The transcription factor, signal transducer and activator of transcription (Stat) 6, regulates TH2-lymphocyte activity by controlling the expression and responsiveness to interleukin (IL)–4, which plays a key role in numerous allergic maladies. Therefore, we sought to use a phosphorothiolate cis-element decoy to target disruption of Stat6 transcriptional activity. Here we showed that the Stat6 decoy potently ablated the messenger RNA expression and production of IL-4, but not of several other cytokines. The Stat6 decoy functionally disrupted IL-4–inducible cell proliferation of murine TH2 cells and primary human CD4+ T lymphocytes. Specificity of the decoy was demonstrated by its ability to directly block Stat6 binding to a cis-element probe and transactivation, but not affect Stat6 tyrosine phosphorylation or expression of the IL-4 receptor chains. Moreover, the decoy failed to inhibit non–Stat6-dependent signaling pathways since IL-2 was competent to induce cell proliferation and activation of Stats 1, 3, and 5a/b. With the use of laser scanning confocal microscopy, fluorescently tagged Stat6 decoy was detectable in the cytoplasm and nucleus; however, greater levels of oligonucleotide were present in the latter following IL-4 treatment. Taken together, these data suggest that IL-4–driven TH2 cell activity can be preferentially restricted via targeted disruption of Stat6 by a novel and specific decoy strategy that may possess gene therapeutic potential.
Functionally distinct T-helper cell subsets, known as TH1 and TH2 cells, are characterized by the patterns of cytokines they secrete.1-3 Different pathological states can be mediated by selective activation of either TH subset.4,5 For example, release of TH1 cytokines can promote disease based on cell-mediated immunity, including multiple sclerosis, insulin-dependent diabetes mellitus, and allograft rejection. In contrast, overproduction of TH2 cells and their products is characteristic of allergic maladies (eg, asthma, allergic rhinitis, atopic dermatitis) and is also reported to promote susceptibility to infectious agents, such as Leishmania major, leprosy, and human immunodeficiency virus.6 7 Therefore, blocking the production of Th-subset cytokines and its consequent cellular activities (ie, growth, differentiation, or cytokine production) by antigene strategies or pharmaceuticals would be expected to have therapeutic potential for lymphoid- and myeloid-derived pathologies.
The development of TH1/TH2 functional subsets appears to be tightly regulated by specific transcription factors.8-11 The signal transducer and activator of transcription (Stat) proteins are a family of cytokine-activated, tyrosine-phosphorylated transcription factors. Stat6 is one member reported to play a significant role in the coordinated transcription of various cytokine genes.12 Stat6 has been postulated to mediate TH2-specific expression of interleukin (IL)–4 through an autocrine mechanism by binding and transactivating the IL-4 promoter.13,14 As IL-4 is a potent inducer of TH2 development, transcription factors activated by IL-4 are potential candidates for regulating IL-4 gene expression and TH2 phenotype development.15 A critical role for Stat6 in TH2 development was first demonstrated by the targeted disruption of the Stat6 gene in mice. Similar to IL-4−/− mice,16 Stat6-deficient (Stat6−/−) mice also lack IL-4–mediated activities, including TH2-cell differentiation, expression of cell surface markers, and immunoglobulin class switching toimmunoglobulin (Ig) E.17-19
While in vivo targeted disruption of transcription factors such as Stat6 has provided a unique pattern of phenotypic deficiencies, some difficulties can arise and have done so. Unconditional deletion of some specific transcription functions can lead to lethal embryogenesis.20-22 These consequences can make the study of certain transcription factors in fully developed cells or animals difficult to assess. Moreover, targeted disruption of transcription factors by genetic deletion has not been as successful in vivo as in human cells. Here, we present a novel strategy for in vitro targeted disruption of Stat6 with several distinct advantages and therapeutic potential.
Transfection of cis-element double-stranded oligodeoxynucleotides (ODNs), referred to as “decoy” ODNs, has beenreported to be a powerful tool that provides a new classof antigene strategies for gene therapy and for the study of gene transcription.23-28 Synthetic ODNs act as decoy cis-elements to block the binding of nuclear factors to promoter regions of targeted genes, resulting in the inhibition of gene transactivation in vitro and in vivo. Therefore, the decoy approach may permit treatment of human diseases by modulation of endogenous transcription gene.29-33 For example, an NF-κB decoy has been shown to reduce myocardial reperfusion injury by inhibiting the protein expression of cytokines (IL-6 and IL-8) and adhesion molecules in aortic endothelial cells.26,34Treatment of hypertension by means of decoy ODNs for angiotensinogen gene–activating elements was also reported.35 It has been shown that intrarenal arterial perfusion of E2F decoy ODNs inhibited mesangial cell proliferation.36 37 However, little is known about the effect of transcription factor decoys on TH cells, which have a significantly shorter half-life than the above-mentioned cells.
In order to investigate the efficacy of cis-element decoy ODN against Stat6 binding site (Stat6 decoy ODN) for TH2 cell activity, we provide novel evidence that fluorescent dye N, N, N′, N′-tetramethyl-6-carboxyrhodamine (TAMRA)–labeled double-stranded Stat6 decoys were successfully transfected into TH2 cells with the use of a cationic liposome-mediated method of gene transfer. We found that the decoy ODN could effectively block Stat6 binding to its specific cis-element by electrophoretic mobility shift assay (EMSA) and inhibit IL-4–induced Stat6 promoter transactivation detected by a luciferase reporter construct containing the Stat6 binding element. Furthermore, our results revealed that the Stat6 decoy ODN could potently and effectively ablate messenger RNA (mRNA) expression and production of IL-4. Stat6 decoy ODN was also found to inhibit IL-4–mediated cell proliferation in TH2 cells and primary human T cells, but not affect IL-2–mediated T-cell activity. Taken together, these data suggest that Stat6 decoy ODN provides rapid and efficient means to assess the roles of specific transcription factors in T-cell development and has genetic therapeutic potential that could be applied to human cells.
Materials and methods
First, ODN was synthesized and sequence targets were selected. The Stat6 decoy ODN is a double-stranded phosphorothioate 28mer that exhibits a high sequence-specific binding affinity to the transcription factor Stat6. Sequences utilized were as follows: Stat6 decoy ODN, 5′-GAT CAA GAC CTT TTC CCA AGA AAT CTA T-3′ and 3′-CAT GTT CTG GAA AAG GGT TCT TTA GAT A-5′; scrambled decoy ODN, 5′-CGA AAA TTC GTT AAA TCA CTA GCT TAC C-3′ and 3′-GCT TTT AAG CAA TTT AGT GAT CGA ATG G-5′. Synthetic ODNs were dissolved in sterile TE buffer (10 mmol/L Tris, 1 mmol/L EDTA, pH 8.0), purified by high-performance liquid chromatography and quantitated by spectrophotometry (Operon Tec, Alameda, CA). Each pair of single-stranded ODN was annealed for 3 hours, during which time the temperature was reduced from 90°C to 25°C.
To create a cell culture, the TH2 cell line, D10, was maintained in RPMI-1640 medium containing 10% fetal calf serum (Sigma, St Louis, MO), 2 mmol/L L-glutamine and penicillin-streptomycin (50 IU/mL and 50 μg/mL, respectively), IL-1 (2 U/mL; PeproTech, Rock Hill, NJ), recombinant human IL-2 (25 U/mL; Hoffmann-LaRoche, Nutley, NJ), Concanavalin A (2 μg/mL; Sigma), 35 μmol/L β-ME and 6 mmol/L HEPES. The TH2 cell clone was a generous gift from Dr Minute Li-Weber. Fresh human T lymphocytes were obtained from normal donors and purified by isocentrifugation and activated for 72 hours with phytohemagglutinin (PHA) in RPMI-1640 medium containing 10% fetal calf serum, 2 mmol/L L-glutamine and penicillin-streptomycin (50 IU/mL and 50 μg/mL, respectively), and then made quiescent by washing and incubating for 24 hours in RPMI-1640 medium containing 1% fetal calf serum. Human CD4+ T cells were purified from the above quiescent T cells by the use of human T-cell CD4 subset column kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions before transfection. More than 95% of the cells were CD4+as assessed by fluorescence-activated cell sorter.
