Abstract
High molecular weight kininogen (HK) and its cleaved form (HKa) have been shown to bind to neutrophils. Based on studies using monoclonal antibodies (mAbs), we postulated that CD11b/CD18 (Mac-1) might be the receptor on the neutrophils for binding to HK/HKa. However, the direct interaction of HK/HKa and Mac-1 had not been demonstrated. We therefore transfected HEK 293 cells with human Mac-1. Cell binding assays using fluorescein isothiocyanate-labeled HKa showed increased binding to the Mac-1 transfected cells compared with the control transfected cells. The binding was specific because unlabeled HKa, Mac-1–specific antibody, and fibrinogen can inhibit the binding of biotin-HKa to Mac-1 transfected cells. HKa bound to Mac-1 transfected cells (20 000 molecules/cell) with a Kd = 62 nmol/L. To demonstrate directly the formation of a complex between HKa and Mac-1, we examined the interaction of HKa and purified Mac-1 in a cell-free system using an IAsys resonant mirror optical biosensor. The association and dissociation rate constants (kon and koff, respectively) were determined, and they yielded a dissociation constant (Kd) of 3.2×10−9mol/L. The functional significance of direct interaction of HKa to Mac-1 was investigated by examining the effect of HKa on cellular adhesion to fibrinogen and intercellular adhesion molecule-1 (ICAM-1), molecules abundant in the injured vessel wall. HKa blocked the adhesion of Mac-1 transfected cells to fibrinogen and ICAM-1 in a dose-dependent manner. Thus, HKa may interrupt Mac-1–mediated cell–extracellular matrix and cell–cell adhesive interactions and may therefore influence the recruitment of circulating neutrophils/monocytes to sites of vessel injury.
High molecular weight kininogen (HK) is an abundant plasma protein (670 nmol/L) coded for by a gene with 10 exons containing 6 domains (Mr = 20 kd).1 HK, together with 2 other plasma proteins, prekallikrein and factor XII, are called the contact system in the blood coagulation cascade because they have been found to require contact with artificial, negatively charged surfaces for activation of the zymogens in vitro. Besides its essential role in the activation of coagulation when blood contacts foreign surfaces, such as in cardiopulmonary bypass,2 HK is a multifunctional protein. HK can interact with blood and vascular cells including platelets,3 neutrophils,4 monocytes, and endothelial cells.5,6 On each cell type, HK serves a discrete biologic function. It prevents the activation of platelets by inhibiting the calcium-activated cysteine protease calpain,7 and it prevents the binding of thrombin.8 Platelets have been shown to contain HK, which can be expressed on the exposed membrane surface of activated platelets.9-11 HK that circulates with plasma prekallikrein in a binary complex serves as an acquired receptor for kallikrein on the surfaces of neutrophils,12 allowing kallikrein to stimulate neutrophil aggregation13 and degranulation.14 Endothelial cell-bound HK is a substrate for plasma kallikrein, which cleaves HK to a 2-chain disulfide-linked molecule, HKa, and releases the nonapeptide bradykinin. It has been shown previously that HK/HKa can compete with an adhesive protein, fibrinogen, for binding to neutrophils,12 and that, as a consequence, it inhibits the adhesion of neutrophils to fibrinogen-coated surfaces under radial flow conditions.15It has also been shown that HK/HKa binds specifically, saturably, and reversibly to neutrophils in the presence of Zn2+.4 However, the cell surface receptor for HK/HKa binding to neutrophils is unclear. Although it is suggested to be the integrin Mac-1 (CD11b/CD18) based on the results of antibody blocking studies,12 a direct interaction between HK/HKa and Mac-1 integrin has not been demonstrated. Moreover, the occurrence of another receptor on neutrophils for HKa, the urokinase receptor, made it important to examine further the hypothesis that HK/HKa directly binds to Mac-1.
Integrins are a family of adhesion molecules serving functions involved in cell–cell and cell–extracellular matrix interactions. Mac-1 (CD11b/CD18), LFA-1 (CD11a/CD18), and p150,95 (CD11c/CD18), are leukocyte integrins. Mac-1, also known as complement receptor type 3 (CR3), is a heterodimeric receptor that is primarily expressed on monocytes, macrophages, neutrophils, and natural killer cells. The function of Mac-1 was initially described as the ability to bind iC3b (a cleaved form of C3b), and therefore to mediate the phagocytosis and lysis of iC3b-coated erythrocytes,16 and to contribute to elevated natural killer cell activity against iC3b-coated target cells.17 In addition to the function of Mac-1 in the immune defense system through binding to its ligand, iC3b, Mac-1 mediates a number of cell–cell and cell–extracellular matrix interactions in which iC3b is not involved, such as neutrophil aggregation18 and chemotaxis,19 and neutrophil adhesion to human umbilical vein endothelial cells.20 These observations suggested that Mac-1 is a multifunctional receptor. Through its ability to recognize multiple and unrelated ligands, including iC3b,16,17 fibrinogen,21 factor X,22 and the counter–receptor intercellular adhesion molecule-1 (ICAM-1),23 Mac-1 plays critical roles in cell adhesion, migration, and invasion, which are central to inflammation, immune responses, vascular biology, hemostasis, and thrombosis. The binding of Mac-1 to fibrinogen/fibrin results in the adhesion of neutrophils/monocytes to the sites of fibrin deposition, and the binding of Mac-1 to ICAM-1 causes the adhesion of neutrophils/monocytes to the endothelium. After binding to the zymogen of factor X, Mac-1 coordinates the activation of factor X independent of tissue factor and factor VII; this is followed by rapid fibrin formation.24Therefore, the ability to interfere with Mac-1–mediated leukocyte adhesion functions offers many opportunities for therapeutic intervention in diseases as diverse as thrombosis, inflammation, and cancer.
In this report, we show, for the first time, that cleaved high molecular weight kininogen, HKa, binds directly to Mac-1 both on cells and in a purified system, and we demonstrate that HKa inhibits Mac-1–mediated adhesion to fibrinogen and ICAM-1. This study may provide additional information for drug design in anti-adhesion therapy.
Materials and methods
Materials
Cleaved human high molecular weight kininogen, HKa, and human fibrinogen were purchased from Enzyme Research Laboratories (South Bend, IN). Recombinant human ICAM-1 was obtained from R&D Systems (Minneapolis, MN). Phosphate-buffered saline (PBS) and Hanks' balanced salt solution without calcium chloride, magnesium chloride, magnesium sulfate, and sodium bicarbonate were purchased from GIBCO BRL (Grand Island, NY). Reactive biotin analog, NHS-LC-biotin, was obtained from Pierce Chemical (Rockford, IL). Fluorescein isothiocyanate (FITC) on celite for fluorescent labeling of proteins and FITC-conjugated avidin were purchased from Sigma (St Louis, MO). 5-Chloromethylfluorescein diacetate for cell labeling was purchased from Molecular Probes. FITC-conjugated goat antimouse IgG (heavy and light chains) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). A monoclonal antibody, integrin αMm (44), specific for the integrin αμ subunit, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). 2LPM19c, a monoclonal antibody against the αμ subunit of Mac-1, was purchased from DAKO (Carpenteria, CA). A monoclonal anti-pan cytokeratin mixture (clone C-11) and a monoclonal anti-cytokeratin 4.62 were all purchased from Sigma.
