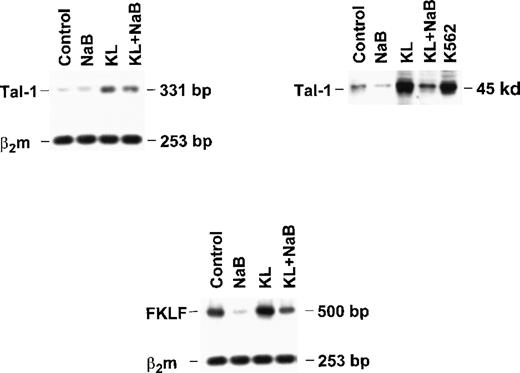
Finally, to explore the molecular mechanisms underlying γ-globin modulation induced by KL and HD inhibitors, we evaluated the expression of Tal-1 and FKLF in minibulk erythroid cultures grown under standard conditions (control) or in the presence of saturating levels of KL ± NaB (Figure 7). Semiquantitative RT-PCR showed that KL caused a significant increase in Tal-1 mRNA expression (top left panel), which was paralleled by a marked increase of Tal-1 protein levels (top right). Conversely, NaB caused a drop of Tal-1 mRNA and protein, which was rescued by combined NaB + KL treatment (top). Evaluation of fetal Krüppel-like factor (FKLF) mRNA levels showed a moderate increase in KL-supplemented cultures, in contrast to the marked drop induced by NaB, which was rescued by combined NaB + KL treatment (bottom panel).
Tal-1 and FKLF expression in bulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± NaB (0.5 mmol/L).
Tal-1 expression was evaluated in terms of RT-PCR (right top panel) and Western blotting (left top panel). FKLF expression was analyzed only by RT-PCR (bottom panel).
Tal-1 and FKLF expression in bulk HPC erythroid cultures supplemented or not with KL (100 ng/mL) ± NaB (0.5 mmol/L).
Tal-1 expression was evaluated in terms of RT-PCR (right top panel) and Western blotting (left top panel). FKLF expression was analyzed only by RT-PCR (bottom panel).
Discussion
Human HbF reactivation has been consistently observed in bulk cultures of normal adult erythroid progenitors.39-41However, elucidation of the underlying mechanisms was hampered by major methodology limitations, namely, (1) the variable effects of different FCS batches, (2) the rarity and heterogeneity of the HPCs, and (3) the large number of coplated accessory cells releasing endogenous cytokines. HbF reactivation was almost completely abolished in HPC FCS-free cultures,23,25,42 indicating that FCS directly and/or indirectly enhances HbF production. Thereafter, HbF reactivation was analyzed in bulk cultures of 90% to 95% purified HPCs, thus eliminating the accessory cell bias.27 Despite these advances, the heterogeneity of progenitors still represented a significant hurdle, particularly with respect to result interpretation (see below). In this regard, we developed a system for unilineage erythroid culture in liquid suspension FCS-free medium.37In this model, purified HPCs undergo a gradual and homogeneous wave of differentiation/maturation along the erythroid lineage up to terminal cells (ie, at day 14 to day 16, ≥ 97% cells are orthochromatic normoblasts). Thereafter, we developed a system for unicellular culture, whereby single sibling BFU-Es are grown in parallel in erythroid-specific medium and then comparatively analyzed at the level of their progeny.38 Specifically, while a small fraction (less than 20%) of the 4 sibling clones undergo apoptosis in this culture system, the other sibling progenitors generate pureerythroblast colonies and show an equivalent proliferative/ differentiative capacity.38
In the current studies, we used the sibling BFU-E approach to investigate the effects of IL-3, GM-CSF, and particularly KL in the reactivation of the HbF synthesis program. Four sibling BFU-Es, generated by a single progenitor and endowed with an equivalent HbF synthesis potential (Figure 4) were grown in a single-cell culture, and the resulting erythroid colonies were comparatively analyzed. Specifically, the control sibling BFU-E was untreated, whereas the other 3 siblings were treated with KL ± HD inhibitor at 3 graded concentrations; this allowed to perform a rigorous dose response with an internal negative control. Furthermore, the progeny of each sibling BFU-E was analyzed at the stage of terminal erythroblast, thus excluding that the positive effects on HbF synthesis were mediated by insufficient maturation of the erythroblasts. It is also noteworthy that GF treatment only slightly diminished the fraction of clones undergoing apoptosis (ie, from less than 20% to less than 10%).
