CD40 ligand (CD40L) has a great potential as a novel treatment for B-cell lymphoma (BCL). It has previously been demonstrated that a nonvirulent strain of Salmonella typhimurium mutant (ST) can be used not only as a vehicle in oral genetic immunization via the intestinal mucosa, but also as an enhancer of interferon γ– and tumor necrosis factor –mediated immunity. After confirming that human CD40L can up-regulate expression of Fas, B7-1, and B7-2 molecules on murine BCL cells in vitro, we transfected the human CD40L gene intoS typhimurium mutant (ST40L), which was administrated orally to determine whether it was able to prevent the growth of BCL in mice. Expression of human CD40L was confirmed immunohistochemically with protein being detected in the Peyer's patches of mice immunized with ST40L. Moreover, human soluble CD40L had been detectable until 7 to 8 weeks after oral administration of ST40L. Although ST alone exhibited some protective effects, ST40L demonstrated a significantly greater protection against the development of CD40 positive BCL compared with the control. In the surviving mice that had been treated with ST40L, a small and hard nodule was formed at the injection site, which was found to be composed of infiltrating lymphocytes expressing Fas ligand. These results have the potential to be a simple, effective, and above all, safe immune-gene therapy against BCL.
CD40 ligand (CD40L) is expressed on the surface of activated CD4+ T cells, basophils, and mast cells.1 Binding of CD40L to its receptor, CD40, on the surface of B cells stimulates B-cell proliferation, homotypic adhesion, differentiation, and immunoglobulin (Ig) class switching.1Moreover, CD40L is also required to activate antigen presenting cells (APCs), including B cells, macrophages, and dendritic cells (DCs).2 This results in an up-regulation in cytokine secretion and the expression of CD80 (B7-1) and CD86 (B7-2) on the APCs, which, in turn, engages the CD28 receptor on T cells, resulting in a reciprocal amplification of antigen specific T cells, as well as cytotoxic T cells and natural killer cells.3-7 CD40 is also expressed on the surface of malignant cells derived from the B-cell lineage.8,9 CD40 ligation up-regulated expression of not only adhesion molecules, MHC class I/II, and costimulatory molecules (B7 family),9 but also the Ku antigen10 and possibly tumor antigens on malignant B cells, which may trigger tumor-specific immunity.11,12 Indeed, CD40L has a growth suppressive effect on malignant B cells both in vivo and in vitro in contrast to the effects of CD40L on normal immune cells.13-16 Therefore, immune-gene therapy using CD40L may be a potential treatment for the B-cell lymphoma (BCL).
Genetic immunization has recently provided promising new approaches to vaccination, such as subcutaneous (SC) injection of isolated plasmid DNA.17 Recently, Darji et al18 demonstrated the efficient vaccination against Listeria monocytogenesis by the use of an oral somatic transgene by using attenuated Salmonella typhimurium (ST). Using live but attenuated ST allows the bacterium to penetrate through the epithelial M cells and to reach the Peyer's patches, causing the activation of APCs by bacterial uptake.19 An auxotrophic mutant that requires metabolites not available in vertebrate tissues is unable to grow in this environment and would therefore be nonvirulent.20 During the process of phagocytosis of ST in Peyer's patches, a plasmid containing the encoded gene in the ST would be released into the cytosol and integrated into the nucleus; this may then lead to expression of the encoded gene in the host cells under the control of the eukaryotic promoter.18 This opens up the exciting possibility of oral CD40L gene therapy against BCL, using ST as vehicle.
In the current study, we transfected the CD40L gene into ST and administered it orally to mice to induce CD40L expression in the intestinal immune system. This oral gene therapy resulted in a high degree of protection against the development of a CD40 positive BCL. These results suggest that the administration of oral CD40L using attenuated ST may work as a simple, effective, and safe immune-gene therapy against BCL.
Materials and methods
Cells and mice
A20 is a BCL cell line derived from BALB/c mice and expressing CD40 as well as the major histocompatibility complex (MHC) class I and class II h-2d, IgG, and Fc receptor (American Type Culture Collection [ATCC], Rockville, MD).21 The level of B7-2 on the surface of A20 cells was observed to be low with B7-1 being undetectable.22 The wehi3 leukemia cell line is also derived from BALB/c mice but does not express CD40 on the surface, this was used as a negative control.22 A20 cells and wehi3 cells were cultured in 90% RPMI1640 with L-glutamine medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 atmosphere. SC injection of 105 A20 cells was sufficient to form lymphoma, being lethal in all injected BALB/c mice (Japan Crea, Tokyo, Japan) during the period of 26 to 35 days after injection. SC injection of 105 wehi3 cells was lethal in all injected mice within 25 days. This is in concordance with previous reports.22Animal care was in accordance with institutional guidelines.
