The development and evaluation of drugs to elevate fetal hemoglobin in the treatment of the genetic diseases of hemoglobin would be facilitated by the availability of reliable cell assays. We have used real-time, quantitative polymerase chain reaction (PCR) analyses of globin messenger RNA (mRNA) levels in a biphasic, erythropoietin-dependent primary culture system for human adult erythroid cells in order to assay compounds for their ability to modulate levels of adult (β) and fetal (γ) globin mRNA. Complementary DNA synthesized from total RNA extracted at timed intervals from aliquots of cells were assayed throughout the period that the culture was studied. γ-globin mRNA levels were found to be much lower (less than 1%) than β-globin mRNA levels. At concentrations of agents chosen for minimal effect on cell division, we find that the 3 drugs studied, 5-azacytidine (5μmol/L), hydroxyurea (40μmol/L), and butyric acid (0.5mmol/L), significantly increase γ-globin mRNA levels. Interestingly, hydroxyurea also had a small stimulatory effect on β-globin mRNA levels, while butyric acid caused a twofold inhibition of β-globin mRNA levels, and 5-azacytidine had little effect on β-globin mRNA levels. The net result of all 3 drugs was to increase the γ/(γ + β) mRNA ratios by threefold to fivefold. These data suggest that the mechanism is distinct for each drug. The profile of butyric-acid–induced changes on globin gene expression is also quite distinct from changes produced by trichostatin A, a known histone deacetylase inhibitor. Quantitative PCR analyses of human erythroid cells should prove useful for studying the mechanism(s) of action of known inducers of γ-globin and identifying new drug candidates.
Expression of the globin genes is developmentally regulated during vertebrate ontogeny. Two major switches characterize hemoglobin production in humans1: embryonic hemoglobins switch to fetal hemoglobins (HbF) after the first 2 months of gestation, and these switch at birth to production of the adult hemoglobins (HbA and HbA2). These changes are believed to be due primarily to changes in the transcriptional control of the individual globin chain messenger RNAs (mRNAs).
An increase in HbF ameliorates the clinical symptoms in both sickle cell anemia and β-thalassemia.2 In sickle cell anemia, HbF-containing red blood cells have lower concentrations of sickle hemoglobin (HbS), and HbF itself inhibits HbS polymerization, which decreases the potential for intracellular polymerization in such cells.3 In β-thalassemia, increased levels of γ-globin chains decrease the chain imbalance due to decreased β-globin protein levels and the resultant destruction of cells during erythropoiesis. Such compounds as 5-azacytidine4-6 and hydroxyurea7,8 as well as, more recently, butyric acid and its analogues9,10 have been studied in great detail. (See reviews by Rodgers and Rachmilewitz,2 Saleh and Hillen,11 and Swank and Stamatoyannopoulos.12) The detailed molecular mechanism(s) by which these pharmacologic agents produce increases in HbF is as yet unclear, but obviously such information would greatly assist in the design of new drugs that might have a lower toxicity as well as greater efficacy in stimulating HbF. In general, it is also assumed that the major effects of these drugs will be on transcription rates, although effects on mRNA processing, stability, or other posttranscriptional mechanisms cannot be excluded.
The recent introduction of real time, fluorescence-based quantitative polymerase chain reaction (PCR) as a rapid, sensitive technique for precise quantitation of nucleic acid template13,14 now allows for detailed monitoring of gene expression. This approach is based on the 5′ nuclease activity of Thermus aquaticus (Taq) polymerase15 to hydrolyze a dual fluorescently labeled oligonucleotide probe.16Emission of a reporter dye at the 5′ end of the intact sequence-specific probe is quenched by resonance energy transfer to a second fluorophore at the 3′ end. Probe hydrolysis by Taqpolymerase during primer extension releases the 5′ reporter, which is detected in real time as an increase in fluorescence intensity within the tube. Quantitation is possible over a considerable range of starting template concentrations since the fluorescence intensity is directly proportional to the quantity of amplified copies. Absolute quantitation is accomplished with the use of a standard curve with an identical target of known quantity.
