Mantle cell lymphoma (MCL) is an aggressive neoplasm characterized by the deregulated expression of cyclin D1 by t(11;14). The molecular mechanisms responsible for MCL's clinical behavior remain unclear. The authors have investigated the expression of p53, E2F-1, and the CDK inhibitors p27 and p21 in 110 MCLs, relating their expression to proliferative activity (Ki-67). For comparison, they have similarly analyzed low-grade (12 MALT, 16 CLL/SLL) and high-grade (19 DLCL) lymphomas. p53 was detected more frequently in large-cell MCL (l-MCL; 5 of 7) than in classical MCL (s-MCL; 13 of 103) and DLCL (8 of 19). In MCL and DLCL, the percentage of E2F-1+ nuclei was high, correlating with high Ki-67 expression. Most MCLs (91 of 112) and DLCLs (12 of 19) showed a loss of p27; MALT and CLL/SLL, however, were p27 positive. Reverse transcription–polymerase chain reaction and in vitro protein degradation assays demonstrated that MCLs have normal p27 mRNA expression but increased p27 protein degradation activity via the proteasome pathway. Correlation of MCL p53 and p27 expression with clinical data showed an association between reduced overall survival rates and the overexpression of p53 (P = .001), the loss of p27 (P = .002), or both. Loss of p27 identified patients with a worse clinical outcome among p53 negative cases (P = .002). These findings demonstrated that MCL has a distinct cell cycle protein expression similar to that of high-grade lymphoma. The loss of p27 and the overexpression of p53 in MCL are prognostic markers that identify patients at high risk. The demonstration that low levels of p27 in MCL result from enhanced proteasome-mediated degradation should encourage additional clinical trials. (Blood. 2000;95:619-626)
Mantle cell lymphoma (MCL) is a lymphoproliferative disorder that, by morphologic, immunophenotypic, and genotypic findings, is thought to be derived from the mature B cells of the follicular mantle of the follicles (ie, the “mantle zone”). At the molecular level, MCL is characterized by the juxtaposition ofbcl-1 and the heavy chain immunoglobulin gene (PRAD-1/CCND1), resulting in the deregulation of bcl-1 expression.1-10 Based on morphologic and genetic features and its unique clinical behavior, MCL is now recognized as a distinct type of non-Hodgkin's lymphoma (NHL) with a very aggressive course. In fact, patients with MCL (along with T-lymphoblastic and peripheral T-cell lymphoma) have one of the lowest 5-year survival rates among all types of lymphomas.11
Most cases of MCL have a strikingly similar appearance, characterized by a uniform population of relatively small neoplastic cells with irregular nuclear contours, dispersed chromatin, and relatively little cytoplasm. However, the morphologic spectrum of MCL is wide. The architectural features comprise a growth pattern that can be perifollicular, nodular, or diffuse; the latter is associated with a worse prognosis.12-17 Furthermore, though uncommon, several cytologic variants have been described, all of which share the classic MCL immunophenotype/genotype (IgM+, IgD±, CD5+, CD10−, CD23−, cyclin D1 overexpression, and t(11;14) association) but demonstrate a distinct clinical course.12,18-21 These variants include the blastoid variant, characterized by slightly larger cells than classical s-MCL, with fine dusty chromatin, inconspicous nucleoli, and a high proliferative rate 12; the pleomorphic variant, characterized by large cells with a pleomorphic nucleus, rarefied chromatin, a single central nucleolus and scant cytoplasm18; and the large-cell variant, characterized by a high proliferation rate, a more aggressive clinical course, and decreased median survival time.12,18 22
Only a few studies have investigated the molecular differences among these MCL subtypes. We and others have reported a higher incidence of p53 anomalies18,23-25 and of bcl-1 translocations of the major translocation cluster locus among the MCL variants.19,22 Recently, loss-of-function mutations in cell cycle-negative regulatory elements, including point mutations and deletions or rearrangements of the cyclin-dependent kinase (CDK) inhibitors p15, p16, and p18 genes, have been described in a subset of MCLs and have been associated with an aggressive clinical course, blastic morphology, and extranodal dissemination.26 27
Deregulated expression of cell cycle genes plays a significant role in oncogenesis. In the past few years, many genetic aberrations of cell cycle regulators, such as cyclin D1, the retinoblastoma gene (pRB), p53, and CDK inhibitors p15 and p16 have been described in a variety of malignancies, including lymphoma.28 The role of cyclin D1 in the pathogenesis of MCL is strongly indicated by its overexpression/deregulation in this neoplasm. Cyclin D1–Cdk4 functions as a sensor for mitogenic signals. The only known major substrate of cyclin D1–Cdk4 is pRb, which, when phosphorylated, allows the release and activation of E2F.29,30 Cyclin/Cdks complexes are negatively regulated by two families of inhibitors—the INK4 family, which includes p15, p16, p18, and p19, and the CIP family, which includes p21, p27, and p57. Of the latter group, p21 is induced by the tumor suppressor p53 in response to DNA damage.31,32p27 can produce cell-cycle arrest in response to inhibitory stimuli such as transforming growth factor-β or cyclic adenosine monophosphate, lack of adhesion, and cell-contact inhibition.33 Recently, the deregulation of p27 protein expression has been demonstrated in several human neoplasms, and its low levels correlate with poor survival rates,34-37high-grade neoplasms, or both.
