Sepsis is defined as the systemic inflammatory response to infection. Phospholipase A2 (PLA2) plays an important role in inflammation processes by initiating the production of inflammatory mediators. The role of cytosolic PLA (cPLA2) has not yet been identified in inflammatory and infectious disease clinical settings. The aim of the present research was to determine whether cPLA2 activity has a role during sepsis. Since neutrophil activation has been documented during sepsis, these cells were chosen as a model to evaluate the function of cPLA2 in this clinical setting. cPLA2 was studied at 3 levels: activity, protein expression, and messenger RNA (mRNA). Neutrophils from 32 septic patients with and without bacteremia were examined. cPLA2 activity was measured using labeled phosphatidyl choline vesicles as a substrate, and total PLA2 was determined by the release of labeled arachidonic acid from prelabeled cells. A significant increase in cPLA2activity, protein expression, and total PLA2 activity in neutrophils was detected during sepsis. mRNA levels, detected by reverse transcriptase–polymerase chain reaction, were significantly higher during sepsis, indicating that the increase in the amount of cPLA2 is regulated on the mRNA level. The significant elevation of cPLA2 activity and expression in neutrophils during sepsis suggests that this enzyme plays a major role in neutrophil function in this clinical setting.
Sepsis is recognized as a common cause of morbidity and mortality, particularly among elderly, immunocompromised, and critically-ill patients.1 Sepsis may be regarded as a constellation of signs and symptoms representing the host's response to infection, whereby cytokines (or substances triggered by cytokines) are responsible for most of the clinical manifestations.1,2Bacterial invasion of the host is the usual setting for sepsis to occur. The most consistent virulence factor of gram-negative organisms is bacterial endotoxin or lipopolysaccharide (LPS). The presence of microorganisms or bacterial products triggers an inflammatory response. Many pathogenic mediators of sepsis have been described, including tumor necrosis factor– (TNF-), interleukin 1 (IL-1), IL-6, IL-8, and platelet activating factor (PAF) in mononuclear phagocytes and endothelial cells.2-5 Phospholipase A2(PLA2) generating arachidonic acid (AA) plays an important role in inflammation by initiating the production of inflammatory mediators such as leukotriens, thromboxane, and prostaglandins.6 These AA metabolites have been shown to have a number of biological actions including vasodilitation, regulation of cytokine secretion from macrophages and T-lymphocytes, stimulation of platelet aggregation and neutrophil activation.7-9
In the last decade, several mammalian PLA2s have been identified, purified, and cloned in phagocytic cells. The secreted nonpancreatic PLA2s (sPLA2s) are all low-molecular mass enzymes (approximately 14 kd). The PLA2s do not manifest significant fatty acid selectivity in vitro, but they exhibit a requirement for millimolar calcium concentrations and are sensitive to reducing conditions.10 The PLA2group IIA11,12 has been found to be present in neutrophil and monocyte microsomes.13 Two new types of human sPLA2s were recently described, group V14 and group × ,15 and were found to be present in neutrophils.16 The calcium-independent PLA2 (40 kd), which was found in muscle cells,17 has recently been identified in the mouse macrophage cell line, P388D.18 The 85 kd cytosolic PLA2 (cPLA2), which is insensitive to reducing conditions present in the cytosol, has been purified from U937 cells.19 The 85 kd cPLA2 has high specificity for phospholipids that contain sn-2 arachidonate.20 cPLA2, whose translocation to the membranes is calcium-dependent,21,22 is activated by phosphorylation on serine residue 505 that is mediated by MAP-kinase.23
Several clinical studies have reported that the activity and the concentration of sPLA2 in serum is markedly elevated in patients with infection, especially those with sepsis or bacteremia,24-26 while the role of cPLA2 has not as yet been identified in such clinical settings. Since neutrophil activation during sepsis24 has been documented, these cells were chosen as a model to evaluate the function of cPLA2. cPLA2 protein expression and activity in neutrophils from patients with sepsis or with both sepsis and a blood culture–documented infection (bacteremia) were examined in order to determine its potential role during these clinical situations.