Cationic liposomes (Boehringer Mannheim, Indianapolis, IN) were used to transfect double-stranded decoy ODN into D10 cells. Liposome-ODN complexes were formed by mixing 30 μg of lipidin HEPES buffer, 20 mmol/L, pH 7.4, to a final volume of 100 μL while 5 μg ODN were diluted to a concentration of 0.1 μg/μL in HEPES buffer, 20 mmol/L, pH 7.4. The solution was gently mixed and incubatedat room temperature for 15 minutes to allow liposomes to form. D10 cells (1.5 × 106) in 5 to 6 mL OPTI-MEM (Life Technologies, Gaithersburg, MD) were mixed gently with liposome-ODN mixture or control liposome without ODN and then incubated for 6 hours at 37°C. The medium was then changed to fresh D10 cell–culture medium. We confirmed that thisquantity of DNA and cationic liposomes had no toxic effect on cell viability, which was judged to be more than 90% viable on the basis of trypan blue dye exclusion.
To assess ODN uptake, cells were transfected with double-stranded ODN tagged with TAMRA at their 3′ end, then at different time points fixed with an equal volume of 5% paraformaldehyde, neutralized by one-tenth volume of 1 mol/L Tris-HCl (pH 7.2), and centrifuged onto a glass slide by means of a cytospin apparatus.36 The plates were allowed to air dry. Dried plates were then stained with 2 μg/mL 4,6-diamidino-2-phenylindole (DAPI) and examinedwith the use of a laser scanning confocal microscope (Model 310, Carl Zeiss, Thornwood, NY). TAMRA (565 nm) and DAPI (UV 364 nm) images (red and blue, respectively) were prepared for each specimen. Blue and red images were superimposed onto the Nomarski image. Photographs were taken with the use of a Sony color video printer, UP5200 MD Mavigraph. Quantitative measurement of nuclear/cytoplasmic fluorescence was also performed.
After transfection with the Stat6 or scrambled decoy ODN, D10 cells (108 cells/mL) were solubilized in lysis buffer.38 Cell lysates were rotated end over end at 4°C for 60 minutes, and insoluble material was pelleted at 12 000g for 20 minutes. The supernatants were incubated with 5 μg/mL rabbit polyclonal α-Stat6 (R&D Systems) for 2 hours at 4°C. Antibodies were captured by incubating for 30 minutes with protein A-Sepharose beads (Pharmacia, Piscataway, NJ). Precipitated material was eluted by boiling in sodium dodecyl sulfate (SDS) sample buffer for 4 minutes and subjected to 7.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. All proteins were transferred to Immobilion-P (PVDF) membrane as previously described.38 Analysis was done by Western blotting with monoclonal antiphosphotyrosine or α-Stat6 antibodies that were diluted 1:1000 in blocking buffer as described.38
D10 cells were washed with the hypotonic buffer (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 0.5 mmol/L DTT), then lysed in the same buffer supplemented with 1% NP-40 and incubated for 20 minutes on ice. The nuclei-containing pellet was resuspended in equal volumes of low-salt buffer (10 mmol/L HEPES, 25% glycerol, 1.5 mmol/L MgCl2, 20 mmol/L KCl, 0.5 mmol/L DTT, 0.2 mmol/L EDTA) and high-salt buffer (low-salt buffer containing 800 mmol/L KCl). The nuclear extract was centrifuged at 4°C for 15 minutes. Supernatants were saved as nuclear protein extract and stored at −70°C. For the EMSA, end-labeled [32P]-Stat6 oligonucleotide probes corresponding to the Cε element sequence 5′-AGT CAA GAC CTT TTC CCA AGA AAT CTA TC-3′),39 Stat5 probes corresponding to the β-casein gene sequence 5′-AGATTTCTAGGAATTCAATCC-3′),40 and Stat1/3 probes corresponding to the m67 SIE gene sequence 5′-AGCTTGTCGACATTTCCCGTAAATCGTCGAG-3′)41 42were used to detect DNA binding activities of the above transcriptional factors. The probe was then incubated with 5 μg of nuclear extracted proteins in 15 μL of binding cocktail (50 mmol/L Tris-Cl, pH 7.4, 25 mmol/L MgCl2, 0.5 mmol/L DTT, 50% glycerol) at 4°C for 15 minutes. For supershift assay, the nuclear extracts were preincubated with 1 μg of either normal rabbit serum or antisera specific to Stat6 at 4°C for 30 minutes. The DNA-protein complexes were resolved on a 5% polyacrylamide gel. The dried gels were exposed to x-ray film.
An oligonucleotide consisting of 3 copies of IL-4 nuclear-activated factor Stat6 binding promoters of the murine Cε (−119 to −104)39 or Stat1/3 binding promoter of the SIE from c-fos gene in a direct repeat was synthesized with SacI and XhoI overhangs and ligated into pGL3 luciferase reporter vector (Promega, Madison, WI). The correct reporter construct sequence was confirmed by DNA sequencing. Plasmid DNA was prepared with the use of Wizard (Promega) maxipreps DNA purfication system vector.
Cationic liposomes were used to co-transfect the Stat6 or SIE reporter plasmid and decoy ODN into D10 cells with the use of 3 μg of plasmid with 20 μg of lipid according to the manufacter's instructions. After 6 hours of transfection, cells were resuspended and cultured in fresh medium for 24 hours. Cells were then stimulated in the presence or absence of IL-4 for another 24 hours at 37 °C. Cell extracts were prepared with the use of the reporter lysis buffer in theLuciferase Assay System (Promega) and centrifuged at 12 000g for 2 minutes at 4°C. Supernatants were transferred to new tubes. 20 μL of lysate was mixed with 100 μL luciferase assay reagent in cuvettes and measured by a luminometer (Monolight 3010; PharMingen, San Diego, CA) according to the manufacturer's instructions. To correct for variations in transfection efficiencies, the luciferase valueswere normalized against protein concentration.
D10 cells were transfected as described above with Stat6 or scrambled decoy ODN, or treated with 200 ng/mL of antimouse IL-4 or antimouse IL-3 (Biosource, Camarillo, CA). Total RNA was isolated from treated or control cells with the use of TRIzol (Life Technologies, Gaithersburg, MD). Cytokine RNA-message was examined by ribonuclease (Rnase) protection assay with the use of 20 μg of total RNA hybridized to 2 × 106 cpm of [33P]-labeled probe corresponding to mCK-1 or mCR-1 (PharMingen) overnight at 56°C. Unhybridized RNA was digested with RNase T1 and RNase A for 45 minutes at 30°C, then digested with proteinase K for 15 minutes at 37°C. After phenol/chloroform extraction and sodium acetate/ethanol precipitation, hybridized RNA probes were denatured at 90°C for 3 minutes and electrophoresed on a 5% polyacrylamide gel. The dried gels were exposed to x-ray film.
