Bacterial DNA and synthetic CpG-oligodeoxynucleotides (ODNs) derived thereof have attracted attention because they activate cells of the immune system in a sequence-dependent manner. Here we investigated the potential of CpG-ODNs to cause proliferation, cytokine production, and regulation of surface molecules in human B-chronic lymphocytic leukemia (CLL) cells. CpG-ODN induced proliferation in both B-CLL cells and normal B cells; however, only B-CLL cells increased proliferative responses when CpG-ODN was added to co-cultures of CD40-ligand transfected mouse fibroblasts (CD40LF) and B cells. Production of interleukin-6 and tumor necrosis factor was detectable at borderline levels, using CpG-ODN as the only stimulus. In contrast, when CpG-ODN was added to co-cultures of B cells and CD40LF, a strong increase in cytokine production occurred in B-CLL cells as well as in normal B cells. The surface molecules CD40, CD58, CD80, CD86, CD54, and MHC class I molecules were up-regulated in B-CLL cells, whereas CD95 expression was not influenced by CpG-ODN stimulation. The same pattern of surface molecule regulation was observed in normal B cells, but up-regulation of CD40 was significantly stronger in B-CLL cells. Costimulation with CpG-ODN and CD40LF resulted in further up-regulation of CD58, CD80, CD86, and MHC class I molecules. In contrast, CD95 expression induced by CD40-ligation was inhibited by CpG-ODN. CpG-ODN activated B-CLL cells acquired a strong stimulatory capacity toward T cells in allogeneic mixed lymphocyte reaction. This effect was completely inhibited by a combination of anti-CD80 and anti-CD86 monoclonal antibody. Taken together, these findings suggest the possible use of CpG-ODN for immunotherapeutic strategies in patients with B-CLL.
B-cell chronic lymphocytic leukemia (CLL) is the most common leukemia in the western hemisphere and is characterized by the progressive accumulation of CD5+ B cells with a low proliferative index but prolonged cell survival. Although the prognosis for early stage B-CLL is excellent, many patients require treatment and suffer complications from immune manifestations of this disease that cannot be cured with the use of conventional chemotherapy.1 The leukemic cell population exhibits marked hyporesponsiveness to proliferative signals that activate normal B cells, including the ligation of the CD40 antigen and the crosslinking of the B-cell antigen receptor complex.2,3 The proliferative hyporesponsiveness by B-CLL cells does not seem to be an intrinsic defect of the malignant cell population as it can be circumvented by direct contact with activated T helper cells.4
The diagnosis of B-CLL is confirmed by fluorescence flow cytometry of lymphocytes expressing cell surface antigens CD5, CD19, and CD23.1 Because of normal expression of MHC class I and II molecules and surface immunoglobulin at variance to immunoglobulin expressed by normal B cells5 and because of normal functions of T cells from patients with B-CLL,6 the leukemic cells might be susceptible to host immune recognition. However, B-CLL cells are poor stimulators of T cells even in allogeneic mixed lymphocyte reactions, in part because they lack important costimulatory accessory molecules necessary for efficient T-cell activation.7,8 As a consequence, attempts have been made to enhance the immunogenicity of B-CLL cells by up-regulation of B7.1 and B7.2 expression with the use of stimulation via the CD40-CD40L pathway.9 Kipps and colleagues10 demonstrated autologous immune recognition of CD40-ligand transfected B-CLL cells. Reports of the first in vivo application of such modified tumor cells have been presented recently.11
Evidence exists that bacterial DNA and certain CpG-oligonucleotides (CpG-ODNs) stimulate cells of the immune system. Prokaryotic (bacterial) and eukaryotic DNA differ in structure. In eukaryotic DNA, the dinucleotide motif 5′-CpG-3′ is suppressed and mostly methylated.12 Tokunaga et al13 observed that mycobacterial DNA and the palindromic sequences derived thereof stimulate murine NK-cells. Subsequently, it was reported that CpG-ODNs directly activate murine APCs such as macrophages, dendritic cells,14,15 and B cells.16 Cell activation requires endosomal uptake of CpG-ODN, resulting within minutes in activation of the stress kinase pathways17 and NF-κB.18 As a consequence, murine B cells enter the cell cycle, secrete immunoglobulin in vitro and in vivo,19 and are rescued from spontaneous or Fas-mediated apoptosis.19,20 In the 38C13 B-cell lymphoma model, CpG-ODNs are effective as immune adjuvants in tumor antigen immunization21 and enhance antibody-dependent cellular cytotoxicity.22 Human B cells proliferate, produce immunoglobulin, and up-regulate surface expression of CD25 and CD86 on stimulation with active CpG-ODNs.23 24
Because CpG-ODNs have been characterized to be potent stimulators of murine and human B cells, we investigated the effects regarding proliferation, cytokine production, and the expression of surface molecules in B-CLL cells. Furthermore, we compared it with the well-characterized CD40 system.