Materials and methods
Patients
The study included 32 patients, with sepsis and with or without bacteremia, who were admitted to Soroka Medical Center between 1996-1998. The patients were divided into 2 groups: Group I comprised 17 patients with sepsis (mean age, 51 years; range, 21-62), and group II had 15 patients with sepsis and positive blood culture (bacteremia) (mean age, 48 years; range, 24-65). The site of infection and isolated bacteria are shown in Table 1. The type of bacteria isolated from patients' blood were: Escherichia coli(11 patients), Staphylococcus aureus (2 patients),Pseudomonas aeruginosa (1 patient), Acinetobacter baumanii (1 patient), and Stenotrophomonas maltophilia (1 patient).
Sepsis was defined as a condition showing clinical evidence of infection plus evidence of a systemic response to infection. This systemic inflammatory response syndrome (SIRS) is determined by the fulfillment of 2 or more of the following criteria, as defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee:27 temperature, > 38°C or < 36°C2; heart rate, > 903; respiratory rate, > 20/minute or PaCO2 < 32 mmHg4; leukocyte count, > 12 × 109/L or < 4 × 109/L or 10% immature forms. cPLA2 protein expression and activity were examined during and after the sepsis episode. Blood was drawn from patients upon admission to the hospital as well as 2 to 3 weeks after complete recovery. The rare patients admitted without prior antibiotic therapy were included in the study in order to exclude the possible effect of antibiotic treatment on cPLA2. Antibiotic treatment was not administered to 29 patients, and 3 patients from group II received antibiotics for 48-72 hours before drawing blood. All patients gave informed consent, and the study was approved by the Human Research Ethics Committee of the Soroka Medical Center. Each patient was analyzed in parallel to a healthy age-matched and sex-matched blood donor.
Neutrophil separation
Neutrophils (95% purity) were separated by Ficoll/Hypaque (Sigma, St Louis, MO) centrifugation, dextran sedimentation, and hypotonic lysis of erythrocytes as described previously.28 The percent of immature neutrophils ranged between 2%-24% with the mean ± SEM of 10.3 ± 5.3.
Opsonized zymosan (OZ) was prepared as follows: 20 mg of zymosan (Sigma, St Louis, MO) were incubated with 1 mL of human pooled serum for 1 hour at 37°C and washed 3 times with Hanks balanced salt solution.
Dithiothreitol-resistant cPLA2 activity
cPLA2 activity was determined in neutrophil lysates using sonicated dispersions of 1-stearoyl-2-[14C]arachidonyl phosphatidyl choline (PC) (30 μmol/L, 50 000 DPM/assay) and sn-1,2-dioleoylglycerol (molar ratio 2:1) in a modified assay mixture containing 5 mmol/L dithiothreitol (DTT), as described earlier.29 Briefly, the assay mixture contained the phospholipid substrate in: potassium chloride, 80 mmol/L; calcium dichloride, 5 mmol/L; DTT, 5 mmol/L; bovine serum albumin, 1 mg/mL; ethylene diamine tetra-acetic acid (EDTA), 1 mmol/L; and HEPES, 10 mmol/L (pH 7.4) (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid). The reaction was started by the addition of 50 μg cytosolic protein (within the linear protein range of the assay) and incubated at 37°C in a shaking water bath for 20 minutes.
Immunoblot analysis
Immunoblot detection of cPLA2 was performed as described earlier.30 Cell lysates were prepared using Triton X-100, 1%; HEPES, 50 mmol/L (pH 7.5); sodium chloride, 150 mmol/L; EDTA, 1 mmol/L; ethylene glycol diamine tetra-acetic acid (EGTA), 1 mmol/L; glycerol, 10%; sodium fluorine, 25 mmol/L; zinc dichloride, 10 μmol/L; phenylmethyl-sulfonyl fluoride, 1mmol/L; and leupeptin, 100 μmol/L. Using electrophoresis, 100 μg protein was separated from cell lysate on 7.5% polyacrylamide SDS gels and blotted to nitrocellulose. The detection of cPLA2 protein was performed using rabbit antibodies raised against a glutatione S-transferase–fusion with cPLA2, as described earlier.30 The relative changes of the proteins were quantitated using densitometry in a reflectance mode (Hoefer; Hoefer Scientific Instruments, San Francisco, CA). Densitometry units are arbitrary units of density with higher numbers representing darker bands on the immunoblot. These measurements are adequate to determine the changes of cPLA2 protein in the same immunoblot.