Cell culture and transfection
To generate human embryonic kidney 293 cells (HEK 293) stably expressing Mac-1 (Mac-1–HEK 293), wild-type CD11b and CD18 subunits of Mac-1 in pCDM8 and plasmid pRSVneo were co-transfected into HEK 293 cells using lipofectin reagent according to the manufacturer's instructions (GIBCO BRL). Plasmid pRSVneo allowed for the selection of stable transfectants in medium supplemented with G418 sulfate. A single G418-resistant clone was selected, cloned, and screened for cell surface Mac-1 expression by flow cytometry. Stable transfected cell lines (Mac-1–HEK 293) were maintained in complete Dulbecco's modified eagle's medium containing 500 μg/mL G418. For control transfection, plasmid pRSVneo alone was transfected into HEK 293 cells using lipofectin reagent according to the manufacturer's instructions.
Flow cytometry and cell sorting
Cells were incubated with Mac-1–specific antibody LM2/1 (ascitic fluid, mouse IgG1, antihuman CD11b)25 for 45 minutes on ice. After they were washed 3 times with cold Hanks' balanced salt solution buffer with 1% bovine serum albumin (BSA), the cells were incubated with FITC-conjugated goat antimouse IgG for 30 minutes on ice. After they were washed 4 times, the cells were fixed with 1% paraformaldehyde in PBS overnight and then analyzed on a flow cytometer. For cell sorting, the cells were directly analyzed and sorted on a flow cytometer without fixation with paraformaldehyde.
Labeling of kininogen
For binding of cleaved human high molecular weight kininogen (HKa) to HEK 293 cells, HKa was labeled with FITC or biotin. Briefly, a total reaction volume of 1 mL containing 1 mg HKa, 1 mg celite–FITC (Sigma) or 1 mg NHS-LC–biotin was adjusted to pH 8.0 with 5% sodium bicarbonate solution. The reaction mixture was incubated for 20 minutes at room temperature with intermittent vortexing for labeling with FITC or for 2 hours on ice for labeling with biotin. The labeled HKa (FITC–HKa or biotin–HKa) was purified by Sephadex G-25 spin column (Bio-Rad Laboratories, Hercules, CA) and eluted with PBS. The labeled protein retained more than 95% of its procoagulant activity.
Binding assays
Unstimulated HEK 293 cells (4×106/mL), transfected with Mac-1 or control, were washed with binding buffer (10 mmol/L HEPES, pH 7.2, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.1% gelatin) and incubated with FITC–HKa at indicated concentrations for 30 minutes at room temperature. Nonspecific binding was measured by including 10 mmol/L EDTA in the binding assay. After they were washed, the cells were fixed with 1% paraformaldehyde in PBS and then analyzed on an Epics Elite flow cytometer (Coulter Diagnostics, Hialeah, FL). Alternatively, the binding reactions were carried out in wells of a filtration 96-well plate containing polyvinylidene difluoride (PVDF) membrane (pore size, 1.2 μm; Millipore, Bedford, MA). After filtration, the fluorescence of cell bound FITC–HKa was measured on a Cytofluor 2350 fluorescence plate reader (Millipore) with a 485-nm excitation filter and a 530-nm emission filter.
Inhibition of biotin–HKa binding to Mac-1–HEK 293 cells
Mac-1–HEK 293 cells were eluted from a confluent monolayer culture dish with PBS containing 5 mmol/L EDTA and washed with binding buffer (10 mmol/L HEPES, pH 7.2, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, 1 mmol/L MgCl2, 1 mmol/L CaCl2, and 0.1% gelatin). Cells (4×106/mL) in the same buffer were incubated with biotin–HKa (10 nmol/L) for 45 minutes at room temperature in the absence or presence of unlabeled HKa, antibodies, or fibrinogen at indicated concentrations. After the addition of avidin–FITC and continued incubation for 10 minutes, 3 aliquots from each reaction were transferred to the wells of a filtration 96-well plate containing PVDF membrane (pore size, 1.2 μm; Millipore). The wells were pre-wet with binding buffer for 2 hours. After filtration, the fluorescence of cell bound biotin–HKa was measured on a Cytofluor 2350 system (Millipore). The relative amount of biotin–HKa bound to the cells in the presence of inhibitors was determined by comparison with biotin–HKa alone.
Adhesion assays
HEK 293 cells transfected with Mac-1 used for adhesion assays were labeled with 5-chloromethylfluorescein diacetate (CMFDA). Briefly, confluent cells were eluted from dishes and resuspended in serum-free medium containing CMFDA (10 μmol/L) and incubated at 37°C for 30 minutes. Free probes were removed by washing with buffer used for adhesion assays (10 mmol/L HEPES, pH 7.2, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, 1 mmol/L MgCl2, 1 mmol/L CaCl2, and 0.1% gelatin). Ligands, fibrinogen (50 μg/mL), ICAM-1 (10 μg/mL), or fibronectin (15 μg/mL) were immobilized on 96-well Immulon II microtiter plates (Dynatech Laboratories, Chantilly, VA) at room temperature for 2 hours. The wells were then blocked with gelatin. Labeled cells (3-4 × 105/well) were added to wells and incubated in the presence or absence of indicated HKa for 60 minutes. Unbound cells were removed by washing with adhesion buffer 3 times. Adherent cells were lysed in 0.1 N NaOH, 0.2% sodium dodecyl sulfate (SDS), and 0.5% Triton X-100. The plates were read on a Cytofluor 2350 system (Millipore).
Purification of Mac-1 from human neutrophils
Mac-1 was purified from human neutrophils with slight modifications to the immunoaffinity purification procedure previously described.26 Briefly, human granulocytes were obtained from normal, healthy volunteers by leukopheresis using a Fenwal CS3000 blood cell separator (Baxter Healthcare, Deerfield, IL) followed by dextran density gradient. The enriched neutrophil product was then lysed with 0.05 mol/L Tris, pH 8.0, 2 mmol/L MgCl2, 1.0% Triton X-100, 0.15 mol/L NaCl, 5 mmol/L DFP, and 0.2 TIU/mL aprotinin for 1 hour at 4°C by gentle stirring before the resultant lysate was centrifuged at 50 000g to remove insoluble material. The clarified lysate was loaded by batch onto LM2/1 immunoaffinity resin (10 mL CNBr-activated Sepharose (Pharmacia Biotech, Piscataway, NJ) coupled with 5 mg LM2/1/mL resin) and incubated 16 hours at 4°C with gentle rotation. The resin was then loaded into a column (1.0×13 cm), and sequentially washed with 20 column volumes (CV) lysis buffer, 10 CV lysis buffer containing 1.0 mol/L NaCl, and 10 CV lysis buffer substituting 1.0% n-octyl glucoside for the Triton X-100. Mac-1 was eluted with 5 CV 0.1 mol/L sodium acetate, pH 4.0, 2 mmol/L MgCl2, 0.15 mol/L NaCl, and 1.0% n-octyl glucoside by collecting 1.0-mL fractions in polypropylene microcentrifuge tubes containing neutralizing buffer (10% by volume of 2 mol/L Tris, pH 9.0). Aliquots of the eluate fractions were immediately assessed by SDS–polyacrylamide gel electrophoresis (4%-15% gradient gels), and they showed 2 bands of equal density (MWt, 160 000 and 95,000). Peak fractions were pooled and underwent dialysis against 10 mmol/L HEPES, 0.137 mol/L NaCl, 4 mmol/L KCl, 11 mmol/L D-glucose, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 0.005% Tween-20 for 16 hours at 4°C. The final protein preparation was sectioned into aliquots and stored at −80°C until used. Protein concentration was determined using amino acid analysis.
