The authors studied the role that interleukin (IL)-11 plays during the early stages of megakaryocyte (MK) development by investigating its in vitro effects on cell subpopulations enriched for bone marrow primitive progenitor cells and early and late committed progenitor cells. Progenitor subpopulations were isolated from bone marrow of normal or 5-fluorouracil (5FU)-treated mice and separated by sorting based on the surface antigens Sca-1, c-kit, and CD34. Functional analysis of the cell subpopulations, 5FU Lin−Sca-1+c-kit+ or normal bone marrow (NBM) Lin−Sca-1+c-kit+CD34−cells, indicated that exposure of these cells to recombinant human (rh)IL-11 in combination with steel factor (SF) stimulates the formation of colonies in methylcellulose and their proliferation in single cell-containing liquid cultures. Kinetic studies of MK progenitor generation, in response to SF and rhIL-11, demonstrated that a significant number of the progenitors produced are committed to the MK lineage. RhIL-11 also synergized with both SF and IL-3 to stimulate MK colony growth from NBM Lin−Sca-1+c-kit+ cells (early progenitors) and NBM Lin−Sca-1−c-kit+ cells (committed late progenitors). In the presence of IL-3, NBM, Lin−Sca-1−c-kit+ cells responded more strongly to rhIL-11 than SF. Consistent with these results is the observation that IL-11 receptor chain mRNA is present in all the progenitor cells from which the MKs are derived. This cell culture and RNA analysis suggest that murine bone marrow primitive progenitor cells and early and late progenitor cells are direct targets of rhIL-11 and that rhIL-11 has the potential to promote megakaryocyte development at several very early stages. (Blood, 2000;95:503-509)
Interleukin (IL)-11, a cytokine originally identified in primate bone marrow-derived stromal cells, has been shown to stimulate human and murine hematopoiesis.1 Most studies have focused on the effects of IL-11 on lymphohematopoietic stem cells and on committed megakaryocyte (MK) progenitors, and they suggest that it plays a role in the proliferation and differentiation of these cells. The first observations of the effects of recombinant human (rh)IL-11 on early progenitor cells were those of Ogawa et al.2,3 In a blast colony assay with bone marrow (BM) or spleen cells from 5-fluorouracil (5FU)-treated mice, rhIL-11 synergized with IL-3, IL-4, or steel factor (SF) to enhance blast cell and colony-forming unit granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) proliferation. In studies with enriched lymphohematopoietic progenitors, rhIL-11 synergized with SF and IL-3 in promoting colony growth in methylcellulose, with CFU-GEMM comprising more than half the colonies. 2-6 RhIL-11 has also been shown to act on human progenitor hematopoietic cells.7,8 In combination with SF, IL-3 or GM-CSF, and erythropoietin, rhIL-11 induced a synergistic or additive increase in the number of CFU cells derived from CD34+ and CD34+CD33-DR- cells. Increased commitment of stem cells into a multipotential progenitor subpopulation was observed when rhIL-11 was added to human and murine long-term bone marrow cultures.9
RhIL-11 has been shown to have synergistic effects on committed MK progenitors and direct effects on MKs.7,10 It synergizes with IL-3 and SF to support human and murine MK colony formation.7,11-13 Alone, rhIL-11 has no influence on murine MK colony growth under serum-free conditions. However, rhIL-11 enhanced CFU-MK and CFU-GEMM derived colony growth when combined with suboptimal or optimal concentrations of IL-3.12 Human BFU-MK-derived colony formation was augmented when CD34+DR− bone marrow cells were exposed to rhIL-11 plus IL-3,13 and, in the presence of SF, rhIL-11 supported the development of large macroscopic CFU-GEMM colonies from purified human CD34+ cells.9 Synergy between rhIL-11 and Tpo has recently been shown in the support of MK colony formation from murine bone marrow cells in serum-containing cultures.14 As a single agent, rhIL-11 is sufficient to induce the maturation of committed MKs. Human and murine MK ploidy and cell size increased when the MKs were exposed to rhIL-11 alone.10 RhIL-11 appears to act directly on these cells; functional IL-11 receptor is expressed on megakaryocytes.15
IL-11 is a member of a family of cytokines that includes IL-6, leukemia inhibitory factor, oncostatin M, and ciliary neurotropic factor that signals through a common receptor subunit, gp130.16Ligand-binding specificity has been shown to be conferred by the recently cloned IL-11 receptor α chain.16,17 When expressed by itself, the IL-11 receptor α chain binds IL-11 with low affinity. High-affinity binding to IL-11 requires coexpression of the α chain and gp130.16 The initiation of signal transduction by cytokine-induced association of the α chain and gp130 activates the JAK/TYK tyrosine kinase and the MAPK serine/threonine kinase families, which in turn activates downstream-signaling molecules including STAT1 and STAT3. 18
To date, little is known about the actions of IL-11 during the very early stages of MK development. In the current study, we investigated the relationship between the effects of rhIL- 11 on primitive, early, and late bone marrow progenitor cells and on megakaryocyte development. We report that rhIL-11 stimulated MK development at very early stages. In combination with SF, it acted on highly enriched murine 5FU and normal bone marrow primitive progenitor cells to generate blast and CFU cells. A significant proportion (20%) of these newly formed cells were MK progenitors. RhIL-11 also enhanced SF and IL-3 induced MK colony growth from normal murine Lin−Sca-1+kit+ and Lin−Sca-1− c-kit+progenitor cells. These effects by rhIL-11 are likely to be direct because IL-11 receptor α chain mRNA was detected in all these bone marrow subpopulations.
