The optimal regimen of intravenous deferoxamine for iron overload in high-risk homozygous β-thalassemia is unknown because only short-term follow-up has been described in small patient groups. We report the outcome over a 16-year period of a continuous 24-hour deferoxamine regimen, with dose adjustment for serum ferritin, delivered via 25 indwelling intravenous lines for 17 patients. Treatment indications were cardiac arrhythmias, left ventricular dysfunction, gross iron overload, and intolerability of subcutaneous deferoxamine. Cardiac arrhythmias were reversed in 6 of 6 patients, and the left ventricular ejection fraction improved in 7 of 9 patients from a mean (± SEM) of 36 ± 2% to 49 ± 3% (P = .002, n = 9). The serum ferritin fell in a biphasic manner from a pretherapy mean of 6281 ± 562 μg/L to 3736 ± 466 μg/L (P = .001), falling rapidly and proportionally to the pretreatment ferritin (r2 = 0.99) for values >3000 μg/L but falling less rapidly below this value (at 133 ± 22 μg/L/mo). The principal catheter-related complications were infection and thromboembolism (1.15 and 0.48 per 1000 catheter days, respectively), rates similar to other patient groups. Only one case of reversible deferoxamine toxicity was observed (retinal) when the therapeutic index was briefly exceeded. An actuarial survival of 61% at 13 years with no treatment-related mortality provides evidence of the value of this protocol.
The benefits of long-term discontinuous subcutaneous (sc) chelation therapy with deferoxamine (DFO) in extending survival and diminishing cardiac complications in transfusion-dependent β-thalassemia are well-established.1-4 For the majority of patients, the administration of 8- to 12-hour sc infusions, at 30-50 mg/kg body weight, 5-6 nights a week titrated against serum ferritin levels5 or hepatic iron concentration6 is current practice and unlikely to result in chelator-induced toxicity if used with these precautions.7 However, a subgroup of patients, who by reason of poor compliance with or delayed commencement of recommended sc regimens, becomes massively iron overloaded and is at risk of early death, principally from cardiac complications.1 2 For such high-risk patients, intensive intravenous (IV) chelation is indicated, but the optimal regimen remains uncertain. In particular, it is not clear whether 24-hour continuous treatment is necessary or whether discontinuous treatment with shorter infusions is equally effective. Currently, no published data are available on the effects of intervention with 24-hour continuous treatment on long-term cardiac outcome and survival in this group of patients.
Previous reports of the use of IV DFO have either used discontinuous regimes whereby DFO is infused <24 hours each day8,9 or have involved relatively small numbers of patients and relatively short follow-up periods.10-12 It is clear from these studies that IV DFO given by a variety of regimens can decrease the serum ferritin and improve left ventricular function in some cases, but the optimal regime to reverse cardiac complications of iron overload is not known. The effect of DFO infusion on serious cardiac arrhythmias, which are common in severely iron-overloaded thalassemia patients and have poor prognostic significance, has also not been convincingly demonstrated. Furthermore, a detailed analysis of risk and benefit of continuous DFO infusion has not been possible because of the small numbers, short follow-up, and the different regimens employed hitherto. For these reasons, in contrast to sc therapy, no consensus exists as to what should constitute standard treatment in high-risk cases.
To establish the optimal regimen in high-risk patients or to select those who may best benefit from long-term IV chelation, 3 areas of knowledge need to be enhanced. First, it is necessary to know how effective the regimen in question is at decreasing iron stores, at eliminating toxic iron pools such as nontransferrin bound iron (NTBI), and at reducing the morbidity and mortality arising from these conditions. Second, it is necessary to understand whether the potential toxic effects of DFO are best minimized with the use of discontinuous therapy8,9 at relatively high doses or with the use of continuous therapy12 at relatively low doses. Finally, it is important to know the complication rate of the indwelling catheters themselves in this group of patients and how these complications can be minimized.
In this paper, we describe the response, survival, and complications associated with long-term continuous IV DFO therapy administered via indwelling central venous catheters over a 16-year period. The treatment protocol employed continuous rather than intermittent DFO, with the aim of clearing toxic iron species for the maximum practical duration, and the DFO dose was guided by the therapeutic index (mean daily dose of DFO in mg/kg divided by the serum ferritin in μg/L) to minimize DFO-related toxicity.5
Patients and methods
Patient selection
Between March 1983 and October 1998, 25 IV devices (22 Port-A-Cath and 3 single-lumen Hickman) were inserted into 17 patients with transfusion-dependent β-thalassemia (11 male, 6 female; age range: 14-43 years) attending the Hematology Department of the University College London Hospitals (Table 1).
