Phospholipid asymmetry is well maintained in erythrocyte (RBC) membranes with phosphatidylserine (PS) exclusively present in the inner leaflet. The appearance of PS on the surface of the cell can have major physiologic consequences, including increased cell-cell interactions. Because increased adherence of PS-exposing RBCs to endothelial cells (ECs) may be pathologically important in hemoglobinopathies such as sickle cell disease and thalassemia, we studied the role of PS exposure in calcium ionophore-treated normal RBC adherence to human umbilical vein endothelial cell (HUVEC) monolayers. When HUVEC monolayers were incubated with these PS-exposing RBCs, the ECs retracted and the RBCs adhered primarily in the gaps opened between the ECs. A linear correlation was found between the number of PS-exposing RBCs in the population and the number of adhering RBCs to the monolayer. Pretreatment of RBCs with annexin V significantly decreased adherence by shielding PS on the RBCs. Similarly, PS-containing lipid vesicles decreased RBC binding by competing for the PS binding sites in the monolayer. PS-exposing RBCs and PS-containing lipid vesicles adhered to immobilized thrombospondin (TSP) and matrix TSP, respectively, and adherence of PS-exposing RBCs to EC monolayers was reduced by antibodies to TSP and to its EC receptor, vβ3. Together, these results indicate a role for PS and matrix TSP in the adherence of PS-exposing RBCs to EC monolayers, and suggest an important contribution of PS-exposing RBCs in pathologies with reported vascular damage, such as sickle cell anemia.
The distribution of phospholipids in the normal erythrocyte (RBC) membrane is highly asymmetrical, with phosphatidylserine (PS) located exclusively in the inner leaflet.1 The loss of membrane asymmetry and the exposure of PS can affect the hemostatic balance,2 and leads to recognition and removal of aging red cells by macrophages.3In patients with sickle cell anemia or thalassemia, a small but significant subpopulation of RBCs can be found that expose PS on their surface.4-6 Adherence of RBCs to the endothelium may play a significant role in vasoocclusive events in sickle cell disease,7 and PS exposure in RBCs could be important in this process.1,8 Although previous reports on abnormal adherence of PS-exposing RBCs to endothelial cell (EC) monolayers suggest a role for PS in adherence,9-11 the mechanism remains to be elucidated.
Phagocytotic macrophage recognition of PS-exposing cells has been extensively studied. Recognition of aged neutrophils was shown to be charge dependent and blocked in a stereo-specific and dose-dependent manner by PS-containing liposomes.12 Similarly, phagocytosis of mouse lymphocytes was inhibited by PS-containing liposomes,13 suggesting that macrophages can specifically recognize PS. Phagocytosis of apoptotic, PS-exposing neutrophils was found to be mediated by macrophage αvβ3integrin. However, blocking experiments suggested that the recognition mechanisms involving αvβ3 and PS were mutually exclusive.14 Subsequently, the participation of thrombospondin (TSP) as a molecular bridge binding to both CD36 and αvβ3 was shown.15
Although many mechanisms have been implicated in the binding of sickle red cells to the endothelium,16,17 one of the most documented, and probably significant, interactions between sickle RBCs and the endothelium is mediated by TSP.18,19 This large multifunctional adhesive protein is synthesized and secreted by many different cell types, including ECs and activated platelets. TSP is present in soluble form in the plasma and can be found in the basement membrane of ECs and in the extracellular matrix of cultured ECs, where it functions in cell-cell and cell-matrix interactions. The versatility of TSP lies in its many domains, with binding sites for heparin, calcium, fibrinogen, fibronectin, collagen V, plasminogen, histadine-rich glycoproteins, and sulfated glycolipids.20Calcium ions regulate the ligating properties of TSP, the transition from its adhesive to its nonadhesive conformer being modulated by calcium depletion. In addition, the physical state of TSP affects its conformation, influencing its interaction with other molecules.21
Abnormal levels of soluble plasma TSP have been found in sickle patients.22 This soluble TSP appears to be instrumental in bridging sickle RBC adherence to ECs.18,23 On RBCs, TSP interacts with CD36, sulfated glycolipids,24 and a normally cryptic domain of the dominant membrane protein, band 3, which is subject to rearrangement in hematologic disorders.11,25 TSP binding to ECs occurs through interactions with the integrin, αvβ3,26 heparin sulfate,23,27 and an integrin-associated protein.26 In addition to a role for soluble TSP, sickle RBC adherence to immobilized TSP 28-30 suggests a role for matrix TSP, exposed by vascular injury, in sickle cell pathology.