Materials and methods
Cytokines
The following cytokines produced by Genetics Institute (Cambridge, MA) were used in this study: purified Escherichia coli-derived rhIL-11, purified Chinese hamster ovary cell-derived steel factor and Chinese hamster ovary cell-derived recombinant murine (rm)IL-3 in conditioned medium (1 U activity is defined as the reciprocal of the dilution of conditioned medium needed to stimulate half-maximal proliferation of RB5 cells). Baculovirus-infected Sf9 cell-derived murine IL-3 was purchased from PharMingen (San Diego, CA). Mouse NSO myeloma cell-derived murine Tpo was purchased from R&D Systems (Minneapolis, MN).
Hematopoietic progenitor cell isolation
Bone marrow cell suspensions were prepared from normal female 8- to 12-week-old C57Bl/6J mice or from mice treated 2 days earlier with 5FU (150 mg/kg body weight intravenously) by gentle crushing of whole femurs and tibias in a ceramic mortar using phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal bovine serum (FBS; JRH BioSciences, Lenexa, KS). Cells were layered over Nycodenz (Nycomed, Oslo, Norway) with a density of 1.077 g/mL and centrifuged for 30 minutes at 1000g. The band of low-density cells at the interface was removed, washed once in PBS/2%FBS, and resuspended in a cocktail of purified rat antibodies recognizing the lineage-specific antigens CD11b/Mac-1, CD45R/B220, Ly-6G/Gr-1, CD4, CD8, and Ter119 (PharMingen). After a 30-minute incubation on ice, the cells were washed twice and reincubated with goat antirat antibody-conjugated magnetic beads (Miltenyi Biotec, Sunnyvale, CA) for an additional 30 minutes. Antibody/bead-coated cells were depleted using a VarioMACS BS column (Miltenyi Biotec) with 23-gauge needle to restrict flow. The lineage-depleted cells were further stained with allophycocyanine (APC)-conjugated goat antirat antibody to detect residual lineage-positive cells, washed, and incubated with 10-fold excess normal rat immunoglobulin. Finally, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated D7 (anti-Sca-1) and phycoerythrin (PE)-conjugated anti-c-kit (Pharmingen) for 30 minutes on ice. In some experiments, the APC-labeled, lineage-depleted cells were stained with anti-Sca-1-FITC, anti-c-kit-PE, and biotinylated 49E8 (anti-CD34; Pharmingen), followed by streptavidin-RED613 (Life Technologies, Grand Island, NY).
Lineage-negative (APC-negative) cells were divided into various subpopulations based on Sca-1, c-kit, and CD34 staining on a dual-laser FACStar Plus (Becton Dickinson, San Jose, CA). Gated subpopulations were sorted directly into tubes containing 300 μL complete medium or lysis buffer RLT (Qiagen, Chatsworth, CA) or into microtiter plates containing complete medium or single-cell reverse transcription–polymerase chain reaction (RT-PCR) lysis buffer using an automatic cell deposition unit and CloneCyt software (Becton Dickinson).