Materials and methods
Cell samples
After informed written consent, peripheral blood was obtained from 11 patients with a diagnosis of B-CLL, according to clinical and immunophenotypic criteria. Patients were either untreated or had not received cytoreductive chemotherapy for a period of at least 3 months before investigation. At the time of analysis, all patients were clinically stable, free from infectious complications, and undergoing routine clinical outpatient review. For control experiments, blood samples were collected from healthy volunteers.
Reagents, antibodies, and cell lines
All ODNs were used single stranded, phosphorothioate stabilized, and synthesized by TibMolBiol (Berlin, Germany). The following ODNs were used: DSP30 (TCGTCGCTGTCTCCGCTTCTTCTTGCC),23 its control DSP30K (TGCTGCCTGTCTCCCCTTCTTCTTGCC),24 pZ2 (CTCCTAGTGGGGGTGTCCTAT),17 1668 (TCCATGACGTTCCTGATGCT),19 and its control 1720 (TCCATGAGCTTCCTGATCCT). All ODNs were used at 2 μM. Murine monoclonal antibodies (mAbs) specific for CD80, CD86, and CD40; FITC conjugated anti-CD40 mAb; PE conjugated anti-CD86 mAb; and appropriate isotype-controls were purchased from Pharmingen. FITC conjugated anti-CD25 mAB, anti-CD54 mAB, anti-CD58 mAB, anti-CD80 mAb, anti-MHC class I mAb, and appropriate isotype controls were purchased from Coulter-Immunotech. NIH3T3 cells stably expressing the human CD40 ligand (CD40LF) were kindly provided by O. Ottmann (Frankfurt, Germany).
Separation procedures
Peripheral blood mononuclear cells (PBMNCs) were isolated from heparinized blood samples by centrifugation over a Ficoll-Hypaque layer (Biochrom, FRG) of 1.077 g/mL density. For separation of CLL B-cells, PBMNCs were incubated with anti-CD2 and anti-CD14 magnetic beads (Dynabeads M450, Dynal, Oslo, Norway) according to the manufacturer's instructions. Such prepared B cells from patients with CLL were > 98% pure as assessed by direct immunofluorescence with the use of a Coulter Epics XL (Coulter, Hamburg, Germany). Nonmalignant B cells did not constitute a meaningful fraction of the total cells isolated because 99% of these cells coexpressed CD5 and CD19 before and after stimulation with CpG-ODN. B cells from normal control subjects were separated by positive selection with the use of CD19-coated magnetic beads (Dynal) and Detachabead (Dynal), according to the manufacturer's instructions to a purity of > 98%. T cells for mixed lymphocyte reactions were isolated from PBMNCs by incubation with anti-CD14 and anti-CD19-coated magnetic beads, resulting in > 97% CD2-positive cells.
Culture conditions and proliferation assay
Purified normal and leukemic B cells were cultured in RPMI 1640 medium (Biochrom, Berlin, Germany), supplemented with 10% fetal calf serum (Biochrom), penicillin/streptomycin 50 IU/mL, Na-pyruvate 1 μmol/L, L-glutamine 2 μmol/L, L-asparagine 20 μg/mL, 2-mercaptoethanol 0.05 μmol/L, HEPES 10 μmol/L, and MEM nonessential amino acids 0.7 × (Biochrom) at 37° and 5% CO2 in a fully humidified atmosphere in 24 well plates at 106 cells in a total volume of 1 mL. For culture in the CD40 system, 105 CD40LF was irradiated (5000 rad) and seeded as a feeder layer before adding the B cells.
Purified B cells were cultured at 5 × 104 cells/200 μL in round-bottom plates with or without 5 × 103irradiated CD40LF or ODN for induction of B-cell proliferation. After 72 hours, the cells were pulsed with 3H-thymidine (Du Pont, Paris) and harvested in a PHD-Cell Harvester (Dunn Labortechnik) after 16 hours. Thymidine incorporation was quantified in a ß-counter (Beckmann, Munich).
Allogeneic mixed lymphocyte reaction
Along with irradiated B-CLL cells, 2 × 105purified CD3+ T cells were cultured at a 1:2 stimulator to responder ratio for 5-7 days. Before the MLR, B-CLL cells were cultivated with or without ODN, CD40LF, or both for 48 hours. Cells were washed two times, irradiated (2000 rad), and cultivated with freshly separated allogeneic T cells. During the last 16 hours of culture, the cells were pulsed with 1 μCi 3H-thymidine (Du Pont, Paris). For inhibition experiments, anti-CD80 mAb, anti-CD86 mAB, or anti-CD40 mAB were added from the beginning of the culture period.