Salmonella strain and CD40L
The auxotrophic ST aroA− strain SL5000 was kindly donated by Dr Bruce A. D. Stocker (Stanford University School of Medicine, Stanford, CA) and used as a gene carrier.20 The full-length human CD40L gene was cloned into pcDL-SR using a CMV promoter (ATCC).23 The auxotrophic ST aroA− strain SL5000 was grown in 100 mL L broth (Sigma Diagnostics, St Louis, MO) to A600 of 0.6, chilled on ice, and harvested by centrifugation (15 minutes, 1000g at 4°C). The pellet was suspended in a final volume of 200 μL in 10% glycerol. Aliquots (40 μL) were mixed with 1 to 2 μL DNA in a chilled microcentrifuge tube and transfected to chilled cuvettes (0.2 cm electrode gap). A single pulse of 12.5 kV/cm (2.5 kV, 200 O, 25 μF) was applied and 1 mL of prewarmed SOC was immediately added as previously reported.24 The bacteria were transferred to 17 × 100 mm polypropylenetubes and shaken for 1 hour at 37°C before being plated onto LB agar with ABPC (50 ng/mL). Groups of 6 to 8 female BALB/c mice were fed with 0.5 mL phosphate-buffered saline (PBS) containing 109 colony-forming units (CFU) of ST with or without CD40L gene. None of the mice exhibited any overt signs of illness during immunization.
CD40L gene transfected NIH3T3 cells
NIH3T3 cells transfected with human CD40L gene in pcDL-SR expression vector (NIH3T3/CD40LT) and NIH3T3 cells transfected with vector alone (NIH3T3/vt) were prepared by electroporation. Transfectants were selected by growth in 400 μg/mL G418 and then subcloned. The cells were harvested at 70% confluence, washed 3 times in PBS (Sigma) to remove G418, and fixed in 1% formalin (Sigma) for 10 minutes at room temperature. After further washing with PBS, the cells were cultured with A20 cells (100 A20 cells/1 NIH3T3 cell) for 48 hours in 10% FBS plus RPMI1640.
Flowcytometric analysis
Phenotypic changes of A20 cells (1 × 106/mL) treated with NIH3T3/ D40LT (1 × 104/mL) or NIH3T3/vt (1 × 104/mL) for 48 hours were examined using flow cytometric analysis, as previously described.25Antibody-coated cells were enumerated by flow cytometric analysis using an EPICS V cell sorter (Coulter Electronics, Hialeah, FL). The following antibodies (Abs) were used: FITC-conjugated hamster antimouse CD80 (B7-1) Ab (hamster IgG)(Pharmigen, San Diego, CA), FITC-conjugated rat antimouse CD86 (B7-2) Ab (IgG2a, k)(Pharmigen), and FITC-conjugated hamster antimouse CD95 (Fas) Ab (hamster IgG)(Pharmigen).
Immunohistochemistry
Paraffin-embedded specimens were used for hematoxylin and eosin (HE) staining and Fas ligand (FasL) (Santa Cruz Biotechnology, Santa Cruz, CA) immune-staining as previously described.26 Moreover, anti-CD4 and CD8 Abs (Pharmigen) were used for immunostaining combined with HE staining. For immunohistochemical staining, frozen tissue sections were treated with antihuman CD40L Ab (Santa Cruz).
Measurement of soluble human CD40L in sera
Soluble human CD40L levels in the sera of BALB/c mice were quantified using the soluble CD40L enzyme-linked immunosorbent assay (ELISA) kit (Chemicon International Inc, Temecula, CA), which used a sandwich-based immunoassay design. The minimal detection level was 0.16 ng/mL of soluble CD40L. Two types of murine monoclonal Abs used in the ELISA system do not detect mouse soluble CD40L protein in the sera.
Results
We first examined the expression of Fas, B7-1, and B7-2 on A20 cells cultured with formalin fixed NIH3T3/vt or NIH3T3/CD40LT cells by immunofluorescence flow cytometry, as shown in Table 1. Levels of Fas and B7-2 on the surface of A20 cells were found to be low, and B7-1 was undetectable when A20 cells were cultured with NIH3T3/vt (control). In contrast, NIH3T3/CD40LT cells upregulated the expression of Fas as well as B7-1 and B7-2 molecules.