We report the combined use of a primary erythroid culture system17,18 with the fluorescence-based, real-time quantitative PCR technique to determine the effects of these 3 drugs, 5-azacytidine, hydroxyurea, and butyric acid, on fetal (γ) and adult (β) globin gene expression in human erythroid cells. The ex vivo culture of primary adult erythroid cells provides a convenient source of immature progenitors and is divided into 2 phases. In the first, an erythropoietin-independent phase, peripheral blood cells are cultured in the presence of a combination of growth factors. In this phase, early erythroid-committed progenitors (burst-forming units) proliferate and differentiate into colony-forming unit–like progenitors. In the second phase, the cells are cultured in an erythropoietin-supplemented medium, where they continue to proliferate and differentiate into more mature erythroid progenitors, such as orthochromatic normoblasts.19 This procedure provides an experimental tool for studying various aspects of erythroid cell development as well as modulation of Hb production by pharmacological agents. It is anticipated that this combination of culture and assay system will provide a convenient, yet powerful method of screening potential drug candidates that affect gene expression as well as of investigating the underlying molecular mechanisms by which such drugs work.
Materials and methods
Two-phase liquid cultures of erythroid progenitor cells from normal donors were established as previously described.17,18 Mononuclear cells from at least 3 (and as many as 6) normal donors were pooled in culture for these analyses in order to minimize the influence of individual variation in response. Briefly, peripheral blood mononuclear cells were isolated by centrifugation on a cushion of Lymphocyte Separation Medium (ICN, Aurora, OH), washed twice with Dulbecco's phosphate buffered saline (Life Technologies, Grand Island, NY), and resuspended in α-minimum essential medium (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (FCS) (Intergen, Purchase, NY), 1 μg/mL cyclosporin A (Sandoz, Basel, Switzerland), 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Biofluids, Gaithersburg, MD), and 10% conditioned medium obtained from cultures of the 5637 bladder carcinoma cell line.20 Cultures were incubated at 37°C in an atmosphere of 5% CO2 in air with extra humidity. The nonadherent cells were harvested from this phase I culture by centrifugation, washed twice in α-medium (without supplements), and resuspended in fresh α-medium with 30% FCS, 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 10% deionized bovine serum albumin, 10−5 mol/L β-mercaptoethanol, 10−6mol/L dexamethasone, 33 μg/mL holo-transferrin (Sigma, St. Louis, MO), and 1 U/mL human recombinant erythropoietin (Ortho Pharmaceutical, Raritan, NJ). Hydroxyurea, 5-azacytidine, and butyric acid were obtained from Sigma. Viable cell counts were performed with the use of the trypan-blue–exclusion technique. The number of Hb-containing cells was determined by means of the benzidine-HCl procedure.21Total RNA was isolated with the use of the RNeasy mini-kit (Qiagen, Santa Clarita, CA). Superscript II reverse transcriptase (Life Technologies Inc) was used to synthesize complementary DNA (cDNA) after priming with oligodeoxythymidine.
Quantitative real-time PCR assay of transcripts was carried out with the use of gene-specific double fluorescently labeled probes in a 7700 Sequence Detector (PE Applied Biosystems, Norwalk, CT). We used 6-carboxy fluorescein (FAM) as the 5′ fluorescent reporter while we added tetramethylrhodamine (TAMRA) to the 3′ end as quencher. The following primer and probe sequences were used: β-globin forward primer, 5′-CTCATGGCAAGAAAGTGCTCG-3′; β-globin reverse primer, 5′-AATTCTTTGCCAAAGTGATGGG-3′; β-globin probe, 5′-FAM-CGTGGATCCTGAGAACTTCAGGCTCCT-TAMRA-3′; γ-globin forward primer, 5′-GGCAACCTGTCCTCTGCCTC-3′; γ-globin reverse primer, 5′-GAAATGGATTGCCAAAAC-GG-3′; γ-globin probe, 5′-FAM-CAAGCTCCTGGGAAATGTGCTGGTG-TAMRA-3′. All probes are designed to span exon junctions in the fully processed message in order to prevent reporting of amplification of any possible contaminating genomic DNA. All primers and probes were made with the use of reagents from Glen Research (Chantilly, VA) on an ABI 394 synthesizer (PE Applied Biosystems).