After transfection with the Stat6 or scrambled decoy ODN, D10 cells were grown to approximately 2.5 × 106 cell/mL and transferred to 25-mL flasks. Supernatants were collected after 24 hours and assayed for murine cytokines (IL-4, IL-5, IL-6, IL-10, IL-13) by means of enzyme-linked immunosorbent assay (ELISA) Endogen kits (Wolburn, MA), according to the manufacturer's instructions, by the Clinical Services Department, Frederick Cancer Research and Development Center, Frederick, MD.43
After transfection with the Stat6 decoy or scrambled ODN, quiescent cells (50 × 103/well) were plated in flat bottom 96-well microtiter plates in 200 μL of growth media (described above), supplemented with 5% fetal calf serum and stimulated with 1 nmol/L of IL-4, or IL-2, or media alone for 16 hours, then pulsed for the remaining 4 hours of the assay with [3H]-thymidine (0.5 μCi/200 μL) and harvested onto glass-fiber filters. [3H]-thymidine incorporation was analyzed by liquid scintillation counting as previously described.38
Results
In order to study the efficacy of transcription factors decoy on T-lymphocyte function, we chose the D10 cell line as a model system. This cell line represents the prototypic TH2 cell, which secretes and responds to IL-4.43 Using cationic liposome gene transfer, we first verified that double-stranded ODN tagged with TAMRA at the 3′ end could be efficiently introduced into D10 cells. TAMRA-labeled ODNs produce red fluorescence when excited at 565 nm, whereas DAPI, a nuclear fluorescence marker, produces blue fluorescence when UV excited. With the use of laser scanning confocal microscopy combined with DAPI staining, it was seen that transfection of TAMRA-labeled ODNs for 6 hours resulted in nearly 60% of the D10 cells exhibiting intense red cytoplasmic fluorescence, but a weak nuclear signal (Figure 1A). After IL-4 stimulation (Figure 1B), red fluorescence staining was increased in both the nuclei and the cytoplasm of cells. This effect was further confirmed by quantification of nuclear/cytoplasmic fluorescence intensity. The ratio of nuclear to cytoplasmic fluorescence in IL-4–stimulated cells (31.62 ± 2.96%) was compared with non–IL-4–stimulated cells (1.20 ± 0.18%) from 3 sample sets (n = 100). In the absence of cationic liposomes, D10 cells did not significantly take up TAMRA-labeled ODN (data not shown). These data suggest that double-stranded ODN can be successfully transfected into TH2 cells via cationic liposomes. Moreover, detectable levels of TAMRA-labeled ODN were elevated in nucleus following IL-4 stimulation.
TAMRA-labeled (ODN) uptake in D10 cells.
Phase-contrast (left panel) and fluorescence (right panel) confocal photomicrographs of D10 cells that were transfected with TAMRA-labeled double-stranded ODN (2 μmol/L) for 6 hours with the use of cationic liposomes. Cells were then stimulated without (A) or with (B) 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were fixed with 5% paraformaldehyde and co-loaded with DAPI and examined by laser scanning confocal microscope.
TAMRA-labeled (ODN) uptake in D10 cells.
Phase-contrast (left panel) and fluorescence (right panel) confocal photomicrographs of D10 cells that were transfected with TAMRA-labeled double-stranded ODN (2 μmol/L) for 6 hours with the use of cationic liposomes. Cells were then stimulated without (A) or with (B) 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were fixed with 5% paraformaldehyde and co-loaded with DAPI and examined by laser scanning confocal microscope.
Stat6 decoy markedly reduces mRNA expression of IL-4 but not other cytokines in D10 cells. Because Stat6 transactivation is necessary for driving IL-4 transcription in TH2 cells, we next investigated whether the Stat6 decoy could block IL-4 mRNA expression. For this experiment, total RNA was isolated from cells treated with the corresponding Stat6 decoy for 24 hours and probed for cytokine message with the use of RPA (Figure2). The IL-4 transcript was found to be greatly reduced in Stat6 decoy–treated cells (lane c) as compared with control cells, which were IL-4–stimulated (lane b), unstimulated (lane a), or scrambled decoy-ODN–treated (lane d). In contrast, pretreatment of D10 cells with antibodies to the Stat6–activating cytokine IL-4 (lane f) displayed no significant inhibitory or stimulatory effects as compared with anti–IL-3 antibody (lane e) or nontreated control (lane g) samples. Densitometric analysis of IL-4 mRNA message was standardized against L32/GAPDH messages. The IL-4 transcript reduced by the Stat6 decoy was greater than 90% as compared with controls, as measured by densitometric analysis. This evidence suggests that Stat6 decoy can specifically block transcriptional regulation of IL-4, since no detectable loss in mRNA expression of IL-5, IL-6, IL-9, IL-10, IL-13, or IFNγ was observed.
Stat6 decoy inhibits mRNA expression of IL-4 but not other cytokines.
RNA for RPA analysis was obtained from untreated control samples (lanes a, b, g), Stat6 decoy (lane c), scrambled (lane d) ODN-transfected D10 cells, or cells pretreated with anti–IL-3 or anti–IL-4 antibodies (lanes e and f, respectively). Cells were stimulated with IL-4 (lanes b through d). RNA was isolated and hybridized with [33P]-labeled RNA probes corresponding to transcripts for murine cytokines (mCK1). The RNase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 90% reduction in Stat6 decoy–treated samples.
Stat6 decoy inhibits mRNA expression of IL-4 but not other cytokines.
RNA for RPA analysis was obtained from untreated control samples (lanes a, b, g), Stat6 decoy (lane c), scrambled (lane d) ODN-transfected D10 cells, or cells pretreated with anti–IL-3 or anti–IL-4 antibodies (lanes e and f, respectively). Cells were stimulated with IL-4 (lanes b through d). RNA was isolated and hybridized with [33P]-labeled RNA probes corresponding to transcripts for murine cytokines (mCK1). The RNase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 90% reduction in Stat6 decoy–treated samples.
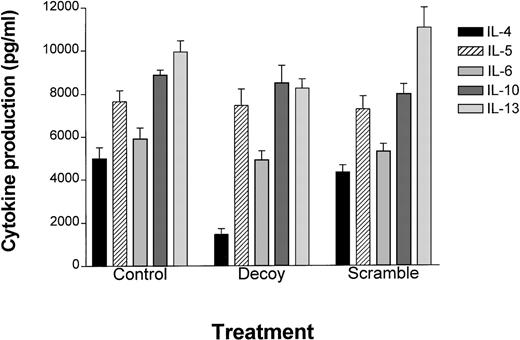
Stat6 decoy decreases IL-4 protein production in TH2 cells. To determine if the Stat6 decoy could inhibit TH2 cell function, ELISA was used to measure the production of TH2 cytokines in D10 cells after transfection with the Stat6 or scrambled decoy ODN. Following transfection, D10 cells were cultured in fresh medium for 24 hours, and supernatants were collected and assayed for TH2 cytokines (Figure3). IL-4 production was dramatically reduced (approximately 70%), while little effect was observed on the remaining TH2 cytokines (ie, IL-5, IL-6, IL-10, IL-13). These data indicated that the Stat6 decoy selectively blocks IL-4 production over other TH2 cytokines.
Stat6 decoy treatment of TH2 clone D10 cells preferentially inhibits IL-4 production.
D10 cells were transfected with Stat6 decoy or scrambled ODN at 37°C for 6 hours, then resuspended and cultured in normal D10 medium for 24 hours. ELISA was performed for each cytokine indicated (n = 3) and plotted as the mean ± the standard error (abscissa) based on ELISA values (pg/mL) (ordinate). Actual values obtained were as follows: For control cells, IL-4 (4980 ± 518), IL-5 (7646 ± 511), IL-6 (5904 ± 518), IL-10 (8860 ± 240), and IL-13, (9926 ± 516). For Stat6 decoy ODN–transfected D10 cells, IL-4 (1461 ± 264), IL-5 (7448 ± 760), IL-6 (4906 ± 423), IL-10 (8479 ± 808), and IL-13 (8233 ± 431). For scrambled decoy ODN–transfected D10 cells, IL-4 (4308 ± 339), IL-5 (7260 ± 609), IL-6 (5276 ± 366), IL-10 (7972 ± 448), and IL-13 (10 996 ± 947).
Stat6 decoy treatment of TH2 clone D10 cells preferentially inhibits IL-4 production.
