We evaluated the effect of β1-integrin receptor engagement on the expression and activity of cell cycle regulatory proteins in CD34+ cells under conditions that mimic the steady-state marrow microenvironment and in the presence of supraphysiological concentrations of interleukin-3 (IL3) and stem cell factor (SCF). Adhesion of CD34+ progenitors to fibronectin (FN) was similar whether IL3 or SCF was present or absent. Engagement of β1-integrins blocked S-phase entry of CD34+ cells in the absence of IL3 or SCF, whereas addition of 10 ng/mL IL3 or SCF prevented such a block in S-phase entry. In the absence of IL3 or SCF, cyclin-E levels were significantly lower and p27KIP1 levels significantly higher in FN-adherent than in FN-nonadherent cells, or than in poly-L-lysine (PLL)–adherent or (PLL)–nonadherent cells. Cyclin-dependent-kinase (cdk)-2 activity was decreased and levels of cyclin-E–cdk2 complexes were lower in FN-adherent than in PLL-adherent cells. In contrast, cyclin-E and p27KIP1 protein levels and cdk2 activity in cells adherent to FN in the presence of IL3 or SCF were similar to those in PLL-adherent and FN-nonadherent or PLL-nonadherent cells. In conclusion, under physiological cytokine conditions, integrin engagement prevents S-phase entrance of CD34+ cells, which is associated with elevated levels of the contact-dependent cyclin kinase inhibitor p27KIP1. Supraphysiological concentrations of IL3 or SCF prevent p27KIP1 elevation and override the integrin-mediated inhibition of entry into S phase.
β1-integrins on human CD34+ cells are responsible for their adhesion to fibronectin (FN) and to vascular cell adhesion molecules.1-3 In addition, a number of studies from our laboratory have shown that adhesion of normal human CD34+ colony-forming cells (CFC) to FN or direct engagement of β1-integrins on CD34+ cells with adhesion-blocking antibodies prevents CFC from entering the S phase of the cell cycle and inhibits expansion of CFC and more primitive long-term culture initiating cells (LTC-IC) in long-term bone marrow (BM) cultures.4-7 The mechanism through which engagement of β1-integrins inhibits CD34+ progenitor proliferation is not known.
Integrin-mediated signaling has been extensively studied in cells of mesenchymal origin and in platelets.8-15 Integrins are divalent cation-dependent cell surface glycoproteins consisting of an α and a β chain. They are responsible for cell–extracellular matrix (ECM) and cell-cell adhesion events. Integrins have a large, heterodimeric extracellular domain responsible for ligand recognition and binding, a short transmembrane domain, and a short cytoplasmic tail. The cytoplasmic tails of integrins have no intrinsic kinase activity. However, engagement of integrins results in the assembly of focal contacts in which a number of kinases become activated.8-15 Integrins activate the focal adhesion kinase (Fak) or the related kinase, Pyk-2.16,17 Phosphorylated Fak or Pyk-2 serve to bind and activate a number of Rous sarcoma (Src)-homology domain SH2- and SH3-containing adaptor proteins, including Src, paxillin, CrkL, Grb-2, p130Cas, p120Cbl, and the p85 subunit of phospho-inositol-3 kinase.18-24 Although the exact mechanism or mechanisms through which engagement of integrins affects cell proliferation, differentiation, or survival are not known, integrins can activate the phospho-inositol-3-kinase pathway23 and the Ras/mitogen–activated protein kinase pathway,24 both of which mediate signals regulating growth. Further, integrin engagement induces immediate-early inflammatory response genes.24
Growth of adherent cells such as fibroblast requires signals not only from growth factor receptors but also from integrins.13-15,25 Recent studies have shown that integrin-mediated adhesion of fibroblasts is associated with increased levels of cyclin-D1, leading to hyperphosphorylation of the retinoblastoma protein (Rb) and transition of the cell through the restriction-point (R) in the G1/S phase of the cell cycle.26,27 Other studies have shown that adhesion of fibroblasts to ECM also results in up-regulation of cyclin-E/cdk2 activity owing to decreases in the cdki's p21CIP1 and p27KIP1, and up-regulation of cyclin-A levels.26 27 It is not known whether or how these observations made in mesenchymal cells relate to signals emanating from integrins following their engagement on human hematopoietic progenitors, which are nonadherent cells.
Signals initiated by integrins can be modified or enhanced by costimulation of cells with cytokines or growth factors.15In the hematopoietic system, a number of investigators have examined the effect of cytokine stimulation on integrin-mediated adhesion.28-34 Cytokines, including interleukin 3 (IL3), granulocyte-macrophage (GM)–colony stimulating factor (CSF), and stem cell factor (SCF), may increase at least short-term integrin-mediated adhesion of cytokine-starved CD34+ to FN. Other studies show that IL3 or SCF do not affect adhesion of progenitor cells. Another group of reports has demonstrated a synergistic effect of cytokines and FN on the expansion of progenitors when cultured in the absence of stromal feeders, thus linking signals initiated following integrin and cytokine receptor stimulation.
In this study, we show that engagement of β1-integrins on normal human CD34+ progenitors results in the inhibition of S-phase entry/progression and increased levels of the cyclin-kinase inhibitor p27KIP1, which inhibits cyclin-E–cdk2 complex formation and cdk2 activity. However, costimulation of cells with cytokines, such as IL3 or SCF, prevents accumulation of p27KIP1, leading to continued S-phase entry/progression in progenitors even though they are adherent to FN.
Materials and methods
We obtained antibodies and reagents from various sources. Human plasma FN was purified as a by-product of Factor VIII. Poly-L-lysine (PLL) and bovine serum albumin (BSA; 98%) were purchased from Sigma Chemical Co (St. Louis, MO).
Blocking antibodies against the integrins α4 (P4C2), α5 (P1D6), β1 (P4C10), and α2 (P1E6) were purchased from Gibco-BRL (Grand Island, NY). The activating anti–β1-antibody, 8A2, was a kind gift from Dr Kovach, University of Washington (Seattle, WA).35 Antibodies against CD34 and CD62L were purchased from Becton Dickinson (Mountain View, CA), and mouse immunoglobulin G (IgG) was purchased from Sigma. FITC-conjugated goat antimouse antibody was obtained from Biosource International, Camarillo, CA.
For the flow-cytometric assessment of the cell cycle, unconjugated antibodies against p21CIP1, p27KIP1, p16INK4A, and cyclin-E and fluorescein (FITC)–conjugated antibodies against cyclin-A and cyclin-D1 + 2 + 3 were obtained from Pharmingen Inc (San Diego, CA). FITC-coupled anti-PCNA antibodies were obtained from DAKO Inc. Secondary goat antimouse FITC antibodies as well as isotype-control antibodies were also purchased from Pharmingen Inc.
For use in immunoprecipitation and Western blot, antibodies against p21CIP1, p27KIP1, Cyclin-E, Cdk2, and β-actin were obtained from Pharmingen Inc. Secondary goat antimouse horseradish peroxydase (HRP)–conjugated antibodies were obtained from Pharmingen Inc.
The following cytokines were purchased from R&D Systems (Minneapolis, MN): IL3, IL6, leukemia inhibitory factor (LIF), and macrophage-inflammatory protein (MIP)–1α. SCF was a kind gift from Amgen Inc (Thousand Oaks, CA); fetal liver tyrosine kinase-3 ligand (Flt3-L) was a kind gift from Immunex Inc (Seattle, WA). GM-CSF was purchased from Immunex, and erythropoietin and G-CSF were purchased from Amgen.