Cytokine measurements
Cytokine levels were determined with the use of commercially available enzyme-linked immunosorbent assay kits (TNFα and IL-6 Duoset), according to the instructions of the manufacturer (Genzyme, Germany). Each value shown represents the mean of duplicate values.
Immunophenotyping
Cells were washed in PBS containing 2% fetal calf serum and incubated with saturating amounts of fluorochrome-conjugated mAB. After 30 minutes at 4°C, the cells were washed with PBS/2% fetal calf serum and analyzed via flow cytometry with the use of a Coulter Epics XL cytofluorometer, acquiring 5000 events. Data were analyzed with the use of WinMDI 2.7 FACS software. The relative expression of surface antigen is described as the mean fluorescence intensity ratio (MFIR). MFIR equals the MFI of cells stained with a fluorochrome-conjugated antigen-specific mAb divided by the MFI of cells stained with a fluorochrome-conjugated isotype control mAb. MFIR induction was calculated with the use of the equation: MFIR (stimulated)/MFIR (untreated).
Statistical analysis
Data from individual experiments are presented as mean ± SEM. Statistical significances were determined with the use of the Wilcoxon signed rank test and the Mann-Whitney test, as appropriate. A Pvalue < .05 was considered to be statistically significant. TheP value was adjusted with the use of the Bonferroni-Holm correction when multiple comparisons were performed.
Results
Proliferation of B-CLL cells in response to CpG-ODNs
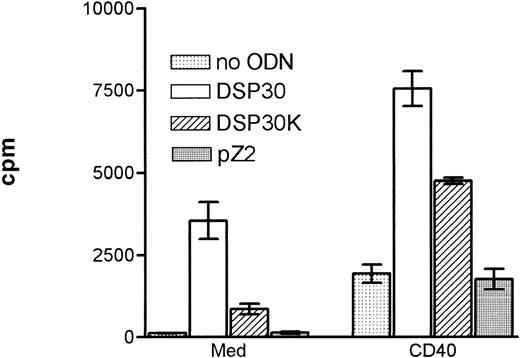
Purified B-CLL cells and normal peripheral blood B cells derived from healthy donors were cultured with the immunostimulatory CpG-ODN DSP30,23 its control CpG-ODN DSP30K (with an inverted GpC motif), and an unmethylated control ODN lacking the CpG dinucleotide (pZ2). In addition, B-CLL cells were stimulated with CD40LF or a combination of CD40LF and CpG-ODN. In all patients with CLL tested, the proliferative response induced by DSP30 was stronger than with its control ODN DSP30K (Figure 1). No thymidine uptake was observed, using the non-CpG-ODN, pZ2. Proliferation of B-CLL cells could be induced with DSP30 at concentrations as low as 20 nmol/L, but the optimal proliferative responses were seen with 2 μmol/L (data not shown). A similar pattern of response was observed with the CpG-ODN 1668 and its control 1720 (data not shown). Ligation of CD40 on B-CLL cells led to a moderate and heterogeneous proliferative response that was strongly enhanced when stimulatory ODNs were added to the culture (Figure 1). This pattern of stimulation was consistently observed with B cells from all patients tested.
Proliferation of chronic lymphocytic leukemia (CLL) B cells activated by DSP30, DSP30K, and pZ2.
5 × 104 CLL-B cells were stimulated in 96-well U-bottom plates with different oligodeoxynucleotides (ODNs) at 2 μmol/L with or without a monolayer of CD40LF.3H-thymidine incorporation was measured after 3 days of culture in triplicates. Results are expressed as mean ± SEM of one representative experiment of 5 performed.
Proliferation of chronic lymphocytic leukemia (CLL) B cells activated by DSP30, DSP30K, and pZ2.
5 × 104 CLL-B cells were stimulated in 96-well U-bottom plates with different oligodeoxynucleotides (ODNs) at 2 μmol/L with or without a monolayer of CD40LF.3H-thymidine incorporation was measured after 3 days of culture in triplicates. Results are expressed as mean ± SEM of one representative experiment of 5 performed.
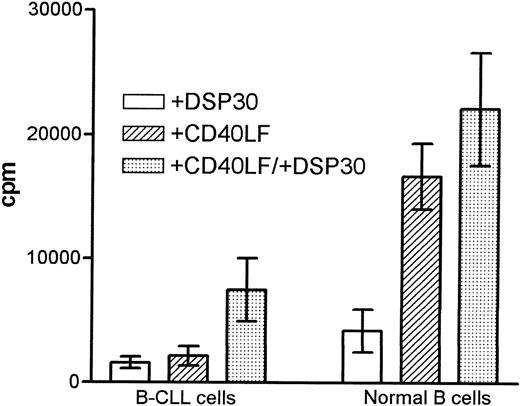
Stimulation with DSP30 seemed to induce a stronger response in normal B cells as compared with B-CLL cells, although the difference was not statistically significant (Figure 2). CD40-ligation resulted in a stronger proliferation in normal B cells (P = .002). In malignant but not in normal B cells, the addition of DSP30 strongly enhanced the proliferative response induced by CD40-ligation, resulting in a higher proliferative index in patients with CLL (4.8 ± 1.6; 1.4 ± 0.2, P = 0.03). No induction of thymidine incorporation was seen when CD40LF was cultured with DSP30 alone (data not shown).