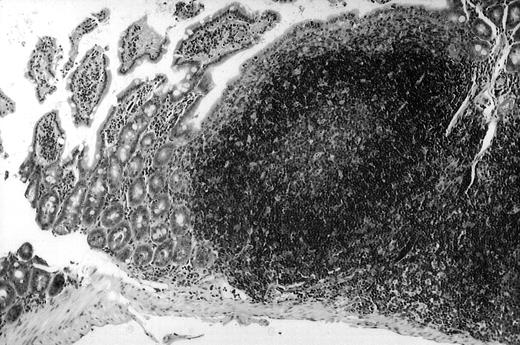
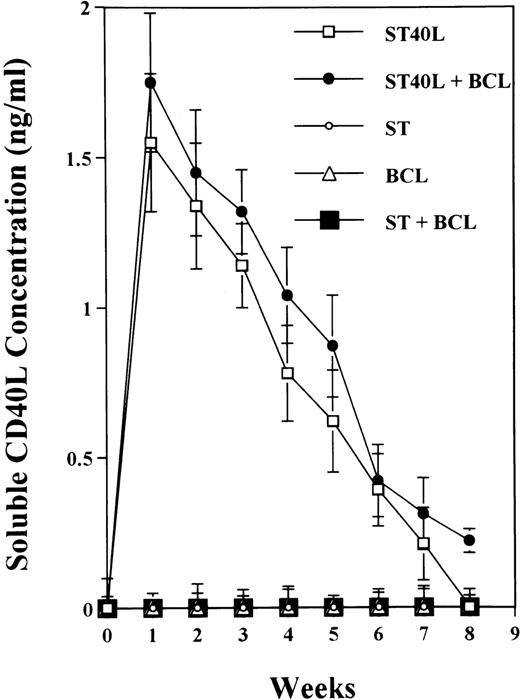
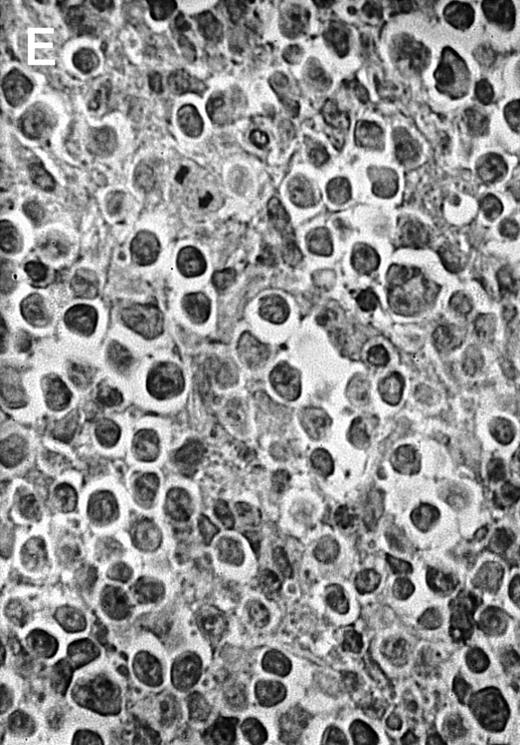
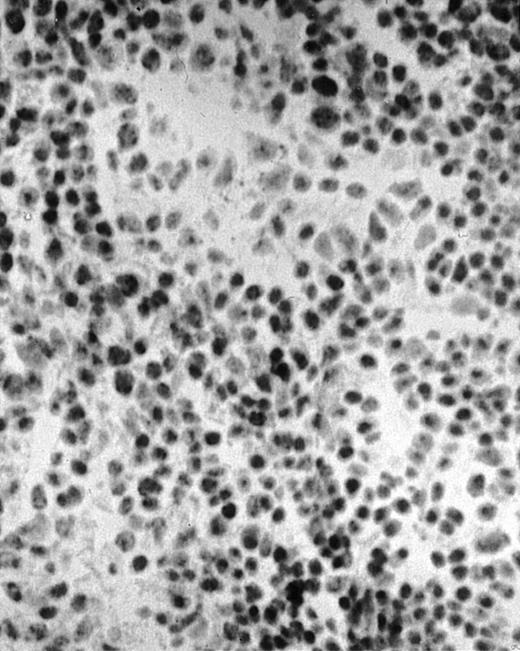
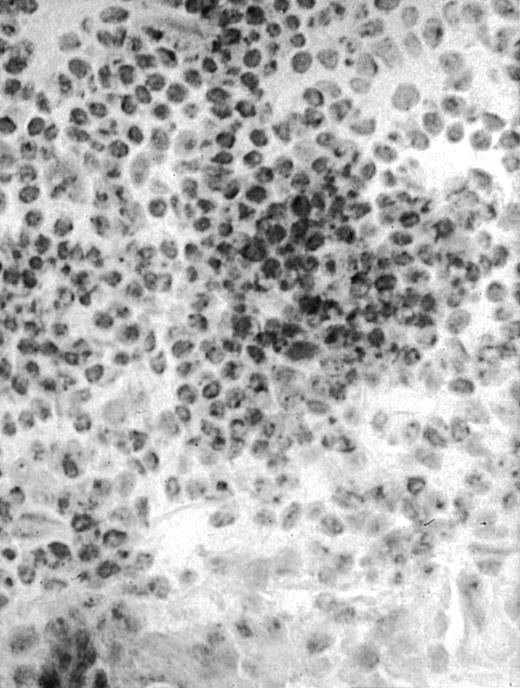
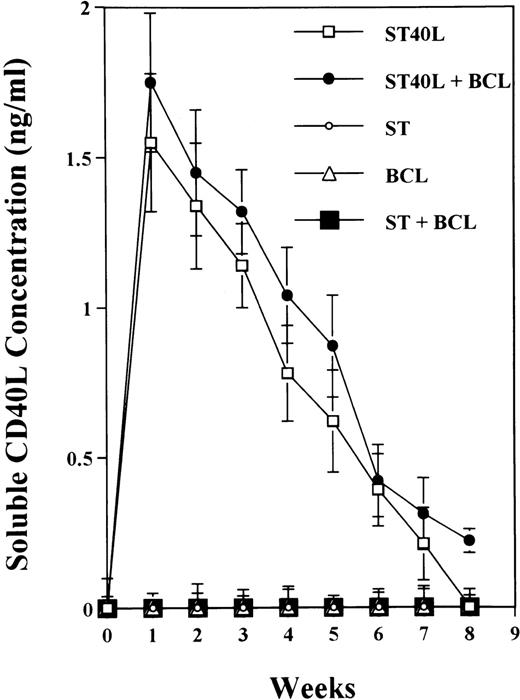
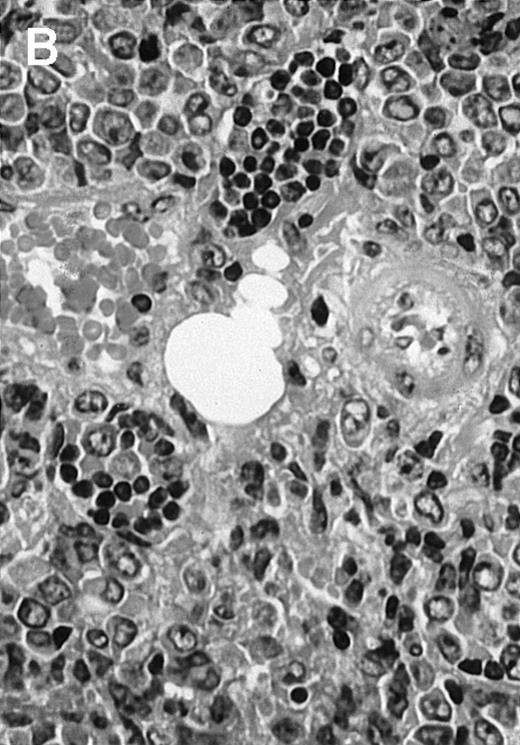
To analyze expression of the human CD40L protein in murine tissues, samples from the small intestine, colon, liver, and spleen were analyzed by HE staining as well as immunohistochemistry. We found that in mice immunized with ST40L, the Peyer's patches were prominent (Figure 1A), and the majority of cells in the Peyer's patches could be seen to express the human CD40L protein (positive: brown or yellow color; negative: blue color) (Figure 1B). There were a few CD40L+ cells in spleen, but not in liver. In contrast, human CD40L was not detectable in the Peyer's patches of mice treated with ST (Figure 1C). To further confirm the secretion of human soluble CD40L by transfected murine cells into the sera, we next examined it by ELISA (Figure 2). Human soluble CD40L protein was detectable only in BALB/c mice treated with ST40L with or without administration of BCL cells, but not detectable in mice treated with ST and/or BCL cells. The level of soluble CD40L protein in the sera peaked at 1 week after oral administration and was detectable until 7 to 8 weeks.
Presence of transduced human CD40L protein in mice treated with ST40L.
(A) HE staining of intestine ( × 100) of BALB/c mice killed 1 week after oral administration of ST40L. (B) Immunostaining of Peyer's patches using antihuman CD40L Ab (×400) of BALB/c mice killed 1 week after oral administration of ST40L. (C) Immunostaining of Peyer's patches using antihuman CD40L Ab (×400) of BALB/c mice killed 1 week after oral administration of ST.