Real-time bimolecular interaction assay
Analysis of Hka–Mac-1 interaction was carried out with purified human Mac-1 on an IAsys resonant mirror optical biosensor (Affinity Sensors, Cambridge, UK). In this assay, biotinylated HKa was immobilized on the sensor chip surface by using an IAsys biotin cuvette (FCB-0401; Affinity Sensors) and the accompanying protocol. Briefly, the biotin surface of the cuvette was washed with PBS/Tween-20 buffer, and streptavidin was added at a concentration of 10 μg/mL. After streptavidin was captured on the surface, Mac-1 was added to test whether there was any nonspecific binding of Mac-1 to streptavidin. No binding of Mac-1 to streptavidin was observed. Biotinylated HKa was then added to the streptavidin-captured surface, and the amount of immobilized HKa was equivalent to a signal of 400 arc seconds. The maximum signal (Rmax) expected on Mac-1 binding for the saturation of 90% of HKa binding sites was calculated from the molecular weight ratio of analyte (Mac-1)/captured ligand (HKa) and a stoichiometry of 1. The calculated Rmaxis 800 arc seconds. Because of the random biotinylation and steric hindrance factors that may reduce the stoichiometry of 1 to a fraction, a lower effective Rmax is expected (see “Results”). This cuvette immobilized with HKa was stored refrigerated in binding buffer and used in subsequent experiments for examining the interaction of Mac-1 and HKa. Purified Mac-1 was added at the indicated concentration, and the association was monitored. The binding buffer used was: 10 mmol/L HEPES, pH 7.2, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.05% Tween-20. In the dissociation phase, Mac-1 solution was replaced with the binding buffer, and the dissociation of the binding was monitored. The HKa surface was regenerated by the addition of 10 mmol/L EDTA and 0.5 mol/L NaCl and was followed by washing with binding buffer.
The kinetics of binding was evaluated from linear transformations of the binding signal.27 28 For a bimolecular interaction,
The rate equation for above reaction is shown in equation 2.
The signal from the biosensor (R) is proportional to the amount of complex (AB), and the maximum signal (Rmax) is proportional to the concentration of immobilized protein B, so equation 2 can be rewritten as:
Here, kon and koff are on and off rate constants, respectively, and [A] is the concentration of analyte injected into the sensor cuvette. A plot of dR/dt versus R has a slope (−ks) equal to −(kon[A] + koff). Straight lines were fit to the initial linear portion of the transformed association phase data to obtain ks. A second association phase is evident at high [A]. This phase, which could not be interpreted with the single bimolecular kinetic model, may be a consequence of increasing mass (aggregates) of analyte on the saturated surface. Replot of ks versus [A] yields a slope of kon. During the dissociation phase [A] = 0, so from equation 3:
Integrating equation 4 gives:
The dissociation phase of the highest concentration of analyte [A] was transformed by plotting ln(R0/R) versus time, and koff was calculated from the slope of a straight line over the initial dissociation phase.
Results
Binding of HKa to HEK 293 cells transfected with Mac-1
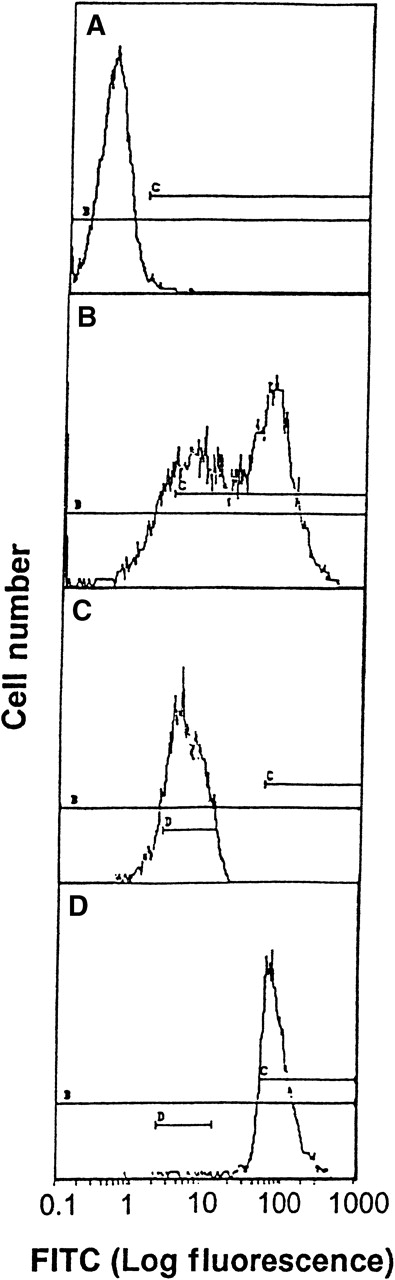
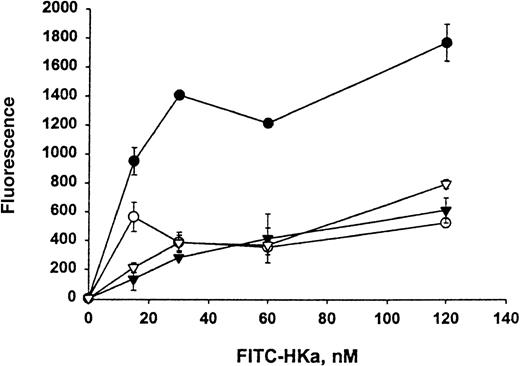
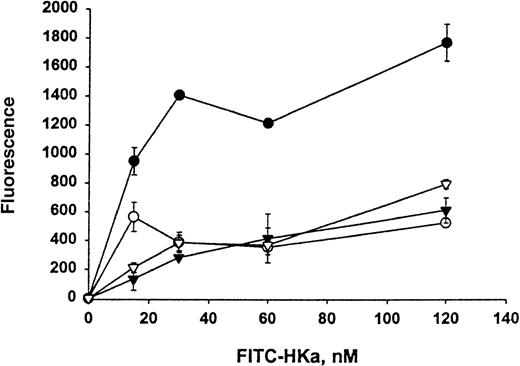
The direct interaction between HKa and Mac-1 was examined using HEK 293 cells stably transfected with a vector containing the cDNA for human Mac-1. Little signal was detected on control transfected HEK 293 cells with monoclonal antibody LM2/1, specific for Mac-1, as demonstrated by flow cytometry (Figure 1A). In contrast, the transfected HEK 293 cells contained 2 populations of cell surface Mac-1 expression (Figure 1B). HEK 293 cells expressing high and low levels of surface human Mac-1 were separated using FACSorting technique, as shown in Figure 1C,D. HEK 293 cells expressing high levels of surface human Mac-1 were used in subsequent experiments, and control transfected HEK 293 cells were used in control experiments. A concentration-dependent binding of FITC–Hka to the Mac-1 transfected HEK 293 cells was observed but was not found using the control transfected cells when varying concentrations of FITC–HKa were used in a cell-binding assay (Figure2). FITC–HKa specifically bound 3 times more to the Mac-1 transfected HEK 293 cells than to the control transfected cells (Figure 2). As a control, FITC-labeled BSA showed no binding to HEK 293 cells transfected with Mac-1 in the same type of binding assays (data not shown). In the presence of 10 mmol/L EDTA, the binding of FITC–HKa to Mac-1 transfected HEK 293 cells was inhibited 70% (Figure 2), indicating that the binding was metal dependent. Using the Scatchard plot of bound/free versus bound, we found evidence for a single class of binding site with Kd = 62 nmol/L and Bmax = 3.1 fmoles/105 cells (20 000 molecules per cell) in the Mac-1 transfected HEK 293 cell system.