Murine progenitor cell assays
Single-sorted stem and progenitor cells were cultured in 96-well U-bottom microtiter plates in Iscove's modified Dulbecco's medium (IMDM) containing 20% FBS in the presence of 100 ng/mL rmSF alone or with 100 ng/mL rhIL-11 or 100 ng/mL rmTpo for up to 10 days. At various time points during the culture period, the number of viable (refractile) cells in each well was determined by phase microscopy using an inverted light microscope.
Colony-forming cells were assayed by incubating 100 to 500 cells in 0.8% methylcellulose medium containing a-MEM (Stem Cell Technologies, Vancouver, BC), rmSF (50 ng/mL), rmIL-3 (20 ng/mL), rhIL-11 (50 ng/mL), and rhEpo (2 U/ml) for 14 days. Colonies with diameters smaller than or larger than 2 mm were scored separately and represented low-proliferative capacity colony-forming cells (LPP-CFC) and high-proliferative capacity colony-forming cells (HPP-CFC), respectively. The majority of HPP-CFC colonies contained more than 1 cell type with various combinations of neutrophils, monocytes, mast cells, megakaryocytes, and erythroid cells represented (data not shown).19,20 Lymphohematopoietic potential was defined as the ability of primary colonies grown for 8 to 12 days in rmSF + rhIL-11 to produce secondary colonies containing B220+ pre-B cells in rmSF + rmIL-7 supplemented methylcellulose cultures on replating.5
Murine megakaryocyte colony assay
CFU-MK were assayed in semisolid agar culture using a modification of the technique described by Metcalf, et al.21 1 to 5 hundred sorted or cultured murine bone marrow progenitor cells were plated in 24 well tissue culture plates (Corning Costar Corp., Cambridge, MA) in IMDM, 20% fetal calf serum (FCS; JRH Biosciences, Lenexa, KS), 0.365% agar (Difco, Detroit, MI), 0.2 mmol/L L-glutamine (Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cytokines were added to the cultures as specified. After 7 days of incubation at 37°C, 5% CO2, the cultures were air dried, fixed, and stained for the detection of acetylcholinesterase (AChE) activity.22 Clusters of 3 or more stained cells were scored as a CFU-MK-derived colony.
Single-cell RT-PCR analysis
Single cells were sorted directly into lysis buffer, and cDNA was prepared and amplified as previously described.23 24 PCR was then carried out for 50 amplification cycles (1 minute at 94°C, 2 minutes at 42°C, and 6 minutes at 72°C with a 10-second extension/cycle) with 5 μmol/L (dT)24 × primer (ATG TCG TCC AGG CCG CTC TGG ACA AAA TAT GAA TTC dT24), and 5 U Taq polymerase (Perkin-Elmer Cetus/Roche Molecular Systems, Branchburg, NJ). Cell-free and reverse transcriptase-free samples were used as negative controls. The PCR DNA fragments were electrophoresed through 1% agarose, stained with ethidium bromide, and transferred to a nylon membrane. The membranes were hybridized at 42°C for 18 hours to a murine IL-11 receptor α chain cDNA radiolabeled probe (2 × 106 cpm/mL). Membranes were exposed for 18 hours to Fuji imaging plates developed in a FUJIX BAS 2000 Bio Imaging Analyzer (Fuji, Tokyo, Japan). Single cells from specified lines were included as negative and positive controls.