Release of radiolabeled arachidonic acid
Assays of incorporation and release of tritum radiolabeled arachidonic acid [3H]AA were performed as previously reported.31 Neutrophils (108 cells/mL) were incubated for 60 minutes at 37°C in Ca++–free and Mg2+–free phosphate-buffered saline containing 1 μCi of [3H]AA. Labeling strategies primarily incorporate AA into 1-acyl-2-AA-sn-phosphatidyl choline and 1-acyl-2AA-sn-phosphatidylinositol, without incorporating AA into the largest AA containing phospholipid pools in the neutrophils, 1-alk-1enyl-2-AA-glycerophosphoethanolamine and 1-alkyl-2-Aaglycerophosphocholine.32 After appropriate washes, neutrophils (107 cells/mL) were stimulated, and the release of [3H]AA was determined in the linear range of the reaction.
Reverse transcription–polymerase chain reaction
Reverse transcriptase–polymerase chain reaction (RT-PCR) of neutrophil cPLA2 was performed with some modification, as described earlier.31 Total cellular RNA was extracted from 107 cells by the RNAzol B method of RNA isolation. The RNA pellet was precipitated with isopropanol and washed twice with 70% ethanol, the pellet was reprecipitated with 10% sodium acetate (3 mol/L) and 70% ethanol. Total RNA was reverse transcribed into cDNA at 37°C for 1 hour using Moloney murine leukemia virus RT (GibcoBRL Life Technologies, Grand Island, NY) and primer p(dT)15 potassium salt (Boehringer Mannheim, Frankfurt, Germany). The RT was then heat inactivated at 65°C for 10 minutes, and the cDNA was cooled to 4°C. The cDNA was amplified via PCR using Thermus aquaticus DNA polymerase in conditions found to amplify cDNA molecules in a linear fashion. The cPLA2 primer pair was constructed according to the cDNA sequence of cPLA2.19 It amplified a 628 base pair (bp) using an upstream primer: 5′-CTCTTGAAGTTTGC TCATGCCCAGAC-3′; and a downstream primer: 5′-GCAAACATCAGCTCTGAAA CGTCAGG-3′. The β-actin primer pairs amplified a 269 bp using an upstream primer: 5′-GGGTCAGAAGGATTCCTATG-3′ and a downstream primer: 5′-GGTCTCAAACA TGATCTGGG-3′.
The PCR amplification was performed in a microprocessor-controlled incubation system (Crocodile II; Appligene, Pleasanton, CA). The reaction was carried out with 1 μmol/L of 5′ and 3′ primers in 50 μL of reaction mixture using a step program (94°C, 1 minute; 55°C, 30 seconds; 72°C, 2 minutes) followed by a 10-minute final extension at 72°C for 25, 35, and 45 cycles. An 8-μL sample of the completed reaction mixture was run on a 2% agarose gel stained with ethidium bromide. The relative changes of the PCR bands were quantitated using densitometry in a reflectance mode (Hoefer Scientific Instruments).
Statistical analysis
The mean differences were analyzed by the Student t test. The plots were drawn as least-square regression lines and tested by analysis of variance (ANOVA).
Results
cPLA2 activity in neutrophil lysates was determined using 1-stearoyl-2-[1-14C] arachydonyl phosphatidylcholine as a substrate. As presented in Figure1, cPLA2 activity was significantly higher during sepsis or sepsis+bacteremia as compared with cPLA2 activity after complete recovery. Of the 17 patients with sepsis, 15 showed significantly higher cPLA2activity (P < 0.001) during the sepsis episode as compared with the activity after recovery, and in 2 patients, cPLA2activity was not elevated during the disease. Of the 15 patients with sepsis+bacteremia, 11 showed significantly increased cPLA2activity (P < 0.001) during the disease than after recovery. In 4 patients from this group, cPLA2 activity did not change during sepsis; 3 of these 4 patients received a course of antibiotics 48 to 72 hours prior to the first blood sampling.
cPLA2 activity in neutrophil lysates of patients with sepsis or with sepsis+bacteremia.