We obtained 50 mL of heparinized BM from normal donors under steady-state conditions. To obtain mobilized peripheral blood (PB) progenitors, we selected normal donors using the standard criteria of the American Association of Blood Banks for blood donors. The donors received a daily dose of 10 μg/kg/d G-CSF subcutaneous for 5 days. On day 6 donors underwent an apheresis procedure, as previously described.36 All donors signed an informed consent according to the guidelines from the Committee for the Protection of Human Subjects at the University of Minnesota.
Steady-state BM- and G-CSF–mobilized PB mononuclear cells were separated by Ficoll Hypaque centrifugation (specific gravity, 1077) (Sigma). CD34+ cells were selected either by 2 passages over the MACS CD34 Isolation Kit (Miltenyi Biotec, Sunnyvale, CA) or by sequential selection with the Ceprate SC device for clinical scale stem cell concentration (CellPro, Bhotell, WA) followed by the MACS CD34 Isolation Kit.36 CD34+ populations were more than 95% pure.
As a low-dose cytokine, serum-free medium, we used Iscove's Modified Dulbecco's Medium (IMDM, Gibco) containing 20 mg/mL BSA, 10 μg/mL insulin (Sigma), 200 μg/mL transferrin (Sigma), 10-4mol/L 2-mercapto-ethanol (Bio-Rad, Hercules, CA), 100 U/mL penicillin and 100 U/mL streptomycin (Gibco), and the following cytokines37: 200 pg/mL GM-CSF, 1000 pg/mL G-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 200 pg/mL MIP-1α, and 1000 pg/mL IL-6.
Integrins were engaged in two ways. One way was to cause them to adhere to FN. In this method, CD34+ cells suspended in serum-free IMDM with or without cytokines were plated onto wells coated with 100 μg/mL FN or 10 μg/mL PLL in a humidified atmosphere at 37°C.3,5,7 Adherent and nonadherent cells were collected after 2 hours to assess adhesion and at 12 to 16 hours to assess cell cycle status as described.3,5 7
Integrins were also engaged in solution. In this method, CD34+ cells were incubated in IMDM, with or without cytokines and with adhesion-blocking antibodies against the β1-, α2-, α4- and α5-integrins, CD62L (all at 10 μg/mL), or mouse IgG control for 30 minutes at 37°C.7 Cells were washed and incubated with goat antimouse antibody (1:500 dilution) for 8 to 12 hours at 37°C in a humidified atmosphere.
We assessed cell adhesion in two ways. In the 51Cr labeled adhesion assay, CD34+ cells were labeled with 0.1 mCi51Cr (specific activity 734.5 mCi/mg; NEN, Boston, MA) for 1 hour at 37°C and washed twice. 51Cr-labeled CD34+ cells suspended in IMDM with or without cytokines were plated in ligand-coated dishes for 2 hours. Nonadherent cells were collected. Adherent cells were lysed with triton-X-100 (Sigma), wells were harvested, and 51Cr emission was counted with the use of a Gamma 4000 Counting System (Beckman Instruments, Irvine, CA).37 The percentage of adhesion was calculated as follows: % = (cpm emission in adherent cells − cpm background) / (cpm emission in all cells − cpm background) × 100.
We also assessed cells for adherence to CFC. In this method, CD34+ cells incubated in IMDM with or without cytokines were plated in ligand-coated dishes for 2 hours. Nonadherent cells were collected in 3 washes, as described.3,5 Adherent cells were collected after trypsinization for 7 minutes. The percentage of adhesion of CFC was determined by replating adherent and nonadherent CD34+ cells in methylcellulose assay and enumerating CFC in the adherent and nonadherent portion.3 5The percentage of adhesion was determined as follows: % adhesion = (CFC in adherent cells) / (CFC in adherent + nonadherent portion) × 100.
We performed various procedures to assess cell cycle and cell cycle regulatory elements. A flow-cytometric evaluation of cell cycle38 was performed in the following way: CD34+ cells incubated in IMDM with or without cytokines were plated in ligand-coated dishes for 12 to 16 hours. Nonadherent cells were collected in 3 washes, as described.3,5 Adherent cells were collected after trypsinization for 7 minutes. We have previously shown that trypsin does not affect assessment of cell cycle.5 Freshly collected CD34+ cells or adherent or nonadherent CD34+ cells recovered after 12 hours of adhesion to BSA, PLL, or FN were fixed in 75% ethanol and stained with propidium iodide (50 μg/mL propidium iodide Σ, and 10 μg/mL RNase Σ in phosphate buffer saline (PBS) [Gibco]).39 Cells were analyzed with a FACS-Calibur flow cytometer (Becton Dickinson). Cell cycle phase distribution was calculated with the use of ModFit LT software (Verity Software House Inc). In some experiments, CD34+ cells selected from G-CSF–mobilized PB were cocultured with BSA, FN, or PLL in the presence of low-dose cytokines with or without IL3 or SCF. After 60 hours, cell cycle status was evaluated by fluorescence-activated cell sorter (FACS).
Flow cytometric evaluation of cell cycle proteins was performed as follows: CD34+ cells recovered in the adherent and nonadherent portion of adhesion assays were fixed in 75% ethanol, then incubated overnight at −20°C.40 For assessment of cyclin-D, cells were fixed in 1% formaldehyde (Sigma) for 15 minutes before fixation in ethanol. Cells were washed and permeabilized with 0.15% Triton X-100 (Sigma) for 5 minutes, washed, and then incubated with antibodies directed at PCNA, cyclin-D1 + 2 + 3, cyclin-E, cyclin-A, p16INK4A, p21CIP1, and p27KIP1 or with isotype control antibody at room temperature for 30 minutes. When unconjugated antibodies were used, cells were washed and incubated with FITC-conjugated goat antimouse Ig for 30 minutes in the dark. Cells were washed and resuspended in 50 μg/mL propidium iodide. Cells were analyzed with a FACS-Calibur flow cytometer with the use of Cell Quest and ModFit LT software. In some experiments, CD34+ cells selected from G-CSF–mobilized PB, which had not been cultured with low doses of cytokines to induce cell proliferation, were cocultured with FN or PLL in the presence of low-dose cytokines with or without IL3 or SCF. After 60 hours, levels of p27KIP1 were assessed by FACS and Western blot.
A thymidine suicide assay was performed as follows: CFC proliferation was assessed in 3H-thymidine suicide assays as described.5 7 (1) Adherent and nonadherent CD34+ cells recovered after 12-hour adhesion to BSA, PLL, or FN or (2) CD34+ cells incubated with blocking or nonblocking anti-integrin antibodies or control antibodies for 12 hours were incubated at 37°C in serum-free IMDM for 30 minutes with or without 5 mCi 3H thymidine (specific activity, 6.7 Ci/mmol, NEN), washed with excess cold thymidine (500 mg/mL Σ in IMDM plus 20% FCS), and plated in methylcellulose assays. The percentage of CFC in S phase was calculated as follows: % CFC in S-phase = [(number of CFC without3H-thymidine − Number of CFC with3H-thymidine) × 100] / [number of CFC without 3H-thymidine].