Results
Characterization of stem/progenitor subpopulations
Hematopoietic primitive progenitor cells were isolated from normal bone marrow (NBM) or from marrow of 5FU-treated C57Bl/6 mice after the depletion of mature myeloid and lymphoid cells. Cell subpopulations were tested in several assays to establish their positions in the progenitor cell differentiation pathway (Table1). Results of these assays show that the NBM Lin−Sca-1−c-kit+subpopulation does not give rise to lymphohematopoietic colonies and contains a limited number of HPP-CFC, suggesting that it is composed primarily of myeloid-committed progenitors. In contrast, the NBM Lin−Sca-1+c-kit+subpopulation contains many multilineage lymphohematopoietic progenitors and is greatly enriched for HPP-CFC. The 2d 5FU BM Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+c-kit+CD34−subpopulations appear to be equivalent in nature and to contain significant numbers of HPP-CFC. This result is in agreement with previous data demonstrating their enrichment for LTRA cells.25-27
Effects of rhIL-11 on murine bone marrow stem cell-enriched subpopulations
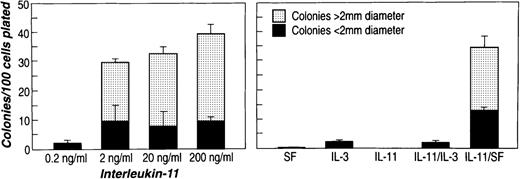
The effect of rhIL-11 was tested on the murine bone marrow subpopulations described above. Figure 1shows the results of exposing 2d 5FU BM Lin−Sca-1+c-kit+ cells to rhIL-11. One hundred cells were plated in methylcellulose with cytokines, and cultures were scored for colonies containing more than 50 cells on day 10 or day 11. IL-3 alone and in combination with rhIL-11 promoted the growth of a small number of LPP-CFC (smaller than 2-mm diameter) (Figure 1, right). SF alone did not stimulate colony growth, but rhIL-11 synergized with SF to stimulate extensive colony formation from these cells. This stimulation was dependent on the rhIL-11 dose because a minimal amount of colony formation was observed at 0.2 ng/mL, and at 200 ng/mL colony formation was maximal (40% clonogenicity). Most (75%) of the colonies formed were HPP-CFC (larger than 2-mm diameter) (Figure 1, left).
Effects of rhIL-11 on colony formation of murine bone marrow 2d 5FU Lin−Sca-1+c-kit+ cells.
Sorted bone marrow cells (100-200 cells) were plated in 0.8% methylcellulose containing aMEM medium and 30% FCS in a total volume of 1.5 mL. (left) Culture contained 100 ng/mL rmSF. (right) Culture was supplemented with purified cytokines at the following concentrations: 100 ng/mL rhIL-11 and rmSF and 20 ng/mL rmIL-3. Target cell population in each panel was Lin−Sca-1+c-kit+ cells from 2d 5FU-treated C57Bl/6 mice. Colony number and size were scored after 10 to 12 days of incubation.
Effects of rhIL-11 on colony formation of murine bone marrow 2d 5FU Lin−Sca-1+c-kit+ cells.
Sorted bone marrow cells (100-200 cells) were plated in 0.8% methylcellulose containing aMEM medium and 30% FCS in a total volume of 1.5 mL. (left) Culture contained 100 ng/mL rmSF. (right) Culture was supplemented with purified cytokines at the following concentrations: 100 ng/mL rhIL-11 and rmSF and 20 ng/mL rmIL-3. Target cell population in each panel was Lin−Sca-1+c-kit+ cells from 2d 5FU-treated C57Bl/6 mice. Colony number and size were scored after 10 to 12 days of incubation.
The ability of rhIL-11 to promote directly the proliferation of cells comprising the primitive progenitor/LTRA-enriched subpopulations was examined in serum-containing liquid cultures with SF and rhIL-11. Table2 shows the frequency of single 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+c-kit+CD34−cells that responded to SF + rhIL-11. SF or rhIL-11 alone (data not shown) was incapable of supporting the growth of these subpopulations of cells. After 5 days of culture, approximately 45% and 22% of the wells responded with colonies of more than 2 and more than 50 cells per well, respectively. After 10 days of culture with SF and rhIL-11, 40% and 36% of the wells gave rise to more than 2 and more than 50 cells, respectively. The results reveal that rhIL-11, in combination with SF, acted directly on primitive hematopoietic progenitors to support growth.
Effects of rhIL-11 on the generation of MK progenitors in murine primitive progenitor-enriched subpopulations
To determine whether the effects of rhIL-11 plus SF on primitive progenitor cell growth includes the generation of MK progenitors, the ability of the 2 cytokines to promote the formation of MK progenitors from BM primitive progenitor cell subpopulations was investigated. Sorted Lin−Sca-1+c-kit+ cells from BM of mice treated with 5FU and Lin−Sca-1+c-kit+CD34−cells from normal murine BM were preincubated for 2, 4, and 6 days in liquid cultures containing SF + rhIL-11. To determine the extent of MK progenitor generation that occurred during the preincubation, MK colony assays were performed on both the initial 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+c-kit+CD34− cells and on cells removed from the liquid cultures. Cells preincubated in SF alone were assayed for MK colony formation after 2 days in liquid culture because longer incubations in SF alone resulted in the progressive loss of cell viability.