Proliferation of B chronic lymphocytic leukemia (CLL) cells and normal B cells in response to DSP30 and CD40LF.
Purified B-CLL cells and B cells from normal control subjects were stimulated with DSP30 at 2 μmol/L with or without 5 × 103 CD40LF. Results are expressed as mean ± SEM of 11 patients with CLL and 7 normal control subjects. Proliferation in medium controls was < 100 cpm for normal B cells and B-CLL cells.
Proliferation of B chronic lymphocytic leukemia (CLL) cells and normal B cells in response to DSP30 and CD40LF.
Purified B-CLL cells and B cells from normal control subjects were stimulated with DSP30 at 2 μmol/L with or without 5 × 103 CD40LF. Results are expressed as mean ± SEM of 11 patients with CLL and 7 normal control subjects. Proliferation in medium controls was < 100 cpm for normal B cells and B-CLL cells.
Cytokine production of B-CLL cells in response to ODNs and CD40-ligation
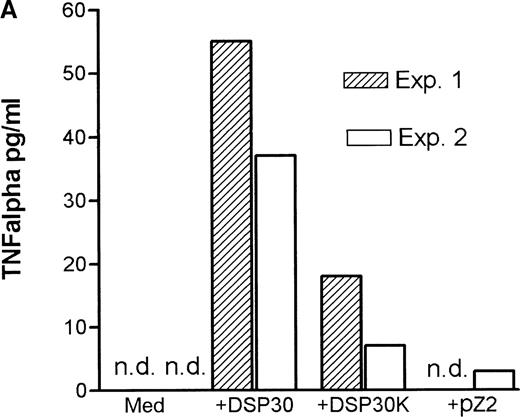
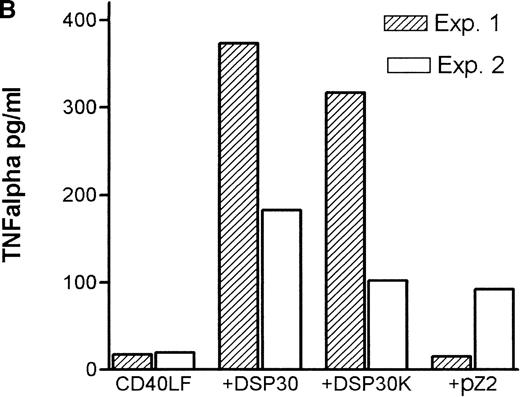
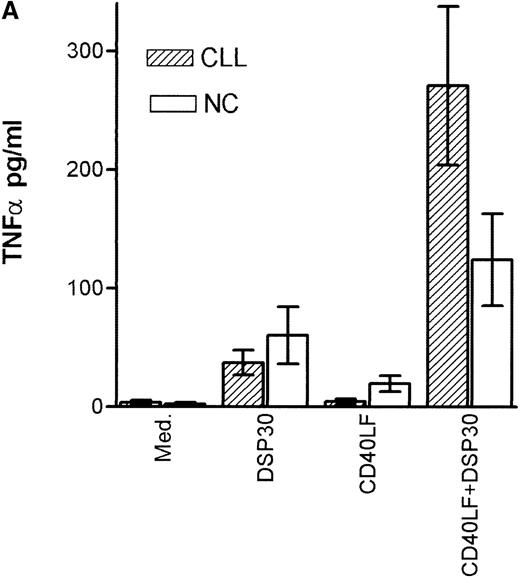
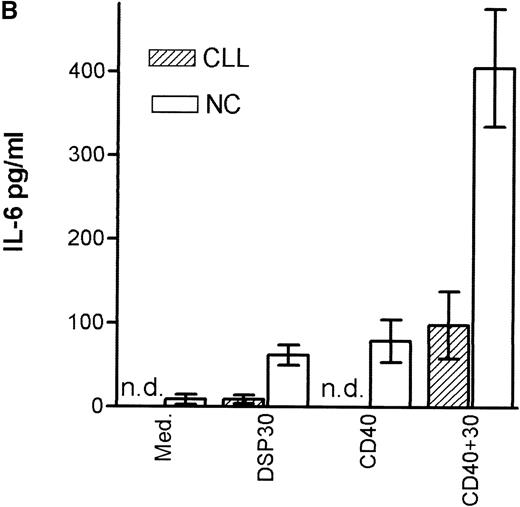
Tumor necrosis factor α (TNF-α) release could be detected in response to DSP30 and to a lesser extent to DSP30K. No significant TNF-α production was found when the control ODN pZ2 was used as the only stimulus. Figure 3A shows the results of two independent experiments. Costimulation with DSP30 and CD40LF resulted in a strong increase in cytokine production. A similar but weaker effect was observed when DSP30K was added to co-cultures of B-CLL cells and CD40LF. In contrast to the proliferation data, the ODN pZ2 was able to costimulate TNF-α release in one of the two patients tested (Figure 3B). A similar pattern of response could be observed with interleuken-6 (IL-6) production but no IL-6 release was detected in response to ODN alone in two independent experiments (data not shown). Figure 4A shows TNF-α production of nine patients with CLL and seven normal control subjects in response to DSP30 with or without CD40LF. TNF-α release could be detected in normal and malignant B cells after 48 hours of culture with the CpG-ODN DSP30. However, addition of CpG-ODN DSP30 to co-cultures of CD40LF and B-CLL cells or normal B cells resulted in strongly augmented TNF-α production. Of note, the addition of DSP30 to co-cultures of B-CLL cells and nontransfected 3T3 fibroblasts did not result in increased TNF-α release (data not shown). The pattern of IL-6 production was essentially comparable to TNF-α production. Again, strong synergistic effects of DSP30 and CD40-ligation were observed. In contrast to TNF-α, the IL-6 levels produced by normal B cells were significantly higher compared with B-CLL cells. In fact, only the costimulation of CD40-ligation and DSP30 resulted in measurable levels of IL-6 in B-CLL cells (Figure 4B).