The aim of this study was to compare the expression of key cell cycle regulators in different subsets of NHL to identify unique protein expression profiles that may correlate with biologic and clinical features. We specifically studied the function of p21, p27, p53, and E2F-1 gene expressions because they play a key role in G1-S transition and they ultimately regulate cell proliferation and cell growth. Moreover, we investigated whether the expression patterns of p53 and p27 could allow the identification of a subset of patients with MCL whose clinical outcomes are worse.
Materials and methods
Pathologic samples
A panel of 157 well-characterized cases of NHL were selected from among those processed in the surgical pathology laboratories of the New York University School of Medicine, the University of Leuven, Belgium, and the University of Verona, Italy. The lymphoproliferative disorders characterized in this study included MALT-type lymphoma (12 cases), chronic lymphocytic leukemia/prolymphocytic leukemia/small lymphocytic lymphoma (16 cases), high-grade large B-cell lymphoma (19 cases of DLCL), and 110 cases of MCL. The l-MCL cases included 1 transformed large, 2 blastoid, and 4 pleomorphic variant cases that were classified using morphologic, immunophenotypic, and genotypic criteria, according to criteria previously described.12,18,19,38 The lymphomas were classified according to the International Lymphoma Study Group,20 based on hematoxylin-eosin staining and immunoperoxidase stains for B- and T-cell markers (CD3, CD5, CD10, CD20, and CD23 antigens; DAKO, Santa Barbara, CA). In select cases, frozen tissue sections also were stained (kappa, lambda, IgG, IgD, and IgM), and extensive flow cytometric analysis was performed. Gene rearrangement18 and/or cytogenetic studies38 39were performed in all cases of MCL to demonstrate the presence ofbcl-1-hIg chimeric products or t(11;14) translocation. Overexpression of Bcl-1 was documented by immunohistochemistry (anti-cyclin D1; Biotechnology, Santa Cruz, CA) or by Western blot analysis in several cases.
Monoclonal antibodies and immunohistochemical staining
The monoclonal antibodies (mAb) used in this study were anti-p21 (WAF-1, 1:25; Calbiochem, La Jolla, CA); anti-p27 (KIP-1, 1:1000; Transduction Laboratories, Lexington, KY), anti-p53 (DO-1, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Ki-67 (MIB-1, 1:1000; Immunotech, Marseilles, France), and anti-E2F-1 (E2F-1, 1:25; tissue culture supernatant KH95, kindly provided by Dr Kristian Helin40 41).
All mAbs required antigen retrieval by microwave pretreatment for 20 minutes in citrate buffer (10 mmol/L, pH 6). The immunostaining was performed on formalin-fixed or B5-fixed, paraffin-embedded tissue using the avidin-biotin-peroxidase complex (ABC) method and semiautomated immunostainers (Optimax; BioGenex, San Ramon, CA; Ventana-ES; Ventana, Tucson, AZ).42 43 For E2F-1 staining, a modification of the ABC technique was used to enhance the signal detection (Tyramide Signal Amplification Products; New England Nuclear, Boston, MA). This modification comprised two additional steps. Specifically, after incubation with ABC complex, the slides were incubated with tyramide (1:100, 8 minutes at room temperature [RT]) followed by 15 minutes of a second ABC incubation step at RT. The DAB was subsequently applied (5 minutes at RT), and the slides were washed and counterstained with hematoxylin.
Purification of neoplastic B cells
Cryopreserved mononuclear cells (1 × 107/mL) from selected patients with CLL/SLL or MCL, containing a high percentage of neoplastic B cells (ratio of neoplastic to normal B cells, >50:1) were incubated with anti-CD19 magnetic beads (80 μL; Immunotech) for 45 minutes on ice, harvested using a magnet, washed twice, and harvested again. The percentage of positive B cells was determined by flow cytometry using anti-CD3 and anti-CD20 mAbs (Becton-Dickinson, Mountain View, CA). Harvested populations always contained more than 95% B cells.
Western blot and immunoprecipitation analysis
Enriched B cells (1 × 106/sample) were lysed (20 mmol/L Tris-HCl, pH 8, 150 mmol/L NaCl, 1% Triton X100, 5 mmol/L EDTA, 1 mmol/L Na3Vo4 and 1 mmol/L PMSF) and spun. Ten microgram of total protein cell lysates were electrophoresed in SDS-PAGE gel and transferred to nitrocellulose membranes. Filters first were blocked (5% low-fat milk in PBS with 0.1% Tween 20) and subsequently incubated with anti-p27 (1:250; Transduction, Lexington, KY; 1-hour RT) or CDK2 (1:500) antibody.44 After three washes, the filters were incubated with HRPO-conjugated goat antimouse antibody (1:2000; Amersham, Arlington Heights, IL; 1 hour at RT) or with HRPO-conjugated goat antirabbit antibody (1:2000; Armesham; 1 hour at RT). The detection of immunocomplexes was performed with chemiluminescence (ECL; Amersham).