Selection of patients for insertion of Port-A-Caths was based on the presence of one or more of the following four criteria: deteriorating left ventricular function with or without clinical heart failure, development of a clinically significant arrhythmia, a persistently high serum ferritin value associated with poor DFO compliance, and intolerance of DFO by the sc route because of severe local reactions. The 17 patients entered into the study form a small minority of the 140 patients followed in the thalassemia clinic over this period. Five patients had been referred from other hospitals specifically for consideration of IV therapy.
Left ventricular dysfunction was judged to be present when at least a 5-point drop in the resting left ventricular ejection fraction (LVEF) had occurred to a value below the reference range (45%-55%) for radionuclide ventriculography. Patients showing symptoms and signs of heart failure who were judged well enough to have a Port-A-Cath inserted for long-term use were included in the study. By its nature, this study excluded patients who were admitted in extremis and died from heart failure rapidly before a Port-A-Cath could be inserted for long-term use. A significant arrhythmia was defined as one that, by causing or having the potential to cause cardiovascular instability, posed a threat to life. Atrial fibrillation was the most common significant arrhythmia recorded in this study, but nonsustained ventricular tachycardia and supraventricular tachycardia were also observed in 2 patients (Table 1). The serum ferritin trigger for inclusion in this study was a level persistently >3000 μg/L in the presence of noncompliance with or intolerance of sc DFO. Good compliers with no evidence of cardiac disease were excluded even if their ferritin levels exceeded this value from time to time.
Patient monitoring
Cardiac follow-up was done yearly unless cardiac disease was evident when the frequency of reviews was dictated by individual patient needs. Resting LVEF measurements were obtained by multigated acquisition (MUGA) scanning after in vivo labeling of the patients' own red cells with the use of IV injections of 10-15 μg/kg body weight of stannous ion (as the pyrophosphate) and 20 μCi of technetium-99m pertechnate given 20 minutes apart.13 Data acquisition was by means of IGE SFV and Optima gamma cameras (General Electric Medical Systems, Milwaukee, WI), respectively interfaced with Informatek SIMIS 3 and IGE S4000 computers. MUGA scans were performed routinely on a yearly basis unless deterioration in LVEF was noted, in which case measurements were performed as clinically indicated. A change in LVEF readings of 5 points or more was considered to be clinically significant. Cardiac arrhythmias were assessed by both resting 12-lead electrocardiography and 24-hour Holter monitoring with the use of the Pathfinder 3 Analysis System (Reynolds Medical Ltd, Hertford, UK).
Measurements of serum ferritin were carried out generally monthly by enzyme immunoassay with the use of IMX and AxSYM analyzers (Abbott Diagnostics, Maidenhead, UK), and all values are referable to the WHO Ferritin 80/602 First International Standard. Although 24-hour urinary iron excretion was determined in most patients, this result was not used as a criterion for treatment modification. Pure tone audiometric assessments were undertaken yearly as previously described5to examine for high frequency sensorineural deficits not attributable to pre-existing middle ear disease. Electroretinography was performed at Moorfields Eye Hospital, London annually (or more frequently if abnormalities were detected or if the patient was symptomatic), according to the protocol developed by Arden et al.14
DFO infusion regimen
Doses of DFO were calculated with reference to the serum ferritin with a view to maintaining the therapeutic index (mean daily dose of DFO in mg/kg divided by the serum ferritin in μg/L) < 0.025.5 The usual DFO regime was 6-7 days of continuous treatment. Patients were instructed to report immediately any symptoms that might be due to DFO toxicity, in particular visual and auditory disturbances. Only 8 patients received mean daily doses exceeding 50 mg/kg at any one time (Table 2). The maximum DFO dose given to each patient has decreased since the commencement of the study in 1983, and since 1994 only one patient has received a mean daily dose exceeding 50 mg/kg. Battery-operated CADD pumps (Pharmacia Deltec Inc, Minnesota, MN), which allow continuous treatment for up to a week at a time, were used for the most part of the study but were superseded in 1995 by disposable balloon pumps (Baxter Healthcare Ltd, Newbury, UK). These disposable infusers have recently been evaluated15 and are generally preferred by patients because they are lightweight, silent, unobtrusive, and easy to use. To encourage patient compliance further, a home-care delivery system was established, whereby the disposable infusers were delivered directly to the patients' homes.
Intravenous therapy was continued in individual patients until the catheter had to be removed on account of complications. The decision about whether to insert a second catheter for further IV treatment was based on the continuing presence or absence of high-risk features, such as demonstrable heart disease or persistently high serum ferritin levels, but the decision ultimately depended on patient acceptance. Eight patients complied with these recommendations, but one of them refused to have a third catheter inserted (Table 1). We have recently introduced continuous 24-hour sc DFO infusions with disposable balloon pumps in some patients who find this an acceptable alternative to continuous IV therapy.