Cytokine release of chronic lymphocytic leukemia (CLL) B cells activated by DSP30, DSP30K, and pZ2.
After 48 hours of culture at 106 cells/mL, supernatants of cells cultured in medium alone or stimulated with DSP30, DSP30K, and pZ2 with (B) or without (A) CD40LF were tested for production of tumor necrosis factor α with the use of an enzyme-linked immunosorbent assay system. Data of two independent experiments (exp. 1, exp. 2) are presented as the mean of duplicate values; n.d. means not detectable.
Cytokine release of chronic lymphocytic leukemia (CLL) B cells activated by DSP30, DSP30K, and pZ2.
After 48 hours of culture at 106 cells/mL, supernatants of cells cultured in medium alone or stimulated with DSP30, DSP30K, and pZ2 with (B) or without (A) CD40LF were tested for production of tumor necrosis factor α with the use of an enzyme-linked immunosorbent assay system. Data of two independent experiments (exp. 1, exp. 2) are presented as the mean of duplicate values; n.d. means not detectable.
Cytokine production of chronic lymphocytic leukemia (CLL) B cells and normal B cells in response to DSP30 with or without costimulation with CD40LF cells.
Purified B-CLL cells and B cells from normal control subjects were stimulated with DSP30 at 2 μmol/L with or without 1 × 105 CD40LF. Supernatants were tested for tumor necrosis factor α (A) or interleuken-6 (B) with the use of an enzyme-linked immunosorbent assay system. Results are expressed as mean ± SEM of 11 patients with B-CLL and 7 normal control subjects; n.d. means not detectable.
Cytokine production of chronic lymphocytic leukemia (CLL) B cells and normal B cells in response to DSP30 with or without costimulation with CD40LF cells.
Purified B-CLL cells and B cells from normal control subjects were stimulated with DSP30 at 2 μmol/L with or without 1 × 105 CD40LF. Supernatants were tested for tumor necrosis factor α (A) or interleuken-6 (B) with the use of an enzyme-linked immunosorbent assay system. Results are expressed as mean ± SEM of 11 patients with B-CLL and 7 normal control subjects; n.d. means not detectable.