Purified recombinant histidine-tagged p27 was incubated (30°C, 1 hour) with HeLa extract obtained as described above. The reaction mix contained 0.1 μmol/L histidine-tagged p27, 0.2 μg/μL HA-tagged Ubiquitin, 20 mmol/L Tris-HCl (pH, 7.2), 2 mmol/L dithiothreitol 0.25 mmol/L EDTA, 0.2 mmol/L adenosine triphosphate, 10 mmol/L creatine phosphate, 70 U/mL creatine phosphokinase, 100 μmol/L hemin, and 20 μg cellular extract. The reaction products were immunoprecipitated with a rabbit anti-HA antibody (3 μg/mL; Santa Cruz) or with a rabbit anti-ubiquitin (2 μL/mL)45 followed by immunoblotting with the p27 mAb.
RNA extraction and cDNA preparation
Total mRNA was obtained from CD19 positive B cells (1 × 106) using a total RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was obtained from total RNA (1–5 μg) after reverse transcription using hexanucleotide random primers and Moloney murine leukemia virus reverse transcriptase (Gibco-BRL, Gaithersburg, MD). Briefly, genomic DNA was digested with DNAse (Boehringer-Mannheim, Indianapolis, IN) in the presence of MgCl2 (1 mmol/L) for 10 minutes at room temperature. Total RNA first was heated in the presence of oligoprimers (50 ng for 10 minutes, at 70°C) and then quenched on ice (2 minutes). The volume of the RNA/primer mixture was adjusted to 20 μL, giving the following final concentration: 0.5 mmol/L each dATP, dCTP, dGTP, and dTTP; 10 mmol/L dithiothreitol; 50 mmol/L Tris-HCl, pH 8.3; 3 mmol/L MgCl2; 75 mmol/L KCl; and 200 U Moloney murine leukemia virus RNAse H- reverse transcriptase (Gibco-BRL). The reaction mixture was incubated at 42°C for 2 hours and then the reverse transcriptase was inactivated at 70°C for 10 minutes.
Polymerase chain reaction analysis
The efficiency and quality of each cDNA preparation was tested by polymerase chain reaction (PCR) amplification using specific oligonucleotides recognizing human β2-microglobulin.46The presence of p27 mRNA transcripts were investigated using specific oligonucleotides recognizing p27 (p27-forward: 5′-ATGTCAAACGTGCGAGTGTCT, bp 1-21; p27-backward: 5′-TTACGTTTGACGTCTTCTGA, bp 577-597, NM_004064, giving a PCR product of 597 bp) and spanning the intron area within the first and second exons. Two microliters cDNA was amplified under appropriate conditions (10 pmol/L each primer, 250 μmol/L dNTP, 10 mmol/L Tris-HCl, pH 8.8, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.01% gelatin, 0.5 U Taq polymerase, in a final volume of 25 μL) for 10, 25, and 30 cycles (denaturing 94°C, 30 seconds; annealing 57°C, 1 minute; extension 72°C, 1.5 minutes) using a Cetus Perkin-Elmer (Norwalk, CT) thermocycler apparatus.
In vitro p27 protein degradation assay
Each frozen lymphoid tissue sample (10 different cases of MCL) was sectioned and quickly disrupted by nitrogen decompression in 100 mol/L lysing buffer (50 mmol/L Tris-HCl, pH 8.3, 5 mmol/L MgCl2, and 1 mmol/L dithiothreitol). The lysates were spun at 15 000 rpm, and supernatants were collected and frozen at −80°C. Histidine-tagged p27 (100 ng) was incubated at 37°C for different intervals in 60 μL degradation mix (containing 30 μg protein tissue homogenates, 50 mmol/L Tris-HCl, pH 8, 5 mmol/L MgCl2, 1 mmol/L dithrothreitol, 2 mmol/L adenosine triphosphate, 70 U/mL creatine phosphokinase, and 10 mmol/L creatine phospatase).47Degradation of p27 was analyzed by immunoblotting with anti-p27 mAb. The optical intensity of the protein bands was calculated using ID Image Analysis Software (Kodak Digital Science; Eastman Kodak, Rochester, NY) and scored (ratio of band intensity of p27 at time 0 hours/band intensity of p27 at time 6 hours: <1.25 = −; 1.25 to 2 = +; >2 = ++. The inhibition of proteasome activity was achieved by incubating the degradation mixture with hemin (100 μmol/L).48
Score and statistical analysis
Neoplasms were considered positive or strongly positive when nuclear staining was detected in at least 15% or more than 50% of the tumors cells, respectively. With the E2F-1 mAb, a discrete subpopulation of neoplastic cells showed moderate to high-density cytoplasmic staining. In these instances, the neoplasms were considered positive only when nuclear staining also was observed in at least 15% of the tumor cells.
p27 nuclear positivity was scored by counting the percentage of positive nuclei, after correction of the percentage of nonneoplastic CD3+ T cells on serial sections and by evaluating the intensity of anti-p27 immunostaining (compared to internal control positive cells). We also decided to score the relative nuclear intensity of p27 because a partial loss of p27 protein expression may have a pathogenetic role in tumorigenesis.49 In our semiquantitative scoring system, neoplastic cells were scored as negative (−) when nuclear immunostaining was negative or barely evident within the neoplastic cells. Neoplastic cells clearly expressing nuclear p27, but at levels intermediate between negative and normal cells, were scored ±, and cells at levels comparable to those seen in normal lymphoid elements were scored as +. Statistical significance was calculated using the Fisher exact test and the Kaplan-Meier (Mantel-Cox) method.