In Western blotting and immunoprecipitation,39 cells recovered in the adherent and nonadherent portion of adhesion assay were lysed in NP-40 lysis buffer (50 mmol/L Tris HCl, pH 7.4, 250 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid, 1% NP-40, 1 mmol/L phenylmethyl–sulfonyl fluoride (PMSF), 10 μg/mL aprotinin, 10 μg/mL leupeptin, 50 mmol/L NaFl, 0.1 mmol/L Na3VO4, all from Sigma), and lysates were recovered by centrifugation. Total protein from each sample to be used in Western blots or immunoprecipitations was normalized with the use of the Bradford assay.
In Western blotting, protein lysates from more than 2 × 106 adherent, nonadherent, or unmanipulated cells were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose with the use of a Semidry Transfer Apparatus (Bio-Rad). Immunoblots were blocked in freshly prepared TBS (Pierce, Rockford, IL) containing 5% nonfat dry milk. Blots were incubated with 1 ng/mL primary antibody in blocking buffer for 2 hours at room temperature. After 4 washes in TBST (TBS supplemented with 0.05% Tween 20), blots were incubated for 1 hour with a goat antimouse HRP-conjugated antibody (1:10 000 dilution). Bands were visualized with the use of an ECL detection system (E.I. du Pont de Nemours & Co, Boston, MA). Quantitative differences in protein levels in different conditions were evaluated by scanning images with a GS-700 Imaging Densitometer (Bio-Rad); the images were then quantitated with the use of Molecular Analyst software (Bio-Rad).
In immunoprecipitation, protein lysates were precleared with 50 μL protein-G-agarose (Boehringer Mannheim, Indianapolis, IN) for at least 3 hours on a rocking platform, and nonbound material was recovered by centrifugation. We added 1 μg/mL anti–cyclin-E or anti-cdk2 antibody, and the mixture was gently rocked for at least 2 hours at 4°C. Soluble immune complexes were incubated with 100 μL protein-G-agarose beads for 3 hours and bead/protein complexes recovered by centrifugation. Beads were washed 3 times for 20 minutes with lysis buffer, and bound material was eluted by boiling in 1% SDS. The immune complexes were resolved by SDS-PAGE and blots probed as described above. Differences were evaluated with the use of a GS-700 Imaging Densitometer (Bio-Rad), and the images were then quantitated with the use of Molecular Analyst software.
A histone H1 kinase assay was performed as follows: Cdk2-associated kinase activity was assayed in cdk2- or cyclin-E–immune complexes. Cell lysates were prepared as described above, and cyclin-E or cdk2 was immunoprecipitated from similar quantities of protein. Bead/protein complexes were washed 3 times with lysis buffer and twice with kinase buffer (50 mmol/L Tris-HCl [pH 7.5], 10 mmol/L MgCl2, and 1 mmol/L dithiothreitol). Then, 5 μg histone H1 (Boehringer Mannheim), 1 μM ATP, and 10 μCi [r-32P] were added to the kinase buffer for 30 minutes at 30°C. The reaction was stopped by adding Laemmli sample buffer and boiling for 3 minutes. Reaction products were resolved by SDS-PAGE. The gel was dried and exposed to X-ray film. Differences were evaluated by scanning images with the use of a GS-700 Imaging Densitometer (Bio-Rad); the images were then quantitated with the use of Molecular Analyst software.
Results of experimental points obtained from multiple experiments were reported as the mean ± SEM. Significance levels were determined by a 2-sided Student t test.
Results
Cell cycle status of mobilized PB progenitors
We have shown that 25% to 30% of CFC present in normal, steady-state BM are in S phase,5,7 and that entry in S phase is inhibited following coculture with FN5 or when β1-integrins are engaged directly by adhesion-blocking antibodies.7 We now show that 95% to 99% of mobilized PB CFC and CD34+ are in G0/G1(n = 5); this is consistent with other published reports.41,42 Culture for 48 hours with cytokines at concentrations found in the BM microenvironment (200 pg/mL GM-CSF, 1000 pg/mL G-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 200 pg/mL MIP-1α, and 1000 pg/mL IL-6)37 caused 23 ± 3% of mobilized PB CD34+ cells (n = 4) and 29 ± 4% of mobilized PB CFC (n = 14) to enter S phase. We then evaluated whether G-CSF–mobilized PB CD34+ cells undergo similar adhesion-mediated proliferation inhibition as CD34+ cells in steady-state BM.
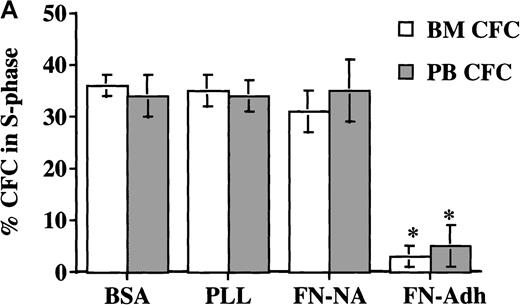
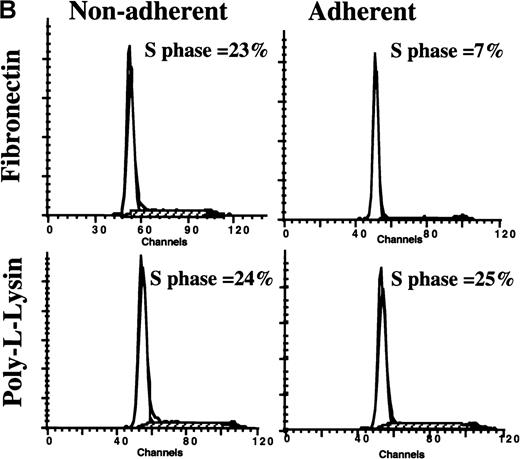
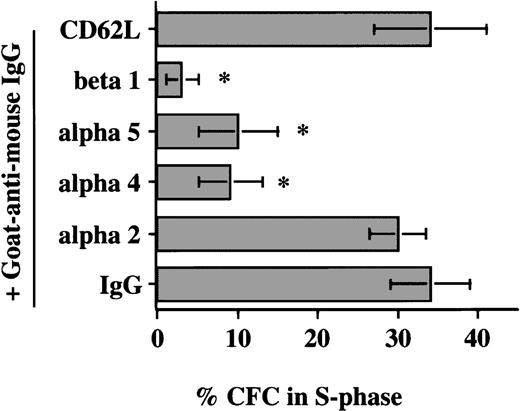
In a first set of experiments, CD34+ cells were induced to proliferate by culturing in low doses of cytokines for 48 to 72 hours. Cultured PB CD34+ cells were then plated for 12 hours in FN-, BSA-, or PLL-coated wells. We have previously shown that although mobilized PB CD34+ cells express fewer α4β1 integrins, and therefore interact less well with fibronectin, expression of α4β1 increases to normal levels after 24-to-48-hour culture with cytokines ex vivo. Coculture with FN, but not with BSA or PLL, resulted in a significant decrease in the portion of CFC (8.5 ± 3%) (Figure 1A) and CD34+ cells (11.5% ± 2%) (Figure 1B) adherent to FN in S phase. Several observations indicate that this is not due to selective adhesion of G0/G1 cells: (1) The percentage of CFC in S phase in the adherent and the nonadherent populations combined was significantly lower for cells cocultured with FN than for cells cultured with either BSA or PLL; (2) Incubation of CD34+ cells with the β1-integrin–activating antibody 8A2 increased CD34+ cell adhesion (without 8A2, 10 ± 1%; with 8A2, 40 ± 1.4%). The cell cycle status of CD34+ cells present in the FN-adherent and FN-nonadherent portions of assays performed in the presence or absence of 8A2 was, however, equivalent (FN-adherent CD34+ cells: without 8A2 = 11 ± 3% S-phase, n = 3; with 8A2 = 11.5 ± 1.6% S phase, n = 9; FN-nonadherent CD34+ cells: without 8A2 = 21 ± 4% S phase, n = 3; with 8A2 = 22.5 ± 2% S phase, n = 9); (3) Engagement of β1-integrins (4 ± 2.4% S phase), α5-integrins (10 ± 5.8% S phase), and α4-integrins (10.5 ± 6.8% S phase), but not the α2-integrin or CD62L, with adhesion-blocking antibodies and cross-linking with a secondary goat antimouse antibody decreased the portion of CFC in S phase (Figure2). Although the portion of CFC exposed to anti–α4-b anti–α5-b or anti–β1-integrin antibodies and FN-adherent CFC that were in S phase was similar, the percentage of CFC cultured in FN-coated wells in S phase (ie, in FN-adherent plus FN-nonadherent portions) was higher than in antibody-exposed CFC. This is consistent with the fact that most CD34+ cells express β1-integrins, and antibody-mediated engagement of β1 therefore suppresses S-phase entry in the majority of cells, whereas only 10% to 15% adhere to FN in the absence of 8A2. Adhesion via β1-integrins therefore inhibits cell cycle progression in the adherent portion only. Thus, these studies are consistent with the concept that block in cell cycle progression in FN-adherent cells is caused by engagement of integrins by FN and not by selective adhesion of G0/G1 cells.