MK colony formation from 2d 5FU Lin−Sca-1+c-kit+ cells preincubated with SF + rhIL-11 and replated into MK colony assays with SF plus Tpo is shown in Figure 2A. Before preincubation with cytokines, approximately 3% of the cells formed MK colonies. SF alone maintained, but did not increase, the potential of 5FU Lin−Sca-1+c-kit+ cells to form MKs (3 MK colonies/100 cells) after 2 days of suspension culture. The addition of rhIL-11 to SF-containing suspension cultures dramatically increased the generation of MK progenitors from these cells, with 20% forming MK colonies after 2 days of preincubation. No overall expansion of the cells occurred during the first 2 days of liquid culture, though a minimal amount of cell death was observed in some experiments. After 4 and 6 days of preincubation with SF + rhIL-11, a 10-fold and 200-fold expansion in cell number was detected, respectively (data not shown). Little more than 1% of the cells had MK potential after 4 days. Figure 2C shows the absolute number of MK progenitors produced from 500 5FU Lin−Sca-1+c-kit+ cells during a 6-day preincubation with SF + rhIL-11. The cells were replated into the MK colony assay with SF + rhIL-11, SF + Tpo, IL-3 + IL-11, or IL-3 + Tpo. The number of MK progenitors responsive to SF + Tpo was greatest after 2 days of preincubation. Preincubation with SF + rhIL-11 for 6 days resulted in a large expansion (100-fold) of MK progenitors, with many more of the progenitors responding to IL-3 than to SF.
Effects of rhIL-11 on MK progenitor formation from murine bone marrow primitive progenitor-enriched subpopulations.
Sorted subpopulations were isolated from the bone marrow of 5–fluorouracil-treated (A,C) and normal (B) mice as described in “Materials and Methods.” Two thousand Lin−Sca-1+c-kit+ (A,C) and Lin−Sca-1+c-kit+CD34−(B) cells/mL were plated in liquid cultures with 50 ng/mL SF and 50 ng/mL rhIL-11. Cells were removed from the cultures on specified days and were replated as follows: 400 cells/mL on days 0 and 2; 2000 cells/mL on day 4; and 40 000 cells/mL on day 6 in semisolid agar medium with either designated cytokine combinations (50 ng/mL SF, 20 ng/mL IL-3, 10-50 ng/mL rhIL-11, 50-100 ng/mL Tpo) (C) or 50 ng/mL SF plus 50 to 100 ng/mL Tpo (A,B). After 7 days of incubation at 37°C, 5% CO2, plates were dried, fixed, and stained for the detection of acetylcholinesterase activity (MK cells). Clusters of 3 or more stained cells were scored as MK colonies. Absolute numbers of MK progenitors (C) are based on culturing 500 sorted cells. Results are representative of 3 to 5 separate experiments.
Effects of rhIL-11 on MK progenitor formation from murine bone marrow primitive progenitor-enriched subpopulations.
Sorted subpopulations were isolated from the bone marrow of 5–fluorouracil-treated (A,C) and normal (B) mice as described in “Materials and Methods.” Two thousand Lin−Sca-1+c-kit+ (A,C) and Lin−Sca-1+c-kit+CD34−(B) cells/mL were plated in liquid cultures with 50 ng/mL SF and 50 ng/mL rhIL-11. Cells were removed from the cultures on specified days and were replated as follows: 400 cells/mL on days 0 and 2; 2000 cells/mL on day 4; and 40 000 cells/mL on day 6 in semisolid agar medium with either designated cytokine combinations (50 ng/mL SF, 20 ng/mL IL-3, 10-50 ng/mL rhIL-11, 50-100 ng/mL Tpo) (C) or 50 ng/mL SF plus 50 to 100 ng/mL Tpo (A,B). After 7 days of incubation at 37°C, 5% CO2, plates were dried, fixed, and stained for the detection of acetylcholinesterase activity (MK cells). Clusters of 3 or more stained cells were scored as MK colonies. Absolute numbers of MK progenitors (C) are based on culturing 500 sorted cells. Results are representative of 3 to 5 separate experiments.