Results
Cell cycle regulatory protein expression
Our goals were to study the expression in MCL of 4 key cell cycle regulators (p21, p27, p53, and E2F-1), which primarily regulate the G1 phase of the cell cycle, or S-phase entry, or both, to identify any aberrant expression pattern(s) and to compare the cell cycle profile of MCL with that of other NHL. Because these proteins are expressed in a well-defined stage of the cell cycle, we first investigated the overall fraction of tumor cells committed to proliferation (G1b) or proliferating (S and G2-M). This was accomplished using the MIB-1 mAb, which specifically recognizes a protein (Ki-67) expressed by all cells within G1 and G2-M phases. As previously described,17,18 50 most MCLs and high-grade lymphomas had a large number of proliferating cells. However, the analysis of Ki-67 positive s-MCL versus l-MCL tumors demonstrated a significant difference (P = .004) when strongly positive tumors (>50% positive cells) were compared. In contrast, only a small subset (25%) of low-grade lymphomas was Ki-67 positive, with positivity primarily restricted to the proliferation centers (>80%) in CLL/SLL and prolymphocytic lymphoma. When the percentage of positive cases among low-grade lymphomas was compared with that of high-grade lymphomas, a statistically significant difference was identified (P < .001).
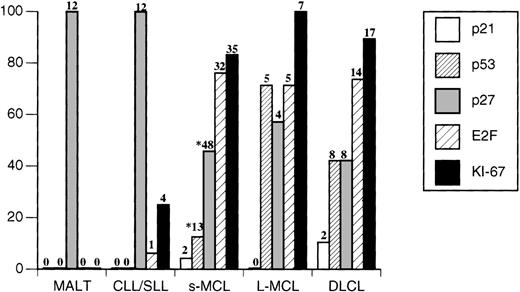
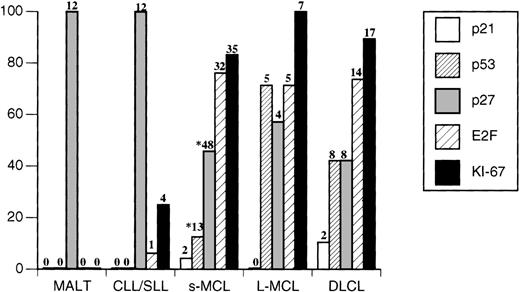
Immunohistochemical analysis for p27 protein expression demonstrated that most MCLs showed a total loss (54%) or a partial loss (29%) of detectable p27. Only in a minority of cases (17%) did the neoplastic cells display p27 levels similar to those seen in the nuclei of normal surrounding CD3+ T cells (Table 1). Expression of p53 was highest in l-MCL. Among 7 cases of 1-MCL with aggressive cytology, 5 cases demonstrated p53 overexpression (1 blastoid, 2 transformed, and 2 pleomorphic cases; 71%) compared with only 13 of 103 cases of s-MCL (12.6%; P = .02). No MCL expressed detectable p21 nuclear staining. When comparisons were made between p27 and Ki-67 expression or between p27 and p53 expression, no significant correlation(s) could be demonstrated in MCL. However, when the same analyses were performed including all lymphoma cases, p27 and Ki-67 expression showed a significant reverse correlation (P = .003). Furthermore, E2F-1 was highly expressed in most MCLs (38 of 50 cases; 76%).
When a similar analysis was performed in DLCL, these tumors overall showed a profile similar to that of MCL, particularly when l-MCL and DLCL were compared. More than 60% of cases of DLCL displayed low or negative levels of p27 (12 of 19). Interestingly, neoplastic cells of DLCL demonstrated a slight degree of intratumoral heterogeneity for p27 nuclear expression in contrast to MCL tumors cells, which showed a more homogeneous pattern of expression. Similarly to l-MCL, a substantial subset (8 of 19) of DLCL overexpressed p53. In only two cases were p21 and p53 concomitantly expressed. Finally, more that 70% (14 of 19) of these neoplasms showed strong nuclear staining for E2F-1 (Figures 1 and2); only 2 cases of all DLCL failed to express any detectable E2F-1 protein despite their high rates of proliferation.
Expression of p21, p53, and E2F in 102 cases of B-cell non-Hodgkin's lymphoma.
Neoplasms expressing the indicated antigens in >15% of the nuclei were considered positive. Numbers of positive cases are indicated on the top of each bar. *p27 and p53 analysis were performed in 103 s-MCL.