D10 cells were transfected with Stat6 decoy or scrambled ODN at 37°C for 6 hours, then resuspended and cultured in normal D10 medium for 24 hours. ELISA was performed for each cytokine indicated (n = 3) and plotted as the mean ± the standard error (abscissa) based on ELISA values (pg/mL) (ordinate). Actual values obtained were as follows: For control cells, IL-4 (4980 ± 518), IL-5 (7646 ± 511), IL-6 (5904 ± 518), IL-10 (8860 ± 240), and IL-13, (9926 ± 516). For Stat6 decoy ODN–transfected D10 cells, IL-4 (1461 ± 264), IL-5 (7448 ± 760), IL-6 (4906 ± 423), IL-10 (8479 ± 808), and IL-13 (8233 ± 431). For scrambled decoy ODN–transfected D10 cells, IL-4 (4308 ± 339), IL-5 (7260 ± 609), IL-6 (5276 ± 366), IL-10 (7972 ± 448), and IL-13 (10 996 ± 947).
Stat6 decoy inhibits IL-4–mediated proliferation of D10 cells and primary human CD4+ T cells. Since IL-4 plays a key role in the differentiation and autocrine expansion of TH2 cells, we asked whether the Stat6 decoy could block IL-4–mediated TH2 cell growth. For this assay, Stat6 or scrambled decoy ODN was used to transfect D10 cells. Cells were then cultured in growth medium with or without 1 nmol/L IL-4. As shown in Figure4 (upper panel), the Stat6 decoy markedly suppressed IL-4–inducible [3H]-thymidine incorporation (61%) compared with the unstimulated control cells, whereas scrambled ODN alone did not affect cell proliferation. By contrast, the Stat6 decoy did not block IL-2–inducible cell proliferation of D10 cells (Figure 4, middle panel). Interestingly, the same inhibitory effect of the Stat6 decoy was also observed on IL-4–inducible proliferation in PHA-activated primary human CD4+ T cells (Figure 4, lower panel). The D10 and human T cells used for the proliferation assay were judged to be more than 90% viable on the basis of trypan blue dye exclusion (data not shown). These findings suggest the Stat6 decoy effectively inhibits IL-4 growth–promoting signals in TH2 cells lines and primary human CD4+ T cells.
Stat6 decoy inhibits IL-4–inducible cell proliferation on a TH2 cell line and human CD4+ T cells.
Quiescent D10 cells (upper and middle panel) or human CD4+T cells (lower panel) (50 × 103/well) were transfected with Stat6 decoy or scrambled decoy ODN for 6 hours and cultured in 200 μL of growth media in the presence of IL-4 (▪, upper and lower panel) or IL-2 (▪, middle panel) or their absence (▨) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi/200 μL) for an additional 4 hours and incorporation of radiolabeled probe plotted on the abscissa (expressed as total cpm for 6 samples).
Stat6 decoy inhibits IL-4–inducible cell proliferation on a TH2 cell line and human CD4+ T cells.
Quiescent D10 cells (upper and middle panel) or human CD4+T cells (lower panel) (50 × 103/well) were transfected with Stat6 decoy or scrambled decoy ODN for 6 hours and cultured in 200 μL of growth media in the presence of IL-4 (▪, upper and lower panel) or IL-2 (▪, middle panel) or their absence (▨) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi/200 μL) for an additional 4 hours and incorporation of radiolabeled probe plotted on the abscissa (expressed as total cpm for 6 samples).
Treatment of D10 cells with Stat6 decoy does not alter mRNA expression of IL-4 receptor chains. To investigate whether the Stat6 decoy mediated loss of IL-4–regulated cell proliferate owing to reduced expression of IL-4 receptor chains, we performed RNase protection assays. For this analysis, total mRNA was isolated from control, Stat6 decoy–treated, or scrambled ODN–treated cells (as described in “Materials and methods”) and hybridized against [33P]-labeled receptor probes. RNase-protected samples were then electrophoretically separated by PAGE, dried, and autoradiographically shown (Figure 5). The IL-4–receptor mRNA message from non–IL-4–stimulated control cells (lanes a, e through g) and IL-4–stimulated (lanes b through d), Stat6 decoy (lane c), and scrambled ODN (lane d) failed to show a loss in cytokine receptor expression. Similarly, pretreatment of D10 cells with anti–IL-4 antibody (lane f) or anti–IL-3 antibody (lane e) and untreated control samples also did not display a significant change in expression of IL-4 receptor α or γc chains compared with the control housekeeping genes, L32/GAPDH. From this data we conclude that the loss of IL-4–mediated cell growth and IL-4 message following Stat6 decoy treatment is not due to a significant reduction in IL-4Rα or γc expression but likely occurs at a site distal to the receptor.
Stat6 decoy does not alter mRNA expression of IL-4 receptors.
Freshly isolated mRNA was obtained from control (lanes a, b, g), Stat6 decoy (lane c), scramble ODN-transfected D10 cells (lane d), or anti–IL-4 antibody treated (lane f) or anti–IL-3 antibody treated (lane e) cells in the absence (lanes a, e, f) or presence of IL-4 (lanes b through d). RNA was then hybridized with [33P]-labeled RNA probes corresponding to transcripts for individual murine γc-receptors (mCR-1) according to PharMingen protocol (see “Materials and methods”). The Rnase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray film.
Stat6 decoy does not alter mRNA expression of IL-4 receptors.
Freshly isolated mRNA was obtained from control (lanes a, b, g), Stat6 decoy (lane c), scramble ODN-transfected D10 cells (lane d), or anti–IL-4 antibody treated (lane f) or anti–IL-3 antibody treated (lane e) cells in the absence (lanes a, e, f) or presence of IL-4 (lanes b through d). RNA was then hybridized with [33P]-labeled RNA probes corresponding to transcripts for individual murine γc-receptors (mCR-1) according to PharMingen protocol (see “Materials and methods”). The Rnase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray film.
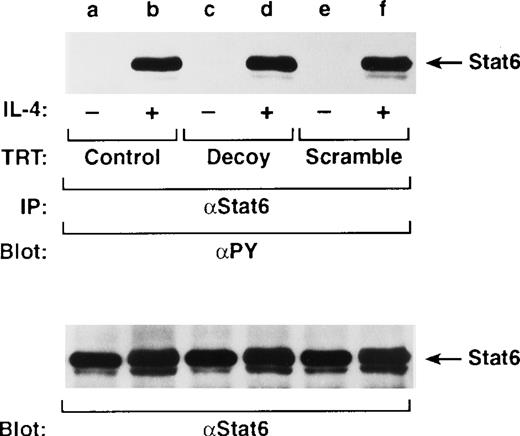
Stat6 decoy does not affect IL-4–dependent Stat6 tyrosine phosphorylation in vivo. It is well established from Stat6 knockout mice studies that this transcription factor provides a key role in T-cell development, IL-4 responsiveness, and TH2 differentiation.19 44 To clarify whether the Stat6 decoy affects IL-4–dependent Stat6 tyrosine phosphorylation, cells were transiently transfected with decoy and stimulated with or without IL-4 for 10 minutes and assayed by Western analysis for tyrosine phosphorylated Stat6. Tyrosine-phosphorylated Stat6 was observed in exogenous IL-4–stimulated cells (Figure 6, lanes b, d, f), but not in lysates from unstimulated cells transfected with Stat6 (lane d) or scrambled decoy ODN (lane f). Immunoblotting of Stat6 (indicated beneath phosphorylation blots) verified equivalent loading and no loss of protein expression. These data suggest that the Stat6 decoy does not affect IL-4–dependent Stat6 tyrosine phosphorylation and directly competes for its ability to bind DNA.
Stat6 decoy does not affect Stat6 tyrosine phosphorylation.
D10 cells were treated with Stat6 decoy (lanes c, d) or scrambled ODN (lanes e, f) for 6 hours and then stimulated with or without 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were lysed, immunoprecipitated with anti-Stat6 (α-Stat6), and then Western blotted with αPY (upper panel) or anti-Stat6 (lower panel) to verify equivalent loading. Arrows indicate location of Stat6.
Stat6 decoy does not affect Stat6 tyrosine phosphorylation.