Transfection of HEK 293 with Mac-1.
Flow cytometry analysis and FACSorting of HEK 293 cells transfected with Mac-1 (A to D). (A) Immunofluorescence assay using mAb LM2/1 with control transfected HEK 293 cells as a control. (B) Immunofluorescence assay using mAb LM2/1 with Mac-1 transfected HEK 293 cells. (C) Low level of expression of Mac-1, after FACSorting of Mac-1 transfected HEK cells. (D) High level of expression of Mac-1, after FACSorting of Mac-1 transfected HEK cells.
Transfection of HEK 293 with Mac-1.
Flow cytometry analysis and FACSorting of HEK 293 cells transfected with Mac-1 (A to D). (A) Immunofluorescence assay using mAb LM2/1 with control transfected HEK 293 cells as a control. (B) Immunofluorescence assay using mAb LM2/1 with Mac-1 transfected HEK 293 cells. (C) Low level of expression of Mac-1, after FACSorting of Mac-1 transfected HEK cells. (D) High level of expression of Mac-1, after FACSorting of Mac-1 transfected HEK cells.
Specific binding of HKa to HEK 293 cells transfected with Mac-1 (Mac-1–HEK 293).
FITC–HKa (at indicated concentrations) was incubated with HEK 293 cells either transfected with Mac-1 or control transfected in binding buffer containing 50 μmol/L ZnCl2 for 30 minutes at room temperature, as described in “Materials and methods.” The same reaction was also performed in the presence of 10 mmol/L EDTA to determine the nonspecific binding. Concentrations of FITC–HKa used were 0, 15, 30, 60, and 120 nmol/L. Reactions were carried out in wells of a filtration 96-well plate containing polyvinylidene difluoride membrane. After filtration, the fluorescence of cell-bound FITC–HKa was measured on a Cytofluor 2350 system. (•) HKa binding to Mac-1 transfected HEK 293 cells. (▾) HKa binding to untransfected HEK 293 cells. (○) HKa binding to Mac-1 transfected HEK 293 cells in the presence of 10 mmol/L EDTA. (▿) HKa binding to untransfected HEK 293 cells in the presence of 10 mmol/L EDTA. The data are the mean ± SE of triplicate reactions.
Specific binding of HKa to HEK 293 cells transfected with Mac-1 (Mac-1–HEK 293).
FITC–HKa (at indicated concentrations) was incubated with HEK 293 cells either transfected with Mac-1 or control transfected in binding buffer containing 50 μmol/L ZnCl2 for 30 minutes at room temperature, as described in “Materials and methods.” The same reaction was also performed in the presence of 10 mmol/L EDTA to determine the nonspecific binding. Concentrations of FITC–HKa used were 0, 15, 30, 60, and 120 nmol/L. Reactions were carried out in wells of a filtration 96-well plate containing polyvinylidene difluoride membrane. After filtration, the fluorescence of cell-bound FITC–HKa was measured on a Cytofluor 2350 system. (•) HKa binding to Mac-1 transfected HEK 293 cells. (▾) HKa binding to untransfected HEK 293 cells. (○) HKa binding to Mac-1 transfected HEK 293 cells in the presence of 10 mmol/L EDTA. (▿) HKa binding to untransfected HEK 293 cells in the presence of 10 mmol/L EDTA. The data are the mean ± SE of triplicate reactions.
Inhibition of biotin–HKa binding to Mac-1 transfected HEK 293 cells
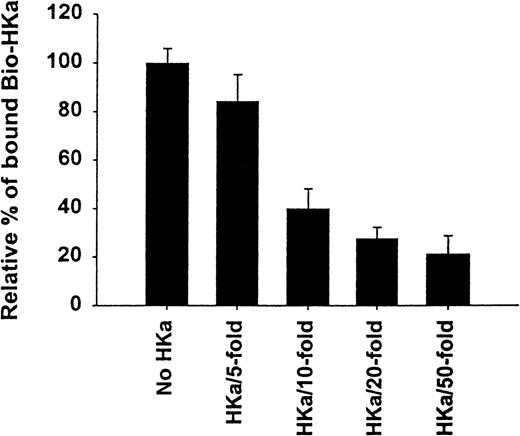
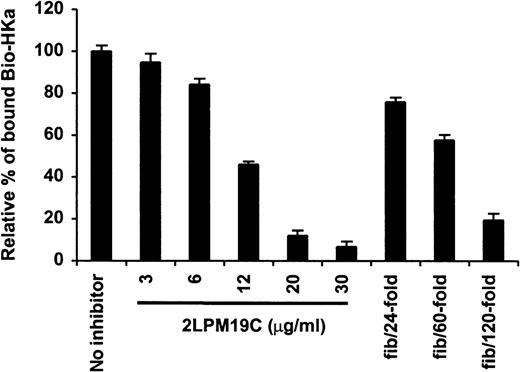
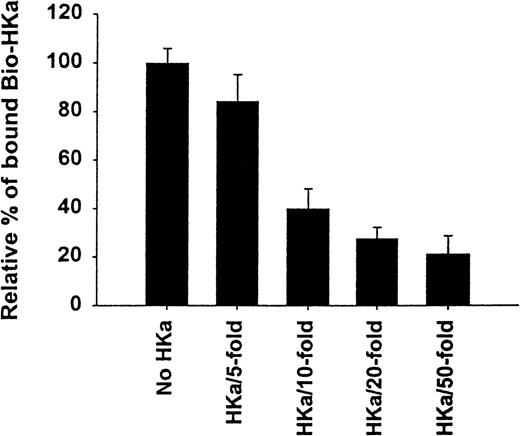
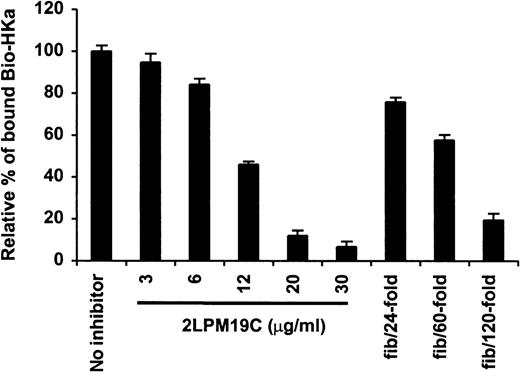
The specificity of biotin–HKa binding to Mac-1 transfected cells was examined using nonbiotinylated HKa in a competition binding assay. Figure 3 showed a concentration-dependent inhibition of biotin–HKa binding to Mac-1 transfected cells by unlabeled HKa. In the presence of 50-fold excess of unlabeled HKa, the binding of biotin–HKa to Mac-1 transfected cells was reduced to 20%. Fibrinogen is a known ligand for binding to Mac-1.21 A concentration-dependent inhibition of biotin–HKa binding to Mac-1 transfected cells by fibrinogen was observed (Figure 4), indicating that fibrinogen competed with biotin–HKa in binding to Mac-1 transfected cells. Antibody 2LPM19c, a monoclonal antibody against the αμ subunit of Mac-1, inhibited the binding of biotin–HKa (10 nmol/L) to Mac-1–HEK cells by more than 90% with a 50% inhibitory concentration of 10 ± 3 μg/mL (Figure 4), indicating that the interaction between HKa and Mac-1 transfected cells was mediated primarily by the αμ subunit of Mac-1.