Expression of p21, p53, and E2F in 102 cases of B-cell non-Hodgkin's lymphoma.
Neoplasms expressing the indicated antigens in >15% of the nuclei were considered positive. Numbers of positive cases are indicated on the top of each bar. *p27 and p53 analysis were performed in 103 s-MCL.
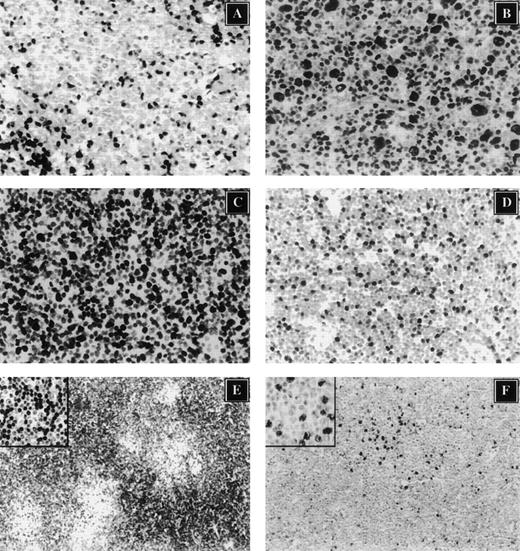
Immunohistochemical characterization of cell cycle regulators in non-Hodgkin's lymphoma.
(A) p27 expression analysis in l-MCL demonstrates that the neoplastic cells are negative and that rare intratumor reactive cells show strong nuclear reactivity. (B) The same case also shows overexpression of p53. The expression of Ki-67 (C) in l-MCL and E2F-1 (s-MCL, Panel D) are shown. (E) p27 is highly expressed in resting CLL/SLL cells. However, E2F-1 is detected primarily in proliferating cells of CLL/SLL within proliferation centers (inset; magnification ×400).
Immunohistochemical characterization of cell cycle regulators in non-Hodgkin's lymphoma.
(A) p27 expression analysis in l-MCL demonstrates that the neoplastic cells are negative and that rare intratumor reactive cells show strong nuclear reactivity. (B) The same case also shows overexpression of p53. The expression of Ki-67 (C) in l-MCL and E2F-1 (s-MCL, Panel D) are shown. (E) p27 is highly expressed in resting CLL/SLL cells. However, E2F-1 is detected primarily in proliferating cells of CLL/SLL within proliferation centers (inset; magnification ×400).
CLL/SLL and MALT cases displayed similar profiles that differed from the corresponding profiles of MCL and DLCL. All cases expressed very high levels of p27 (Table 1), and strong nuclear p27 staining was detected in >90% of the tumor cells on average. Paraimmunoblasts and prolymphocytes within proliferation centers proved to be an exception; they were only weakly positive or negative (Figure 1). Detectable staining was never observed in more than 15% of the neoplastic cells for p21 and p53 antigens. Among CLL/SLL cases, only one (classified as a paraimmunoblastic variant) expressed E2F-1 in >50% of the cells. When all neoplasms were compared, overall E2F-1 expression showed a positive correlation with Ki-67 (P < .0001) and a negative correlation with p27 (P = .0003). This was confirmed by the reverse pattern of expression in normal and neoplastic cells within normal germinal centers and proliferation centers of CLL/SLL, in which Ki-67 expression was high and p27 expression was low.
p27 expression and protein degradation in mantle cell lymphoma
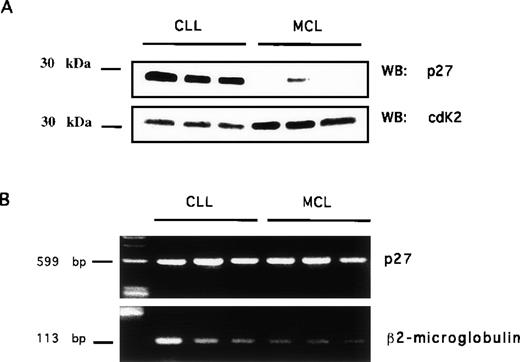
To study the mechanism leading to the decrease or loss of p27 expression in MCL, we studied the presence of p27 protein and mRNA expression in highly enriched neoplastic B cells (>95% CD19+ B cells) by Western blotting and RT-PCR, respectively. The relative amount of p27 protein was normalized with that of CDK2 protein expression. We used CDK2 because its levels are stable along the cell cycle.44 By Western blot analysis, p27 protein levels in MCL cells were undetectable or considerably lower than p27 protein levels in cells comprising CLL/SLL (Figure3A) and in normal T cells (data not shown). These results confirmed our immunohistochemical findings and demonstrated that the low levels of p27 detected by immunohistochemistry were not caused by possible steric inhibition by other cellular proteins (such as cyclin D1). In view of the fact that p27 protein expression can be regulated at a transcriptional level, we performed semiquantitative RT-PCR using purified CD19+ MCL cells. No difference could be demonstrated among representative MCL and CLL control cases (Figure 3B). Similar data were found when cDNA was subjected to a subliminar amplification (15 and 20 cycles only, data not shown).
p27 protein and mRNA expression in CLL and MCL B cells.