D10 cells were treated with Stat6 decoy (lanes c, d) or scrambled ODN (lanes e, f) for 6 hours and then stimulated with or without 100 nmol/L IL-4 at 37°C for 10 minutes. Cells were lysed, immunoprecipitated with anti-Stat6 (α-Stat6), and then Western blotted with αPY (upper panel) or anti-Stat6 (lower panel) to verify equivalent loading. Arrows indicate location of Stat6.
Stat6 decoy specifically inhibits IL-4–induced Stat6 DNA binding. To provide evidence that the Stat6 decoy ODN prevented the binding of endogenous Stat6 to its target sites, we performed an electrophoretic mobility-shift assay in the presence of the Stat6 decoy or the scrambled control ODN, both in vitro and in vivo. First, we examined whether the Stat6 decoy can compete for binding the sequence-specific DNA binding proteins. As shown in Figure7A, IL-4–induced Stat6 DNA binding was abolished by preincubating nuclear extracts with an excess of unlabeled Cε oligonucleotide probe and Stat6 decoy ODN (lanes e, c). However, no effect was observed when this was performed with the unlabeled scrambled ODN (lane d). By contrast, the Stat6 decoy failed to interfere with IL-2–induced Stat1, Stat3, and Stat5 DNA binding activities (Figure 7B, 7C, lane d).
Binding site specificity of Stat6 decoy.
(A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.
Binding site specificity of Stat6 decoy.
(A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.
We next determined the ability of the Stat6 decoy to penetrate cells and compete with the native Stat6 transcription activity in vivo. Nuclear extracts prepared from D10 cells transfected with either Stat6 decoy ODN or scrambled decoy ODN or prepared from control cells were tested for their ability to bind a radiolabeled Cε oligonucleotide probe (Figure 8). Control extracts readily displayed IL-4–modulated Stat6 DNA binding (lanes b through d); however, equivalent protein from nuclear extracts of Stat6 decoy ODN–treated cells showed greatly diminished DNA binding capacity (lanes e, f). Control scrambled decoy ODN had no effect (lanes g, h). To confirm that the identity of Stat6 DNA binding activity was IL-4 dependent, complexes were incubated with anti-Stat6 antibody or normal rabbit serum. Only the anti-Stat6 antibody (lane c) could supershift the IL-4–inducible protein-DNA complex, unlike normal rabbit serum (lane d), thus verifying the identify of the radiolabeled band. These findings suggest that the Stat6 decoy is a highly specific competitor for activated Stat6.
Stat6 decoy specifically blocked Stat6 DNA binding activity.
Control (lanes a, b, c, d) and Stat6 decoy ODN–treated (lanes e, f) or scrambled decoy ODN–treated (lanes g, h) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were obtained, and 5 μg of protein were incubated in the absence of antibody (lanes a, b, e through h), α-Stat6 (lanes c), or normal rabbit serum (nrs; lane d) and then with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Arrows indicate migrational location of each nonsupershifted Stat6-DNA complex or free probe.
Stat6 decoy specifically blocked Stat6 DNA binding activity.
Control (lanes a, b, c, d) and Stat6 decoy ODN–treated (lanes e, f) or scrambled decoy ODN–treated (lanes g, h) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were obtained, and 5 μg of protein were incubated in the absence of antibody (lanes a, b, e through h), α-Stat6 (lanes c), or normal rabbit serum (nrs; lane d) and then with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Arrows indicate migrational location of each nonsupershifted Stat6-DNA complex or free probe.
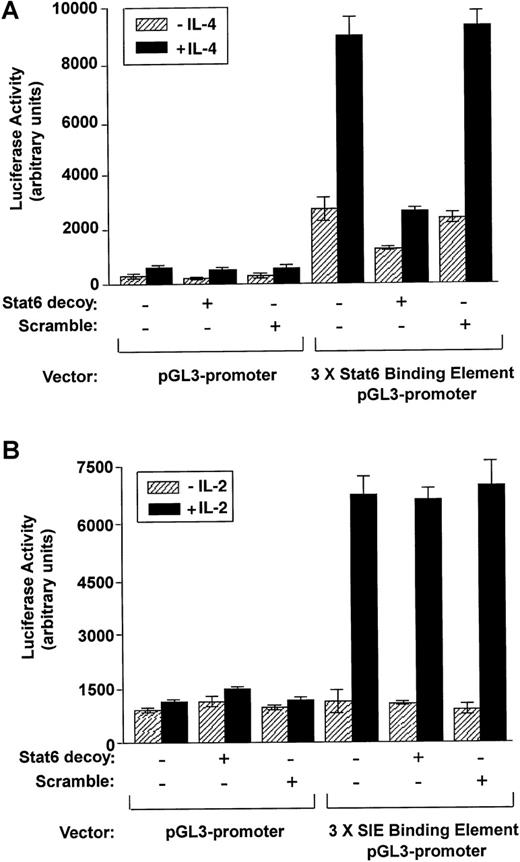
Stat6 decoy inhibits IL-4–stimulated Stat6 transactivation. We used the Stat6–luciferase reporter gene construct to quantitatively assess the effect of the Stat6 decoy on IL-4–stimulated transcriptionalactivation. As shown in Figure 9(upper panel), Stat6 luciferase activity of IL-4–stimulated cells was substantially reduced in Stat6 decoy–treated samples as compared with the control and the scrambled ODN–treated cells. Interestingly, basal levels of luciferase activity were higher for cells transfected with the Stat6 binding element construct than for cells transfected with control plasmid, but was attenuated in the presence of the Stat6 decoy. Specificity of the decoy was further assessed by measuring IL-2–inducible transactivation of Stat1/3 to an SIE reporter. IL-2 activation of the SIE-reporter construct was not blocked by the decoy (Figure 9, lower panel). These observations suggest that the ODN decoy can specifically inhibit constitutive Stat6 promoter transactivation potential and subsequent production and response to IL-4.
Effects of Stat6 decoy ODN on transactivation of Stat6 compared with Stat1/3 in cultured D10 cells.
(A) D10 cells treated with Stat6 decoy ODN or scrambled decoy ODN were co-transfected with a 3 × Stat6 binding element pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. After transfection, cells were stimulated with or without IL-4 (100 nmol/L). Cells were harvested after an additional 24 hours, and luciferase activity was measured and normalized against protein concentration. (B) D10 cells treated with Stat6 decoy ODN, or scrambled decoy ODN were co-transfected with a 3 × SIE binding element pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. After transfection, cells were stimulated with or without IL-2 (100 nmol/L). Cells were harvested after an additional 24 hours, and luciferase activity was measured and normalized against protein concentration.
Effects of Stat6 decoy ODN on transactivation of Stat6 compared with Stat1/3 in cultured D10 cells.
(A) D10 cells treated with Stat6 decoy ODN or scrambled decoy ODN were co-transfected with a 3 × Stat6 binding element pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. After transfection, cells were stimulated with or without IL-4 (100 nmol/L). Cells were harvested after an additional 24 hours, and luciferase activity was measured and normalized against protein concentration. (B) D10 cells treated with Stat6 decoy ODN, or scrambled decoy ODN were co-transfected with a 3 × SIE binding element pGL3 promoter–luciferase construct or with pGL3 promoter–luciferase construct alone. After transfection, cells were stimulated with or without IL-2 (100 nmol/L). Cells were harvested after an additional 24 hours, and luciferase activity was measured and normalized against protein concentration.
Discussion
IL-4 is a pleiotropic cytokine that plays a prominent role in driving inflammatory and cell-mediated responses in numerous types of immune cells.44-48 IL-4 is thehallmark cytokine produced by TH2 cells49 and is critical for TH cell differentiation. While Stat6 plays an essential role in TH2 differentiation, its role in IL-4–mediated transcription remains unclear.12 50 We propose that IL-4 is a target gene for the Stat6 decoy, which in turn ultimately blocks TH2-cell activity. The results of the present study clearly demonstrated that TH2 cells transfected with the Stat6 decoy ODN showed significantly lower Stat6 transcriptional activity and significantly lower production of IL-4 than TH2 cells transfected with the scrambled ODN. Thus, blocking Stat6 may be useful for inhibiting IL-4–derived TH2 cell activity and proliferation.