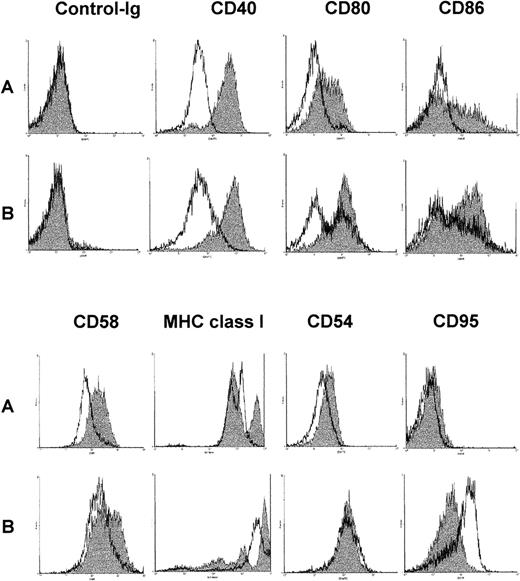
Up-regulation of surface molecules in response to ODNs
After a 48-hour culture period with either DSP30, CD40LF, or the combination of both, the expression of surface molecules CD40, CD58, CD80, CD86, MHC class I, CD54, and CD95 was examined by FACS analysis. The results of a typical FACS analysis are shown in Figure5. Tables1A and 1Bgive the pooled results from 11 B-CLL samples and 7 normal control samples for MFIR-induction when using DSP30, CD40LF, or both as stimuli. DSP30 stimulation of B-CLL cells and normal B cells up-regulated the expression of CD40, CD54, CD58, CD80, CD86, and MHC class I surface molecules in all samples tested, whereas CD95 expression was not changed. However, DSP30 was significantly stronger in up-regulation of CD40 expression in B-CLL cells (P < .002) as compared with normal B cells (Table 1A vs Table 1B). Overall, the degree of CD58, CD80, CD86, and MHC class I up-regulation was not different when comparing stimulation by CpG-ODN versus CD40-ligation. Compared with CD40-ligation, CpG-ODN was a stronger up-regulator of CD40 (P = .002) in B-CLL cells but not in normal B cells (P = .13). As compared with CpG-ODN, CD40-ligation strongly affected CD54 expression in B-CLL cells (P = .004) and normal B cells (P = .03). Stimulation by co-culture of CpG-ODN and CD40LF resulted in an enhanced up-regulation of CD58, CD80, and CD86 as compared with either stimuli alone in B-CLL cells. Similar effects were observed in normal B cells. Expression of MHC class I molecules was further increased in B-CLL samples but not in normal B cells (Table 1A, 1B). Of note, CD95 was up-regulated by CD40-ligation, but addition of CpG-ODN inhibited CD95 up-regulation in normal B cells (P = .03) and B-CLL cells (P = .004). In accordance with the proliferation assays and cytokine measurements, the control non-CpG-ODN DSP30K induced a similar but weaker response, and the unrelated ODN pZ2 had no effect on surface molecule expression (data not shown).
Change of surface antigen phenotype of chronic lymphocytic leukemia (CLL) B cells stimulated with DSP30, CD40LF, or both.
106 B cells were cultured for 48 hours in medium alone (A, unshaded histograms), with DSP30 at 2 μmol/L (A, shaded histograms), with 105 CD40LF (B, unshaded histograms), or with DSP30 and CD40LF (B, shaded histograms).
Change of surface antigen phenotype of chronic lymphocytic leukemia (CLL) B cells stimulated with DSP30, CD40LF, or both.
106 B cells were cultured for 48 hours in medium alone (A, unshaded histograms), with DSP30 at 2 μmol/L (A, shaded histograms), with 105 CD40LF (B, unshaded histograms), or with DSP30 and CD40LF (B, shaded histograms).
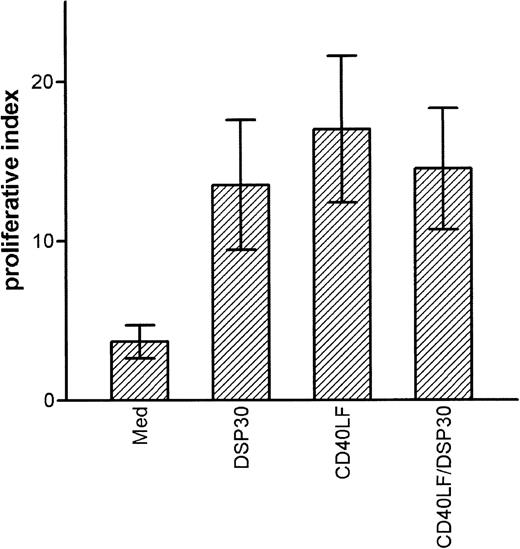
CpG-ODN activated B-CLL cells activate allogeneic T cells in a mixed lymphocyte reaction
B-CLL cells are poor stimulator cells in allogeneic mixed lymphocyte reactions. Because CpG-ODN activated B-CLL cells to up-regulate expression of costimulatory molecules, we evaluated whether CpG-ODN activated B-CLL cells could act as stimulator cells in an allogeneic MLR. We also examined the stimulatory capacity of B-CLL cells that had been cultured with CD40LF in the presence or absence of CpG-ODN. After 48 hours of culture, cells were washed twice, irradiated, and co-cultured with freshly isolated allogeneic T cells at a stimulator to responder ratio of 1 : 2. After 5 days, cell proliferation was assessed by incorporation of 3H-thymidine. The results of 4 independent experiments are depicted in Figure6 and show that CpG-ODN-stimulated B-CLL cells were as effective as CD40LF-activated cells in stimulating proliferative responses in allogeneic T cells. Although unstimulated B-CLL cells caused a proliferative index (3H-thymidine incorporation of stimulated T cells/3H-thymidine incorporation of T cells alone) of 3.7 ± 1.5, CpG-ODN-stimulated B-CLL cells caused an index of 13.5 ± 4.1. Overall, CpG-ODN activation was as efficient as activation with CD40LF or with a combination of CD40LF and CpG-ODN (Figure 6). A mitogenic effect of residual DSP30 was excluded because addition of DSP30 to co-cultures of allogeneic T cells and irradiated leucemic B cells did not result in increased proliferation.