Highly enriched neoplastic B cells from patients with CLL and MCL were used to prepare total protein lysates and total RNA (see Material and Methods). Total protein lysates were electrophoresed, blotted, and incubated with anti-p27 (A top row) or with anti-CDK2 (A, bottom row). cDNA transcribed from total RNA was amplified using oligonucleotides recognizing p27 (B, top row) or β2-microglobulin (B, bottom row).
p27 protein and mRNA expression in CLL and MCL B cells.
Highly enriched neoplastic B cells from patients with CLL and MCL were used to prepare total protein lysates and total RNA (see Material and Methods). Total protein lysates were electrophoresed, blotted, and incubated with anti-p27 (A top row) or with anti-CDK2 (A, bottom row). cDNA transcribed from total RNA was amplified using oligonucleotides recognizing p27 (B, top row) or β2-microglobulin (B, bottom row).
To investigate whether the low levels of p27 in MCL were caused by enhanced proteasome-mediated degradation, as in colon and lung carcinomas,35,36 the degradation kinetics of recombinant p27 in the presence of protein lysates derived from 10 MCL fresh-frozen tissue samples were examined. The percentages of neoplastic B cells and normal intratumor T cells first were calculated by immunohistochemistry on fresh-frozen tissue samples. Using this approach, we were able to demonstrate that extracts from p27-negative MCL were able to degrade recombinant p27 rapidly and efficiently (Table2 and Figure4A). Conversely, extracts from those neoplasms with high p27 expression displayed considerably slower degradation kinetics. Intermediate kinetics were observed primarily in those cases containing a large number of p27-positive T cells. To study whether the p27 degradation of MCL was caused by a proteasome-dependent pathway, we analyzed the effect of hemin, a specific proteasome inhibitor.48 Recombinant p27 rapidly disappeared over time in the presence of hemin. Conversely, new polyubiquitinated p27 products were readily identified (Figure 4B). To confirm the presence of polyubiquinated high-molecular-weight p27 products, recombinant histidine-tagged p27 was incubated in the presence of HA-ubiquiting over time and then immunoprecipated with anti-HA or anti-ubiquitin mAbs. Ubiquitaned p27 products were successfully identified using anti-p27 mAb (Figure 4C).
Kinetics of p27 degradation in MCL.
Purified recombinant p27 was incubated for the indicated intervals with the extracts from three representative MCL cases (a, high p27 expression; b, low expression of p27 by the tumor cells, but the lymphoid tissue sample contained a relative large number of p27+ reactive T cells; c, low expression of p27 by the tumor cells, and the lymphoid tissue sample contained a small number of p27+ reactive T-cells) (A). To demonstrate that p27 degradation is mediated by proteasome the MCL lysates were incubated with and without hemin (B). p27 was polyubiquitinated before proteosome degradation (C). Purified recombinant p27 was incubated for 1 hour with the cell extract (HeLa) in the presence of HA-ubiquitin and subsequently was immunoprecipated with an anti-HA or anti-ubiquitin mAbs. After transfer, polyubiquitinated p27 forms were identified using anti-p27 mAb.
Kinetics of p27 degradation in MCL.
Purified recombinant p27 was incubated for the indicated intervals with the extracts from three representative MCL cases (a, high p27 expression; b, low expression of p27 by the tumor cells, but the lymphoid tissue sample contained a relative large number of p27+ reactive T cells; c, low expression of p27 by the tumor cells, and the lymphoid tissue sample contained a small number of p27+ reactive T-cells) (A). To demonstrate that p27 degradation is mediated by proteasome the MCL lysates were incubated with and without hemin (B). p27 was polyubiquitinated before proteosome degradation (C). Purified recombinant p27 was incubated for 1 hour with the cell extract (HeLa) in the presence of HA-ubiquitin and subsequently was immunoprecipated with an anti-HA or anti-ubiquitin mAbs. After transfer, polyubiquitinated p27 forms were identified using anti-p27 mAb.
Loss of p27 protein expression is a predictor for overall survival
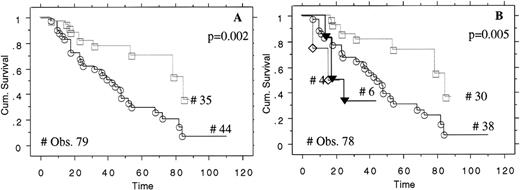
To analyze the prognostic significance of p27 expression on overall survival, MCLs were stratified based on the intensity of p27 expression (group 1, 44 patients; group 2, 35 patients). Clinical data were obtained in 79 patients with MCL with a minimum follow-up of 4 months. The mean follow-up was 39 months (SD, ±24 months; range, 106 months; minimum, 4 months; maximum, 110 months). No significant differences were demonstrated when the age, sex, LDH level, and stage (stage 3 vs. stage 4; P = .5) of these two groups were compared. However, the overall survival times from the date of diagnosis were significantly shorter in patients with tumor cells expressing no detectable p27 (median survival time, 44 months) than in patients with tumors in which the neoplastic cells expressed on intermediate or high levels of p27 expression (median survival time, 67 months; P = .002). We also compared the overall survival times of patients with MCL overexpressing p53 (10 of 78). Despite the relatively small number (10), p53+ patients showed a survival time considerably shorter than p53− patients (P = .001), regardless of p27 expression (P = .7). Finally, when p27 expression and survival of p53− patients were correlated, loss of p27 was found to be associated with a decreased overall survival time (P = .002) in patients with p53-negative tumors (Figure5B).