The MK potential of NBM Lin−Sca-1+c-kit+CD34−cells preincubated in liquid culture with SF + rhIL-11 over a period of 6 days is presented in Figure 2B. The response of this primitive progenitor-enriched population to preincubation with SF + rhIL-11 was similar to that observed with 2d 5FU Lin−Sca-1+c-kit+ cells. Approximately 4% of freshly isolated Lin−Sca-1+ c-kit+CD34−cells formed MK colonies when replated into agar medium containing SF + Tpo. After preincubation for 2 days with SF + rhIL-11, an increase in MK potential was observed with 15% of the cells now able to form MK colonies. After 4 days of culture with the 2 cytokines, 1% of the Lin−Sca-1+c-kit+CD34−cells exhibited MK potential.
RhIL-11-induced MK formation during different stages of lineage development was investigated by studying the effects of rhIL-11 on MK colony growth from cells that represented primitive/early progenitor cells and myeloid-committed progenitors, NBM Lin−Sca-1+c-kit+ and NBM Lin−Sca-1−c-kit+, respectively. RhIL-11 alone did not support MK colony growth, whereas IL-3 and SF supported the formation of MK colonies from Lin−Sca-1− c-kit+cells. The addition of rhIL-11 to cultures containing either IL-3 or SF enhanced the number of colonies formed from both progenitor subpopulations. The combination of SF and rhIL-11 was more effective in supporting MK colony growth from Lin−Sca-1+ c-kit+ cells, whereas IL-3 and rhIL-11 were more effective with Lin−Sca-1−c-kit+ cells (data not shown).
Expression of IL-11 receptor
The ability of the murine bone marrow primitive, early, and late progenitor subpopulations to respond directly to rhIL-11 was evaluated by examining the expression of the rhIL-11 receptor α chain in these subpopulations. Hematopoietic progenitor cells were isolated from murine bone marrow after the depletion of mature myeloid and lymphoid cells, and single cells were isolated by sorting into microtiter wells. Complementary DNA was generated from single-cell mRNA and then amplified nonspecifically by a modified PCR protocol. The cDNA was probed by Southern blot analysis for IL-11 receptor α chain expression. Fragments corresponding to IL-11 receptor α chain are generated in all cells from both primitive progenitor/LTRA cell-enriched subpopulations, 2d 5FU Lin−Sca-1+c-kit+ (Figure3, top) and NBM Lin−Sca-1+ c-kit+CD34−Figure 3, middle). IL-11 receptor α chain is expressed in all the cells from the early progenitor-enriched subpopulation, NBM Lin−Sca-1+c-kit+CD34+(Figure 3, bottom) and in more than 90% of the cells from the NBM Lin− Sca-1+c-kit+subpopulation, a more expansive subpopulation enriched for early progenitor cells (data not shown). All cells from the late progenitor-enriched subpopulation NBM Lin−Sca-1−c-kit+expressed IL-11 receptor α chain (data not shown).
Expression of IL-11 receptor chain mRNA in single murine bone marrow primitive progenitor-enriched subpopulations.
Total RNA was prepared from single murine bone marrow cells, and first-strand cDNA was synthesized and amplified as described in “Materials and Methods.” The bottom frame shows the ethidium bromide-stained amplified cDNA transferred to a nylon membrane, probed with the IL-11 receptor α chain cDNA, and subjected to Fuji bio-imaging analysis (top frame). 10 5FU Lin−Sca-1+ c-kit+ cells (upper panel), NBM Lin−Sca-1+ c-kit+CD34−cells (middle panel), and NBM Lin−Sca-1+c kit+CD34+ cells (lower panel) were analyzed for IL-11 receptor α chain expression to avoid individual cell artifacts. Cell differences may represent heterogeneity in each subpopulation. T10 cells were used as a positive control for IL-11 receptor α chain, and FDC P1 cells were used as a negative control. The amplification protocol produces cDNA of variable lengths from any given transcript, so that smears rather than bands result when the membranes are hybridized to a corresponding probe.24
Expression of IL-11 receptor chain mRNA in single murine bone marrow primitive progenitor-enriched subpopulations.