Catheter care
Patients with indwelling lines were trained in all aspects of catheter use and care, but the majority preferred to have them accessed by nurses in the hematology day care unit. Although this entailed a weekly visit to the hospital, it provided more opportunities for early intervention if any complications were found. Individuals on 6-day regimens were advised to remove the Huber needles of their Port-A-Caths on completion of the last infusion, thus allowing a 24-hour needle-free rest period. Entry sites for the Huber needles were rotated as much as possible to further lessen the risk of infection, and catheters were flushed with heparinized saline solution each time they were accessed.
At each visit, patients were asked about symptoms that might indicate a catheter infection (fever, exudate, pain, erythema, swelling), and the port site and catheter track were examined for signs of infection. If an infection was suspected, appropriate swabs were taken and blood samples were drawn from the catheters and a peripheral vein for culture. The tips of all catheters removed were sent for culture. Organisms were identified by standard microbiological methods. The laboratory diagnosis of catheter-related sepsis depended on the isolation of identical organisms from blood drawn from a peripheral vein and from the catheter itself. Catheter-related infections were treated with a 7-day course of IV antibiotics via a peripheral vein, followed by a further 7 days through the catheter itself with antibiotic locking. Antibiotic treatment of infected lines was considered successful if cultures from the line remained repeatedly negative for at least 1 month afterward with associated resolution of clinical signs.
Patients were also assessed for thromboembolic complications at each visit and were investigated as appropriate with the use of Doppler ultrasonography, venography, ventilation-perfusion scanning, and spiral computed tomography.
Data and statistical analysis
All determinations are expressed as the mean ± standard error unless otherwise stated. Differences between means have been analyzed by the Student t test and by P values <.05 reported as statistically significant. Rates of decline of serum ferritin in response to IV chelation have been calculated by linear regression analysis. For ferritin data showing biphasic kinetics, the initial and subsequent rates of decline (K1 and K2, respectively) have been determined separately by linear regression, having first identified the points of inflexion of the individual curves. The duration of the initial phase for each curve was defined by the x-axis intercept of the K1 plot for that particular curve. Calculations of actuarial survival have been done by the Kaplan-Meier method.
Results
Left ventricular ejection fraction
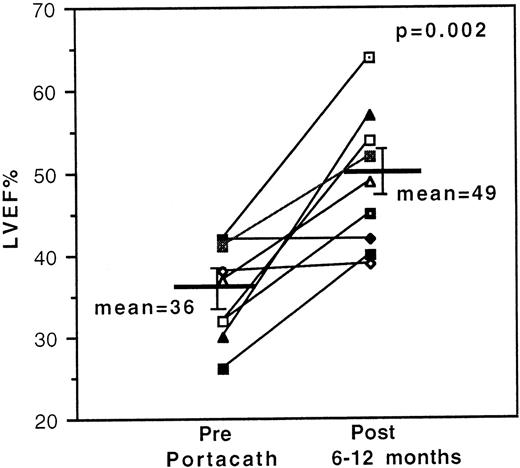
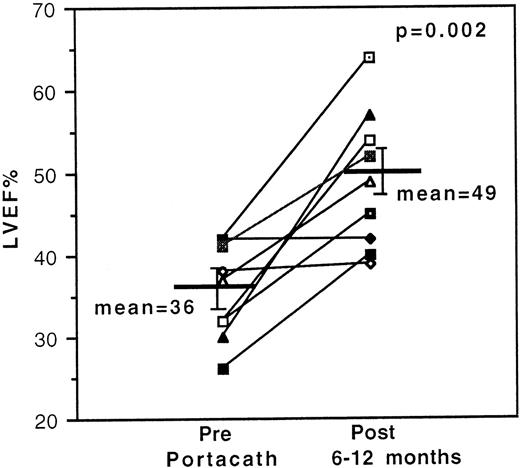
Resting LVEFs improved significantly in 7 of 9 evaluable cases with previously documented deterioration in LVEF and stabilized in the remaining 2 cases. Mean initial and final LVEFs of all 9 evaluable cases were 36 ± 2% and 49 ± 3%, respectively (P = .002) [Figure 1]. Analysis of the serial data (not shown) on individuals with improved LVEFs revealed a >10% increase in LVEF readings in 2 cases after 3 months and in 3 cases after 6-8 months of treatment. An 11% increase was recorded in 1 case after 14 months of treatment, although the dose of DFO had been reduced from 80 mg/kg/d to 26 mg/kg/d during this period on account of DFO-related toxicity. Serial data were incomplete for the seventh case. Long-term follow-up measurements confirm that the improvements in resting LVEF have been sustained with standard sc regimens after cessation of IV therapy.
Effect of 24-hour continuous intravenous deferoxamine infusion on resting left ventricular ejection fraction.