Loss of p27 is associated with poor survival in mantle cell lymphoma.
Kaplan-Meier curve for overall survival of p27+ (open squares) and p27− (open circles) in MCL. Survival curves in p27+/p53− (open squares), p27−/p53− (open circles), p27−/p53+ (full triangles), and p27+/p53+ (open diamonds) in patients with MCL (panel B).
Loss of p27 is associated with poor survival in mantle cell lymphoma.
Kaplan-Meier curve for overall survival of p27+ (open squares) and p27− (open circles) in MCL. Survival curves in p27+/p53− (open squares), p27−/p53− (open circles), p27−/p53+ (full triangles), and p27+/p53+ (open diamonds) in patients with MCL (panel B).
Discussion
In this study, we investigated the expression of multiple cell cycle regulators in MCL and in a representative panel of B-cell NHL, including low- and high-grade neoplasms. Overall, our findings demonstrated that MCL has an expression pattern similar to that observed in DLCL (high Ki-67, p53, E2F-1; low p27); decreased levels or total loss of p27 protein expression are common features of MCL; low protein levels of p27 in MCL do not correlate with proliferation rates and are caused by ubiquitin- and proteasome-dependent degradation; and loss of p27 levels correlates with a decreased rate of overall survival. Taken together, these findings indicate that, in combination with p53 overexpression, p27 expression may allow a more precise characterization of patients with MCL.
To ascertain whether the expression of key cell cycle regulators in MCL could allow a better stratification of these neoplasms, we investigated the expression of p53, p21, p27, and E2F-1 in MCL and compared the expression of these regulators with that seen in low- and high-grade NHLs. In keeping with data published by other investigators, MCLs are characterized by high proliferation rates as determined by the high number of Ki-67- and E2F-1-positive tumors. We investigated the expression of E2F-1 because it is a key regulator of cell growth.29 Members of the E2F transcription factor family (E2F-1–E2F-5) act as critical positive regulators of cell cycle progression.29 Two groups independently have reported that E2F-1 knockout mice show a high incidence of tumors, including large-cell lymphoma.51,52 Relatively little is known about E2F-1 expression and its correlation with other key cell cycle regulators in lymphoma.53 54 Here, a positive correlation between the proliferation rate and E2F-1 expression was demonstrated in cases of MCL and DLCL. We observed a similar correlation when we compared the expression of Ki-67 and E2F-1 of proliferation centers in CLL/SLL (Figures 2E, 2F) and in normal residual germinal centers. In contrast, none of the MALT-type lymphomas and only 2 of the CLL/SLL cases showed a significant number of E2F-1-positive tumor cells. Moreover, we identified rare cases of DLCL in which the neoplastic cells did not express detectable E2F-1 despite high proliferation rates. Characterization of the E2F-1 locus in these neoplasms is to demonstrate whether deletions or somatic mutations ultimately are responsible for these findings. Alternatively, other mechanisms acting upstream from E2F-1, or posttranscriptional mechanisms, may be operational. One may speculate that an aberration involving cellular protein degradation may alter E2F-1 levels because, like p27, levels of E2F-1 also are regulated by ubiquitination and proteasome degradation.
Overexpression of p53 is the most common defect identified in human tumors. It often is seen within high-grade NHL, in which it is associated with cellular transformation. We and others18,23,25 have demonstrated that p53 is a prognostic indicator in MCL and that its overexpression is more frequently seen in MCL variants than in classical s-MCL18,23,24 (71% in l-MCL vs. 11% in s-MCL; P = .002). We also have confirmed, despite the relatively small number of cases, that p53 expression in l-MCL does not appear significantly different from p53 protein expression in DLCL. The considerably lower incidence of p53 overexpression in s-MCL compared with l-MCL and DLCL tend to indicate that p53 somatic mutations may occur during the transformation of MCL and therefore may be associated with a more aggressive clinical course. The fact that only a relatively small subset of s-MCL carries p53 genetic aberrations suggests that factors other than p53 anomalies may be responsible for their aggressive clinical course. Our data demonstrate that the level of p27 protein expression may have a crucial role in the pathogenesis and biologic behavior of these neoplasms. In fact, total or partial loss of p27 appears to be a consistent feature of MCL and one that differentiates MCL from low- and even high-grade lymphomas. Because p27 expression appears to correlate inversely with the cell proliferation index, an observation supported by our findings in normal germinal centers and in the proliferation centers of CLL/SLL, the fact that high p27 expression is seen in low-grade NHL is not surprising if one considers that in these neoplasms only a small number of cells is committed to cell division. That a larger percentage of MCLs than DLCLs loses p27 is intriguing because the fraction of proliferating cells in MCL is approximately the same or even less than that seen in most DLCLs. In addition, though classical and variant MCLs do not differ significantly regarding the percentage of p27 positive cases (P = .7), these two groups are considerably different regarding their percentages of proliferating tumor cells (classical MCL, MIB-1 >50 = 14.3%; variant MCL, MIB-1 >50 = 71.4%). Thus, the deregulation of p27 in MCL appears not to be correlated with the cell cycle proliferation.50 On the other hand, the neoplastic cells of some DLCL appear to express different levels of p27, indicating that in these cases p27 protein levels are still under the physiological regulation of cell cycle progression.