Inhibition of biotin-HKa binding to Mac-1 transfected cells by HKa.
Biotin–HKa (10 nmol/L) was incubated with Mac-1 transfected HEK 293 cells (4 × 106/mL) in binding buffer containing 50 μmol/L ZnCl2 for 45 minutes at room temperature in the presence of indicated HKa concentrations. The fluorescence of cell-bound biotin–HKa was measured, and the percentage of biotin–HKa bound to the cells in the presence of HKa was determined by comparing that with biotin–HKa alone, which was 100%. Data are the mean ± SE of 3 separate experiments.
Inhibition of biotin-HKa binding to Mac-1 transfected cells by HKa.
Biotin–HKa (10 nmol/L) was incubated with Mac-1 transfected HEK 293 cells (4 × 106/mL) in binding buffer containing 50 μmol/L ZnCl2 for 45 minutes at room temperature in the presence of indicated HKa concentrations. The fluorescence of cell-bound biotin–HKa was measured, and the percentage of biotin–HKa bound to the cells in the presence of HKa was determined by comparing that with biotin–HKa alone, which was 100%. Data are the mean ± SE of 3 separate experiments.
Inhibition of biotin–HKa binding to Mac-1 transfected cells by antibody and fibrinogen.
Biotin–HKa (10 nmol/L) was incubated with Mac-1 transfected or control transfected HEK 293 cells (4 × 106/mL) in binding buffer containing 50 μmol/L ZnCl2, 1 mmol/L MgCl2, and 1 mmol/L CaCl2 for 45 minutes at room temperature. The reactions were carried out either in the presence or absence of antibody, 2LPM19c, or fibrinogen as indicated. The relative amount of biotin–HKa bound to the Mac-1 transfected cells in the presence of inhibitors was determined by comparing that with biotin–HKa alone. Data are the mean ± SE of 3 separate experiments.
Inhibition of biotin–HKa binding to Mac-1 transfected cells by antibody and fibrinogen.
Biotin–HKa (10 nmol/L) was incubated with Mac-1 transfected or control transfected HEK 293 cells (4 × 106/mL) in binding buffer containing 50 μmol/L ZnCl2, 1 mmol/L MgCl2, and 1 mmol/L CaCl2 for 45 minutes at room temperature. The reactions were carried out either in the presence or absence of antibody, 2LPM19c, or fibrinogen as indicated. The relative amount of biotin–HKa bound to the Mac-1 transfected cells in the presence of inhibitors was determined by comparing that with biotin–HKa alone. Data are the mean ± SE of 3 separate experiments.
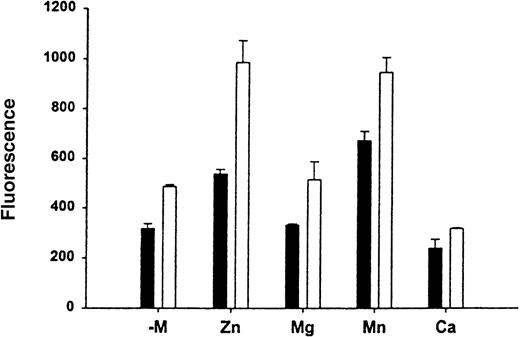
Effect of divalent cations on the HKa binding to Mac-1
To determine the effect of divalent cations on the HKa and Mac-1 interaction, binding assays of FITC–HKa to Mac-1 transfected or untransfected HEK 293 cells were performed in the presence of different divalent cations as indicated. In each binding assay, cells were preincubated with buffer containing 10 mmol/L EDTA for 10 minutes at 37°C and then washed with binding buffer without any divalent cation. As shown in Figure 5, in the presence of Zn2+, the binding of HKa to Mac-1 transfected HEK 293 cells increased 2-fold. In the presence of Mn2+, the binding of HKa to Mac-1 transfected HEK 293 cells was increased; however, the binding of HKa to untransfected cells was also increased. There is no significant difference in HKa binding to Mac-1 transfected cells with or without the presence of Mg2+.
Effect of divalent cation on the HK-Mac-1 interaction.
To determine the effect of divalent cations on the HK–Mac-1 interaction, binding assays of FITC–HKa (60 nmol/L) to Mac-1 transfected (open bars) or untransfected (black bars) HEK cells were performed in the presence of different divalent cations as indicated. Cells were preincubated with 10 mmol/L EDTA for 10 minutes at 37°C, then washed with binding buffer without any divalent cation (−mol/L). One of the divalent cations was added to each assay as follows: MgCl2, MnCl2, and CaCl2 at 1 mmol/L, and ZnCl2 was added at 50 μmol/L.
Effect of divalent cation on the HK-Mac-1 interaction.
To determine the effect of divalent cations on the HK–Mac-1 interaction, binding assays of FITC–HKa (60 nmol/L) to Mac-1 transfected (open bars) or untransfected (black bars) HEK cells were performed in the presence of different divalent cations as indicated. Cells were preincubated with 10 mmol/L EDTA for 10 minutes at 37°C, then washed with binding buffer without any divalent cation (−mol/L). One of the divalent cations was added to each assay as follows: MgCl2, MnCl2, and CaCl2 at 1 mmol/L, and ZnCl2 was added at 50 μmol/L.