On the basis of these observations, we postulated that PS exposure in RBCs leads to PS-mediated RBC adherence to matrix TSP in damaged endothelial monolayers. In this study we used calcium-loaded RBCs to show a linear increase of RBC adherence with PS exposure and to elucidate a mechanism that explains this phenomenon.
Materials and methods
Reagents
Gelatin, fibronectin from human plasma, bovine serum albumin (BSA), glutaraldehyde, Hanks' buffered saline solution (HBSS), HEPES buffer, thrombin, histamine, EDTA, EGTA, calcium ionophore A23187, phosphatidylcholine (PC) from egg yolk, and L-α-phosphatidyl-L-serine from bovine brain were obtained from Sigma Chemicals Co (St. Louis, MO). Calcium, magnesium-free phosphate buffered saline (PBS) was from the University of California San Francisco cell culture facility. Endothelial Cell Growth Medium (EGM) was from Clonetics Corp (San Diego, CA). Dispase II was obtained from Boehringer Mannheim Corp (Indianapolis, IN). NBD (7-nitro-2-1,3-benzoxadiozol-4-yl)–labeled phosphatidylcholine was purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Nucleopore filters were from Corning Costar Corp (Cambridge, MA). Eight-chambered culture slides were obtained from NUNC (Naperville, IL). Biomeda Gel Mount was from Biomeda Corp (Foster City, CA), and Vectashield Mounting Medium was from Vector Labs (Burlingame, CA). TSP was a gift from Dr Jack Lawler, Beth Israel Deaconess Medical Center, Boston, MA; band 3 peptides and monoclonal antibody (MAb) 1F4 were gifts from Dr Irwin W. Sherman, University of California Riverside; polyclonal antibody to CD59 was a gift from Dr Samuel Test, Children's Hospital Research Institute, Oakland, CA. Monoclonal antibodies, OKM5 to CD36 were from Coulter Immunotech, Inc (Westbrook, ME); L230 to αv and AP3 to β3 were from American Type Culture Collection (Rockville, MD); polyclonal antibodies to thrombospondin were from Calbiochem (San Diego, CA). Phycoerythrin conjugated goat antimouse IgG and phycoerythrin conjugated donkey antirabbit IgG were purchased from Jackson ImmunoResearch Laboratories, Inc (Westgrove, PA); Biotinylated antirabbit IgG and Streptavidin Texas Red were from Amersham Pharmacia Biotech (Piscataway, NJ). FITC-labeled annexin V was prepared as described previously.4
Erythrocytes
Blood samples were collected in citrate after informed consent from healthy donors or sickle cell patients of Children's Hospital Oakland. Red cells were isolated by centrifugation, washed 3 times in HBSS, and the buffy coat was removed after each wash. Phospholipid organization was scrambled in normal RBCs by incubation in 0.5 mmol/L CaCl2 in the presence of 2 μmol/L calcium ionophore A23187.4 After incubation, the calcium ionophore was removed by back extraction with BSA. These PS-exposing RBCs were mixed with untreated red cells to obtain samples with 5% to 40% PS-exposing red cells. Alternatively, back extraction of ionophore was omitted, the PS-exposing cells were mixed with normal cells to a mixture of 20% treated, 80% untreated RBCs and subsequently incubated in 2.5 mmol/L MgCl2 and 8 mmol/L EGTA, followed by extraction of the ionophore with BSA. This treatment reverses the exposure of PS.31 The percentage of PS-exposing cells in erythrocyte samples was measured by annexin V-FITC labeling, using flow cytometry on a Becton Dickinson FACScan.4
Phospholipid vesicles