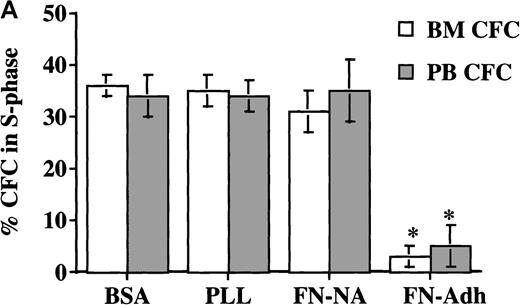
Adhesion to FN.
Adhesion to FN decreases portion of blood and BM CD34+ cells and CFC that are in S phase. CD34+cells from steady-state BM or mobilized PB were cultured for 48 hours in serum-free medium with low-dose cytokines. Cells were then plated in wells coated with PLL or FN for 12 to 16 hours. We collected adherent (Adh) and nonadherent (NA) cells separately. We analyzed the S phase of CFC by thymidine suicide assay and analyzed the S phase of all CD34+ cells by labeling cells with propidium iodide and analysis by FACS. (A) Thymidine suicide assay (n = 4 for BM and n = 4 for PB). Data are shown as mean ± SEM. Comparison between the FN-Adh and the FN-NA portion for PB or BM: * = P < .01. (B) FACS analysis of propidium iodide labeled cells (n = 12). A representative experiment is shown.
Adhesion to FN.
Adhesion to FN decreases portion of blood and BM CD34+ cells and CFC that are in S phase. CD34+cells from steady-state BM or mobilized PB were cultured for 48 hours in serum-free medium with low-dose cytokines. Cells were then plated in wells coated with PLL or FN for 12 to 16 hours. We collected adherent (Adh) and nonadherent (NA) cells separately. We analyzed the S phase of CFC by thymidine suicide assay and analyzed the S phase of all CD34+ cells by labeling cells with propidium iodide and analysis by FACS. (A) Thymidine suicide assay (n = 4 for BM and n = 4 for PB). Data are shown as mean ± SEM. Comparison between the FN-Adh and the FN-NA portion for PB or BM: * = P < .01. (B) FACS analysis of propidium iodide labeled cells (n = 12). A representative experiment is shown.
Antibody-mediated engagement of β1-integrins.
Antibody-mediated engagement of β1-integrins inhibits S-phase entry of cultured blood CFC. Mobilized PB CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with adhesion-blocking antibodies against the α2 (n = 3), α4 (n = 6), α5 (n = 5), or β1 (n = 6) integrins or CD62L (n = 3) or mouse IgG (n = 6). After incubation for 30 minutes at 4°C, cells were washed and incubated with goat antimouse antibody. Cells were incubated for 8 to 12 hours at 37°C, and the percentage of CFC in S phase was assessed by thymidine suicide assay. Data are shown as the mean ± SEM. Comparison between anti-β1, anti-α4b and anti-α5 group and IgG control group: * = P < .01.
Antibody-mediated engagement of β1-integrins.
Antibody-mediated engagement of β1-integrins inhibits S-phase entry of cultured blood CFC. Mobilized PB CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with adhesion-blocking antibodies against the α2 (n = 3), α4 (n = 6), α5 (n = 5), or β1 (n = 6) integrins or CD62L (n = 3) or mouse IgG (n = 6). After incubation for 30 minutes at 4°C, cells were washed and incubated with goat antimouse antibody. Cells were incubated for 8 to 12 hours at 37°C, and the percentage of CFC in S phase was assessed by thymidine suicide assay. Data are shown as the mean ± SEM. Comparison between anti-β1, anti-α4b and anti-α5 group and IgG control group: * = P < .01.
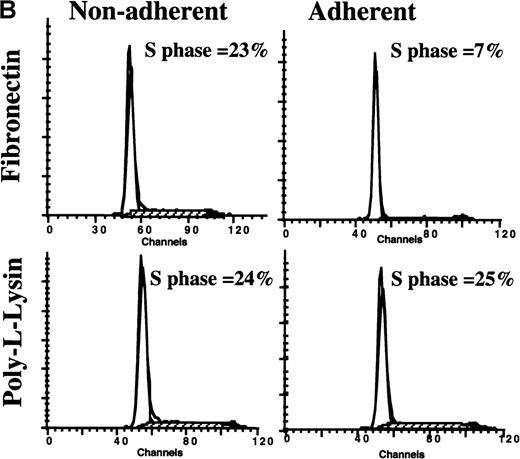
To confirm this further, we tested the hypothesis that culture of freshly isolated PB CD34+ cells, which are in G0/G1, on FN rather than on BSA- or PLL-containing wells would delay entry of CD34+ cells into S phase (Figure 3). Freshly sorted CD34+ cells (n = 2) suspended in low-dose cytokine-containing serum-free medium were cultured in wells coated with FN or PLL. After 24, 48, and 60 hours, adherent and nonadherent cells were collected, and cell cycle status was assessed by FACS. On day 0, 0.9% and 1% of CD34+ cells were in S phase. For PLL-adherent cells, this increased to 5% and 3% after 24 hours, to 17% and 12% after 48 hours, and to 17% and 16% after 60 hours. For cells in the FN-nonadherent CD34+ cell portion, 6% and 4% were in S phase after 24 hours, 17% and 13% after 48 hours, and 18% and 16% after 60 hours. In contrast, for CD34+ cells present in the FN-adherent portion, only 3% and 2% were in S phase after 24 hours, 7% and 4% after 48 hours, and 10% and 4% after 60 hours. This confirms that contact with FN prevents S-phase entry.
Coculture with FN.
Coculture with FN prevents entry into S phase and is associated with elevated p27KIP1 levels. G-CSF–mobilized PB CD34+ cells were analyzed fresh or after coculture with FN or PLL for 60 hours in the presence of serum-free medium supplemented with low concentrations of cytokines. Adherent and nonadherent cells were collected separately and labeled with propidium iodide (FACS analysis cell cycle status, n = 3) or subjected to Western blot (representative example of 2 experiments; levels of p27KIP1) as described in “Materials and methods.” Quantitative differences in protein levels were evaluated by scanning images with the use of a GS-700 Imaging Densitometer, and the images were quantitated using Molecular Analyst software. Values under Western blot represent relative density of each band compared with day 0 levels of p27KIP1.