Interaction of HKa and purified Mac-1
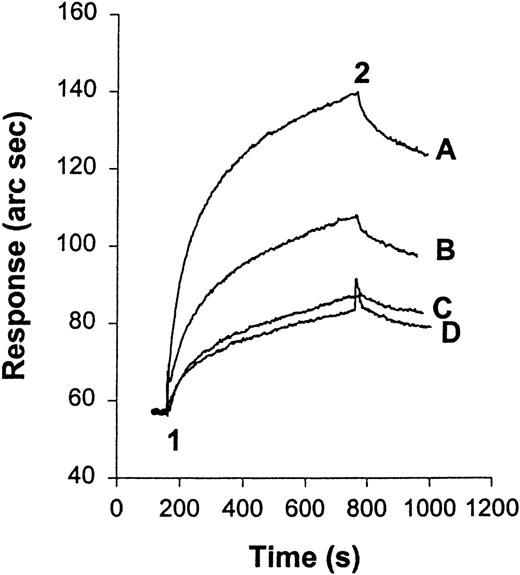
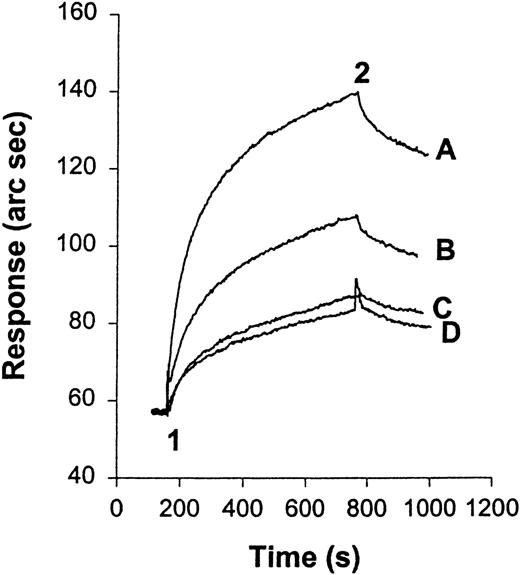
We have examined the interaction of HKa and purified Mac-1 using an IAsys resonant mirror optical biosensor (Affinity Sensors). In this type of binding assay, biotinylated HKa was immobilized on the sensor chip surface by binding to streptavidin that had been pre-captured on the surface of an IAsys biotin cuvette (Affinity Sensors). Before HKa was immobilized on the surface of the cuvette, a control experiment was performed in which purified Mac-1 was added to the streptavidin surface. No interaction was detected between the streptavidin surface and Mac-1 at the highest concentration used in this experiment (data not shown). Purified Mac-1 was then added at indicated concentrations to the Hka-captured surface, and the response (in arc seconds) versus time (in seconds) was recorded. An overlay of sensor-grams for the binding of Mac-1 to the HKa surface is shown in Figure 6A. Sensor-grams show 2 phases: an association phase, detected when purified human Mac-1 was added and was allowed to bind to the immobilized HKa (20-300 seconds), and a dissociation phase, in which the Mac-1 solution was replaced with buffer (300-400 seconds). The association phase was linearized according to equation 3, and a plot of dR/dt versus R is shown in Figure 6B. A replot of the slope of these lines (ks) against Mac-1 concentration gives a straight line (Figure 6C). The slope of this straight line equals the association rate constant (kon) of 5.6 × 106M−1s−1. The dissociation phase was analyzed according to equation 5. Figure 6D shows a plot of ln(R1/Rn) versus time using the dissociation phase data of the highest concentration of Mac-1. This plot did not fit the model of single exponential decay. To estimate the dissociation rate constants, the plot ln(R1/Rn) versus time was divided into fast and slow phases (phases 1 and 2, respectively). The dissociation rate constant (koff) calculated from the slope of the first 20 seconds of this plot (phase 1) was 18.1×10−3s−1. Then the equilibrium dissociation constant (Kd), calculated from the ratio of the rate constants, was 3.2 nmol/L. Independent evaluation of Kdfrom the steady state binding signal resulted in an estimated value of 5.26 nmol/L (R2 = 0.95; Scatchard plot not shown).
A real-time observation of HK-Mac-1 interaction.
(A) Overlay sensor-gram from the IAsys showing the binding of Mac-1 to immobilized HKa. At “1” purified human Mac-1 was added to the cuvette at the following concentrations: 5, 10, 20, 40, 60, 80, 120, and 160 nmol/L. At “2” the cuvette was washed with buffer (10 mmol/L HEPES, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.05% Tween-20). (B) dR/dt plots of association data according to equation 3. Straight lines were fitted to the initial linear portion of the association phase data to obtain ks. (C) Plot of ks versus Mac-1 concentration. The slope of the line gives the association rate constant. (D) Dissociation phase data from 160 nmol/L Mac-1 sensor-gram plotted according to equation 5. A straight line was fitted to the first 20 seconds of the plot for phase 1, and the other straight line was fitted to the data between 20 and 70 seconds for phase 2.
A real-time observation of HK-Mac-1 interaction.
(A) Overlay sensor-gram from the IAsys showing the binding of Mac-1 to immobilized HKa. At “1” purified human Mac-1 was added to the cuvette at the following concentrations: 5, 10, 20, 40, 60, 80, 120, and 160 nmol/L. At “2” the cuvette was washed with buffer (10 mmol/L HEPES, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.05% Tween-20). (B) dR/dt plots of association data according to equation 3. Straight lines were fitted to the initial linear portion of the association phase data to obtain ks. (C) Plot of ks versus Mac-1 concentration. The slope of the line gives the association rate constant. (D) Dissociation phase data from 160 nmol/L Mac-1 sensor-gram plotted according to equation 5. A straight line was fitted to the first 20 seconds of the plot for phase 1, and the other straight line was fitted to the data between 20 and 70 seconds for phase 2.
Mac-1 transfected HEK 293 cells binding to HKa-immobilized surface
Specific binding of HKa to Mac-1 transfected HEK 293 cells was also observed using the IAsys resonant mirror optical biosensor (Affinity Sensors) (Figure 7). In this assay, biotinylated HKa was immobilized on the surface of a cuvette as described in “Materials and methods.” The binding of Mac-1 transfected HEK cells to HKa was significantly greater than that of untransfected cells to HKa at equivalent cell numbers. The binding was Zn2+ dependent because, in the presence of 10 mmol/L EDTA, the binding of Mac-1 transfected HEK cells to HKa was decreased by 50%.
HEK 293 cells transfected with Mac-1 or untransfected binding to immobilized HKa using the IAsys optical sensor.
At “1” cells (105) were added, and at “2” they were washed with buffer (10 mmol/L HEPES, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.5% BSA). (A) Mac-1 transfected HEK 293 cells. (B) Untransfected HEK 293 cells. (C, D) Untransfected and Mac-1 transfected HEK 293 cells in the presence of 10 mmol/L EDTA, respectively. Data are representative of 3 separate experiments.