Presence of transduced human CD40L protein in mice treated with ST40L.
(A) HE staining of intestine ( × 100) of BALB/c mice killed 1 week after oral administration of ST40L. (B) Immunostaining of Peyer's patches using antihuman CD40L Ab (×400) of BALB/c mice killed 1 week after oral administration of ST40L. (C) Immunostaining of Peyer's patches using antihuman CD40L Ab (×400) of BALB/c mice killed 1 week after oral administration of ST.
Presence of transduced human soluble CD40L protein in the sera of BALB/c mice treated with ST40L.
BALB/c mice were orally administered with ST or ST40L, with or without SC injection of A20 cells (105 cells). Sera samples were taken from these mice every week. Human soluble CD40L protein in sera from mice administered with ST40L (□), with ST40L + BCL (•), with ST(○), with BCL (▵), or with ST + BCL (▪) were quantified using the soluble CD40L ELISA kit. Data shown are mean ± SD from 3 independent experiments.
Presence of transduced human soluble CD40L protein in the sera of BALB/c mice treated with ST40L.
BALB/c mice were orally administered with ST or ST40L, with or without SC injection of A20 cells (105 cells). Sera samples were taken from these mice every week. Human soluble CD40L protein in sera from mice administered with ST40L (□), with ST40L + BCL (•), with ST(○), with BCL (▵), or with ST + BCL (▪) were quantified using the soluble CD40L ELISA kit. Data shown are mean ± SD from 3 independent experiments.