Phosphatidylcholine (PC) from egg yolk and phosphatidylserine (PS) from bovine brain were mixed, in a 4:1 molar ratio in chloroform/methanol 2/1, dried under nitrogen and resuspended in HBSS to produce liposomes with a final concentration of 100 mmol/L lipid. Alternatively, pure PC liposomes were made at the same final concentration of phospholipid. Unilamellar vesicles with a mean diameter of 100 nm were generated using extrusion.32 The liposomes were passed 6 times through a Thermobarrel Extruder (Lipex Biomembranes, Vancouver, BC) equipped with two 0.1 mm pore size Nucleopore filters. NBD-labeled vesicles consisting of 10% NBD-labeled PC and 90% PC, or 10% NBD-labeled PC, 40% PC and 50% PS, were prepared as follows. The labeled and unlabeled phospholipids were mixed in chloroform/methanol, dried under nitrogen, and resuspended in HBSS. Subsequently, the lipid mixtures were sonicated at 4°C for 5 minutes at 30W using a microtip Branson sonifier (Branson Sonic Power, Danbury, CT). The supernatant was used after centrifugation at 245 000g for 30 minutes.
RBC receptor labeling
To examine for coexpression of the cytoadherent receptors, CD36 or band 3 peptide 3d, on PS-exposing red cells, PS-exposing RBCs, normal RBCs, or sickle RBCs were resuspended to an hematocrit of 4% in PBS, 1% albumin (PBSA), and labeled with either 100 ng/mL OKM5 (MAb to CD36) or 1 mg/mL 1F4 (MAb to band 3 peptide 3d) for 45 minutes. at 4°C, and subsequently with goat antimouse secondary antibody conjugated to phycoerythrin for flow cytometric analysis.
Endothelial cell culture
Umbilical cords were obtained from anonymous donors at the Labor and Delivery Unit of Alta Bates Medical Center (Berkeley, CA) or San Francisco General Hospital (San Francisco, CA) with approval from the respective Human Research Committees. Human umbilical vein endothelial cells (HUVECs) were isolated within 3 days of cord collection, according to the method of Jaffe et al33 with the following modifications. Enzymatic digestion to free the HUVECs was for 10 minutes at 37°C with 0.15% dispase in M199 medium. Cells were grown at 37°C in 5% CO2 / 95% air in gelatin-coated flasks in EGM. Cobblestone morphology and positive staining by an antibody to von Willebrand factor identified the resulting culture as ECs. At 85% confluence, the cells were subcultured to fibronectin-coated 8-chambered slides. The second and third passages were used for adherence assays no later than 24 hours after HUVECs had reached confluence.
Matrix exposure
To generate exposure of the matrix, HUVECs were grown to confluence in chambered slides as described previously, and treated for 5 minutes with either 10 mmol/L histamine or 0.1 NIH U/mL thrombin, or for 10 seconds with 0.5 mmol/L EDTA in HBSS, 1% BSA, 50 mmol/L HEPES pH 7.4 (HAH). Similar incubation times of HUVECs with HAH served as control. Microscopic observation of gap formation was used to characterize the retraction of the ECs and the exposure of the matrix. The HUVEC monolayers were washed with HAH and subsequently tested for their ability to bind RBCs.
To generate a surface completely devoid of endothelial cells, but with the matrix still present, HUVEC monolayers were washed with PBS and incubated in a modification of a buffer described by Wu et al,34 0.01 mol/L phosphate, 0.15 mol/L NaCl, 5 mmol/L NaHCO3, 10 mmol/L EDTA, 0.1% BSA, 1 mmol/L phenylmethylsulfonyl fluoride, pH 7.2. After 30 minutes at 37°C, ECs were gently washed away with HAH.