Measurements were obtained serially by radionuclide ventriculography in 9 transfusion-dependent β-thalassemia patients with cardiac disease, and the initial and final values are shown.
Effect of 24-hour continuous intravenous deferoxamine infusion on resting left ventricular ejection fraction.
Measurements were obtained serially by radionuclide ventriculography in 9 transfusion-dependent β-thalassemia patients with cardiac disease, and the initial and final values are shown.
Arrhythmias
All 6 cases with cardiac arrhythmias reverted to sinus rhythm with continuous IV DFO therapy. Five of these patients received conventional anti-arrhythmic drugs from the outset, but one patient who had presented with atrial fibrillation cardioverted after 5 days of treatment with DFO alone. The time to documented cardioversion ranged from <24 hours to 12 months. In those who continued to comply with treatment, sinus rhythm was sustained even when conventional anti-arrhythmic agents were discontinued. Two patients who had documented recurrences of atrial fibrillation, coinciding with short periods of erratic compliance, were successfully cardioverted on recommencing continuous DFO infusion. Arrhythmia recurrence in one of this pair had occurred despite prophylaxis with the β blocker, sotalol. A third patient had a recurrence of prolonged palpitations at home when his infusion pump temporarily malfunctioned, although he was taking amiodarone and digoxin at the time. The palpitations did not recur after the malfunction was rectified, and no significant arrhythmia was recorded when a 24-hour electrocardiogram recording was done a few days later.
Serum ferritin
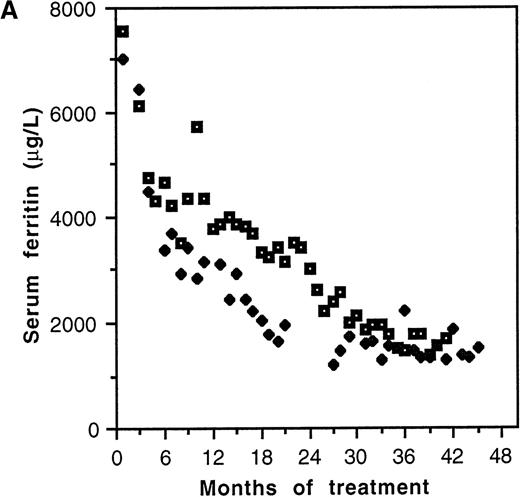
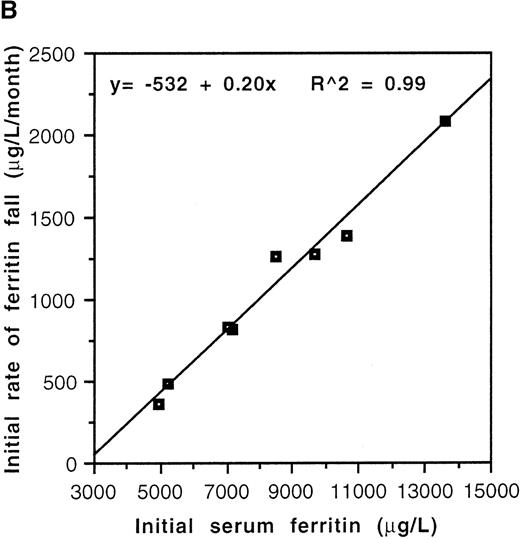
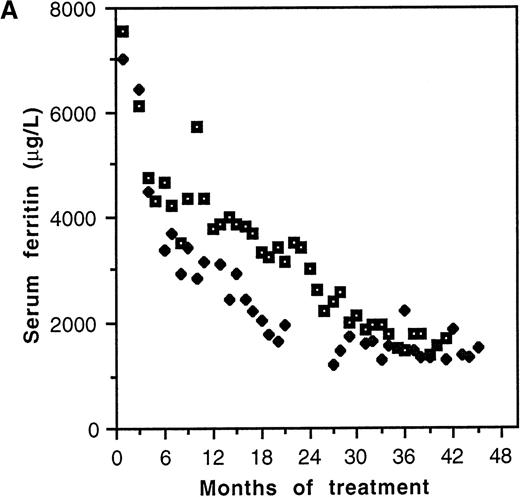
Serum ferritin values fell from a pretherapy mean of 6281 ± 562 μg/L to 3736 ± 466 μg/L (P = .001, n = 25) during the lifetime of the catheters (Table 2). Two distinct patterns of response to chelation were observed. Biphasic kinetics, characterized by an initial rapid fall (K1) in the first few months of treatment followed by a slower rate of decline (K2), were evident in individuals with elevation of serum ferritin more than approximately 3000 μg/L (Figure2A). Mean K1 was 1082 ± 203 μg/L/mo (n = 8), and the median duration of this phase was 4 months (range: 3-6 months). The serum ferritin level at which K1 became zero was 2660 μg/L (Figure 2B). In patients with pretreatment values less than approximately 3000 μg/L, K1 was not discernible, only the slower K2being evident. The mean value of K2 was 133 ± 22 μg/L/mo (n = 12). This slower kinetic phase was evident in patients with pretreatment ferritin values of <3000 μg/L as well as in those patients with higher pretreatment values once levels <3000 μg/L had been achieved. Although K2 was relatively constant, K1 was variable and correlated with the pretherapy serum ferritin value (r2 = 0.99, P < .0001; Figure 2B).