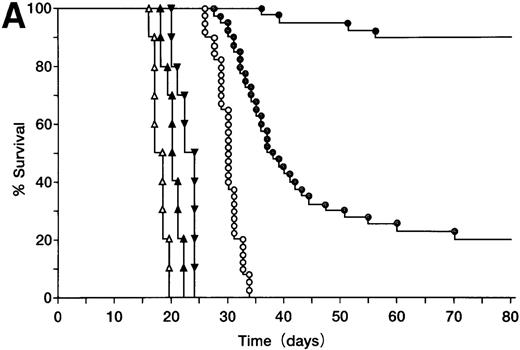
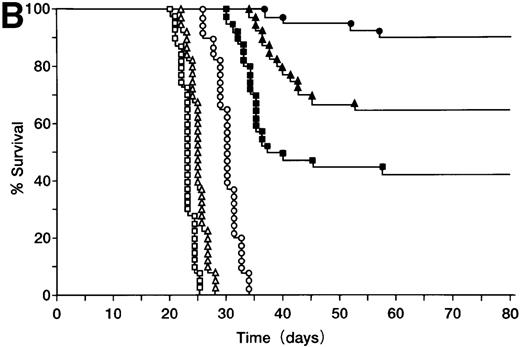
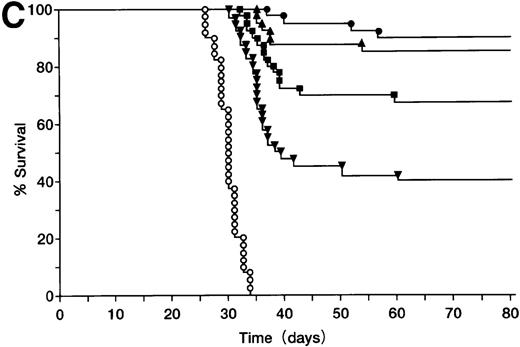
BALB/c mice were injected SC with 105 A20 cells (Figure3A), these mice were then orally administered with ST40L, ST, or PBS alone, and their survival monitored. Mice in the group treated with ST40L were found to have a significantly longer survival than those treated with ST or PBS (Kaplan-Meier method: Mantel-Cox, P < .0001). The mice in the group treated with ST alone also survived for a significantly longer period than those treated with PBS alone (P < .0001). In contrast, SC injection of an equivalent number of CD40 negative wehi3 leukemia cells showed that there was no significant difference between treatments with ST40L, ST, and PBS. When differing numbers of A20 cells (105, 106, or 107) were injected, the survival rates of the mice were 92%, 77%, and 55%, respectively (Figure 3B). ST40L was then administered before or after tumor challenge (Figure 3C and D). When ST40L was administered 1 week before tumor cell challenge (105 A20 cells, SC injection), no significant differences were observed in mice survival compared with simultaneous vaccination by SC injection of A20 cells alone. However, the efficiency of the ST40L was found to be decreased when the mice were immunized at either 3 weeks (52%, P < .01) or 2 weeks (67%, P < .05) before A20 cell (105) SC injection. This effect was also seen when the mice were ST40L immunized at 3 weeks (42%, P < .01), 2 weeks (69%,P < .001), or 1 week after (70%,P < .02) A20 cell (105) SC injection.
Protection against tumor challenge with ST40L.
(A) A20 cells (105) were administered subcutaneously to BALB/c mice with 109 CFU of ST40L (•, upper line), 109 CFU of ST (⊙, middle line), or PBS (○, lower line) being given by simultaneous oral administration. As a control, wehi-3 CD40 negative cells were treated by the administration of ST40L (▴), ST(▾), or PBS(▵), in a similar manner to that above. (B) Differing numbers of A20 cells were subcutaneously injected into BALB/c mice (40 mice for each condition) with either 109 CFU of ST40L (A20 cell number: 105, •;106, ▴;107, ▪) or PBS (A20 cell number: 105, ○;106, ▵;107, □) being given by simultaneous oral administration. (C) BALB/c mice were preimmunized with ST40L 1 week (▴), 2 weeks (▪), or 3 weeks (▾) before A20 challenge (105 SC). The survival curves were compared with simultaneous immunization with ST40L (•) or PBS (○). (D) BALB/c mice were postimmunized with ST40L 1 week (▴), 2 weeks (▪), or 3 weeks (▾) following A20 challenge (105 SC). The survival curves were compared with simultaneous immunization with ST40L (•) or PBS (○).
Protection against tumor challenge with ST40L.