Double fluorescently labeled probes were high performance liquid chromatography (HPLC)–purified on a 250 mm × 10 mm Biovantage C8 reverse phase column (Thomson, Chantilly, VA) fitted to a Gilson HPLC system (Gilson Inc., Middleton, WI) with the use of a 10% to 35% acetonitrile gradient in 0.1 mol/L triethylamine acetate, pH 7, with the detector set to monitor column eluate at 260 nm. Products were lyophilized and characterized by absorption spectroscopy as well as DNAse I treatment to confirm a more than twofold increase in fluorescence. All oligonucleotide primers and probes were quantitated by absorbance at 260 nm. Specific transcripts within cDNA reaction products were quantitated in a reaction mix consisting of 10 mmol/L Tris, pH 8.3; 50 mmol/L KCl; 4 mmol/L MgCl2; 1 mmol/L ethylenediaminetetraacetic acid; 200 μmol/L deoxynucleotide triphosphate; and 0.025 U/μL Platinum Taq polymerase (Life Technologies Inc). Standard curves were constructed with the use of dilutions of an accurately determined plasmid containing the cDNA of interest as template. A dynamic range of 5 log orders of concentration or greater was routinely achieved for each transcript of interest. Data are presented as fold change relative to values immediately prior to the addition of test compounds.
To perform HPLC quantitation of hemoglobin, we separated hemoglobins by cation-exchange HPLC of supernatants from cell lysates as previously described.18 22 Briefly, cells were pelleted and then suspended and lysed in sterile distilled water. After the debris was pelleted, the supernatant was chromatographed on a Synchropak CM 300, 250 mm × 4.6 mm column (Synchron Inc, Lafayette, IN) fitted to a Maxima 820 (Waters, Milford, MA) developed with a sodium acetate gradient in 30 mmol/L Bis—Tris buffer (pH 6.3–pH 6.15). The area under the fetal (HbF) and adult (HbA) globin peaks were integrated with the use of the system software.
Results
The effect of the 3 compounds 5-azacytidine, hydroxyurea, and butyric acid on the number of benzidine-positive cells present in the erythroid cultures 96 hours after their introduction is shown in panels A to C of Figure 1. We added the compounds to the final concentrations shown on the fourth day after adding erythropoietin to the cultured cells. In the range of concentrations tested, hydroxyurea (Figure 1B) does not show a consistent and reproducible impact on cell number. 5-Azacytidine (Figure 1A) and butyric acid (Figure 1C) addition both resulted in reduced cell counts. Hydroxyurea has previously been shown to reduce benzidine-positive cell counts in primary erythroid cultures18 at concentrations 4 times the maximum used in these experiments. There was also a time dependence of this decrease in benzidine-positive cells: addition of the drug 4 days after erythropoietin yielded a greater impact than addition at 7 or 10 days (data not shown).
Effect of 5-azacytidine (A), hydroxyurea (B), and butyric acid (C) on primary adult erythroid cell proliferation.
Each bar represents the number of benzidine-positive cells present 96 hours after addition of the compound or vehicle.
Effect of 5-azacytidine (A), hydroxyurea (B), and butyric acid (C) on primary adult erythroid cell proliferation.
Each bar represents the number of benzidine-positive cells present 96 hours after addition of the compound or vehicle.