DTT-resistant cPLA2 activity is measured by [14C]AA release from PC vesicles using neutrophil lysates from patients with sepsis or sepsis+bacteremia (“During”) and after recovery (“After”).
cPLA2 activity in neutrophil lysates of patients with sepsis or with sepsis+bacteremia.
DTT-resistant cPLA2 activity is measured by [14C]AA release from PC vesicles using neutrophil lysates from patients with sepsis or sepsis+bacteremia (“During”) and after recovery (“After”).
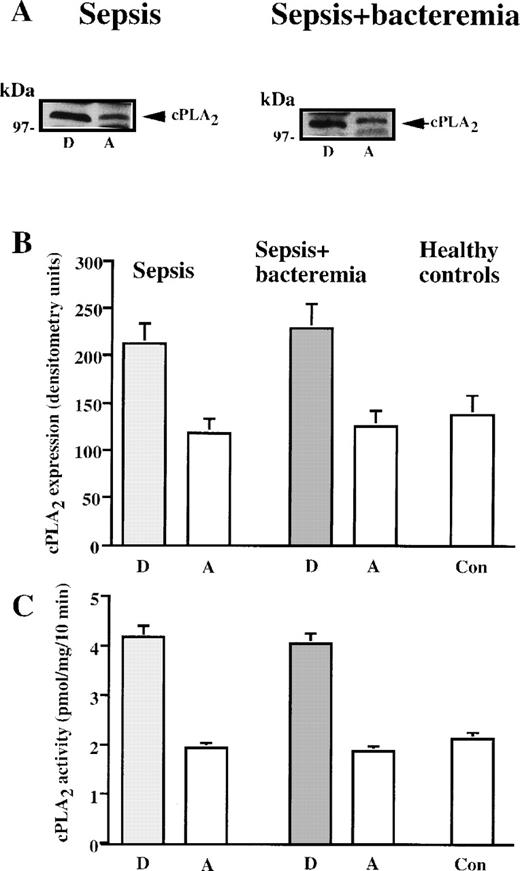
To assess whether the elevated neutrophil cPLA2 activity resulted from increased protein expression, its level was determined by Western blot analysis using polyclonal antibodies. cPLA2levels in neutrophil lysates were determined in all the patients and were compared to cPLA2 levels after recovery. All the patients with sepsis or with sepsis+bacteremia who showed increased cPLA2 activity also demonstrated elevated cPLA2protein expression in neutrophil lysates during the disease compared with the level of cPLA2 after recovery. Immunoblot analysis of cPLA2 protein expression in neutrophils of representative patients with sepsis or with sepsis+bacteremia are presented in Figure 2A. The relative amount of cPLA2 was quantitated by densitometry in neutrophil lysates of the septic patients who showed increased activity and the healthy controls studied in parallel. During the disease, the mean ± SEM in neutrophil lysates was 212 ± 22 units for patients with sepsis and 229 ± 25 units for patients with sepsis+bacteremia. After recovery, the neutrophil lysate count was 118 ± 15 units for patients who had sepsis and 125 ± 17 units for patients who had sepsis+bacteremia (Figure 2B). cPLA2 protein levels were significantly higher (P < 0.001) during sepsis or sepsis+bacteremia than after recovery. The mean ± SEM of cPLA2 expression in neutrophils of healthy controls was 138 ± 20 units, significantly lower (P < 0.001) than that of neutrophils of septic patients and similar to the levels of this protein after recovery. Figure 2C shows the mean ± SEM of cPLA2 activity in neutrophil lysates of the 2 groups of patients who showed increased activity (Figure 1) and of healthy controls studied in parallel. The high correlation between protein level and activity suggests that increased cPLA2 activity in neutrophil lysates is due to induction of this protein during these clinical settings. There were no differences between the presence of gram negative or gram positive bacteria in the blood on induction of cPLA2 protein synthesis.