Kinetics of decline of serum ferritin levels in response to continuous intravenous 24-hour deferoxamine infusion.
(A) Pattern of decline in 2 grossly iron-overloaded patients with homozygous β-thalassemia. (B) Correlation of initial rate of serum ferritin decline, K1, and pretherapy serum ferritin level.
Kinetics of decline of serum ferritin levels in response to continuous intravenous 24-hour deferoxamine infusion.
(A) Pattern of decline in 2 grossly iron-overloaded patients with homozygous β-thalassemia. (B) Correlation of initial rate of serum ferritin decline, K1, and pretherapy serum ferritin level.
Effect on long-term survival
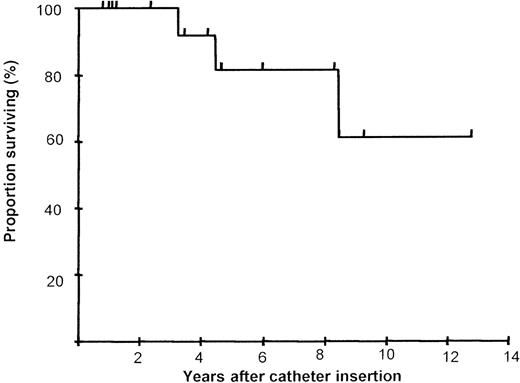
The median follow-up for this study is 54 months (range 9.6-153.6 months), representing the longest follow-up reported to date for this modality of treatment. The actuarial survival is 61% at 13 years in all patients with no patients dying while complying with the prescribed regimen (Figure 3). Compliance with intended treatment was good in all but 3 patients, representing a marked improvement compared with sc therapy as evidenced by a progressive and significant fall in the serum ferritin (Table 2). The reasons for poor compliance were largely psychosocial and were not a consequence of mechanical or physical problems with the regimen.
Long-term survival following 24-hour continuous intravenous deferoxamine infusion.
Kaplan-Meier actuarial survival curve at a median follow-up of 4.5 years for 17 patients with high-risk β-thalassemia is shown.
Long-term survival following 24-hour continuous intravenous deferoxamine infusion.
Kaplan-Meier actuarial survival curve at a median follow-up of 4.5 years for 17 patients with high-risk β-thalassemia is shown.
Actuarial survival in patients with demonstrable cardiac disease was 62% at 13 years (not shown), which is not significantly different from the patient group as a whole (Figure 3). Three patients have died from heart failure since the commencement of this study. The first of these patients did not comply with IV treatment. The second patient died a year after removal of her second Port-A-Cath, having refused further IV therapy. The third patient, who had earlier made a full recovery from an episode of severe cardiac failure while on IV treatment, died 2 years after his infected catheter had been taken out. All the patients who presented with serious arrhythmias and three of the four who had heart failure are alive and leading normal lives.
DFO-related toxicity
DFO-related toxicity was limited to a single early case (patient 5) of reversible retinopathy when the therapeutic index briefly exceeded 0.025 (Table 2). This patient had preexisting diabetes mellitus, and the ferritin had fallen rapidly from a starting value of 7075 μg/L before the start of continuous IV therapy to 3150 μg/L on a mean daily dose of 80 mg/kg of DFO at the onset of visual symptoms. Full resolution of visual field and visual acuity defects occurred over a period of 9 months after reduction in the dose of DFO. No audiometric abnormalities developed in any of the patients.
Catheter-related complications
The catheter-related complications are itemized in Table3. The median life span of the Port-A-Caths (n = 22) was 623 days (range: 71-1851 days) with a median complication-free survival of 516 days (range: 43-1659 days). The Hickman catheters (n = 3) had a shorter survival (median: 285 days; range: 68-536 days), with a median time to first complication of 285 days (range: 68-452 days). For both types of catheters, no significant difference was seen between the time to first complication and the overall life span (P = .516 and .881, respectively), the onset of a complication leading to a greatly shortened catheter survival; median 39 days (range: 0-638 days) for Port-A-Caths and 42 days (range: 0-84 days) for Hickman catheters. The principle Port-A-Cath complications were infection (1.15 per 1000 days of catheter use) and thromboembolism (0.48 per 1000 days of catheter use). Rarer complications included catheter disconnection and migration presenting as ventricular tachycardia (0.06 per 1000 catheter days), perforation of the superior vena cava [previously reported by Russell et al16 (0.06 per 1000 catheter days)], and nonthrombotic catheter occlusion (0.12 per 1000 catheter days). The Port-A-Cath removal rate because of complications was 0.91 per 1000 catheter days. There were no catheter-related deaths.