The Stat6 decoy is a double-stranded phosphorothioate 28mer ODN. As emphasized by Bielinska et al,24,51-53 oligonucleotides with modified phosphodiester bonds, such as phosphorothioate, methyl phosphate, phosphoramidite, or methyl phosphonate derivatives, are relatively resistant to nucleases. Recently, Park et al54 reported that phosphorothioate ODNs can be stable up to 48 hours in cell systems, on the basis of evidence that a 24mer decoy phosphorothioate ODN accumulated in cells at a size consistent with the duplex/hairpin forms. On the basis of these findings, we hypothesized that the Stat6 decoy could be used to transfect cells. To determine the site of action for the decoy, we employed an ODN tagged with fluorescent group TAMRA that could be efficiently introduced and monitored in D10 cells. Using a cationic liposome delivery and laser scanning confocal microscopy, we combined TAMRA (red) with DAPI (blue) and found that the Stat6 decoy ODN could be introduced into TH2 cells without causing cellular toxicity. As shown in Figure 1A, under these transfection conditions, resting levels of TAMRA-labeled ODN were significantly higher in the cytoplasm than in the nucleus. However, following addition of IL-4, we observed a dramatic increase in the ratio of nuclear to cytoplasmic fluorescence intensity, from 1:100 to 1:3 (Figure 1B). These findings suggest that Stat6 dimer may bind the decoy and be translocated into the nucleus as a complex. Another possibility is that the increase in fluorescence intensity following IL-4 stimulation could be due to increased stability of the decoy since dimers of activated Stat6 would be expected to bind and shield the Stat6 ODN core sequence from nucleases. Indeed, using crystallographic evidence, Chen et al55 recently demonstrated that a complex of Stat1 homodimers form a C-shaped complex that surrounds a 15-bp region of the DNA. From either scenario, we conclude that the decoy binds Stat6 with sufficient affinity to compete for IL-4–induced Stat6 DNA binding and disrupt Stat6 activity.
We next investigated the molecular mechanism by which transcription factor Stat6 decoy ODN inhibited the gene expression of cytokine IL-4, resulting in the suppression of TH2 cells. Stat6 is recognized to perform a key role in mitogenic and pleiotropic functional response induced by cytokines such as IL-4.19,44Selective activation of Stat6 through phosphorylation and dimerization results in its translocation to the nucleus, where it activates gene transcription.56 As shown in Figure 5, the Stat6 decoy failed to inhibit IL-4 receptor α-chain expression. While a Stat6 binding element resides in the promoter of this receptor, a role for Stat6 in maintaining or promoting IL-4 receptor expression in committed TH2 cell lines is not readily known. Indeed, convincing results have failed to demonstrate a direct role for the IL-4 receptor pathway in IL-4 gene expression in fully committed TH2 cells.57-59 Using neutralizing anti–IL-4 antibodies, we failed to observe a significant reduction in IL-4 and IL-4 receptor expression (Figures 2 and 5). This suggests that additional effector proteins and/or transcriptional elements activated by IL-4, other than Stat6, are required to maintain IL-4 receptor α-chain expression in committed TH2 cells. On the other hand, Stat6 tyrosine phosphorylation following IL-4 stimulation was not markedly affected by the Stat6 decoy, suggesting that the decoy does not block IL-4 receptor activation of Stat6 and that activated Stat6 is sequestered by the decoy. These results tend to support the imaging results observed from laser scanning confocal microscope in that the Stat6 decoy does not block Stat6 translocation to the nucleus but does compete for its ability to regulate gene transcription such as IL-4 (Figures 2 and 3) or proliferative growth signals (Figure 4).
In order to characterize the specificity of Stat6 decoy on Stat6 DNA binding and transactivation, we employed gel shift analysis and reporter gene assay. First, IL-4–inducible Stat6 DNA binding was abolished by Stat6 decoy ODNs. The binding-site specificity of Stat6 decoy was confirmed since a 100-fold excess of unlabeled double-stranded Stat6 ODN competed away the Stat6 complex, while similar concentration of scrambled ODN did not (Figure 7A). Also, we demonstrate that the decoy did not interfere with IL-2–induced Stat1/3 DNA binding to the SIE-probe (Figure 7C) or with Stat5a/b DNA binding to β-casein probe (Figure 7B). Second, the highly specific effects of the Stat6 decoy on transactivation were found by reporter gene assay. Stat6-luciferase activity was significantly reduced (fivefold) in D10 cells co-transfected with the Stat6 decoy ODN as compared with Stat6-luciferase activity in the scrambled ODN–co-transfected cells (Figure 9), whereas no statistical loss in the transcriptional activation of Stat1/3 (Figures 7 and 9) was detectable. Moreover, specificity of the decoy was supported by its functional inability to affect expression of other TH2 cytokines (Figures 2 and 3) or IL-2–inducible TH2-cell growth (Figure 4B). Therefore, we believe that transfecting a Stat6 decoy corresponding to the cis-sequence may specifically result in the attenuation of an authentic cis/trans interaction, leading to the removal of trans-factors from the endogenous cis-element. This event subsequently inhibits IL-4 gene expression.
While transcription factors play a critical role in the development of mature cell function, functional characterization of these proteins in fully differentiated mammalian cells is not possible if it is based entirely on gene knockout studies. Transcription factor decoys provide a novel strategy for disrupting gene expression and have several substantive advantages over the use of knockout animals. This strategy is a rapid, specific, and cost-effective means to analyze the function of a specific transcription factor in the fully differentiated cells, which suggests that it has therapeutic applications in treating human disease. Sullenger et al31 reported that overexpression of the sequences containing HIV trans-activation response element (TAR decoy) rendered CD4+ human T-lymphoid cells resistant to human immunodeficiency virus replication. Using a similar approach, we have successfully introduced a transcription factor decoy that blocked IL-4–inducible proliferation of PHA-activated primary human CD4+ T cells (Figure 4).
In conclusion, we have identified a cis-element decoy that potently blocks Stat6 signaling pathway in murine and human T cells. Our results indicate that Stat6 decoy ODNs can bind Stat6 with sufficient affinity and scavenge activated Stat6 dimers and inhibit their ability to recognize native DNA binding sites, which can ultimately suppress TH2 cell activity. This approach represents a rapid and specific strategy to investigate transcription factor functions and their roles in gene expression and T cell biology.
Acknowledgments
We thank Dr Minute Li-Weber for generously providing the TH2 cell line, D10. We also acknowledge Dr Joost Oppenheim for critical review of the manuscript.
Funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000 and sponsored in part by the National Cancer Institute, US Department of Health and Human Services, under contract with ABL.