(A) A20 cells (105) were administered subcutaneously to BALB/c mice with 109 CFU of ST40L (•, upper line), 109 CFU of ST (⊙, middle line), or PBS (○, lower line) being given by simultaneous oral administration. As a control, wehi-3 CD40 negative cells were treated by the administration of ST40L (▴), ST(▾), or PBS(▵), in a similar manner to that above. (B) Differing numbers of A20 cells were subcutaneously injected into BALB/c mice (40 mice for each condition) with either 109 CFU of ST40L (A20 cell number: 105, •;106, ▴;107, ▪) or PBS (A20 cell number: 105, ○;106, ▵;107, □) being given by simultaneous oral administration. (C) BALB/c mice were preimmunized with ST40L 1 week (▴), 2 weeks (▪), or 3 weeks (▾) before A20 challenge (105 SC). The survival curves were compared with simultaneous immunization with ST40L (•) or PBS (○). (D) BALB/c mice were postimmunized with ST40L 1 week (▴), 2 weeks (▪), or 3 weeks (▾) following A20 challenge (105 SC). The survival curves were compared with simultaneous immunization with ST40L (•) or PBS (○).
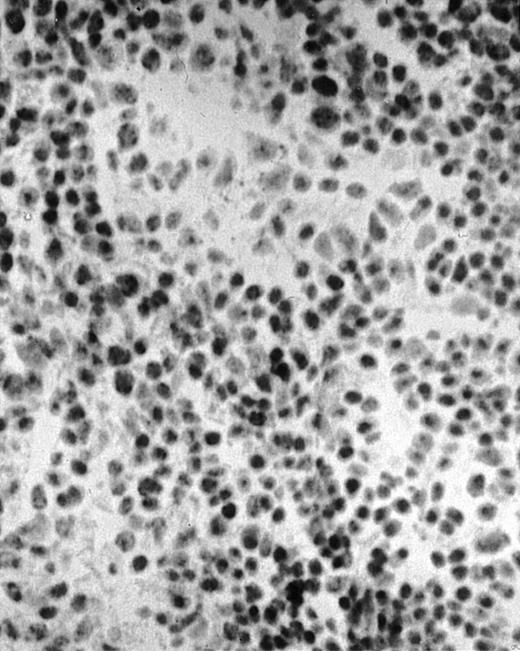
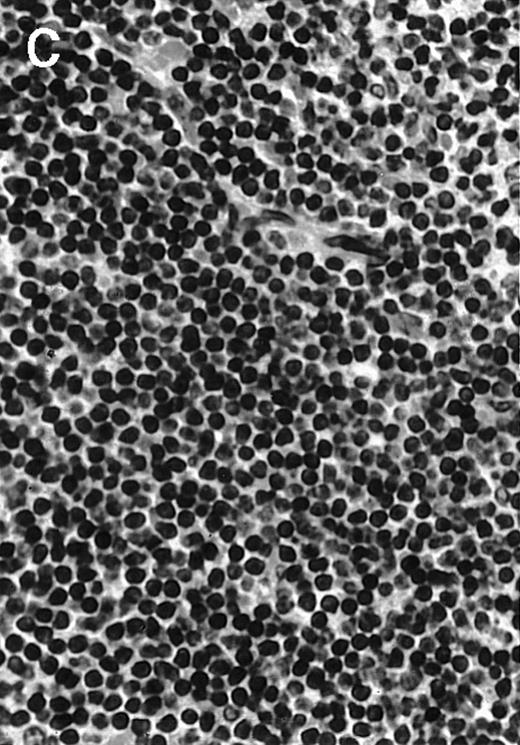
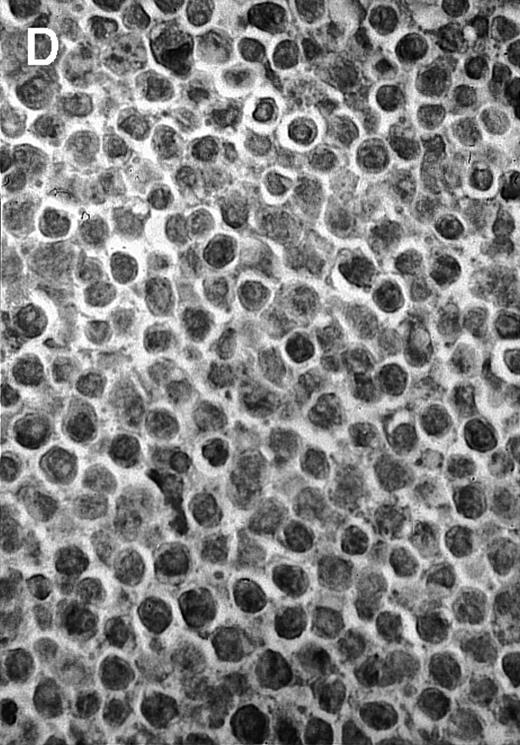
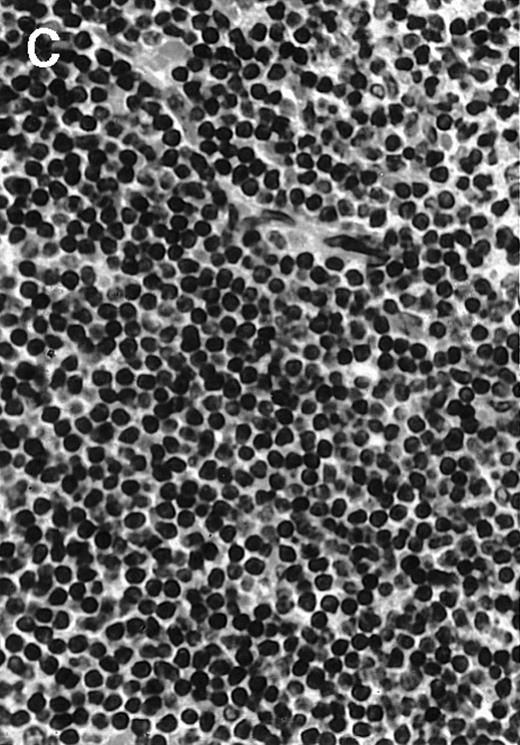
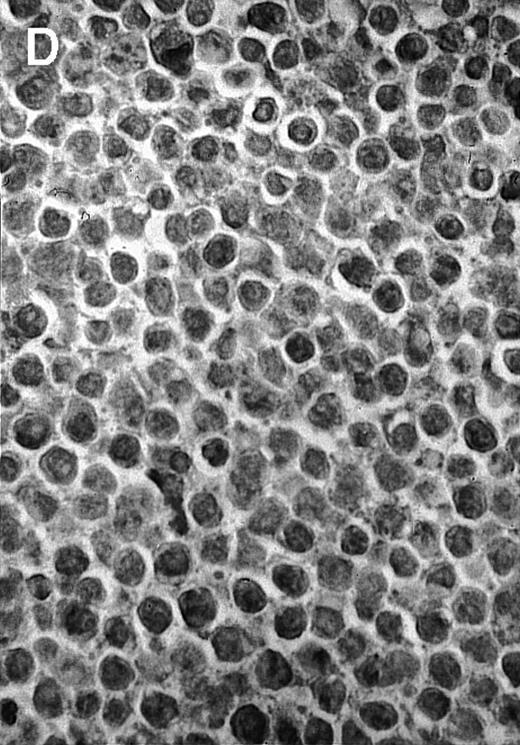
To explore the mechanisms of the protection from BCL growth, histologic analysis was performed on tumor tissue from mice treated with ST40L, ST, or PBS alone. In the mice treated with PBS alone, no cellular infiltrate expressing FasL was observed in the surrounding tissues and inside the BCL region (Figure 4A and D). In contrast, infiltrating lymphocytes expressing FasL were observed around the vessels and also scattered in the smaller tumor tissues in the mice treated with ST (Figure 4B and E). Small