TSP exposure
To visualize the exposure of TSP in the matrix, histamine-treated HUVEC monolayers were fixed for 10 minutes with 5.7% paraformaldehyde in PBS, washed twice with PBSA, and incubated for 1 hour at RT in PBSA. The cells were incubated for 3 hours at room temperature with saturating solutions of polyclonal antibodies to TSP. After three 5-minute washes in PBSA with vigorous shaking, the cells were incubated with biotinylated antirabbit IgG for 45 minutes. The monolayers were washed again 3 times as above and subsequently incubated with Texas red-conjugated streptavidin. Controls with primary antibody omitted were included in each experiment. The slides were washed briefly with PBSA, mounted with Vectashield mounting medium, and photographed at magnification ×400 with a Zeiss Axiovert 135TV microscope (Carl Zeiss, Inc, Thornwood, NY). A quantitative image processing system based on the MicroImager 1400 digital camera (Xillis Technologies, Vancouver, BC) and image analysis software, Xphoto (University of California, Berkeley, CA), was used to acquire and analyze 170 μm optical sections of cells.
Gravity adherence assays
RBC adherence to HUVECs was measured using a modification of the methods described by Sugihara et al.18 Packed RBCs were resuspended to a hematocrit of 1% in HAH. Confluent HUVEC monolayers were washed with MAH to remove traces of serum, covered with RBC suspensions, and incubated at 37°C for 25 minutes. The wells were then filled completely with MAH, sealed with packing tape, and inverted at 37°C for 20 minutes. While still inverted, the well walls and gaskets of the slide chambers were removed. The slides were rinsed in HBSS under standard conditions to remove nonadherent RBCs, fixed in 3% glutaraldehyde in PBS, stained, and mounted. RBC adherence was monitored visually by microscopy. RBCs adherent to HUVEC monolayers in 15 fields marked by a grid (same random fields for each sample) were counted at magnification ×200 and the mean adherence and variance (n = 15) were calculated. The mean of the means and standard error of the means from at least 4 experiments, analyzed in parallel, are plotted as ratios of test relative to control. Absolute values were analyzed for statistical significance by the Student t test.
To evaluate the effect of different components on the adherence of PS-exposing cells to endothelium, RBC suspensions were incubated for 5 minutes with 50 mg/mL 3d peptide or 50 mg/mL 3dS (band 3 control peptide) or with 10 mg/mL annexin V in MAH. Alternatively, RBCs were incubated for 5 minutes in HBSS with phospholipid vesicles to a final concentration of 4.8 mol/L phospholipid before addition of the RBC/vesicle mixture to the endothelial cells. The vesicles were composed of either egg phosphatidylcholine (PC) or a mixture of egg PC and bovine brain PS at a molar ratio of 4:1 (PC:PS).
RBC adherence to immobilized proteins
Flow adherence assays were performed according to the method described by Barabino et al.29 In short, 2.5 μg purified proteins were immobilized on a glass plate at 37°C for 1 hour. After washing each slide with HBSS, a parallel-plate flow chamber was mounted on the glass slide using vacuum to maintain the assembly. RBC suspensions of 1% Hct, sustained at 37°C, were drawn over the slide using a syringe pump (Harvard Apparatus, South Natick, MA) at a controlled flow rate to give a venular wall shear stress of 1 dyne/cm2. Adherent RBCs were visualized using an inverted-phase contrast microscope (DIAPHOT-TMD, Nikon, Garden City, NY) equipped with a charge-coupled device (CCD) video camera (Model 72, Dage-MTI, Michigan City, IN) All experiments were recorded. For each experiment, the protein layer was washed for 2 minutes with HBSS, followed by a 10-minute perfusion with the RBC suspension. The number of adherent RBCs remaining after a 10-minute rinse period were counted in a minimum of 12 fields. The mean of the means and standard error of the means from at least 4 experiments, analyzed in parallel, are plotted as ratios relative to control RBCs to BSA. Statistical comparison of absolute adherence values between PS-exposing and normal RBCs to each of the proteins was by the Studentt test.