We also examined the effects of the 3 compounds on the percentage of HbF, the total Hb, and the amount of Hb per cell. 5-Azacytidine, hydroxyurea, and butyric acid all produced dose-dependent increases in the HbF fraction 96 hours after their addition to primary cultures on the fourth day after erythropoietin addition (Figure2A-C); hydroxyurea and butyric acid yielded the largest effects. Linear correlation coefficients are as follows: r2 = 0.788 for 5-azacytidine (Figure 2A); r2 = 0.915 for hydroxyurea (Figure 2B); and r2 = 0.996 for butyric acid (Figure 2C). Similar results have previously been reported for hydroxyurea.18 However, the total hemoglobin level (Figure 2D-F) was decreased in a dose-dependent manner for both hydroxyurea and butyric acid while 5-azacytidine produced no consistent effect. In order to estimate the effect of these compounds on the hemoglobin content per cell, we used the cell counts after 96 hours' exposure. The results are shown in Figure 2G-I. There was no effect on the hemoglobin content per cell for the lowest concentration of 5-azacytidine used, but higher concentrations resulted in a slightly increased total hemoglobin content per cell. Hydroxyurea tended to decrease levels of hemoglobin per cell, and butyric acid showed a strong dose-dependent reduction in hemoglobin content per cell.
Effect of varying concentrations of 5-azacytidine, hydroxyurea, and butyric acid in erythroid cultures.
(A-C) Effect on fractional HbF content in erythroid cultures. (D-F) Effect on total Hb. (G-I) Effect on the amount of HbF per cell.
Effect of varying concentrations of 5-azacytidine, hydroxyurea, and butyric acid in erythroid cultures.
(A-C) Effect on fractional HbF content in erythroid cultures. (D-F) Effect on total Hb. (G-I) Effect on the amount of HbF per cell.
The interpretation of changes in absolute globin mRNA levels in these primary human erythroid cells is complex for 2 reasons. First, in general, γ-globin mRNA is less than 1% of β-globin mRNA levels. Second, during the course of culture, γ-globin mRNA levels gradually decrease while β-globin mRNA levels simultaneously increase. We compare the effects of the various agents to that of control cultured cells in which these changes are occurring in the absence of drugs.
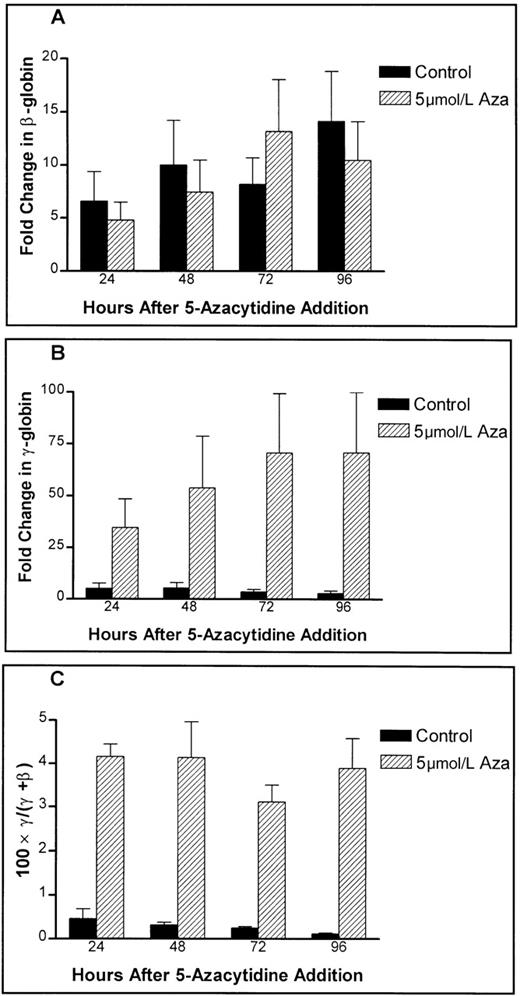
Little net effect of 5-azacytidine is in evidence on β-globin mRNA levels in 24-hour, 48-hour, and 96-hour samples of cells treated with 5 μmol/L 5-azacytidine as compared with control cells (Figure 3A). Expression of γ-globin message, on the other hand, showed a marked increase from 5-azacytidine, and the effect continued to increase with days in culture (Figure 3B) in comparison with the untreated controls. The net effect was a threefold to fourfold increase in the fraction of γ-globin mRNA compared with β-globin message (Figure 3C). This suggests that the mechanism of pharmacologic stimulation of HbF synthesis by 5-azacytidine reported in primates23 and in patients with thalassemia or sickle cell disease4 does indeed involve a specific increase in γ-globin gene transcription.