hard nodules (2-5 mm in diameter) were observed at the SC injection sites of the long-term survival mice that had been treated with ST40L. On histologic analysis, these small nodules were confirmed to be the result of an accumulation of lymphocytes, and not BCL cells (Figure 4C). These lymphocytes were also found to be strongly positive for FasL expression (Figure 4F). On the other hand, lymphocytes infiltrating in the nodules were stained by either CD4+ Ab or CD8+ Ab, but the ratio of CD4+ and CD8+ cells was not 1-sided (data not shown).
Histologic analysis of A20 tumors excised 21 days postchallenge from BALB/c mice immunized with ST40L.
(A) HE stain of a BCL region in a mouse treated with PBS alone. (B) HE stain of a smaller BCL region in a mouse treated with ST. (C) HE stain of a small hard nodule at the SC injection site in a mouse treated with ST40L. (D) Fas ligand staining of a BCL region in a mouse treated with PBS alone. (E) Fas ligand staining of a BCL region in a mouse treated with ST. (F) Fas ligand staining of a small hard nodule at the SC injection site in a mouse treated with ST40L. Original magnifications are ×400.
Histologic analysis of A20 tumors excised 21 days postchallenge from BALB/c mice immunized with ST40L.
(A) HE stain of a BCL region in a mouse treated with PBS alone. (B) HE stain of a smaller BCL region in a mouse treated with ST. (C) HE stain of a small hard nodule at the SC injection site in a mouse treated with ST40L. (D) Fas ligand staining of a BCL region in a mouse treated with PBS alone. (E) Fas ligand staining of a BCL region in a mouse treated with ST. (F) Fas ligand staining of a small hard nodule at the SC injection site in a mouse treated with ST40L. Original magnifications are ×400.
