We read with interest the article by Chelucci et al,1 reporting chemokine receptor 4 (CXCR4) mRNA and protein expression on about 50% of steady-state circulating CD34+ cells. This expression pattern was coincident with that observed in resident normal CD34+ cells from bone marrow, where CXCR4 was found on approximately 40% of CD34+ progenitors2; moreover, bone marrow CD34+CXCR4− and CD34+CXCR4+ cells differ in their colony-forming potential, with CD34+CXCR4+cells originating from CD34+CXCR4− cells and having the capacity to proliferate and differentiate into B and T lymphocytes in vitro, but being incapable of forming myeloid progeny in serum-free culture systems. From these data on steady-state hematopoiesis, it can be asserted that CD34+CXCR4− cells represent a more primitive subset compared with CD34+CXCR4+ cells, which have already initiated their commitment toward the lymphoid lineage.2 Further investigations identified the expression of mRNA for CXCR4 in about 80% of samples of highly enriched CD34+ cells collected by leukapheresis after G-CSF–supported cytotoxic chemotherapy.3 In that study, apparently in contrast with CXCR4-expression pattern on marrow resident precursors, CXCR4 was found predominantly on a more immature subset of circulating progenitors (CD34+/HLA-DR−); similarly, Ruiz et al recently confirmed the presence of primitive CD34+/CD90+/CXCR4+ cells among steady-state circulating precursors.4 The above-mentioned discrepancies between CXCR4 expression patterns in marrow versus steady-state and G-CSF–exposed circulating precursors recall previous findings on peripheral CD34+ progenitors that homogeneously express CD33 and CD38 antigens, unlike bone-marrow-resident CD34+ cells, whose antigens, to the contrary, identify distinct functional subsets.5
To settle the apparent inconsistency of available data on CXCR4 expression on human CD34+ precursors, we examined the expression of CXCR4 protein on highly purified G-CSF–mobilized peripheral blood CD34+ cells from 7 patients with hematological malignancies. Moreover, CXCR4 expression was evaluated during the differentiation of those CD34+ cells along the erythroid (E), granulocytic/monocytic (GM), and megakaryocytic (Mk) lineages in liquid culture; details on cell culture methodology have been already published.6,7 Aliquots of unfractionated peripheral blood progenitor cells (PBPC) or purified/cultured CD34+ cells were incubated with a phycoerythrin (PE)-conjugated anti-CXCR4 monoclonal antibody (mAb) (12G5 clone, Pharmingen, San Diego, CA) and were costained with fluorescein isothyocyanate (FITC)-conjugated mAb directed against CD34, CD33, CD61, CD105, and glycophorin-A.8
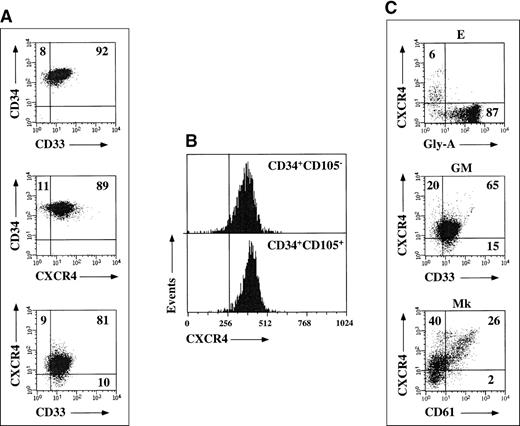
CD34+ cells mobilized by chemotherapy and G-CSF homogeneously expressed CD33, CD13, and HLA-DR antigens, thus confirming previous observations (Figure, A).5 CXCR4 was expressed on the majority of circulating G-CSF–mobilized CD34+ cells (90%, range 86 to 93). CD34+ cells were next fractionated into a CD105+ and a CD105− population, as previously described6; surprisingly, no differences in CXCR4 expression levels were detected when comparing the 2 cell subsets (> 90% CXCR4+; Figure, B). Coreceptor expression was also investigated during unilineage E, GM, and Mk differentiation pathways. CXCR4 expression was rapidly down-regulated during erythroid differentiation, and a negligible percentage of glycophorin-A+ cells were found to coexpress CXCR4 after 7 days of culture (< 2%). CXCR4 levels moderately decreased along the GM pathway; conversely, high levels of coreceptor were found in Mk cultures, and increasing CD61 staining intensities were associated with increasing CXCR4 levels (Figure, C).