Total RNA was prepared from single murine bone marrow cells, and first-strand cDNA was synthesized and amplified as described in “Materials and Methods.” The bottom frame shows the ethidium bromide-stained amplified cDNA transferred to a nylon membrane, probed with the IL-11 receptor α chain cDNA, and subjected to Fuji bio-imaging analysis (top frame). 10 5FU Lin−Sca-1+ c-kit+ cells (upper panel), NBM Lin−Sca-1+ c-kit+CD34−cells (middle panel), and NBM Lin−Sca-1+c kit+CD34+ cells (lower panel) were analyzed for IL-11 receptor α chain expression to avoid individual cell artifacts. Cell differences may represent heterogeneity in each subpopulation. T10 cells were used as a positive control for IL-11 receptor α chain, and FDC P1 cells were used as a negative control. The amplification protocol produces cDNA of variable lengths from any given transcript, so that smears rather than bands result when the membranes are hybridized to a corresponding probe.24
Discussion
It has been shown that rhIL-11 has effects on the proliferation and differentiation of primitive hematopoietic progenitors.28It acts in synergy with a number of early- and late-acting cytokines in vitro to stimulate pluripotent human and murine cell cycle-dormant stem cells.8 RhIL-11 also acts in synergy with SF and IL-3 to enhance the formation of murine and human megakaryocyte colonies from bone marrow-committed MK progenitors.7,11-13 Alone, rhIL-11 is able to increase the size and ploidy of immature megakaryocytes.10 Recently, we investigated the mechanism of action of the effects of rhIL-11 on megakaryocytopoiesis.15 We showed that the effects of rhIL-11 on committed MK progenitors are not mediated through thrombopoietin, the ligand for c-mpl receptor, and that MKs can be direct targets of rhIL-11 action as they express functional IL-11 receptor. In this study, we demonstrated the direct actions of rhIL-11 and the expression of IL-11 receptor on all progenitor cells during the early stages of MK lineage commitment.
Two murine primitive progenitor/LTRA-enriched subpopulations, Lin−Sca-1+c-kit+ cells isolated from bone marrow 2 days after treatment of mice with 5FU and Lin−Sca−1+c-kit+CD34−cells isolated from normal murine bone marrow, were used in this study. These subpopulations were compared with respect to their functional characteristics and were found to be equivalent and similarly enriched for HPP-CFC. This result was consistent with previous reports that describe repopulating activity among murine 5FU Lin−Sca-1+c-kit+cells26,27 and NBM Lin−Sca-1+c-kit+CD34lo/−cells.25
In the current study, we examined the effects of rhIL-11 on murine 2d 5FU Lin-Sca-1+c-kit+ and NBM Lin−Sca-1+ c-kit+CD34−cells, and more specifically, its ability to induce the generation of MKs from these 2 cell populations. We observed that rhIL-11 synergizes with SF to promote the proliferation of cells within these subpopulations. In response to the 2 cytokines, multipotential progenitors (HPP-CFC colonies) were formed in a dose-dependent fashion. SF or rhIL-11 alone did not support colony formation. Analysis of the effects on single cells determined that the actions of rhIL-11 on these stem cell-enriched subpopulations were direct. In the presence of SF plus rhIL-11 approximately 38% of single 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+ c-kit+CD34−cells gave rise to more than 50 cells after 10 days of culture. These findings are consistent with previous investigations that have shown that the combination of SF and rhIL-11 is effective in promoting progenitor cell growth and maintenance.29,30 Holyoake et al30 note that ex vivo short-term incubation of unfractionated BM cells with SF and rhIL-11 produced an expansion of clonogenic progenitors. Neben et al29 and Jacobsen et al31 demonstrate an enhancement of BM progenitor cells that were exposed to SF and rhIL-11 for several days. In contrast to the bone marrow cells used in the other studies, the subpopulations examined in this research are enriched for dormant primitive progenitors.25-27 Most of the 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+ c-kit+CD34−cells are multipotent and exhibit primitive progenitor characteristics in that they preferentially form HPP-CFC.