The precise mechanisms regulating the amount of p27 in tumor cells have been only partially elucidated. The overall consensus is that levels of p27 in normal and neoplastic cells are regulated by multiple mechanisms. These include increased degradation through the ubiquitin-proteasome machinery,47 reduced mRNA expression,55 decreased protein translation,56,57 and the acquisition of rare somatic mutations resulting in premature protein termination.58,59In addition, the loss of the p27 locus has been demonstrated in some cases of acute lymphoblastic leukemia with and without the concomitant presence of p27 somatic aberration(s) on the remaining second allele.58,60-62 Among all these possible mechanisms, an enhanced ubiquitin proteosome-mediated degradation is the most common mechanism regulating the availability of p27 in normal cells.47 In the past few years, several investigators have demonstrated that the deregulation of p27 expression is a relatively common feature of many solid tumors.34-37,55 Total or partial loss of p27 expression in tumor cells may have important implications. This is supported by several studies on breast, colon, and esophageal carcinomas demonstrating that loss of p27 correlates with poor prognosis and high-grade neoplasms.34-37Furthermore, p27 knockout mice63-65 and heterozygous mice (p27+/−), in which the loss of a single p27 allele is associated with decreased levels of p27 protein expression, are more prone to spontaneous and induced tumors.49 Thus, decreased levels of p27 resulting from the loss of heterozygosity may be sufficient to give a growth advantage to tumor cells. Toward this end, recent studies in Rb−/−/p27+/− mice have shown that the loss of p27 allows the growth and escape of cells that have acquired additional genetics defects (biallelic loss of Rb), resulting in the generation of more aggressive neoplasms, higher incidence of neoplastic transformation, or both.66 Thus, it is possible that MCL lymphomas that have acquired p27 deregulation may not only lose the inhibitory action of p27 on cyclin E/cdk2 complex, they may be prone to accumulate additional mutations. The high rate of p53 abnormalities18,23,25 and the loss of p16 function26 27 seen in aggressive forms of MCL tend to support this hypothesis.
In this article, we have shown that levels of p27 mRNA are similar in normal and neoplastic cells (MCL and CLL/SLL), but that in MCL cells, there is a positive correlation between p27 protein levels and the degree of p27 degradation in vitro. This enhanced protein degradation is blocked by a proteasome-specific inhibitor and requires the generation of ubiquitinated forms. Our data not only support the concept that p27 regulation is achieved primarily through proteasome degradation, they expand previous findings by demonstrating for the first time that the loss of p27 in NHL can occur as a result of enhanced protein degradation. Furthermore, we have demonstrated that, as in solid human neoplams, the loss of p27 in MCL is associated with a more aggressive biologic phenotype and decreased overall survival. These findings are particularly important given the relatively few clinically prognostic indicators in MCL. Among the histopathologic criteria, a nodular pattern is associated with a better outcome than is a diffuse pattern; a worse prognosis also is associated with a high proliferation index and with some cytologic MCL variants.12,18,22 More recently, the overexpression of p53 has been shown to be a marker of poor prognosis.23,25However, p53 anomalies occur only in a small subset of MCL and therefore are of limited value. In contrast, the loss of p27 involves most cases of MCL,50 and therefore it appears to be a more useful prognostic marker. Our findings clearly demonstrated that the analysis of p27 expression allows a more reliable and comprehensive stratification of patients with MCL and possibly of patients with other forms of NHL into prognostically significant subgroups suitable for ad hoc chemotherapeutic approaches. We can envision alternative therapeutic protocols designed to modulate pharmacologically the degradation of p27, thereby providing new avenues in the management of MCL.
Acknowledgments
The authors thank Elena Mazzone for her excellent technical assistance. They also thank Kristian Helin for supplying the anti-E2F-1 antibody and J. Tiesinga for helpful discussion and critical review of the manuscript.
Received June 15, 1999; accepted September 3, 1999.
Supported in part by the Dutch Cancer and the Johan Vermeij Foundations and by National Institutes of Health grants CA66229, CA14462, CA76584, CA76584, and GM57587; by HFSPO grant RG0229/98-M; and by the Associazione Italiana Ricerca sul Cancro, Milan, Italy.
R.C. and L.M.B. contributed equally to this work.
Reprint:Giorgio Inghirami, New York University, Department of Pathology and Kaplan Comprehensive Cancer Center, 550 First Avenue, New York, NY 10016; e-mail:inghig01@mcrcr6.med.nyu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.