Activation of allogeneic T cells by B-chronic lymphocytic leukemia (CLL) cells in the mixed lymphocyte reaction.
B-CLL cells were cultured for 48 hours in medium alone, with DSP30 at 2 μmol/L, with CD40LF, or with DSP30 and CD40LF. Cells were washed twice, irradiated, and co-cultured with freshly separated allogeneic T cells. After 5 days, culture at a stimulator to responder ratio of 1:2 cells were pulsed with 1 μCi 3H-thymidine. The proliferative index (3H-thymidine incorporation/3H-thymidine incorporation of T cells alone) was calculated. Data represent mean proliferative index ± SEM of 4 independent experiments.
Activation of allogeneic T cells by B-chronic lymphocytic leukemia (CLL) cells in the mixed lymphocyte reaction.
B-CLL cells were cultured for 48 hours in medium alone, with DSP30 at 2 μmol/L, with CD40LF, or with DSP30 and CD40LF. Cells were washed twice, irradiated, and co-cultured with freshly separated allogeneic T cells. After 5 days, culture at a stimulator to responder ratio of 1:2 cells were pulsed with 1 μCi 3H-thymidine. The proliferative index (3H-thymidine incorporation/3H-thymidine incorporation of T cells alone) was calculated. Data represent mean proliferative index ± SEM of 4 independent experiments.
B7 costimulatory molecules are necessary for the induction of allogeneic T cell proliferative response toward B-CLL cells
The role of B7 costimulatory molecules in inducing an allogeneic T-cell proliferative response toward B-CLL cells activated by CD40-ligation has been described.8 9 Hence, we evaluated whether CpG-ODN-induced expression of B7 costimulatory molecules was responsible for the stimulatory capacity of CpG-ODN-activated B-CLL cells. Inhibition experiments were performed by adding anti-CD80 mAb, anti-CD86 mAB, or a combination of both to the cultures. The addition of anti-CD80 mAb or anti-CD86 mAb to cultures reduced thymidine incorporation by 40%-70%. Combination of both mAbs reduced the proliferative response to baseline levels. Addition of anti-CD40 mAb had no inhibitory effect on T-cell proliferation (data not shown).
Discussion
In this study, we investigated the potential of immunostimulatory CpG-ODN to induce proliferation, cytokine production, and accessory surface molecule expression in B-CLL cells. DSP30 is a CpG-ODN, containing three CG dinucleotides and has been described as an effective CpG-ODN for human B cells.23 To evaluate the importance of CG motifs, we also used DSP30 K (with inverted CG motifs) and the ODN pZ2 (without CG dinucleotides). Proliferation of B-CLL cells was induced with CpG-ODN DSP30 but some 3H-thymidine incorporation could also be measured with the use of the control ODN DSP30K (Figure 1). Similar results were observed with purified tonsillar B cells stimulated with CpG-ODN.24Because no proliferation was observed in response to the phosphorothioate stabilized ODN pZ2, B-CLL-cell activation must be dependent on specific sequences of CpG-ODNs rather than on the phosphorothioate backbone.23 The mechanism of human B-cell activation by specific ODNs is not clear. Studies in the mouse showed activation of the stress kinase pathway and a strict dependency on the CpG motif.25 This activation has been questioned in human B cells because ODNs lacking the CpG motif were active in inducing B-cell proliferation although the entire CpG was necessary for maximal proliferation. It remains possible that the phosphorothioate backbone used to reduce degradation provides a permissive influence that permits sequence-specific B-cell activation as the diester form of DSP30 has a reduced stimulatory capacity.23 Interestingly, the control ODN DSP30K was able to induce low cytokine production, proliferation, and surface molecule regulation despite lacking the CpG motif, whereas the control-ODN pZ2 was not reactive when used alone but induced cytokine production when used together with CD40LF (Figure 3A, 3B).