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Reprints:William L. Farrar, National Cancer Institute, PO Box B, Bldg 560, Room 31-68, Frederick, MD 21702; e-mail:farrar@mail.ncifcrf.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. Stat6 decoy inhibits mRNA expression of IL-4 but not other cytokines. / RNA for RPA analysis was obtained from untreated control samples (lanes a, b, g), Stat6 decoy (lane c), scrambled (lane d) ODN-transfected D10 cells, or cells pretreated with anti–IL-3 or anti–IL-4 antibodies (lanes e and f, respectively). Cells were stimulated with IL-4 (lanes b through d). RNA was isolated and hybridized with [33P]-labeled RNA probes corresponding to transcripts for murine cytokines (mCK1). The RNase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 90% reduction in Stat6 decoy–treated samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439002w.jpeg?Expires=1769097871&Signature=MfqLxnQPZ0Lt6xFO4k4CeRZn8Nheli8ugVrDRGP0dYR6cyd5lun7jrFPnu6vqNLXr9xx6BMqTFVRzEELVzFV5IZXDsExtX-kFWeWJjc7EhyGx3Y4imFQE1LcdXoF7~EQbu-PTN11HfJpLVBXM4PMPZ3pwiaG62MGOB1G~Eepvh5TmPlkxOQJwT1BceiwrihNL3eGCtoq2YS~RzOubQuJcJlNFmBE6waGf5iTjAXF3NwKeLRIX-K5mh~cS1n-3tIMOJq~BoXO9Q7~Vr4hb3Njb020sdpXYDi03t4Bb~KhyDjwNKOAEY87ba3ldQCDEDGs3-2j85lVXXgtu6A2DH6MwQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Stat6 decoy inhibits IL-4–inducible cell proliferation on a TH2 cell line and human CD4+ T cells. / Quiescent D10 cells (upper and middle panel) or human CD4+T cells (lower panel) (50 × 103/well) were transfected with Stat6 decoy or scrambled decoy ODN for 6 hours and cultured in 200 μL of growth media in the presence of IL-4 (▪, upper and lower panel) or IL-2 (▪, middle panel) or their absence (▨) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi/200 μL) for an additional 4 hours and incorporation of radiolabeled probe plotted on the abscissa (expressed as total cpm for 6 samples).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439004x.jpeg?Expires=1769097871&Signature=Q71TGeShwhWHnQPj6KUEsCOxdwMY~x88iWJD363ETMXsqYl1LfhMWsQEKvmn9dJ0iVHek2wFCg7DjXxXmTrxYyyGw57ajjkJ0Jul7rd6ouWqG4cUoKSF9ttTNxTrX-8x4D4arTCwO9bHXxXaJmuq05qNh1nXSvghcUHftJCSBOMUbbtoYazAUnCW5HYmZkdTK0k999ZoSaLo58yHVWLg6Bui5MWoaYqLxSZWe7EDg6R-2L14gfAqCN4bUHTnQ-KCSOv5p~sch3kA78SNuuGTfknrUolxKBiER59cWMN9PW6-2ep1~0-xEh0FEducsuUDdiGB2hebQA042NvOdrCaYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Stat6 decoy does not alter mRNA expression of IL-4 receptors. / Freshly isolated mRNA was obtained from control (lanes a, b, g), Stat6 decoy (lane c), scramble ODN-transfected D10 cells (lane d), or anti–IL-4 antibody treated (lane f) or anti–IL-3 antibody treated (lane e) cells in the absence (lanes a, e, f) or presence of IL-4 (lanes b through d). RNA was then hybridized with [33P]-labeled RNA probes corresponding to transcripts for individual murine γc-receptors (mCR-1) according to PharMingen protocol (see “Materials and methods”). The Rnase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray film.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439005w.jpeg?Expires=1769097871&Signature=l5O2PL79SwZc5VP-aAthYdbqUMbdbbaqV8m6hSQJZJ0kWE3ZrURfRqPlCr9TF2-gQYajNsy2YcxGgo3P3s5Kil2RS9LACd4RiCmgxhrk~kNmchKgvh8nhrkY2M5TbX389aPur03sPv3hjVgwx4mkV9mLhIoDpUbuM7KBYDaj-GLujtuiPGNjn-q8YZV43y0TmJSDYhRoVKxcy3Mn8Et~MG31cxrki~DZS0gdqkiWmXFB0DGg6QBOTbEWIJsvHXo10GA8EPekc5GPdIuhIngeM1DW~bL0YIdw6hXdDsh0~I11liX~jHdN03FE1l61ecVtlWHQXUJj~ynLxjH9VRs5MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Binding site specificity of Stat6 decoy. / (A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439007aw.jpeg?Expires=1769097871&Signature=EczBb-tl2Hw32CgJVyC~Ak6A4K~eb1INXgipnYth2~PDBu~EeMhurCOy3nJefLPZhvm571HAMKXi5zSr5tqSRG3xNFbitVzjgaOYoanD6ORbXLvj6XuigYFB8jofUo3apW7-TQMPlJorlbmirwZeJ9xIhKh8QnQGT6Cd609p3XZ-WLC9em4Nhr-tsUzGWZvZrujYZo1mfvYy-kZ1r0ifDT-vM7w5yDfPwohhAJ8TQdMRTu04eWUwhpd-H4mB9rP4MJRDi0AfRe2WLlCYRjF~dAvlyoyEeESnAJirAgOIthGr0zdCharSfi-6fjI74hDmBODuku3gzhP8JBXZP--z-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Binding site specificity of Stat6 decoy. / (A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439007bw.jpeg?Expires=1769097871&Signature=DAmlYaayEzmuhWwUx5h9AUcQ368kIvZntBCxE8WuabUiToVf36Iz1iFeqzCDVMx5qEmpsD1xwWHpbzrop0cAATV2paJgR7gZtHhh1~-bbSk53JpwokG9dcb~sMXFX8x2ijfWbpDtjpo9m3MtTYLF1OM82lX8T6zWHj2MIF6jpDu7RVgrSFAEc0HwMPSWtIN2UY6vzXtkcMhknmvnfhgIOcGFScnlrjbSxZHs6NmUWJnAU2fjYwSq2zm9dGGQj8TfgWafZI1uqbCj6LWx4um6j~95t~MVV6JLlCQ609KV-cAWQyn4ZTdE6z-unOyaKBY-Bf5KJxXA7rDOPyyRQOiL4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Stat6 decoy specifically blocked Stat6 DNA binding activity. / Control (lanes a, b, c, d) and Stat6 decoy ODN–treated (lanes e, f) or scrambled decoy ODN–treated (lanes g, h) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were obtained, and 5 μg of protein were incubated in the absence of antibody (lanes a, b, e through h), α-Stat6 (lanes c), or normal rabbit serum (nrs; lane d) and then with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Arrows indicate migrational location of each nonsupershifted Stat6-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439008w.jpeg?Expires=1769097871&Signature=ICc4Htnc9QHIiK4NX-QqK1shSBN-SA5Dlyd5j5bZq2dpZj80aScknnUVW4igbSDYp9IB6WiQY1T0iryujcK2YmFNKbnJAqWjoDD64Jh-nZt3yIxEBiEBzCEUENzbCbPkuOaNcKk1xYYDQlKcgUgsCnfTfIpYW5E6ViQ56dPhZtQOVE33p8JpHGetFL7rZxm5hvS5dZxvyJ~zP9zSAAUsdSaN8AemcIOCvFLj0AcNP6GB0g5aMuZ5VEsE41OBObyxCbl4gercjfV8SAtMLl8So0bG4kUKWTGnukcpoiDZ9Kk7EEF66nbzrdghX9aTrQRFLYEGUVH8JBLPadUaK~rOoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 2. Stat6 decoy inhibits mRNA expression of IL-4 but not other cytokines. / RNA for RPA analysis was obtained from untreated control samples (lanes a, b, g), Stat6 decoy (lane c), scrambled (lane d) ODN-transfected D10 cells, or cells pretreated with anti–IL-3 or anti–IL-4 antibodies (lanes e and f, respectively). Cells were stimulated with IL-4 (lanes b through d). RNA was isolated and hybridized with [33P]-labeled RNA probes corresponding to transcripts for murine cytokines (mCK1). The