Time course of 5-azacytidine effects on the absolute levels of globin mRNA in primary human adult erythroid cultures.
Hatched bars show the effect of 5 μmol/L 5-azacytidine on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content. Untreated controls are shown as solid bars.
Time course of 5-azacytidine effects on the absolute levels of globin mRNA in primary human adult erythroid cultures.
Hatched bars show the effect of 5 μmol/L 5-azacytidine on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content. Untreated controls are shown as solid bars.
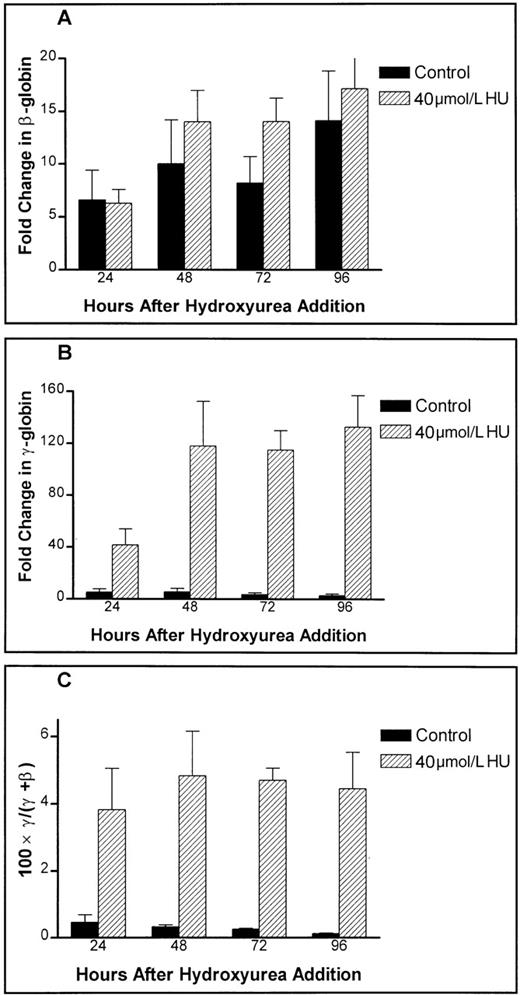
In comparison with the untreated control cells, there is a small increase in β-globin mRNA expression evident 48 to 72 hours after treatment with 40 μmol/L hydroxyurea (Figure4A). In contrast, γ-globin mRNA expression is increased by approximately 20-fold to 50-fold over the equivalent controls (Figure 4B), correlating with the increased HbF expression previously reported for in vitro erythroid cell culture18 and sickle cell patients taking the drug.7 24 In the absence of the drug, γ-globin mRNA levels declined over the period of observation. The resultant effect on the fractional γ-globin mRNA content (γ/[γ + β]) is a significant, time-independent increase over the equivalent controls, as expected, in the fourfold to fivefold range (Figure 4C).
Hydroxyurea effects on absolute globin mRNA levels in primary human adult erythroid cultures.
The effect of 40 μmol/L hydroxyurea on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content are shown. Untreated controls are shown as solid bars, while hydroxyurea-treated samples are shown as hatched bars.
Hydroxyurea effects on absolute globin mRNA levels in primary human adult erythroid cultures.
The effect of 40 μmol/L hydroxyurea on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content are shown. Untreated controls are shown as solid bars, while hydroxyurea-treated samples are shown as hatched bars.