Coculture with FN.
Coculture with FN prevents entry into S phase and is associated with elevated p27KIP1 levels. G-CSF–mobilized PB CD34+ cells were analyzed fresh or after coculture with FN or PLL for 60 hours in the presence of serum-free medium supplemented with low concentrations of cytokines. Adherent and nonadherent cells were collected separately and labeled with propidium iodide (FACS analysis cell cycle status, n = 3) or subjected to Western blot (representative example of 2 experiments; levels of p27KIP1) as described in “Materials and methods.” Quantitative differences in protein levels were evaluated by scanning images with the use of a GS-700 Imaging Densitometer, and the images were quantitated using Molecular Analyst software. Values under Western blot represent relative density of each band compared with day 0 levels of p27KIP1.
G1/S blockade is associated with increased p27KIP1 levels but decreased cyclin-E protein levels and decreased cdk2 kinase activity
We next examined the effect of adhesion to FN on cell cycle protein expression and activity. CD34+ cells were plated for 48 to 72 hours in low-dose cytokine-containing medium to induce entry of cells into S phase. Cells were then plated in dishes coated with FN or PLL for 12 to 16 hours, and adherent and nonadherent cells were collected separately, permeabilized, and stained with antibodies directed at cell cycle–associated proteins. Since no significant differences were seen in the cell cycle status of cells assayed in the presence (FN-adherent: 11.4 ± 3.1% S phase; FN-nonadherent: 26.7 ± 4.9% S phase, n = 3) or absence of low-dose cytokines (FN-adherent: 11.5 ± 2% S phase; FN-nonadherent: 22.5 ± 2.3% S phase, n = 9), or in the presence or absence of the activating antibody 8A2 (see above), results from assays with or without 8A2 or with or without low-dose cytokines during the adhesion assay were pooled.
Cyclin-E protein levels were lower in FN-adherent compared with FN-nonadherent cells, and FN-adherent cells had elevated levels of p27KIP1 compared with FN-nonadherent cells (Figure4, representative example of 5 individual experiments). FN-adherent cells contained slightly less cyclin-A than FN-nonadherent cells, and levels of PCNA, cyclin-D1 + 2 + 3, p16INK4A, and p21CIP1 were similar in FN-adherent and FN-nonadherent cells (not shown). These results were confirmed by immunoprecipitation and Western blot. All blots were analyzed by densitometry to obtain quantitative results. Levels of p27KIP1 were 1.99 ± 0.1-fold higher (P < .01) in FN-adherent compared with FN-nonadherent cells (Figure5, representative experiment of 3 individual experiments). Levels of cyclin-E were 4.4 ± 0.9-fold lower in FN-adherent compared with FN-nonadherent cells (Figure 5). We also immunoprecipitated cdk2 from FN-adherent and FN-nonadherent cells. Immunoprecipitates were then evaluated for the amount of cdk2 present and the activity of the kinase. In FN-adherent cells, cdk2 activity was 3.46 ± 0.16 lower than in FN-nonadherent cells (Figure5). However, the total cdk2-protein level was not significantly different between FN-adherent and FN-nonadherent cells (Figure 5). Further, the amount of cdk2 that coimmunoprecipitated with cyclin-E was 3.8 ± 0.25-fold lower in FN-adherent than FN-nonadherent cells (not shown). No differences between cells that were adherent or nonadherent to PLL were seen in PCNA, cyclin-D1 + 2 + 3, cyclin-E, cyclin-A, cdk2, p16INK4A, p21CIP1, and p27KIP1 protein levels and in cdk2-activity (Figures 4, 5, and not shown).
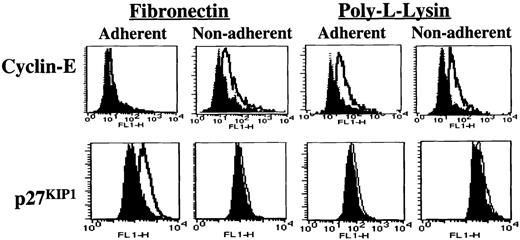
Adhesion to FN.
Adhesion to FN leads to increased levels of p27KIP1 and decreased levels of cyclin-E. Mobilized blood CD34+ cells, cultured for 48 hours in serum-free medium with low-dose cytokines, were incubated with the activating anti–β1-integrin antibody 8A2 for 30 minutes at 37°C and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent and nonadherent cells were collected separately. Cells were fixed, permeabilized, and incubated with antibodies directed at p27KIP1 and cyclin-E (open histogram) or isotype control (closed histogram) antibody at room temperature for 30 minutes followed by FITC-conjugated goat antimouse immunoglobulin for 30 minutes in the dark. Cells were washed and resuspended in 50μg/mL propidium iodide. Cells were analyzed on a FACS-Calibur flow cytometer with the use of CellQuest software. A representative example of 5 individual experiments is shown.
Adhesion to FN.
Adhesion to FN leads to increased levels of p27KIP1 and decreased levels of cyclin-E. Mobilized blood CD34+ cells, cultured for 48 hours in serum-free medium with low-dose cytokines, were incubated with the activating anti–β1-integrin antibody 8A2 for 30 minutes at 37°C and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent and nonadherent cells were collected separately. Cells were fixed, permeabilized, and incubated with antibodies directed at p27KIP1 and cyclin-E (open histogram) or isotype control (closed histogram) antibody at room temperature for 30 minutes followed by FITC-conjugated goat antimouse immunoglobulin for 30 minutes in the dark. Cells were washed and resuspended in 50μg/mL propidium iodide. Cells were analyzed on a FACS-Calibur flow cytometer with the use of CellQuest software. A representative example of 5 individual experiments is shown.
Adhesion to FN.
Adhesion to FN is associated with increased levels of p27KIP1 and decreased cdk2-kinase activity. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2, washed, and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent (Adh) and nonadherent (NA) cells were collected separately, and cells were lysed. A representative example of 3 individual experiments is shown. For p27KIP1, protein extracts were separated by SDS-PAGE, transferred onto nitrocellulose, and incubated with antibodies against p27KIP1 and goat antimouse HRP-conjugated antibody. Cyclin-E was immunoprecipitated from protein-G-agarose beads. Immune complexes were separated by SDS-PAGE and blots probed with anti–cyclin-E antibodies and goat antimouse HRP-conjugated antibody. Protein bands were visualized with the use of the ECL detection system, and cdk2 was immunoprecipitated with the use of protein-G-agarose beads. The immune complexes were separated by SDS-PAGE, and blots were probed with anti-cdk2 antibodies and goat antimouse HRP-conjugated antibody. Cdk2 activity was assayed by adding 5 μg histone and 10 μCi [r-32P]. Reaction products were resolved by SDS-PAGE, and the gel was exposed to X-ray film. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).
Adhesion to FN.