RNase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray. Densitometric analysis of IL-4 RNA message compared with L32/GAPDH indicated a greater than 90% reduction in Stat6 decoy–treated samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439002w.jpeg?Expires=1769125767&Signature=UpZsHKmZCWqQaskVgzsyQH0RQeUfun-26~6WHn4IfPiUlOUxLtmNEP-VKPi4pK01i8DDVotvTFJwFbIKbEOlTZO2Y79NeZ7qaGuAeKAgV~lvFOI5MiGkZmzyBDs~MppytvIPfVrbxbaLBW8WFVSM01AuQAvdC9bHV66EuJnL5HnrzLwWv9spObmc2zBRTS5R4oPgYjZKq3-k73C0gqJDOu6WRhx-3Vb58W8b6jR-DbRfjb1jrBpx3GpMBMHCLAWUZ3AzzfOqTy7IoRBt2t4baGfN~ELYHiwkF5x2IGCpe7tI7bbAM5Ezjzw-PEDdrtcOr8QLj3bT0qIhbbRwd~v7tQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Stat6 decoy inhibits IL-4–inducible cell proliferation on a TH2 cell line and human CD4+ T cells. / Quiescent D10 cells (upper and middle panel) or human CD4+T cells (lower panel) (50 × 103/well) were transfected with Stat6 decoy or scrambled decoy ODN for 6 hours and cultured in 200 μL of growth media in the presence of IL-4 (▪, upper and lower panel) or IL-2 (▪, middle panel) or their absence (▨) for 16 hours at 37°C. Cells were then pulsed with [3H]-thymidine (0.5 μCi/200 μL) for an additional 4 hours and incorporation of radiolabeled probe plotted on the abscissa (expressed as total cpm for 6 samples).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439004x.jpeg?Expires=1769125767&Signature=xq8hZkYFuhOvY5pTpnPYbC8sTv0MTa8Bd7cdfW0eDsIe1Nvd4~1h4MElCRoBDi-HsVK-DbnSDrpF7eZaNlJI4UeWgxIbr-0hsypZzGZv8EpenpSf6ABOdURYWJy4Lu58EznBUkukidioSewmlBgVwoRhO0a05B0xYV~QYAHg9d8rm0bMcWsfobS6AqF7tR4vWTXzuZuuZkQ1xfBF~wNEPDwtYVpO-qHstmn~8lLyhRlNmFjlFIlBeWWj4YpEivzzMyRSHXVrEC6~T0ygUlSEWJiesW~-PpEwGI8M7FBEFla8LdMYbcs7Z1bllb0-vfU925pBDrNFOfcyyCKVY0OJEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Stat6 decoy does not alter mRNA expression of IL-4 receptors. / Freshly isolated mRNA was obtained from control (lanes a, b, g), Stat6 decoy (lane c), scramble ODN-transfected D10 cells (lane d), or anti–IL-4 antibody treated (lane f) or anti–IL-3 antibody treated (lane e) cells in the absence (lanes a, e, f) or presence of IL-4 (lanes b through d). RNA was then hybridized with [33P]-labeled RNA probes corresponding to transcripts for individual murine γc-receptors (mCR-1) according to PharMingen protocol (see “Materials and methods”). The Rnase-protected fragments were separated on 5% PAGE, dried, and exposed to x-ray film.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439005w.jpeg?Expires=1769125767&Signature=TA-f2rchPNVhzihb8PGldjzA5X7l~JjRQeVp6WlH~d13TkVf7NlVCXBiG-LTViN4eizaWBvi-NZcI4Fbcm9yUMix3a~HWhbR19a-TE5uw9ozDfhXpD-Yz7-zSDMnf9vYx~T9UjO4HBNo3nBPy5pYuXHhYHITTY0WPquASf3WF-kTMoiGGfwqr98Y2bi0QIegx04JEBmSOW6s7aC~hyhEt7x59YYDbuvGHT92ra5wEsulhJT1DvpH8p8nPKG~3uIWWd~4BX4U3eTdqQa1DFXiYK-AD6Sp5DVqBmoqU3vCOphFbJsHZO91ezD4z1DURVJWXvf3C494XPCW19a-T92iFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Binding site specificity of Stat6 decoy. / (A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439007aw.jpeg?Expires=1769125767&Signature=PD8qaT3jfQ5I2rjwZ3Ne~3wphSKRp64BSXHkAebwH6eTMwzGnPONI79nh9gVCfQQyBy2OCe0WUy7eJXq7FWJ2S8EMdcEjQEror4d-4QbalyyDSIqTv2nnVMLmXoZfL86-6piqCooEgvVplVLT54I3lt4Y16JHnjEO2xolFQNY6WEkbqjxfPlOKDTg8~Ljtvi6J9uk7FBP2pSCazkoG1ogaG6XOkVJGpKlSpYneQcdilDvRT~AnQc2zxnwRg2z55hNrs5SfppDHWVFf~LnLyAXuA9H~R6h4sWWVKI2O4O-hfqwaSthevbRP4KegbBVXK6vp9k19PVcvddxrg9izxHLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Binding site specificity of Stat6 decoy. / (A) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Competition was performed with 100-fold molar excess of unlabeled Stat6 decoy ODN (lane c), scrambled ODN (lane d), or cold Cε element ODN (lane e). Arrow indicates migrational location of Stat6-DNA complex or free probe. (B) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat5 oligonucleotide probe corresponding to the prolactin response element of the β-casein gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat5 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of Stat5-DNA complex or free probe. (C) D10 cells were incubated with medium (−) or 100 nmol/L IL-2 (+) for 10 minutes at 37°C. Nuclear extracts were incubated with a [32P]-labeled Stat1/3 oligonucleotide probe corresponding to the SIE gene promoter. Competition was performed with 100-fold molar excess of unlabeled cold Stat1/3 ODN (lane c), Stat6 decoy ODN (lane d), or scrambled ODN (lane e). Arrow indicates migrational location of each Stat1- or Stat3-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439007bw.jpeg?Expires=1769125767&Signature=0~4NNJpi1MepqgQkZg50ywFPd4t8OSCCe8CMyK0-e9ICCKx3DI~9ld0UkXDjQ7lWJsrEKU07LRY90AFFfaMDJlZ4yK7myRCe8-Zy~X6XxC1U7BExvigM-nMS3hg2yMm-EMnKyBqhcV5rnfuCbpKyNqFdTuEJdJWyEVWeLAe0~71uQv57DiUwAaZ8rIOAx38ukZs180gSLnK7Io6fBzLFQpyfLnOcVds35LLZe~lYNd4FPJiLnGhVf~qtVP5xEGAS7XpiUFc3dEKP~~G80e0v6uXPhmmK0wnzGt5PNU~5WZeCHl8PWzRs2VyrD18v6iuSI8I94QKTBoI-6o0sTpm-lA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Stat6 decoy specifically blocked Stat6 DNA binding activity. / Control (lanes a, b, c, d) and Stat6 decoy ODN–treated (lanes e, f) or scrambled decoy ODN–treated (lanes g, h) D10 cells were incubated with medium (−) or 100 nmol/L IL-4 (+) for 10 minutes at 37°C. Nuclear extracts were obtained, and 5 μg of protein were incubated in the absence of antibody (lanes a, b, e through h), α-Stat6 (lanes c), or normal rabbit serum (nrs; lane d) and then with a [32P]-labeled Stat6 oligonucleotide probe corresponding to the Cε gene promoter. Arrows indicate migrational location of each nonsupershifted Stat6-DNA complex or free probe.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/4/10.1182_blood.v95.4.1249.004k39_1249_1257/6/m_bloo00439008w.jpeg?Expires=1769125767&Signature=5Kw1u82Z3YkbXxEJMefA7kI4f4KEcp600NVJwp0STxr1z3IGWpb4oI0jxeTgx4Xr~sK8dyRz46h2fYIr3pSGaapBaF1eM5KWKxMdxXCDM4NcvVTQurVgkTjtC4UnFWB1N1Y8beTVVjSJoCGVKI7fm1WlaLp-dEH-EyxYVvLyDS8ME5lNQdhQxkwF-p2BXA175xSpHQ-sTwoCymTLq659vd-HkuiWuTeoeAwTH7n0McBVeAuvEFGy1hpqxMIpTYYfdB4v2wyR3Sgw~NMfl9T2F~JTkqZSFLSy2ixbp~Bf0mjtvYn1CO-mIcBdt0Vyma9JQfK-D69kHaVThgyWME-4Jw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)