In contrast to low responsiveness toward CD40-ligation or crosslinking of the B-cell antigen receptor complex,2,26 the proliferative response of B-CLL cells stimulated by DSP30 was similar to that of normal B cells. Addition of DSP30 to co-cultures with CD40LF resulted in a fivefold increase of thymidine incorporation in B-CLL cells but not in normal B cells. These data suggest that hyporesponsiveness of B-CLL cells to CD40-ligation can, at least in part, be overcome by CpG-ODN (Figure 2A). The following 2 mechanisms might be involved: (1) up-regulation of CD40 is significantly enhanced in B-CLL cells as compared with normal B cells, which may allow a stronger signaling via CD40-ligation; and (2) the increased TNF-α production achieved via costimulation with CD40-ligation and CpG-ODN may stimulate B-CLL cells in an autocrine fashion (Figure 3B, 4A). Autocrine stimulation by TNF-α has been described previously for malignant B cells.27 28
CpG-ODN rapidly activate murine B cells to secrete IL-6,29and high levels of TNF-α can be detected in cultures of CpG-activated murine and human monocytes.15,24 To date, production of IL-6 and TNF-α has not been described by CpG-ODN-activated human B cells. Interestingly, IL-6, which is thought to inhibit the growth of B-CLL cells,30 is produced only in low amounts by B-CLL cells (Figure 4B). In fact, only costimulation with CpG-ODN and CD40LF resulted in measurable levels of IL-6. The reduced potential of B-CLL cells to produce IL-6 has been postulated to play a role in B-CLL progression and in clinical manifestations of the disease.31
Enhanced expression of accessory molecules, such as CD40, CD86, and MHC class I molecules, has been described in human monocytes, tonsillar B cells,24 and B cells separated from PBMC.23Here, we investigated the potential of CpG-ODN to regulate the expression of costimulatory molecules and molecules involved in B-cell T-cell interaction that are known to be expressed on B lymphocytes from chronic B-cell malignancies.32 In contrast to the CD40 antigen that is known to play a central role in T-cell-mediated B-cell activation,33 CD95, another member of the TNF receptor superfamily, was not modulated by CpG-ODN. In fact, CD40LF induced enhanced CD95 expression was inhibited when DSP30 was added to co-cultures of CD40LF and normal B cells or B-CLL cells (Table 1A, 1B). CpG-ODN-activated murine B-lymphocytes are protected against FAS-mediated apoptosis, possibly via down-regulation of CD40-ligation-induced CD95-expression.20 However, B-CLL cells are resistant to FAS-mediated apoptosis,34 35 and we observed no difference in the propensity to apoptosis on adding an agonistic FAS antibody to B-CLL cells activated with CD40LF in the presence or absence of CpG-ODN (unpublished data).
CpG-ODN triggered a strong and consistent up-regulation of CD40, CD80, CD86, CD54, MHC class I, and CD58 surface molecules in B-CLL cells. All these molecules have been described to be of major importance for professional APC function.7,36-38Accordingly, CpG-ODN-activated B-CLL cells proved to be as effective in inducing allogeneic T-cell proliferation as B-CLL cells after CD40-ligation. In spite of the increased expression of B7-costimulatory molecules in cells stimulated with CD40LF and CpG ODN, no further increase in proliferation of allogeneic T cells was noted (Figure 6). As described for the MLR between allogeneic T cells and CD40-stimulated B-CLL cells,9 allogeneic T-cell proliferation could be inhibited with the use of a combination of anti-CD80 and anti-CD86 mAb.
Prior studies have demonstrated the potential for immunotherapy, using tumor cells expressing the accessory molecules CD80 and CD86.39,40 CD40-ligation has been used to up-regulate CD80 and CD86 molecules in chronic lymphocytic leukemia cells,9follicular lymphoma cells,41 and acute lymphoblastic leukemia cells.42 Kato et al10 have demonstrated that CD154 transfection of B-CLL cells is associated with up-regulation of B7 costimulatory molecules in bystander leukemia B cells. With the use of this approach, generation of autologous cytotoxic T lymphocytes has been achieved,10 and the results from therapeutic application of CD154-transfected cells have been presented recently.11 In view of these data, our results suggest the possibility of using CpG-ODN-activated B-CLL cells to generate autologous T-cell responses. The strong and consistent up-regulation of costimulatory molecules, the costimulatory effect of CpG-ODN with CD40-ligation, and the possibility of stimulating cells in vitro and in vivo suggest CpG-ODN as an attractive addition to immunotherapeutic strategies in CLL.
Acknowledgments
We thank K. Götze and W. Spelsberg for critically reading the manuscript.
Supported by a research grant from the German Research Foundation (Sonderforschungsbereich 519, Teilprojekt A3) and a research grant from the Technical University of Munich (KKF H30-97).
Reprints:Christian Peschel, IIIrd Department of Medicine, Technical University of Munich, Ismaninger Str. 15, 81675 Munich, Germany; e-mail: christian.peschel@lrz.tu-muenchen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.