HEK 293 cells transfected with Mac-1 or untransfected binding to immobilized HKa using the IAsys optical sensor.
At “1” cells (105) were added, and at “2” they were washed with buffer (10 mmol/L HEPES, 137 mmol/L NaCl, 4 mmol/L KCl, 50 μmol/L ZnCl2, and 0.5% BSA). (A) Mac-1 transfected HEK 293 cells. (B) Untransfected HEK 293 cells. (C, D) Untransfected and Mac-1 transfected HEK 293 cells in the presence of 10 mmol/L EDTA, respectively. Data are representative of 3 separate experiments.
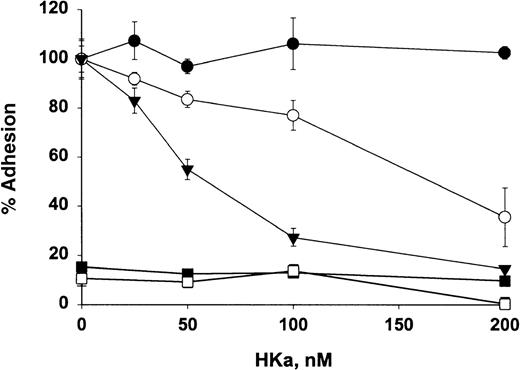
HKa inhibits Mac-1–mediated cell adhesion to fibrinogen and ICAM-1
The functional relevance of HKa directly interacting with Mac-1 was investigated by examining the effect of HKa on cellular adhesion to fibrinogen and ICAM-1, molecules abundant in the injured vessel wall. As shown in Figure 8, HKa blocked adhesion of Mac-1 transfected HEK cells to fibrinogen and ICAM-1 by 62% and 85%, respectively. HKa has no effect on the adhesion of Mac-1 transfected HEK cells to fibronectin, which is mediated by β1 integrin, indicating that HKa specifically inhibits the Mac-1– mediated adhesion. Moreover, untransfected HEK cells failed to adhere to fibrinogen or ICAM-1.
Effect of HKa on cellular adhesion to fibrinogen and ICAM-1.
HEK 293 cells transfected with Mac-1 were labeled with CMFDA, added to wells precoated with the ligands ICAM-1 (▾), fibrinogen, (○) or fibronectin (•), and incubated in the presence or absence of indicated HKa for 60 minutes. As a control, untransfected HEK 293 cells labeled with CMFDA were added to fibrinogen-coated (▪) or ICAM-1–coated (□) wells. After they were washed 3 times, adherent cells were lysed. Plates were read on a Cytofluor 2350 system. Percentage of adherent cells to each ligand in the presence of HKa was determined by comparing that with in the absence of HKa, which was calculated as 100%. Data are the mean ± SE of 3 separate experiments.
Effect of HKa on cellular adhesion to fibrinogen and ICAM-1.
HEK 293 cells transfected with Mac-1 were labeled with CMFDA, added to wells precoated with the ligands ICAM-1 (▾), fibrinogen, (○) or fibronectin (•), and incubated in the presence or absence of indicated HKa for 60 minutes. As a control, untransfected HEK 293 cells labeled with CMFDA were added to fibrinogen-coated (▪) or ICAM-1–coated (□) wells. After they were washed 3 times, adherent cells were lysed. Plates were read on a Cytofluor 2350 system. Percentage of adherent cells to each ligand in the presence of HKa was determined by comparing that with in the absence of HKa, which was calculated as 100%. Data are the mean ± SE of 3 separate experiments.
Discussion
Plasma kallikrein activates human neutrophils,14,15 and, in plasma, prekallikrein circulates in a binary complex with high molecular weight kininogen (HK).29 Bradykinin is released from HK by plasma kallikrein cleavage. Cleaved HK (HKa) consists of a heavy chain and a light chain that remain linked by a single interchain disulfide bond. On the basis of studies of a patient with HK deficiency,12 we suggested that HK might serve as a cofactor for kallikrein binding to neutrophils. Later, we demonstrated that human neutrophils contain and bind HK,4 and further studies of HK binding to neutrophils indicated that both heavy chain (domain 3) and light chain (domain 5) of HK were involved in the binding to neutrophils.30 We have shown that fibrinogen acts as a noncompetitive inhibitor of HK binding to neutrophils.12 Inhibition studies with monoclonal antibodies suggested that Mac-1 might be the HK binding site on neutrophils. However, a direct interaction between HK/HKa and Mac-1 has not been demonstrated. Although both HK and HKa have been used previously for studies of the binding of kininogen to neutrophils, the contamination of HKa in HK preparation is not excluded. In this study, we used HKa and showed for the first time that HKa binds directly to cell surface human Mac-1 integrin and, consistent with this, forms a complex with Mac-1 in a purified system.
To examine the direct interaction of HKa and Mac-1 integrin, we initially used Chinese hamster ovary (CHO) cells stably transfected with human Mac-1 in the binding studies. However, we found no significant difference in HKa binding to Mac-1 transfected CHO cells or control transfected CHO cells (data not shown), indicating that other receptor(s) may be present on the surface of CHO cells and may be bound to HKa. Because it has been shown that 1 binding site for HKa on human endothelial cells is urokinase plasminogen activator receptor (uPAR),31 we postulated that the binding of HKa to control transfected or untransfected CHO cells is probably caused by the binding of HKa to uPAR. Recently, human prourokinase was demonstrated to bind to 50 000 sites on CHO cells with Kd = 1.1 nmol/L. Cloning of the receptor showed a 63% identity with human uPAR,32 consistent with our hypothesis.
Because human embryonic kidney 293 cells (HEK 293) normally do not express uPAR or Mac-1 receptor, we used this cell line to transfect with the human Mac-1 receptor. Our data showed that the binding of HKa to Mac-1 transfected HEK 293 cells was 3-fold greater than the binding of HKa to control transfected HEK 293 cells. The following evidence from this report indicated that the binding of HKa to Mac-1 was specific. Unlabeled HKa inhibited the binding of biotin-HKa to Mac-1 transfected HEK 293 cells in a concentration-dependent manner. A known Mac-1 ligand, fibrinogen, also inhibited the binding of biotin–HKa to Mac-1 transfected cells, but with lower binding affinity to Mac-1 than HKa (Figures 3, 4). This agrees with our previous finding that fibrinogen has a lower binding affinity to human neutrophils than kininogen.12 Antibody against the α subunit of Mac-1 inhibited the binding of biotin–HKa to Mac-1 transfected HEK 293 cells by 50%.