Because studies32-34 show that rhIL-11 has thrombopoietic activities and this study demonstrated that it has direct effects on primitive progenitor cells, we investigated the ability of rhIL-11 in combination with SF to stimulate the formation of MK progenitors from BM primitive progenitor-enriched subpopulations. Exposure of 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+c-kit+ cells to rhIL-11 plus SF significantly increased the MK potential of both these primitive progenitor/LTRA-enriched subpopulations. After 2 days of preincubation, 15% to 20% of the cells were able to form colonies containing MK compared with 3% to 4% in the starting subpopulations. The ability of rhIL-11 to generate MK progenitors appeared to be most potent during the first 2 days of preincubation as the percentage of cells in the expanding cultures with MK potential decreased after this time. Nevertheless, rhIL-11 demonstrated a capacity to expand the subpopulation of MK progenitors during 6 days of culture, with the absolute number increasing 20- and 100-fold after 4 and 6 days of preincubation, respectively. The responsiveness of the MK progenitors to different colony-promoting factor combinations in culture was observed to change with time. This suggests the generation of different classes of MK progenitors in the presence of rhIL-11 and SF. Similar results were obtained with serum-depleted media, indicating that the ability of rhIL-11 to induce MK potential in primitive progenitor cell-enriched populations is independent of factors contained in serum.
Given that a small number of contaminating late progenitor cells existed in the primitive progenitor-enriched subpopulations used in this study and that not all the cells in these subpopulations responded to the combination of rhIL-11 and SF, our data only suggest that primitive progenitors may be direct targets of rhIL-11. Therefore, it was of interest to determine the percentage of cells within the 2 primitive progenitor/LTRA cell-enriched subpopulations with the ability to respond directly to rhIL-11. We did this by investigating IL-11 receptor α chain gene expression in 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+c-kit+CD34−cells. When individual 2d 5FU Lin−Sca-1+c-kit+ and NBM Lin−Sca-1+ c-kit+CD34−cells were examined, all were found to express the IL-11 receptor α chain message. These results suggest that all cells in each primitive progenitor/LTRA cell-enriched subpopulation are capable of responding directly to the actions of rhIL-11 and that they support the potential of rhIL-11 to stimulate hematopoietic primitive progenitor cells directly to form MK progenitors.
We have shown that rhIL-11 has the ability to act directly at all early stages of MK development. Exposure of primitive progenitor/LTRA cell-enriched subpopulations to rhIL-11 resulted in the production of rhIL-11- and Tpo-responsive MK progenitors. In accordance with this finding is the observation that rhIL-11 supports MK colony formation from cells contained within the Lin−Sca-1+c-kit+ progenitor subpopulation. RhIL-11 and Tpo have been shown to act in synergy in vivo to support the recovery of platelets in thrombocytopenic mice (Goldman S, personal communication). Treatment of mice with rhIL-11 and Tpo after the administration of carboplatin and irradiation greatly enhanced the reticulated platelet numbers and completely abolished the thrombocytopenia associated with the severe myelosuppression of this regimen. RhIL-11 was able to stimulate MK development from committed MK progenitors by the direct plating of NBM Lin− Sca-1−c-kit+cells into MK colony assays. This experiment revealed the ability of rhIL-11 to induce the formation of a significant number of CFU-MK-derived colonies from this committed progenitor subpopulation. The effects of rhIL-11 on all these hematopoietic progenitor cells are likely to be direct given that rhIL-11 receptor α chain mRNA expression was detected in more than 90% of the progenitors.
The ability of rhIL-11 to act during the early stages of MK development and to induce the commitment of MK progenitors from primitive BM cells may explain, in part, its effectiveness in the treatment of chemotherapy-induced thrombocytopenia.32,33,35-37 When administered to mice treated with carboplatin and irradiation, rhIL-11 accelerated the recovery of platelets and improved platelet nadirs by approximately 200%.35 Similar results were observed with rhIL-11 in severely thrombocytopenic nonhuman primates.36Clinical studies demonstrate the ability of rhIL-11 to induce the formation of platelets in myelosuppressed patients undergoing chemotherapy and to decrease their need for platelet transfusions.33 37 The results presented in this study are consistent with the potential of rhIL-11 to stimulate megakaryocytopoiesis in myelosuppressed animals and in human patients in whom committed hematopoietic progenitors are damaged or destroyed. RhIL-11 enhances thrombopoiesis by stimulating the generation of MK progenitors from stem and primitive progenitor bone marrow cells. In conclusion, the results obtained in this and previous studies suggest that IL-11 acts directly to promote all stages of megakaryocyte development, from primitive progenitor cells to mature MK.
Acknowledgments
The authors thank Drs J. Kaye, R. E. Ploemacher, and A. M. Gewirtz for their critical reviews of the manuscript.
Reprints:Katherine J. Turner, Genetics Institute, Inc., 87 CambridgePark Drive, Cambridge, MA 02140; email: kturner@genetics.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.