Adhesion to FN is associated with increased levels of p27KIP1 and decreased cdk2-kinase activity. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2, washed, and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent (Adh) and nonadherent (NA) cells were collected separately, and cells were lysed. A representative example of 3 individual experiments is shown. For p27KIP1, protein extracts were separated by SDS-PAGE, transferred onto nitrocellulose, and incubated with antibodies against p27KIP1 and goat antimouse HRP-conjugated antibody. Cyclin-E was immunoprecipitated from protein-G-agarose beads. Immune complexes were separated by SDS-PAGE and blots probed with anti–cyclin-E antibodies and goat antimouse HRP-conjugated antibody. Protein bands were visualized with the use of the ECL detection system, and cdk2 was immunoprecipitated with the use of protein-G-agarose beads. The immune complexes were separated by SDS-PAGE, and blots were probed with anti-cdk2 antibodies and goat antimouse HRP-conjugated antibody. Cdk2 activity was assayed by adding 5 μg histone and 10 μCi [r-32P]. Reaction products were resolved by SDS-PAGE, and the gel was exposed to X-ray film. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).
When freshly isolated CD34+ cells were cultured for 60 hours on PLL- or FN-coated dishes in the presence of low doses of cytokines, similar results were seen: p27KIP1 levels were 1.5-fold higher in the FN-adherent portion compared with FN-nonadherent portion, and 1.8-fold and 2.3-fold compared with PLL-adherent and PLL-nonadherent portions, respectively. Interestingly, the level of p27KIP1 was 2.13-fold higher in CD34+ cells cultured for 60 hours in FN-coated wells in the presence of low doses of cytokines compared with freshly isolated and uncultured CD34+ cells, even though both populations contained only a small portion of cells in S phase. Thus, contact with FN, rather than G0/G1 state of the cell, is associated with increased levels of p27KIP1(Figure 3).
IL3 or SCF does not alter adhesion of CD34+ cells and CFC to FN but overrides p27KIP1-mediated G1/S blockade following β1-integrin engagement
We next examined if supraphysiological concentrations of cytokines known to stimulate progenitor growth would affect integrin-mediated functions. Cells were cultured for 48 to 72 hours in low-dose cytokine-containing medium to induce S-phase entry. Cells were then resuspended in serum-free medium either without cytokines or with 10 ng/mL IL3, GM-CSF, SCF, or Flt-3L. The portion of CD34+ cells or CFC that adhered to FN was similar when adhesion assays were done in the absence or presence of any of the following: IL3, GM-CSF, SCF, or Flt-3L (Table1). In contrast to CFC cultured in contact with FN in the absence of cytokines, contact with FN in the presence of 10 ng/mL IL3, GM-CSF, SCF, or Flt-3L prevented inhibition of G1/S–phase progression of CFC, and contact with FN in the presence of IL3 or SCF also prevented the G0/G1 blockade in CD34+cells (Table 2).
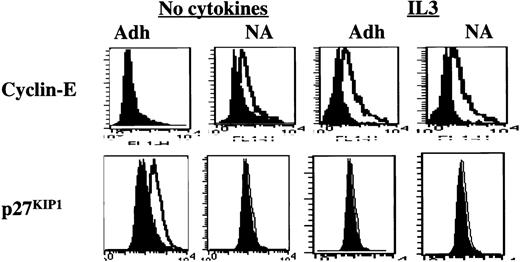
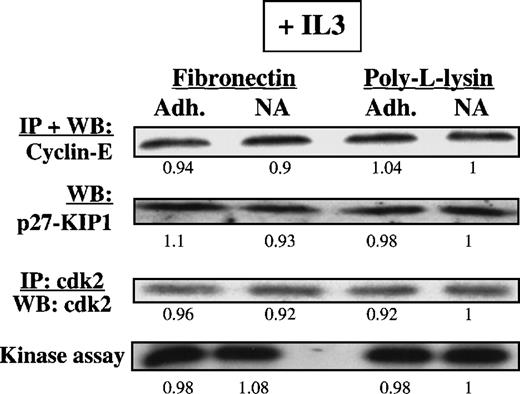
We next analyzed the expression level and activity of cell cycle proteins in FN-adherent or FN-nonadherent cells in cultures supplemented with IL3 or SCF (n = 3). To increase the portion of FN-adherent cells, we added the activating anti–β1-integrin antibody 8A2. Except for an increase in the portion of adherent cells, no differences were seen in the proliferative status of FN-adherent and FN-nonadherent cells or in the levels of cell cycle–associated proteins in the presence or absence of 8A2 (not shown). Levels of cyclin-E were higher in CD34+ cells adherent to FN in the presence of either IL3 (Figure 6) or SCF (data not shown) compared with CD34+ cells adherent to FN in the absence of cytokines. In contrast to cytokine-free assays, p27KIP1levels were not elevated in CD34+ cells adherent to FN in the presence of IL3 (Figure 6) or SCF (data not shown). Levels of the other cell cycle proteins, including PCNA, cyclin-D1 + 2 + 3, p21CIP1, and p16INK4A, were similar in the presence or absence of IL3 or SCF (not shown). Immunoprecipitation and Western blot and kinase assays (n = 3) confirmed that IL3 prevents the accumulation of p27KIP1 and allows activation of cdk2, even when CD34+ cells were adherent to FN (Figure7).
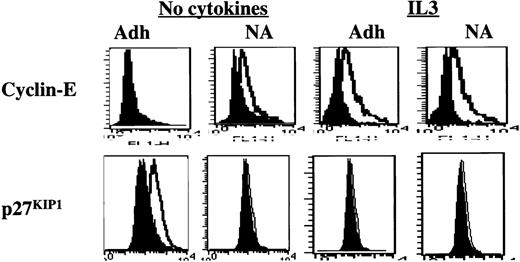
Presence of IL3 during the adhesion assay.
Presence of IL3 during the adhesion assay prevents up-regulation of p27KIP1 and allows up-regulation of cyclin-E. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2 and plated, with or without 10 ng/mL IL3, in PLL- or FN-coated wells for 12 to 16 hours. Adherent and nonadherent cells were collected separately, fixed, permeabilized, washed, and incubated with antibodies directed at p27KIP1 and cyclin-E (open histogram) or isotype control (closed histogram) at room temperature for 30 minutes, followed by FITC-conjugated goat antimouse immunoglobulin. A representative example of 3 individual experiments is shown. Adhesion to PLL in the presence or absence of IL3 did not affect the expression level of cyclin-E or p27KIP1 (not shown). However, up-regulation of p27KIP1 and down-regulation of cyclin-E levels are prevented when IL3 is present during the adhesion assay.
Presence of IL3 during the adhesion assay.
Presence of IL3 during the adhesion assay prevents up-regulation of p27KIP1 and allows up-regulation of cyclin-E. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2 and plated, with or without 10 ng/mL IL3, in PLL- or FN-coated wells for 12 to 16 hours. Adherent and nonadherent cells were collected separately, fixed, permeabilized, washed, and incubated with antibodies directed at p27KIP1 and cyclin-E (open histogram) or isotype control (closed histogram) at room temperature for 30 minutes, followed by FITC-conjugated goat antimouse immunoglobulin. A representative example of 3 individual experiments is shown. Adhesion to PLL in the presence or absence of IL3 did not affect the expression level of cyclin-E or p27KIP1 (not shown). However, up-regulation of p27KIP1 and down-regulation of cyclin-E levels are prevented when IL3 is present during the adhesion assay.