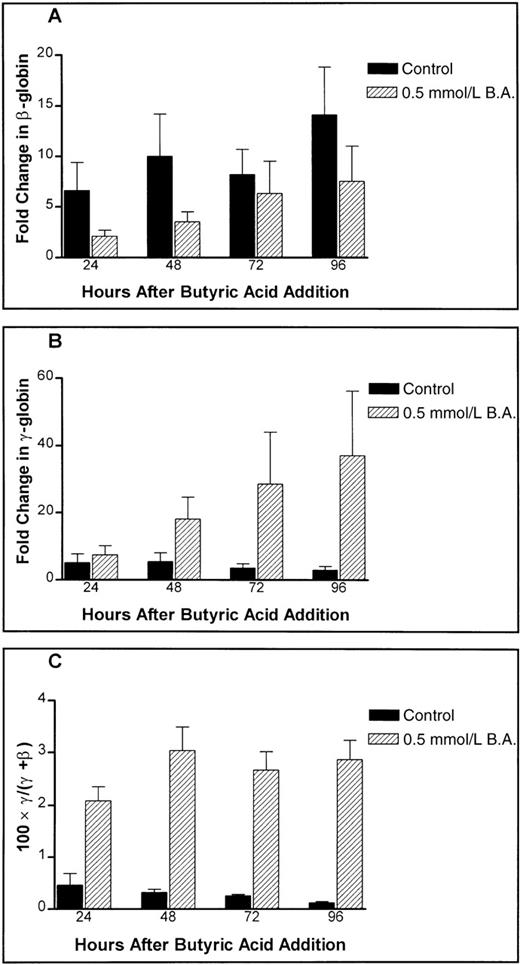
Although there was a monotonic increase of β-globin mRNA expression both in cells treated with 0.5 mmol/L butyric acid and in the untreated controls (Figure 5A), the level of expression was significantly lower in treated cells (P < .05, paired t test), indicating a relative inhibition of β-globin gene expression. In contrast, γ-globin message was expressed at considerably higher levels in treated cells in a time-dependent fashion (Figure 5B). Consequently, fractional γ-globin message expression is increased twofold to threefold in butyric-acid–treated cells (Figure 5C). While these observations are in agreement with previous reports that butyric acid increases fractional and absolute HbF,25 simultaneous lowering of β-globin expression has not been reported.
Butyric-acid effects on absolute globin mRNA levels in primary human adult erythroid cultures.
The effect of 0.5 mmol/L butyric acid on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content are shown. Untreated controls are shown as solid bars while butyric-acid–treated samples are shown as hatched bars.
Butyric-acid effects on absolute globin mRNA levels in primary human adult erythroid cultures.
The effect of 0.5 mmol/L butyric acid on (A) the levels of β-globin message, (B) the levels of γ-globin message, and (C) fractional γ-globin message content are shown. Untreated controls are shown as solid bars while butyric-acid–treated samples are shown as hatched bars.