CPLA2 in neutrophils during sepsis and after recovery.
(A) Immunoblot analysis of cPLA2 in neutrophil lysates of septic patients during infection and after recovery. Shown are the results of a representative patient with sepsis and a representative patient with sepsis+bacteremia during the disease (“D”) and after complete recovery (“A”). (B) cPLA2 expression in human neutrophils during sepsis and after recovery (expressed in densitometry units). The mean ± SEM of the densitometry values in the septic patients who showed increased cPLA2 activity and the controls are shown. (C) cPLA2 activity in neutrophil lysates during sepsis and after recovery. The mean ± SEM of cPLA2 activities in neutrophil of the individual patients who showed increased cPLA2 activity (Figure 1) and of the controls are shown.
CPLA2 in neutrophils during sepsis and after recovery.
(A) Immunoblot analysis of cPLA2 in neutrophil lysates of septic patients during infection and after recovery. Shown are the results of a representative patient with sepsis and a representative patient with sepsis+bacteremia during the disease (“D”) and after complete recovery (“A”). (B) cPLA2 expression in human neutrophils during sepsis and after recovery (expressed in densitometry units). The mean ± SEM of the densitometry values in the septic patients who showed increased cPLA2 activity and the controls are shown. (C) cPLA2 activity in neutrophil lysates during sepsis and after recovery. The mean ± SEM of cPLA2 activities in neutrophil of the individual patients who showed increased cPLA2 activity (Figure 1) and of the controls are shown.
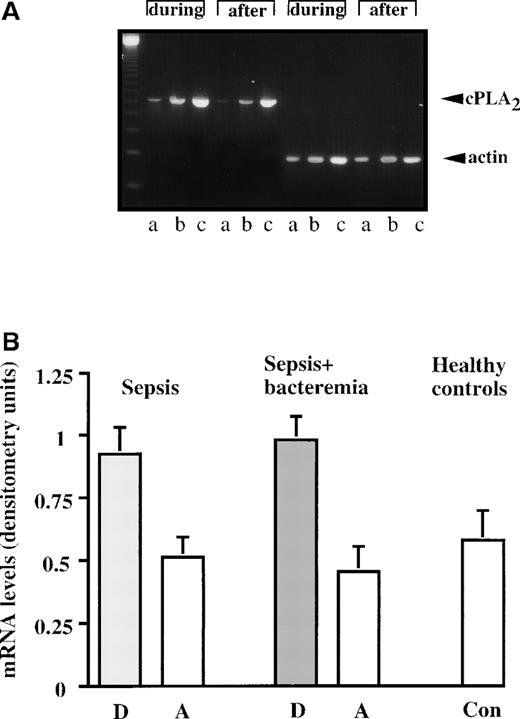
Next, the level of cPLA2 mRNA was studied by the RT-PCR technique. As demonstrated in representative results from a septic patient in Figure 3A, the cPLA2mRNA level was higher during sepsis than after recovery. The levels of cPLA2 mRNA in neutrophils of the septic patients and healthy controls were quantitated by densitometry and divided by the corresponding actin mRNA levels. As shown in Figure 3B, densitometric analysis demonstrated that cPLA2 mRNA levels are significantly higher (P < 0.001) during the disease than after recovery. During the disease, the mean ± SEM of the densitometric analysis of neutrophil cPLA2 mRNA in patients with sepsis was 0.925 ± 0.103 units, and in patients with sepsis+bacteremia the mean was 0.980 ± 0.093 units. After recovery, the patients with sepsis had values of 0.508 ± 0.085 units, and the patients with sepsis+bacteremia had values of 0.453 ± 0.101 units. The mean ± SE of the densitometric analysis of neutrophil cPLA2 mRNA of healthy controls was 0.578 ± 0.122 units, significantly lower (P < 0.001) than cPLA2 mRNA levels during sepsis and similar to the levels of cPLA2 mRNA after recovery.
cPLA2 mRNA levels during sepsis and after recovery.