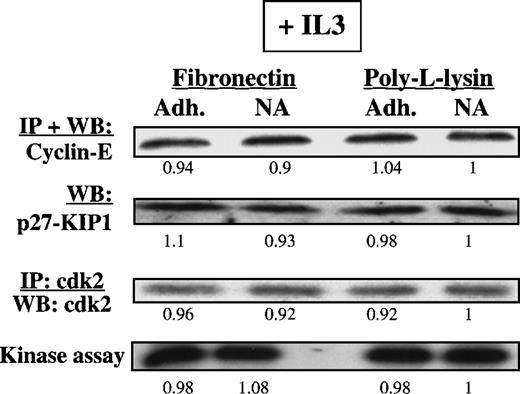
Presence of IL3 or SCF during the adhesion assay.
Presence of IL3 or SCF during the adhesion assay prevents up-regulation of p27KIP1 and inhibition of cdk2-kinase. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2 and plated, with or without 10 ng/mL IL3, in PLL- or FN-coated dishes for 12 to 16 hours. Adherent and nonadherent cells were collected separately, and cells were lysed. Methods used to demonstrate presence and activity of p27KIP1, cyclin-E, and cdk2-kinase activity are as described in legend to Figure 5. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. A representative example of 2 individual experiments is shown. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).
Presence of IL3 or SCF during the adhesion assay.
Presence of IL3 or SCF during the adhesion assay prevents up-regulation of p27KIP1 and inhibition of cdk2-kinase. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2 and plated, with or without 10 ng/mL IL3, in PLL- or FN-coated dishes for 12 to 16 hours. Adherent and nonadherent cells were collected separately, and cells were lysed. Methods used to demonstrate presence and activity of p27KIP1, cyclin-E, and cdk2-kinase activity are as described in legend to Figure 5. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. A representative example of 2 individual experiments is shown. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).
Discussion
We still do not know which signals are responsible for the regulation of hematopoietic progenitor proliferation, quiescence, or differentiation. More than 30 hematopoietic cytokines and growth factors that increase or decrease progenitor proliferation and differentiation have been cloned and characterized. Although the molecular effect of these factors on hematopoietic cells has been extensively studied, it remains unclear how the combination of these factors regulates the hematopoietic process. In vivo hematopoiesis normally occurs in close proximity with BM stromal elements. Coculture of progenitors with BM stromal feeders in vitro inhibits progenitor proliferation through mechanisms that are as yet not understood.4-6,43,44 Progenitors, as well as cytokines, can bind specifically with ligands on cells and extracellular matrix present in the BM,1-3,45,46 resulting in the colocalization of progenitors at a specific stage of differentiation with a specific array of cytokines.47 This is thought to provide one level of regulation of growth and differentiation. There is also mounting evidence that contact interactions between progenitors and BM stromal ligands may be equally, or even more, important for the regulation of the hematopoietic process. Recent studies have shown that engagement of, for instance, selectins48 and mucins49,50may profoundly affect progenitor survival and growth. Likewise, engagement of integrins may enhance progenitor survival in culture.51 We describe here how integrin engagement alters progenitor growth.
We used 2 experimental assays to investigate these questions. Almost all CD34+ cells collected from the PB of individuals treated with G-CSF are in G0/G1. In 1 set of studies, we showed that FN coculture of PB CD34+cells that are in G0/G1 prevents entry into S phase compared with cells cocultured with PLL or BSA, suggesting that engagement of β1-integrins prevents entry into cell cycle. This is consistent with what we have previously shown for steady-state marrow–derived CD34+cells4-7 and what we show here for CD34+ cells present in cultured (and hence proliferating) mobilized PB CD34+ cell populations: when cocultured with FN, but not PLL, the portion of CD34+ cells in S phase declines. Using blocking monoclonal antibodies, we showed that induction of G1/S blockade by adhesion-receptor engagement in human hematopoietic progenitors is β1-integrin specific: in contrast to β1-integrins, engagement of other transmembrane adhesion receptors, such as CD44,7 CD34,7 and CD62L (data shown here), did not affect the proportion of progenitors that is in S phase.
β1-integrin–mediated block in G1/S transition in hematopoietic cells occurs in late G1 and is associated with elevated levels of p27KIP1 and inactivation of cyclin-E/cdk2 complexes. Of interest is the fact that levels of p27KIP1 were significantly less elevated in uncultured CD34+ cells selected fresh from mobilized PB than in CD34+ cells cultured for 60 hours with low concentrations of cytokines in contact with FN. This suggests strongly that elevation of p27KIP1 is not merely a reflection of presence in G0/G1 status but is correlated with engagement of integrins.
This is in contrast to what has been described in the majority of other biological systems: engagement of integrins usually activates cyclin-D/cdk4 and cyclin-E/cdk2 or cyclin-A/cdk2 complexes leading to cell proliferation but not growth arrest.13-15,25-27 In contrast to the CD34+cells studies here, those reports describe the effect on cell cycle proteins in cells that require adhesion for cell growth, such as fibroblasts, endothelial cells, and myocytes. A few examples have been described of adhesion-mediated G1-phase arrest mediated through mechanisms similar to what we show here for hematopoietic cells: growth arrest occurred late in G1 and was due to elevated levels of the “contact” cdki p27 KIP1, which inhibits cyclin-E/cdk2 kinase activity.52-55 When α5β1–mediated adhesion of the FET colon carcinoma cell line to FN was prevented, FET cells were induced to proliferate.51 This was associated with activation of extracellular signal-regulated kinase–1 and extracellular signal-regulated kinase–2 and significantly elevated levels of cdk4, phosphorylation of Rb, and increased cyclin-A/cdk2 and cyclin-E/cdk2–associated kinase activity. Thus, as we show here for hematopoietic cells, α5β1 engagement in FET colon carcinoma cells suppresses cyclin-dependent kinase activity, leading to growth arrest. G1-phase growth arrest was also seen when arterial smooth muscle cells were cultured on polymerized type I collagen.53 This was associated with elevated levels of p27KIP1 and p21CIP1 and decreased cyclin-E–associated cdk2 kinase activity. In contrast, when cultured on monomer collagen, arterial smooth muscle cell proliferation was induced rather than growth arrest. Like FN-adherent human CD34+ cells, which are round even in the adherent state, arterial smooth muscle cells adherent to polymeric collagen adhere in a rounded state and do not spread. These results suggest that, like lateral association and ligand-binding–site occupation,10 cell shape may be an additional parameter that dictates the type of molecules recruited to focal adhesions and therefore the type of signal pathways that are activated or blocked. This is consistent with recent studies by Chen et al54demonstrating that cell death is influenced not only by integrin engagement by a substrate but also by cell shape.
That cell-cell contact causes normal cells to stop proliferating has long been known in normal organ development. More recently it has become clear that such contact-mediated growth arrest is mediated by up-regulation of the cdki, p27KIP1,56,57 which inactivates cyclin-E/cdk2 and cyclin-A/cdk2 complexes. This is illustrated in mutant p27 KIP1−/− mice that display generalized increased body size and a significantly expanded hematopoietic progenitor pool.56,57 This appears to be consistent with our observation that cell-ECM interaction elevates levels of p27 KIP1 and inhibits G1/S progression of CD34+ progenitors. Regulation of p27KIP1 levels is complex.58 59 Elevated levels can be due to increases in transcription, messenger RNA (mRNA) stabilization, or decreased protein degradation. Preliminary results from ribonucleotide protection assays (results not shown) suggest that the regulation of p27KIP1 by integrin engagement on CD34+ cells may not be at the transcriptional level.