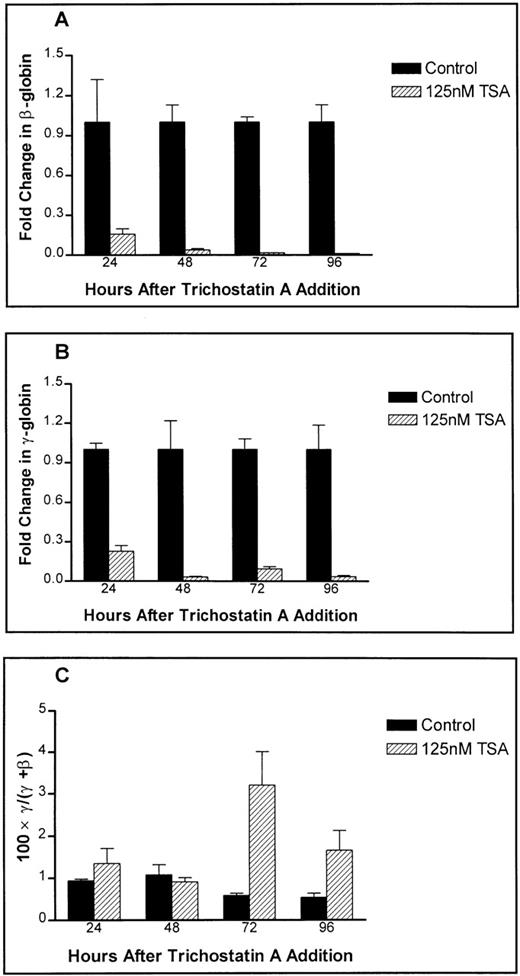
Inhibition of histone deacetylase activity26 has been reported and has recently been proposed as being directly involved in increased γ-globin gene expression by butyric acid.27Trichostatin A, a reversible inhibitor of histone deacetylase activity both in vitro and in vivo,28 was used to treat primary erythroid cells, and the response profile it induced in primary cell cultures was found to be different from that induced by butyric acid. Despite having a relatively small impact on the benzidine-positive cell count (data not shown), hemoglobin protein product was not detectable for cells treated with trichostatin A over a concentration range covering 62.5 nmol/L to 1 μmol/L. A profound decrease in both β- and γ-globin message expression was observed in primary erythroid cells treated with 125 nm trichostatin A as early as 24 hours after addition (samples were normalized to controls at equivalent time points instead of there being one sample taken at time 0) (Figure 6). Despite this, a small relative increase in the fraction of γ-globin mRNA to β-globin mRNA was found.
Effect of trichostatin A on globin expression in primary human adult erythroid cultures.
The fold change in (A) β-globin message levels, (B) γ-globin message levels, and (C) fractional γ-globin message content are shown. Controls are represented by filled bars while trichostatin A treated samples are shown as hatched bars. Fold changes are expressed relative to equivalent controls.
Effect of trichostatin A on globin expression in primary human adult erythroid cultures.
The fold change in (A) β-globin message levels, (B) γ-globin message levels, and (C) fractional γ-globin message content are shown. Controls are represented by filled bars while trichostatin A treated samples are shown as hatched bars. Fold changes are expressed relative to equivalent controls.
Discussion
During growth and differentiation, gene expression undergoes selective activation and repression/silencing.1 Silenced genes can, however, be reactivated and expressed. This is the basis of therapies for certain monogenic genetic diseases, such as Duchenne muscular dystrophy,29 30 and holds promise for the development of therapies for certain other genetic diseases. Reactivation of γ-globin synthesis to increase fetal hemoglobin with the use of 5-azacytidine, hydroxyurea, or butyric acid (or its derivatives) in the treatment of sickle cell disease and thalassemia syndromes is an example of this approach.
The dose-dependent cytotoxicity of benzidine-positive cells observed in this study with butyric acid has not been published previously although similar results were reported for sodium phenylacetate.31Treatment of erythroid cultures with 5-azacytidine, hydroxyurea, or butyric acid resulted in both a 30-fold–to–120-fold increase in expression of γ-globin mRNA and approximately 5-fold increase in γ/(γ + β) fractional mRNA levels. In contrast to the uniform increase in γ-globin mRNA by all 3 compounds, hydroxyurea treatment produced a small stimulatory effect on β-globin mRNA expression. This observation could explain, at least in part, previous reports of increased total Hb expression both in human erythroid cells in culture18 and in patients32treated with hydroxyurea.