(A) A representative RT-PCR experiment assessing cPLA2 mRNA levels of a septic patient manifesting elevated cPLA2protein activity and expression. The amplified products of cPLA2 (628 bp) and the corresponding products of β-actin (269 bp) are shown. The PCR reactions were amplified for 25, 35, and 45 cycles (A, B, and C, respectively). The first lane shows the 123 bp size marker. (B) cPLA2 mRNA levels in human neutrophils during sepsis and after recovery. The levels of cPLA2 mRNA in neutrophils of the septic patients with increased cPLA2and the controls were quantitated by densitometry and divided by the corresponding actin mRNA levels. The mean ± SEM of the densitometry analysis in the septic patients and the controls are shown. The densitometric analysis demonstrates that cPLA2 mRNA levels are significantly higher (P < 0.001) during the disease than after recovery.
cPLA2 mRNA levels during sepsis and after recovery.
(A) A representative RT-PCR experiment assessing cPLA2 mRNA levels of a septic patient manifesting elevated cPLA2protein activity and expression. The amplified products of cPLA2 (628 bp) and the corresponding products of β-actin (269 bp) are shown. The PCR reactions were amplified for 25, 35, and 45 cycles (A, B, and C, respectively). The first lane shows the 123 bp size marker. (B) cPLA2 mRNA levels in human neutrophils during sepsis and after recovery. The levels of cPLA2 mRNA in neutrophils of the septic patients with increased cPLA2and the controls were quantitated by densitometry and divided by the corresponding actin mRNA levels. The mean ± SEM of the densitometry analysis in the septic patients and the controls are shown. The densitometric analysis demonstrates that cPLA2 mRNA levels are significantly higher (P < 0.001) during the disease than after recovery.
The release of labeled AA was studied in parallel to the analysis of cPLA2 levels and activity. [3H]AA release from prelabeled neutrophils stimulated with 50 ng/mL phorbol myristate acetate (PMA) or 1mg/mL OZ was determined in all patients during sepsis or sepsis+bacteremia and after recovery. A high correlation between cPLA2 expression and activity and [3H]AA release from prelabeled neutrophils was observed. All patients with elevated levels and activity of cPLA2 also showed increased [3H]AA release from prelabeled neutrophils during the disease. As demonstrated in Figure 4, [3H]AA release from prelabeled neutrophils was higher during sepsis or sepsis+bacteremia than after recovery, in correlation with the high cPLA2 activity and protein expression.
The release of [3H]AA from prelabeled stimulated neutrophils.
The cells were stimulated with 50 ng/mL PMA (•, ○) or 1 mg/mL OZ (▪,□). Shown are the mean ± SEM of the septic patients during the disease and after recovery.
The release of [3H]AA from prelabeled stimulated neutrophils.
The cells were stimulated with 50 ng/mL PMA (•, ○) or 1 mg/mL OZ (▪,□). Shown are the mean ± SEM of the septic patients during the disease and after recovery.
In order to further support the correlation between cPLA2activity, cPLA2 protein expression, and [3H]AA release from prelabled neutrophils, these 3 parameters were also studied in all patients whose cPLA2activity did not change during sepsis or sepsis+bacteremia. As shown for a representative patient of this group, cPLA2 activity, cPLA2 protein expression, and [3H]AA release from prelabled neutrophils were similar during sepsis and after recovery.