Infection incidence.
Staphylococci were the predominant cause of Port-A-Cath infections, with coagulase-negative strains accounting for 10 of 19 episodes, methicillin-sensitive Staphylococcus aureus for 4 of 19 episodes, and endogenous infection with methicillin-resistant S aureus for 3 of 19 episodes. Infections with gram-negative organisms were much less common; Escherichia coli andAchromobacter spp were each responsible for 1 of 19 infective episodes. The methicillin-resistant S aureus infections in both patients were believed to have arisen as a result of repeated courses of antibiotic treatment for intra-abdominal conditions not directly related to thalassemia. No significant preponderance was seen of localized infections of the sc pocket over bacteremias (10 of 19 vs 9 of 19 episodes), but coagulase-negative staphylococci were involved in a much higher proportion of the former (8 of 10 vs 2 of 9 episodes). Eradication of infection from the lines with antibiotics was achieved in 2 of 2 of the gram-negative infections, in 6 of 10 of coagulase-negative staphylococcal infections, but in only 1 of 7 ofS aureus infections, giving an overall success rate of 9 of 19 (47%). Two Hickman lines were removed on account of infection. The first infection was an exit-site abscess for which microbiology results are unavailable, and the second was a bacteremic episode because of a coagulase-negative staphylococcus. The overall rate of infection of the Hickman catheters was 2.8 per 1000 days of catheter use. No cases of localized or systemic fungal infections were seen during this study.
Thrombosis incidence.
Thromboembolic events included catheter thrombosis in 3 of 8 episodes, pulmonary embolism in 2 of 8, superior vena cava thrombosis in 2 of 8, and thrombosis of the left internal jugular in 1 of 8, respectively. The caval thrombus was complicated by a persistent postphlebitic obstruction syndrome that necessitated a right internal jugular-right atrial bypass operation 14 months after the initial thrombotic event. One patient was found to have a small organized right atrial thrombus 3 years after removal of an infected Port-A-Cath. With the exception of patient 8's second catheter, all catheters complicated by thrombus formation were managed by catheter removal ab initio followed by oral anticoagulation for 3 months. The tip of this catheter, which was located in the right atrium, had a large (4.56 cm × 3.23 cm) thrombus demonstrated by echocardiography and was treated for 3 months with warfarin, following which complete dissolution of the thrombus was documented by echocardiography. The catheter has subsequently been repositioned without complication and remains in use 21 months later.
Discussion
This study shows that continuous IV DFO, administered through indwelling catheters with dose adjustment for serum ferritin levels, can correct left ventricular dysfunction and reverse serious cardiac arrhythmias with acceptable degrees of catheter- and DFO-related complications. Furthermore, provided patients comply with IV therapy until the clinical objectives have been achieved and subsequently comply with conventional sc DFO, long-term survival is good, the actuarial survival being 61% at 13 years even in this high-risk group of adult homozygous β-thalassemia patients. The short-term benefits of continuous IV DFO therapy for patients with cardiac decompensation have been documented previously,10,11 but the effects of such intervention on long-term survival have not been described. Direct comparison of survival in this series with other high-risk groups is not possible because of the limited follow-up data in other studies and because of the heterogeneity of the high-risk patients in this study. However, an indication of the effectiveness of this regimen may be gleaned by comparing survival with other groups having persistently high ferritin values or in groups with demonstrable cardiac disease. All but 2 patients in our study had serum ferritin values consistently above 2500 μg/L before IV therapy, levels which were previously reported to be associated with 13-year cardiac survival of only 25%,2 and these 2 patients (patients 3 and 12) both had demonstrable heart disease. Survival in patients with demonstrable cardiac disease, before DFO was generally available, was only 50% at 1 year.17 More recently, survival in 39 patients with demonstrable cardiac disease was only 29% at 3.6 years.18If we examine only those patients with demonstrable cardiac disease in our study (Table 1), actuarial survival is 62% at 13 years. Thus, while precise comparisons are not possible, examination of the nearest clinically comparable data in patients not receiving this continuous DFO regimen strongly suggests that this regimen has beneficial effects on survival of high-risk patients.