In contrast to the increase in β-globin mRNA observed for hydroxyurea, butyric-acid treatment resulted in a twofold decrease in β-globin mRNA levels. This finding invites speculation on the possibility that the butyrate-induced increase in γ-globin may be due, at least in part, to a compensatory mechanism related to the concomitant decrease in β-globin mRNA levels. The molecular mechanisms underlying the pharmacologic regulation of γ-globin gene expression have been proposed as involving histone deacetylation27 and/or a specific butyrate response element.33 While butyrate response elements have been described in the promoters of several butyrate-inducible genes, they have been only partially defined in the globin genes.34Butyrate was discovered to be an inducer of HbF as a result of an investigation into the reasons behind the delayed fetal-to-adult-hemoglobin switch in infants born to diabetic mothers.35 Although the histone deacetylase inhibitory activity of butyrate is well known and is proposed as responsible for the HbF-induction activity, it is yet to be demonstrated that alteration of acetylation levels of histones packaging the globin locus affects globin gene expression specifically. Interestingly, although treatment of cultures with trichostatin A, a widely used and well-characterized histone deacetylase inhibitor,28 has minimal effect on the viable cell count, it yielded drastic decreases in both β-globin and γ-globin transcripts. In addition, there was no detectable hemoglobin protein product, suggesting the possibility of incomplete (or no) overlap of their cellular targets. This raises the further intriguing possibility that different classses of histone deacetylases, possibly restricted in their locus of activity, act as specific targets for various inhibitors, such as butyric acid and trichostatin A. Alternatively, the difference in activities may reflect a more potent inhibitory effect of trichostatin A on histone deacetylases in primary human erythroid progenitors. In addition to affecting mRNA levels, treatment of cultures with any of the 3 therapeutic compounds also resulted in increases in HbF/(HbF + HbA) parallel to that observed for γ/(γ + β) fractional mRNA values. Despite the use of butyrate for the treatment of some patients with thalassemia and sickle cell disease and the reports of striking inductions of HbF for some of these patients,9 36 possible in vivo correlates of this cytotoxicity should be investigated along with its exact clinical effectiveness.
The proposed mechanisms of action for the 3 therapeutic compounds tested include alteration of the kinetics of erythropoiesis by hydroxyurea,37 direct induction of γ-globin gene transcription by demethylation of regulatory sequences by 5-azacytidine,4 and alteration of the acetylation status of histones packaging the globin genes by inhibition of histone deacetylase activity in the case of butyric acid.27 That perturbations of such widely varying mechanisms can lead to increases in HbF in peripheral blood (as well as in ex vivo cultures of erythroid progenitors from normal donors) suggests that the overall regulation of globin gene expression is likely to comprise many parts and be quite complex. The high risk of carcinogenicity with 5-azacytidine has limited its use to severe cases of homozygous β-thalassemia.38 Hydroxyurea, on the other hand, is believed to present a low risk of carcinogenicity, is administered orally, and is well tolerated by the patient.39 The effect of treatment with hydroxyurea, however, as with the other drugs, is quite transient. When administration of the drug stops, the production of HbF-containing erythrocytes ceases, and HbF in the patient's blood declines. Therefore, the accumulation and maintenance of therapeutic levels of fetal hemoglobin in the blood require continuous treatment with hydroxyurea. Butyrate action appears to allow use of a pulse dosing regimen.10 In addition, it is known that not all patients respond to the now standard hydroxyurea therapy. Among those who do, there is significant variation in the level of HbF induction; some show drug-induced HbF levels near 40% while others show only minor changes.40 The reasons for this variation remain unknown.
An in vitro system that can be used for prediction of treatment outcomes (measured as HbF and/or γ-globin mRNA response) would be of value as a prognostic tool and also as a platform for testing novel potential pharmacologic modulators of hemoglobin production. The biphasic erythroid culture system employed in this study is a potential candidate for such a tool. The quantitative PCR assay provides a sensitive, reproducible, and specific assay of DNA templates and provides a convenient assay of changes in gene expression under various conditions and at varying time points. In combination with the erythroid culture system, the quantitative PCR assay could serve as the basis for development of such a system. Before any prognostic value could be realized, a correlation would have to be established between the response of cells in cultures derived from donors and the response in the donors themselves.
Reprints:Alan N. Schechter, Laboratory of Chemical Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Building 10, Room 9N307, 10 Center Drive, MSC 1822, Bethesda, MD 20892-1822; e-mail:aschecht@helix.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.