CXCR4 expression.
(A) Expression of CXCR4 and CD33 antigens on freshly purified G-CSF mobilized CD34+ progenitors from a representative experiment. Markers were set according to isotypic controls. The percentage of cell coexpressing both antigens is indicated. (B) CXCR4 expression on freshly purified CD34+CD105+ and CD34+CD105− cell fractions from a representative experiment. Markers were set according to isotypic controls. (C) CXCR4 expression during unilineage E, GM, and Mk differentiation. Markers were set according to isotypic controls. The percentage of cells coexpressing CXCR4 and glycophorin-A, CD33, or CD61 differentiation antigens is indicated.
CXCR4 expression.
(A) Expression of CXCR4 and CD33 antigens on freshly purified G-CSF mobilized CD34+ progenitors from a representative experiment. Markers were set according to isotypic controls. The percentage of cell coexpressing both antigens is indicated. (B) CXCR4 expression on freshly purified CD34+CD105+ and CD34+CD105− cell fractions from a representative experiment. Markers were set according to isotypic controls. (C) CXCR4 expression during unilineage E, GM, and Mk differentiation. Markers were set according to isotypic controls. The percentage of cells coexpressing CXCR4 and glycophorin-A, CD33, or CD61 differentiation antigens is indicated.
Chemokine receptors belong to a family of 7 membrane- spanning molecules coupled to G-proteins, and their activation leads to calcium flux and intracellular and extracellular signaling; the surface expression of different chemokine receptors on leukocytes and the biological functions of their cognate ligands have been thoroughly investigated.9 CXCR4 is abundantly expressed on peripheral blood lymphocytes, monocytes, thymocytes, pre-B cells, and dendritic cells; stromal cell-derived factor 1 (SDF-1), the natural ligand for CXCR4, initially characterized as a pre-B-cell stimulating factor, as well as a potent chemoattractant for T lymphocytes, has been shown to potently mediate the chemotaxis of CD34+ progenitors, suggesting a pivotal role in the regulation of hematopoiesis.
Circulating CD34+ cells contain a progenitor pool supporting prompt and durable trilineage hematopoietic reconstitution after myeloablative chemotherapy.8 G-CSF has been recently shown to upregulate CXCR4 expression on peripheral blood CD34+CD38− cells from normal donors10; interestingly, CXCR4 expression is crucial for the engraftment of human stem cells and for the repopulation of NOD/SCID mice.11 The present investigation suggests that G-CSF might up-regulate CXCR4 expression on peripheral blood CD34+ cells, which differ profoundly from their bone marrow counterpart, where CXCR4 is specifically associated with lymphoid commitment2; in addition, the bone marrow microenvironment might easily accomodate immigrating progenitor cells that express high levels of CXCR4 following G-CSF mobilization or stress conditions. The different functional significance of CXCR4 on G-CSF–mobilized cells compared with bone marrow CD34+cells is further suggested by the finding of similar CXCR4 levels on CD34+CD105+ and CD34+CD105− cell subsets, which represent functionally distinct subpopulations of circulating progenitors.6,7 Following the observation that CXCR4 might be required for the migration of CD34+ progenitor cells from the fetal liver to the bone marrow microenvironment during fetal development,2 the dynamic alterations of CXCR4 expression on human CD34+ hematopoietic progenitors during growth factor-induced mobilization compared with steady-state hematopoiesis could confer an enhanced homing capacity and deserve further investigation.