Discussion
The presence and activation of both sPLA2 and cPLA2 have been demonstrated in neutrophils.13,30,33,34 However, the role of these 2 enzymes in the regulation of neutrophil AA mobilization and lipid mediator formation needs further elucidation. The present study clearly demonstrates that cPLA2 activity, protein expression, and mRNA levels are elevated in peripheral blood neutrophils of septic patients, indicating that this type of PLA2 may have a role in such clinical settings. There is a significant correlation between cPLA2 expression and activity and AA release from prelabled neutrophils (Figure 2 compared with Figure 4, and Figure5). These results suggest that cPLA2 makes a major contribution for the release of AA in human neutrophils. Recent cells studied have indicated that both cPLA2 and sPLA2 are involved in eicosanoid production.35,36 However, in accordance with our results, the critical involvement of cPLA2 was demonstrated by recent genetic studies in “knockout” mice lacking this enzyme.37,38 These mice showed a loss in biosynthesis of lipid mediator (prostaglandine E2, leukotriene B4 or C4, and PAF) in peritoneal macrophages, although these cells contain other forms of PLA2. The disruption of the cPLA2 gene in these mice results with protection against endotoxin shock and reduced postischemic brain injury. Experiments conducted in our laboratory demonstrated that the level of sPLA2 type 2A in neutrophils detected by RT-PCR did not change during sepsis (data not shown), but its activity and secretion has to be analyzed. It was recently reported39 that TNF-α priming of human neutrophils caused a marked increase in formyl-methionyl phenylalanine (fMLP)-stimulated AA release in parallel to enhanced sPLA2 activity, although there was no increase in its expression. In addition, the role of other types of PLA2found in these cells, such as the secreted type V or type X, in AA release during sepsis cannot be excluded, and this question is currently under investigation in our laboratory.
cPLA2 protein expression, cPLA2activity, and [3H]AA release.
cPLA2 protein expression (A), cPLA2 activity (B), and [3H]AA release from prelabeled neutrophils stimulated with 50 ng/mL PMA in a representative patient whose activity did not change during sepsis (C). [3H]AA release, expressed as % of total, was determined after 10 minutes. “D” = during the disease; “A” = after complete recovery.
cPLA2 protein expression, cPLA2activity, and [3H]AA release.
cPLA2 protein expression (A), cPLA2 activity (B), and [3H]AA release from prelabeled neutrophils stimulated with 50 ng/mL PMA in a representative patient whose activity did not change during sepsis (C). [3H]AA release, expressed as % of total, was determined after 10 minutes. “D” = during the disease; “A” = after complete recovery.
The present study demonstrates that both clinical situations (sepsis or sepsis+bacteremia) induced an increase in the level and activity of cPLA2 in neutrophils. The presence of bacteria in blood during sepsis did not augment the elevation of either the level or the activity of cPLA2 in neutrophils. These ex vivo studies on the effect of LPS and cytokines on cPLA2 activity and expression in various cell types are in accordance with recent reports of in vitro studies. It has been shown40-42 that preincubation of neutrophils with minute quantities of lipopolysaccharide (LPS) or TNF-α resulted in a preferential hydrolysis of AA containing phospholipids. Exposure of human monocytes to LPS for over 18 hours resulted in a 2-fold increase in the 85-kd PLA2 protein and its activity levels.43TNF-α was observed to induce rapid phosphorylation of cPLA2, which was followed within hours by elevation of cPLA2 mRNA and protein in Hela cells.44 TNF-α priming increased cPLA2activity stimulated by fMLP in human neutrophils.39 It has been reported that in human lung fibroblasts45 and mesangial cells46,47 IL-1 first stimulated phosphorylation of cPLA2 and then, after prolonged incubation (8-24 hours), continued to increase cPLA2 protein levels. Treatment of the rat C6 glioma cell line with IL-1β resulted in an accumulation of cPLA2 mRNA levels.48 Likewise, treatment of synovial cells with IL-1β induced a de novo synthesis of cPLA2 mRNA and protein.49 The macrophage colony-stimulating factor stimulated arachidonic acid release and increased cPLA2 mRNA levels in macrophages.50
In summary, the elevation of cPLA2 activity and protein expression in peripheral blood neutrophils during sepsis implies its importance in this clinical setting. The increased cPLA2activity suggests that this type of PLA2 contributes to the generation of AA in human neutrophils during sepsis. AA metabolites are potent mediators in sepsis-related processes, including endothelial damage, vasodilation, and anti-inflammatory and pro-inflammatory action. The elevated cPLA2 activity and expression documented in neutrophils most likely has implications for other cell types. Using a cPLA2-deficient differentiated PLB-985 cell line,29 we provided evidence for the essential requirement of cPLA2 activity and its metabolite, AA, for activation of NADPH oxidase generating superoxides. The production of these superoxides is one of the most important functions for host defense. However, during altered physiological states, superoxides may promote inflammatory reactions and participate in processes that lead to tissue injury.49 Inhibition of cPLA2 may diminish the formation of inflammatory mediators, resulting in reduced inflammatory manifestations, and may regulate the release of oxygen radicals that also participate in endothelial damage. Thus, this type of PLA2 may be a suitable target enzyme for the development of inhibitory therapeutic agents for the treatment of such inflammatory disorders.