In the studies of HKa binding to Mac-1 transfected HEK 293 cells, we have found that there was some residual binding (20%) of HKa to untransfected HEK 293 cells (data not shown). Recently, Hasan et al33 reported that cytokeratin 1 is a major endothelial cell receptor for kininogens. To examine whether cytokeratin 1 is present on HEK 293 cells, we performed an immunofluorescence assay using the collections of monoclonal anti-cytokeratins because a cytokeratin 1-specific monoclonal antibody was unavailable. We used a monoclonal anti-pan cytokeratin mixture that recognizes human cytokeratins 1, 4, 5, 6, 8, 10, 13, 18, and 19; a monoclonal anti-pan cytokeratin (clone C-11) that recognizes cytokeratins 4, 5, 6, 8, 10, 13, and 18; and monoclonal anti-cytokeratin 4.62, which is immunospecific for cytokeratin 19. Flow cytometry results showed that the first of these antibody mixtures, the only 1 containing cytokeratin 1, was positive, indicating that cytokeratin 1 is expressed on HEK 293 cells (data not shown). Therefore, a possibility for the residual binding is cytokeratin 1. In addition, Herwald et al34isolated a 33-kd protein, which was identified as gC1q receptor,35 on an HK affinity column from EA.hy926 cells, a human umbilical vein endothelial cell line. The binding of HK to gC1q receptor did not require Zn2+, though other investigators36 report that Zn2+ is required for ligand blots. Another explanation for the residual binding is the presence of the gC1q receptor.
With the use of EDTA, the binding of HKa to Mac-1 transfected cells was greatly reduced, indicating that the binding was metal dependent. Examining the effect of divalent cations on the HKa binding to Mac-1 transfected cells showed that, in the presence of Zn2+, the binding of HKa to Mac-1 transfected HEK 293 cells increased 2-fold (Figure 5). The trace element Zn2+ is an important cofactor of several proteins and enzymes, such as transcription factors,37,38 focal adhesion molecules,39 or matrix metalloproteinases.40Previous studies have shown that the binding of HKa to neutrophils, platelets, and human umbilical vein endothelial cells requires Zn2+, and Zn2+ is the only divalent cation that appears to be essential.3-5,31 In plasma, the total Zn2+ concentration is 10 to 25 μmol/L.41 The actual free Zn2+ concentration is significantly lower (1-3 μmol/L) because most of the ion is bound to albumin.42Mg2+ or Ca++ alone does not support the binding of HKa to Mac-1 transfected HEK 293 cells. Altieri43 has reported that Mn2+ ions can dramatically stimulate the adhesive functions of the leukocyte integrin Mac-1. In our experiment, Mn2+ stimulated the binding of HKa to Mac-1 transfected HEK 293 cells 2-fold; however, the nonspecific binding of HKa to untransfected HEK 293 cells was also increased (Figure 5). A structural feature of leukocyte integrins is that each α subunit contains 3 homologous repeats that have putative divalent cation-binding sites. Ca++ and Mg2+ are required for stabilizing the interaction of integrin α and β chains44 and, therefore, are required for the function of integrins. It is unclear whether Zn2+ can play a similar role in stabilizing the interaction of integrin α and β domains.
Using an IAsys resonant mirror optical biosensor (Affinity Sensors), we examined the interaction of HKa and purified Mac-1. With this technique, it is possible to monitor both the association and the dissociation between HKa and Mac-1 in real-time and, therefore, to measure rate constants. Linearization analysis of Hka–Mac-1 binding data showed a good fit to a theoretical 1:1 interaction model for the lower concentration range of Mac-1. The dissociation phase analysis did not fit the model of single exponential decay, especially at high concentrations of Mac-1, as shown by the curvature of the plot in Figure 6D. The Scatchard plots of Req/C (bound/free) versus Req (bound), which were estimated from the extrapolated values of R (response) at dR/dt = 0, showed a single slope with Kd = −1/slope = 5.26 nmol/L. At higher Mac-1 concentrations, the deviation from the 1:1 model may result from the binding of Mac-1 to the lower affinity sites of HKa molecules generated by random biotinylation or from an aggregation of Mac-1 molecules. The equilibrium dissociation constant (Kd), calculated by using the dissociation rate constant for phase 1 and the calculated kon, was 3.2 nmol/L. This Kd agrees reasonably well with the Kd of 9 to18 nmol/L for the binding of HK to neutrophils reported previously by Gustafson et al.4 The equilibrium dissociation constant (Kd) calculated from the slowest dissociation step was 0.33 nmol/L. This value is approximately 1 order lower than that of HK binding to neutrophils.4 However, interactions at a surface with the receptor as the soluble ligand may account for the discrepancy.
Leukocyte integrin Mac-1 recognizes multiple and unrelated ligands, including iC3b, fibrinogen, factor X, and ICAM-1.16,17,21-23 The engagement of Mac-1 with its ligands fibrinogen, factor X, or serum-opsonized zymosan triggered monocyte degranulation and cathepsin G activation of factor X.45Activated platelets express not only P-selectin but also different β2-integrin ligands, including fibrinogen and ICAM-2. Polymorphonuclear adhesion to activated platelets is initiated with a P-selectin–dependent recognition step and is followed by adhesion-strengthening interactions mediated by β2-integrin Mac-1.46 HKa could potentially inhibit these functions of the neutrophils or monocytes. However, since there are multiple receptors on neutrophils or monocytes, it is difficult to prove that inhibition with HKa is brought about by the interaction of HKa with Mac-1.
Here, we report that cleaved high molecular weight kininogen (HKa) is another ligand for Mac-1. HKa can bind directly to Mac-1, both on cells and in a purified system. To investigate the functional significance of Mac-1-HKa interaction, we demonstrated that HKa inhibited the Mac-1–dependent cell adhesion to fibrinogen and ICAM-1, molecules abundant in the acutely injured vessel wall.47 The interaction of Mac-1 to fibrin(ogen) and ICAM-1 results in the adhesion of neutrophils and monocytes to the sites of fibrin deposition and to the endothelium, respectively. In fact, the early response to vascular injury is characterized by the migration of platelets and inflammatory cells, including neutrophils and monocytes, to the injured vessel wall.48,49 Therefore, the finding that HKa directly interacts with Mac-1 and inhibits Mac-1–dependent adhesion may offer an opportunity for the discovery of peptides that can be expected to decrease neutrophil/monocyte activation and adhesion to perturbed endothelial cells. Efforts to uncover such peptides have focused on identifying the binding sites of kininogen on endothelial cells.34,50 More recently, fine mapping has revealed 3 noncontiguous binding regions on kininogen for neutrophils.51 Neutrophils express both Mac-1 and uPAR, and it is known that HKa binds to uPAR.31 Thus, some of the sequences on HKa could be caused by binding to uPAR rather than to Mac-1. Therefore, further characterization of the regions in HKa involved in Mac-1 binding, using direct binding of HKa to Mac-1 on transfected cells or in a purified system, may well provide new approaches to interrupt Mac-1 mediated neutrophil/monocyte adhesion. These studies will ultimately help in designing peptides that could serve as leads for antiadhesive therapeutic intervention.
Supported by National Institutes of Heart Lung and Blood training grant 5T32HL07777 (R.W.C.) and project 2 of PO1 HL 56914 (R.W.C.) and by American Heart Association grant 9730241N (N.S.).
Reprints:Nijing Sheng, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 North Broad Street, Philadelphia, PA 19140; e-mail: nsheng@thunder.ocis.temple.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.