Although previous studies on IL-3 and GM-CSF provided controversial results,25 the current observations unequivocally demonstrate that both multilineage HGFs exert a mild, but significant, stimulatory effect on HbF reactivation, as originally indicated in bulk BFU-E cultures.23 24
The stimulatory action of KL on HbF production in the bulk BFU-E culture is of uncertain significance,27 ie, it may be mediated by an effect on the BFU-E HbF synthesis program or the recruitment of BFU-Es with elevated HbF production potential. The latter mechanism, however, is excluded by the present observations, which demonstrate a direct, dose-related stimulatory effect of KL on HbF reactivation in sibling BFU-Es. It is well established that KL potentiates the erythroid proliferation induced by Epo.30Interestingly, the KL-induced rise of erythroid cell proliferation is strictly correlated with the reactivation of HbF. In human development, the erythroid proliferation rate gradually declines from the fetal through the perinatal and adult period.43 In this regard, fetal liver HPCs are particularly sensitive to KL,44 whose concentration is more elevated in cord than adult blood.45On this basis, it is hypothesized that the perinatal Hb switch may be, at least in part, mediated via a decline of KL activity and hence, erythroid proliferation activity.
Chemical compounds reactivating HbF9-12,14-16,46 may act via different mechanisms: TPX, TSA, and probably butyrate, and derivatives act via HD inhibition,47 whereas HU neither affects bulk histone acetylation, nor activates γ-globin promoter constructs.14 On the other hand, KL induces tal-148 and possibly other transcription factors such as FKLF,36 which may up-modulate γ-globin seemingly via promoter activation.36,48 Tal-1, in cooperation with other transcription factors, interacts with a conserved E-box in the β-globin control region enhancer to stimulate γ-globin gene transcription.49,50 FKLF is preferentially expressed in fetal rather than in adult erythroid tissue, whereas it stimulates γ-globin gene expression in the K562 cell line.36 Our experiments, performed on HPCs grown in erythroid culture in the presence of KL ± NaB, provided evidence that KL, but not NaB, exerted a marked stimulatory effect on Tal-1 expression, and a moderate increase of FLKF mRNA. This suggests that the stimulatory effect of KL on γ-globin gene expression may, at least in part, be mediated through enhanced Tal-1 and possibly FKLF expression. On the other hand, NaB seemingly exerts its stimulatory effect on HbF reactivation by a different molecular mechanism, ie, modification of chromatin structure of the β-globin gene cluster, resulting in increased transcriptional output of the γ-globin gene.51
Agents reactivating HbF synthesis via different molecular mechanisms may act additively or synergistically.52-54 The current studies demonstrate that the HD inhibitors TSA and NaB significantly potentiate the KL-induced reactivation of HbF in human adult BFU-Es. TSA also caused a partial block of BFU-E proliferation/differentiation, which was restored by combined treatment with KL. Similarly, the drop of Tal-1 and FLKF expression induced by NaB was restored by combined treatment with KL. We suggest that the additive effect exerted by KL and the HD inhibitors on HbF reactivation may be mediated via complementary mechanisms, as indicated above.
Finally, it is noteworthy that, in the clinical setting, combined treatment with HD inhibitors and KL may be considered for HbF reactivation in patients with β-globin disorders, as sickle cell anemia or β-thalassemia.
Reprints:C. Peschle, T. Jefferson University, Kimmel Cancer Center, Bluemle Life Science Bldg, 233 S 10th St, Philadelphia PA 19107-5541.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.