Reprints:Rachel Levy, Faculty of Health Sciences, Infectious Disease Laboratory, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel; e-mail: ral@bgumail.bgu.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. cPLA2 activity in neutrophil lysates of patients with sepsis or with sepsis+bacteremia. / DTT-resistant cPLA2 activity is measured by [14C]AA release from PC vesicles using neutrophil lysates from patients with sepsis or sepsis+bacteremia (“During”) and after recovery (“After”).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.660/6/m_bloo00211001x.jpeg?Expires=1769412075&Signature=PcG2cCBvcvVGLLQ-n8uhhVozmcgE3uQ-jhheKvrYRCD-K3E4CilLGoVb7uYWC7GSeceFFjsWr1DePWBJYdNj9nZvYNl0IhRpNnSRWzTrqN1smNTxPlQ9AIsMw1vjWlEnMI1oxVFlfemZuSDxzmrHZ-GohDKkJA7lhSsFLRhYBRZY7gC7w8bEQzDeaCOIX4PGUGllH-ztE-FdL7oqfdMsghhLlqlJOS5BndHMACsZcMkf-W0Bre378cuTvwV8EMH98Ix-5hYBGJZoNJ5XN9nsE5QBnizhr7g72vIyLL2bojOXvTgQ5d5A-u625UlxECdpOzvHBQDPbuflex2wTzQjdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. The release of [3H]AA from prelabeled stimulated neutrophils. / The cells were stimulated with 50 ng/mL PMA (•, ○) or 1 mg/mL OZ (▪,□). Shown are the mean ± SEM of the septic patients during the disease and after recovery.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.660/6/m_bloo00211004x.jpeg?Expires=1769412075&Signature=rOq7nYXZc-imA1iqiDFXBVCwPdVkOy6KXfC2SdnssINP~pcb4oPNQxZgnDKFgNFyvj~zom5cdU3yVP21EsDyKFiJvMHMwOLm8JLlb04lqIYwUzlzH68dCpwSgMTzrkudBnkqGD1n2il7M3~6U6KQoIE08SeMY~cfCh8KuPYka1zOJ31i5dGl4kwwvTK3o7MsrzcUZiOgWNz5A-8MuK3JtB67N5j~8-jxzz2L5Z52L-sysw1ap70sbSE4xDYlddb8zd6o0UBf3~s697FEkWeCihqKyiKeeFi9iJ8mOTaj7endVZewNxXORmLK~EJ1okvdnO6ezJSAz6EJTQ3G5-6Cag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. cPLA2 protein expression, cPLA2activity, and [3H]AA release. / cPLA2 protein expression (A), cPLA2 activity (B), and [3H]AA release from prelabeled neutrophils stimulated with 50 ng/mL PMA in a representative patient whose activity did not change during sepsis (C). [3H]AA release, expressed as % of total, was determined after 10 minutes. “D” = during the disease; “A” = after complete recovery.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.660/6/m_bloo00211005w.jpeg?Expires=1769412075&Signature=rL74zjLpEHwBqOO6yWJ3ufk2dafSiBvgk3cOGGfvpCB44NwyIDcLuRzu5dOkr2Ky7X5SkBXzsvw4N2YQUm5aiJyzUOranvuuQhNgGlgoR75UWPy6HanbwsguLtM9R5U~4VhS5pIz4IlgUv7Iyoa2~BlGJa-~GObBsLQuM~10vr6wqQdoB78hRBaImVVcPhAjXTv6iiMJ3Qe-b~mK-5XXVyt97qX-FY~el7w7FgMD6ZtUd9IK04lp6y8cml4AiJK8850u60KAlRzZ2JQgJ3h6sQrVzGSzle9P6w~8KtVVm6JYIZs~094IrlmMeOdtDStdBxzuZmSmYZ9VU3RXfM9LYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