The 3 deaths occurred in patients who either did not comply with IV therapy or who, after showing initial benefit with IV therapy, subsequently failed to comply with regular sc treatment once the catheter was removed. The common factor associated with improved survival has been good compliance with treatment and is consistent with previous findings that the long-term outcome ultimately depends on compliance with DFO and on liver iron concentrations.3 It is clear from inspection of the results that, although nearly all the patients had poor compliance as an associated or primary factor for commencing IV therapy, once the IV therapy ceased, all but 2 patients complied well with regular sc DFO on its re-introduction. Thus, it appears that most patients undertaking IV therapy are motivated subsequently to comply better with regular therapy. The reasons for improved compliance are likely to be complex but may in part result from the demonstration of a measurable benefit from the therapy (eg, reversal of cardiac dysfunction, suitable rate of fall in serum ferritin) providing longer term motivation. In those that fail, there may be unresolved psychosocial difficulties underlying continued poor compliance.
This is the first study in which sustained reversal of life-threatening arrhythmias with the use of continuous IV therapy has been demonstrated in more than the occasional patient. Successful reversal of cardiac arrhythmias has previously been reported with the use of discontinuous IV therapy in 1 patient8 and continuous therapy in another.11 However, it has not been possible until now to establish whether arrhythmia reversal is consistently achievable and to what extent the long-term prognosis is improved thereby. In all 6 cases commenced on IV DFO on account of arrhythmias, a sustained reversal to sinus rhythm was documented. The observation that conventional anti-arrhythmic agents on their own appeared to provide inadequate prophylaxis against the recurrence of arrhythmias in several of these individuals suggests that DFO is an anti-arrhythmic sine qua non in this group of patients. The response of arrhythmias to continuous DFO provides supportive evidence to experimental models on the pathophysiological basis of cardiac dysfunction in iron-overloaded patients. It is known that low molecular forms of NTBI are taken up rapidly by heart cells in culture, at more than 200 times the rate of iron uptake from transferrin iron,19 and induce abnormal rhythmicity and poor contractile function through their ability to generate highly toxic free hydroxyl radicals.20,21 These effects are both preventable and reversible in vitro by DFO.21 The clinical implication is that removal of NTBI by DFO22 may abrogate the arrhythmogenic effects of NTBI independently of the effects of DFO on storage iron pools that are accessed relatively slowly by DFO.23 This hypothesis is consistent with the clinical observations in this study, namely that objective improvements in arrhythmias often occurred relatively quickly, before iron excretion by DFO could have reduced tissue iron to levels regarded as safe.3 Indeed, rapid and sustained return to sinus rhythm from atrial fibrillation was documented in 1 patient within 5 days of treatment (when the effect of DFO on total body iron would have been minimal), without the need for conventional anti-arrhythmic agents. We suggest that it is, thus, more likely that the beneficial effect of continuous infusion of DFO on cardiac arrhythmias is exerted by continuous removal of a toxic labile iron pool than on reduction of absolute levels of total tissue iron.
The optimal regimen for IV DFO in high-risk patients has not been evaluated prospectively in controlled trials. However, continuous treatment has theoretical advantages over discontinuous treatment for several reasons. First, in view of the rapid return of NTBI after cessation of IV DFO infusions,22 24-hour continuous DFO therapy is likely to be preferable. Second, the evidence for benefit of discontinuous therapy in high-risk patients is limited. In a previous study using discontinuous DFO intravenously at 100 mg/kg for 8 h/d,9 no improvement in cardiac status was seen in 2 patients with proven cardiac disease after 41-43 months, despite the falling ferritin levels. Although the numbers are small in the latter study, the lack of improvement contrasts with the reversal of arrhythmias and improvement in LVEF with the use of continuous treatment in our study. In a further report,8 using discontinuous treatment in 16 iron-overloaded patients, only 1 patient had abnormal LVEF that demonstrably improved with treatment. The optimum dose of DFO has not been prospectively tested in our study, but, during the 16 years, progressively lower maximum doses were used on new patients with cardiac disease and excellent outcomes were still achieved, using maximum doses of 50 mg/kg (Patients 3, 6, 10, 12, and 17; Tables 1 and 2). Indeed, we have no evidence that NTBI is removed more rapidly or that outcome is improved at higher doses.22
The data on serum ferritin kinetics in response to continuous DFO infusions show that the rate and pattern of decline depend on the pretreatment ferritin value. In patients with transfusional iron overload, serum ferritin reflects both increased intracellular synthesis as well as release from damaged cells. A direct relationship with body iron stores cannot be assumed when ferritin levels exceed 4000 μg/L, and above this value there is an increasing contribution from damaged cells to the ferritinemia.24 The biphasic response to DFO observed in grossly iron-overloaded individuals, therefore, may represent effects both on leakage from damaged cells and on ferritin synthesis in response to lowering of intracellular iron levels (Figure 2A). We suggest that the initial rapid phase of decline (K1) in serum ferritin (Figure 2B) indicates the effect of DFO on leaked ferritin, possibly mediated by a restoration of cell membrane or organelle integrity as low molecular weight chelatable iron pools are cleared rapidly. Thus, the greater the initial leakage, the greater the rate of fall as damaged cells recover in response to chelation therapy. We further suggest that the second slower phase (K2) reflects the removal of iron from tissue stores that is known to be a slow process, being only a limited amount of iron from this pool available for chelation at any one time. Of interest, the point of convergence of K1 and K2 in this study is approximately 3000 μg/L (Figure 2B), which is close to the level at which the maximum rate of ferritin synthesis is thought to be reached.24 We have found it helpful to be able to estimate for patients the likely rate of fall in the serum ferritin based on these observations. Figure 2B can be used to predict the approximate rate of fall for each ferritin value above 3000 μg/L. For ferritin values below 3000 μg/L, a fall of 133 μg/L/mo can be used to estimate the likely decrements, as the rate of decline below this value appears to be relatively independent of the serum ferritin. This prediction may help to avoid overoptimistic expectation of the rate of ferritin fall and also allows patients to check their progress against a predicted range of response.