Discussion
In the current study, we have demonstrated an outstanding protection from development of BCL in mice by the oral administration of an ST40L vaccine. SC injection of 105 A20 cells was lethal in all injected BALB/c mice; in contrast, more than 90% of mice survived with simultaneous oral administration of ST40L. The efficiency was dependent on the dose of A20 cells administered and the timing of the immunization. Dilloo et al15 have demonstrated that the CD40L-dependent response generated to a preexisting malignancy is significantly increased by the coinjection of cells secreting IL-2, resulting in antileukemic activity greater than obtained with either molecule alone. French et al16 have shown that anti-CD40 Ab evokes cytotoxic T cells which eradicate BCL in mice, whereas CD40 monoclonal Ab treatment in the absence of BCL cells did not result in an expansion of T cells, suggesting that simultaneous nonspecific stimuli to the immune system may play an important role in the success of CD40L treatment. It is also of interest that the oral administration of ST alone was able to rescue some of the mice injected with BCL. Mutant strains of Salmonella have shown promise as live oral vaccines in humans, capable of stimulating both mucosal and systemic immune responses.27 In particular, the oral administration of an Aro− mutant strain can evoke strong Th1 immune responses dependent on interferon γ.28 These suggest that ST itself may also be a potent inducer of anti-BCL immunity and synergies with CD40L in antitumor effects.
As we have previously reported, ligation of CD40 on multiple myeloma cells up-regulated B7-1 as well as adhesion molecules and MHC, human CD40L enhanced expression of B7-1, and B7-2 on A20 cells in vitro, which may activate tumor-specific cytotoxic T-cell proliferation through CD28.29 Indeed, ectopic expression of the B7 molecule on tumor cells has been shown to be protective in a number of animal tumor models.30-32 Furthermore, CD40L further can induce IL-12 secretion by DCs33 as well as expression of cell surface molecules required for antigen presentation leading to antitumor immunity, which might be superior to the potential of ectopic B7 expression alone. Moreover, a cross-functionality between mouse and human CD40L was demonstrated in this experiment, which is advantageous in that ectopic CD40L expression can be identified by the Abs reacting only to human CD40L. A soluble isoform of CD40L has been shown to exist in the circulation.34 This soluble molecule is a homotrimer of an 18-kd protein exhibiting full activity in B-cell proliferation and differentiation assays, and is able to rescue B cells from apoptosis and bind soluble CD40.35 Soluble CD40L was selectable in the sera of the BALB/c mice treated with ST40L with the level peaking at 1 week after oral administration and being detectable until 7 to 8 weeks after immunization; this further supports the successful transduction of the human CD40 L gene into murine cells.
When A20 cells were stimulated with NIH3T3/CD40LT, Fas expression was found to be up-regulated in vitro. CD40 ligation induces Fas expression on cells and facilitates apoptosis through the FasL/Fas pathway.36 Fas is not always expressed on malignant B cells, but CD40 ligation leads to an up-regulation of Fas antigen and enhances sensitivity to the Fas-mediated death signal in vitro.37 38 ST40L was found to trigger the infiltration of FasL expressing lymphocytes into the BCL tumors, with the degree of infiltration being associated with tumor regression, thus suggesting that ST40L might elicit a strong antitumor immunity via up-regulating Fas on A20 cells and amplifying T cells expressing FasL in tumor.
Previous reports have demonstrated that cytokine-gene therapy, such as that with GM-CSF, may be successful in tumor reduction.39However, most of the gene therapies currently available involve complicated ex vivo cell manipulation,39,40 and only the local administration or ex vivo transfection of the gene is in clinical studies.41 In contrast, the current study using an oral ST40L lymphoma vaccine is characterized by its simplicity of treatment, coupled to a significant efficiency. There are always safety concerns regarding the use of gene therapy and drug delivery systems. However, the safety of the aroA−, live oral ST vaccine has already been confirmed in both human volunteers as well as some other species of animals.42-44 Pawelek et al45,46intraperitoneally injected attenuated S typhimurium with the herpes simplex virus thimidine kinase as an antimelanoma agent, but the use of this could be limited by the potential induction of TNF α–mediated septic shock. Therefore, the oral route may be much safer than systemic administration of ST in clinical use. With respect to the use of the CD40L gene, T-lymphoblastic lymphoma, and other undesirable side effects were observed when the CD40L gene was retrovirally transfected into the bone marrow or thymic cells in CD40L−/− mice.47 However, in this study, no tumor formation was observed in the mice administered ST40L, the type of target cell appeared to be important and therefore cells in Peyer's patches may be safe targets. From a treatment aspect, we suggested that patients in complete remission may be treated with the oral ST40L gene therapy to prevent the relapse of disease.
In conclusion, attenuated ST carrying CD40L can be used for genetic anti-BCL immunization via the oral route, which may provide a new immuno-gene therapy to B-cell malignancies.
Acknowledgment
The authors thank Tsuneo Natori, of SRL Inc (Tokyo, Japan), for the excellent specimen of mouse pathology.
Supported with a grant from Sankyo Foundation for Life Science and the Mother and Child Health Foundation.
Reprints:Mitsuyoshi Urashima, Department of Pediatrics, Jikei University School of Medicine, 3-25-8 Nishi-shimbashi, Minato-Ku, Tokyo, Japan; e-mail: urashimaf@aol.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.