Finally, we found that integrin-mediated block in G1/S transition can be modulated when external conditions change: addition of supraphysiological concentrations of IL3 and GM-CSF, which bind to a common receptor in the hematopoietic receptor binding family,60 or SCF and Flt-3L, which signal via tyrosine kinase receptors,61 counteracts the adhesion-mediated block in G1/S progression. Recent studies from other groups have shown that IL3 and SCF activate protein kinase C (PKC) and tyrosine kinases that lead to enhanced adhesive capacity of β1-integrins present on serum- and cytokine-starved CD34+ cells and CFC.28-30 In contrast, we show that supraphysiological concentrations of IL3, GM-CSF, SCF, or Flt-3L did not affect adhesion of human CFC or CD34+ cells when they were maintained under physiological conditions. Our results are consistent with studies from Strobel et al32 and Schofield et al31 demonstrating that supraphysiological concentrations of cytokines do not alter the adhesive function of integrins under serum/cytokine–replete conditions. Even though adhesion of CD34+ cells to FN was not affected by the presence of IL3 or SCF, presence of these supraphysiological concentrations of cytokines prevented adhesion-mediated increased p27KIP1 levels and prevented adhesion-mediated inhibition of cdk2 activity. Signal pathways activated by cytokine stimulation have been extensively studied. Stimulation of either hematopoietic or tyrosine kinase receptors leads to recruitment and activation of signal and adaptor proteins and to activation of signal pathways that are also recruited/activated by integrin stimulation. Like integrin engagement, stimulation of c-kit leads to phosphorylation of paxillin and CrkL, activation of PI3-kinase, Ras, and MAPK.60,61 Likewise, IL-3 phosphorylates CrkL and paxillin and activates PI3-kinase and MAPK, molecules known to be recruited to and activated in focal adhesions.62-68 Thus, significant overlap exists between signal pathways activated following cell adhesion or stimulation with cytokines. Since the mechanism(s) that underlie adhesion-mediated signaling in hematopoietic cells are unknown, we can only speculate how the combined activation of integrin and cytokine receptors affects hematopoietic cell growth. However, the characterization of conditions that allow adhesion without cell cycle arrest and conditions that induce adhesion with cell cycle arrest should prove useful in deciphering the signal pathways that are activated by engagement of integrins leading to growth arrest of human hematopoietic progenitors.
In conclusion, we demonstrate that cell cycle arrest and reduced proliferation of CD34+ cells, which are adherent to FN under physiological cytokine conditions, are mediated by the cdki, p27KIP1. However, costimulation of FN-adherent progenitors with certain growth-promoting cytokines overrides this integrin-dependent elevation of p27KIP1 and allows S-phase entry of CD34+ cells. Future studies will identify the molecular mechanisms underlying the reversible inhibition of entry in S phase mediated by changes in p27KIP1.
Supported in part through grants from the National Institutes of Health (RO1 HL-49930 and RO1-DK-53673) and the Bone Marrow Transplant Research Fund to C. M. V. and a grant from Fondo De Investigaciones Sanitarias(FIS 98/0863) to F.P. C. M. V. is a scholar of the Leukemia Society of America.
Y. J. and F. P. contributed equally to this paper.
Reprints:Catherine M. Verfaillie, Box 806 UMHC, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: verfa001@tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 5. Adhesion to FN. / Adhesion to FN is associated with increased levels of p27KIP1 and decreased cdk2-kinase activity. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2, washed, and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent (Adh) and nonadherent (NA) cells were collected separately, and cells were lysed. A representative example of 3 individual experiments is shown. For p27KIP1, protein extracts were separated by SDS-PAGE, transferred onto nitrocellulose, and incubated with antibodies against p27KIP1 and goat antimouse HRP-conjugated antibody. Cyclin-E was immunoprecipitated from protein-G-agarose beads. Immune complexes were separated by SDS-PAGE and blots probed with anti–cyclin-E antibodies and goat antimouse HRP-conjugated antibody. Protein bands were visualized with the use of the ECL detection system, and cdk2 was immunoprecipitated with the use of protein-G-agarose beads. The immune complexes were separated by SDS-PAGE, and blots were probed with anti-cdk2 antibodies and goat antimouse HRP-conjugated antibody. Cdk2 activity was assayed by adding 5 μg histone and 10 μCi [r-32P]. Reaction products were resolved by SDS-PAGE, and the gel was exposed to X-ray film. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.846.003k31_846_854/6/m_bloo00331005w.jpeg?Expires=1768078794&Signature=E4fd5TDKmuLQR2b-pm-Y4288PYoZHoctRoR~RAWF-dBUDAuj5rK5FoKYlLezxqcathR2UYgB1VqhslPKFnPuAJfyUeE9iMlh5U4ubTpL87Zw5227SXaRF8rJuSXxI2-vFwF2rUfDVPsmwJGb5CbZekJjtjzbc5tVLgTAsrFoH~E~W0OHGj4gOvArGcgryr5eXC1D1R1fj9rd-IAmdKcGApSuVf6bNS2cus3exrnGV8konhHK1gFzKo6RvVFbF5YXeJ~Km4IhVGpcKYsSXOvOWG1uROcDANoKIBktZooaDc~NcMD28wO35wuaU83L7NBetsM1II2Gx9bNatTXlCga2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 5. Adhesion to FN. / Adhesion to FN is associated with increased levels of p27KIP1 and decreased cdk2-kinase activity. Mobilized blood CD34+ cells cultured for 48 hours in serum-free medium with low-dose cytokines were incubated with 8A2, washed, and plated in PLL- or FN-coated dishes for 12 to 16 hours. Adherent (Adh) and nonadherent (NA) cells were collected separately, and cells were lysed. A representative example of 3 individual experiments is shown. For p27KIP1, protein extracts were separated by SDS-PAGE, transferred onto nitrocellulose, and incubated with antibodies against p27KIP1 and goat antimouse HRP-conjugated antibody. Cyclin-E was immunoprecipitated from protein-G-agarose beads. Immune complexes were separated by SDS-PAGE and blots probed with anti–cyclin-E antibodies and goat antimouse HRP-conjugated antibody. Protein bands were visualized with the use of the ECL detection system, and cdk2 was immunoprecipitated with the use of protein-G-agarose beads. The immune complexes were separated by SDS-PAGE, and blots were probed with anti-cdk2 antibodies and goat antimouse HRP-conjugated antibody. Cdk2 activity was assayed by adding 5 μg histone and 10 μCi [r-32P]. Reaction products were resolved by SDS-PAGE, and the gel was exposed to X-ray film. Differences in protein levels were evaluated by scanning images with a GS-700 Imaging Densitometer and quantitated with the use of Molecular Analyst software. Relative protein levels/kinase activity values are shown below all lanes (PLL-nonadherent is arbitrarily 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.846.003k31_846_854/6/m_bloo00331005w.jpeg?Expires=1768078795&Signature=CyNbWcJYbU1hvz~uyvEXXkRGHHPvw3X8aC2VebbIJPYsld~0X4LYpOQ36BihfjgIXqscOgKy72-vtz2xH0-kRdj7QKdKQRz-8Sxf3jCXp1byuT7YvgV9TGR2FwlkDNG7L~-H5fUH6TCYJfi5gDcq7bwoJaX4jTFAGWVNpNLiJBtMSFIR-QzE0ggMm7zjPqEZZpXVKedsZ9e22q6LOi2HzFbXKWOrem92l4rWaNils33PQwgjW8HkK4PvyZBteSJQOIL8i21p~vIwbXTGZ4zQVACI2KQk4xpTU8r39I358Modp9SJQb4wRcGN3DYjVK8zIIpNS7uMD7q7t8QmGsHU8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)