The benefits of IV therapy described above must inevitably be weighed against the additional potential risks of the indwelling lines. However, this study shows that the incidence of infection and thrombosis of the Port-A-Caths compare favorably with those seen in other patient groups and should not act as a deterrent to this treatment in those at high risk from the complications of iron overload. In a review of 8 studies on the use of similar venous access devices in adult and pediatric oncology patients (306 patients, 314 catheters, 4700-12 797 catheter days), the infection rate ranged between 0.00 and 2.35 per 1000 days of catheter use.25Similarly, coagulase-negative staphylococci and S aureus have been the organisms most frequently implicated in catheter infections in other patient groups.26 Of note, no cases of fungal infections were documented in the present study, in particular mucormycosis that has been described in immunosuppressed thalassemia patients27,28 and in dialysis patients receiving DFO.29 The higher infection rate in the Hickman catheters in our study must be interpreted with caution in view of the very small sample size, but it is of interest to note that the incidence of Hickman infections were also higher than in Port-A-Caths in a previous study of thalassemia patients.8 Novel developments in catheter design, including polymer improvements30 and the incorporation of antiseptics and antimicrobials,31-33 may in the future offer additional strategies to scrupulous aseptic technique in the prevention of catheter sepsis, but their efficacy in patients requiring indwelling catheters for longer than a few weeks is at present unknown.
The rate of thrombotic complications in this study is comparable to the rates in other patient groups. Three studies34-36 in oncology patients reported thrombotic rates of 1.2, 0.97, and 0.53 per 1000 days of catheter use. Although regularly transfused thalassemia patients do not, therefore, appear to be at increased risk of thrombotic complications from indwelling lines, we have recently introduced prophylactic anticoagulation for patients having new lines inserted. The reasons for introducing prophylactic anticoagulation are: first, some of the thrombotic complications have been troublesome, such as superior vena cava and right atrial thrombosis; second, it has been suggested that anticoagulation may also reduce the incidence of catheter infection in other patient groups, as thrombus formation is a well-known etiological factor in catheter infections;37-40 and third, as this study has shown, the onset of infective and thrombotic complications greatly shortens catheter survival on which the success of IV therapy depends. As the effectiveness of fixed mini-dose warfarin at 1 mg daily for the purpose of reducing the risk of central venous catheter-related thrombosis remains unproven,41 our policy is to anticoagulate with adjusted-dose warfarin to a target international normalized ratio of 2.0:3.0 for as long as the lines remain in situ, unless there is a patient-specific contraindication. Insufficient follow-up at this point is available to comment on the effectiveness of this strategy in reducing thrombosis and infection significantly.
The low frequency of catheter-related complications, together with only 1 case of reversible DFO-related complications, shows that, with careful monitoring and use of appropriate dose adjustment as serum ferritin levels decline, continuous DFO infusions are a safe therapeutic option in patients at high risk of cardiac toxicity. The findings of consistent reversal of arrhythmias and left ventricular dysfunction should stimulate further investigation into the mechanisms that underlie these benefits and should argue in favor of continuous therapy in patients with cardiac disease. Finally, the long-term survival and compliance data show that in most patients a secondary benefit is observed following cessation of IV therapy; namely, improved long-term compliance with conventional sc therapy that, in turn, contributes to the long-term survival of these high-risk patients.
Acknowledgments
We wish to thank Sarah Benn-Hirsch and Barbara Bull for their kind assistance with data collection and Goli Taghipour for help with analysis of the survival data. Special thanks goes to Catherine Jarrold for helpful advice regarding the chelation protocol used in this study.
Supported in part by a grant from Cooley's Anemia Foundation, USA.
Reprints:John B. Porter, Department of Hematology, University College London Medical School, 98 Chenies Mews, London WC1E 6HX, UK; e-mail: j.porter@ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.