Phospholipid vesicle adherence to TSP
Confluent EC monolayers were washed and treated with 0.01 mol/L phosphate, 0.15 mol/L NaCl, 5 mmol/L NaHCO3, 10 mmol/L EDTA, 0.1% BSA, 1 mmol/L phenylmethylsulfonyl fluoride, pH 7.234 to detach the ECs. The matrix was then incubated with fluorescent phospholipid vesicles in HBSS, 50 mmol/L HEPES, pH 7.4 in the presence or absence of 0.5 mmol/L CaCl2, for 3 hours at 4°C on a rotator. Nonadherent vesicles were washed off with HAH, and the slides were mounted with Vectashield mounting medium. Vesicle adherence to TSP was monitored by fluorescence microscopy at magnification ×400.
Results
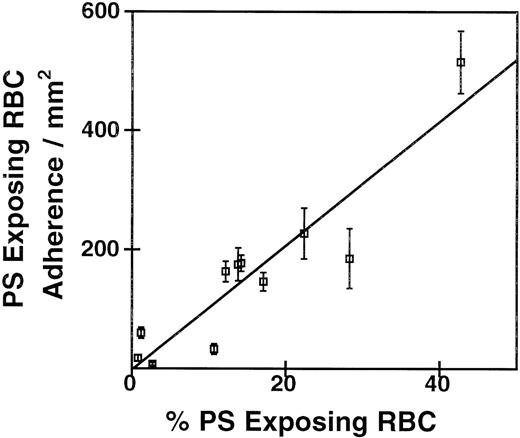
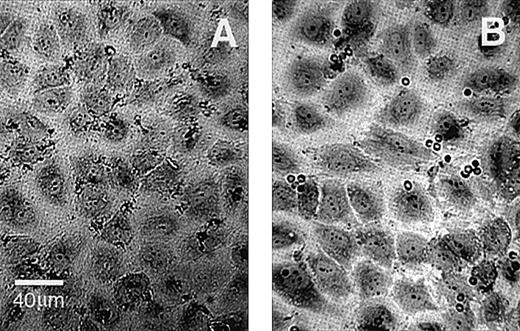
To investigate the adherence of ionophore-treated PS-exposing RBCs to HUVEC monolayers, we generated mixtures of PS-exposing RBCs and control RBCs as described in “Materials and Methods.” The number of PS-exposing cells in these mixtures was determined by annexin V labeling and flow cytometry, and the RBC mixtures were tested for adherence to confluent HUVEC monolayers by our static adherence assay. The correlation between the number of adherent RBCs in these mixtures relative to control and the percentage PS-exposing RBCs in the population is shown in Figure 1. The linear correlation (r = .94, P = .001) shows a strong dependence of RBC adherence on the percentage of calcium ionophore–induced PS-exposing cells in the suspensions. Interestingly, the incubation of confluent HUVEC monolayers with these RBC mixtures resulted in a mild retraction of the ECs, which was more pronounced as the number of PS-exposing cells increased. Figure2 shows a typical result of the adherence of RBCs from a mixture that contained 40% PS-exposing cells. The adherence of RBCs seemed to occur preferentially at the edges of the endothelial cells and in the gaps between cells. Although gap formation was less pronounced when the number of PS-exposing cells in the population decreased, RBCs were preferentially found at the edges of the ECs in all mixtures tested. For all subsequent experiments, we used mixtures of RBCs with 5% to 10% PS-exposing cells.
Correlation of PS exposure with adherence to EC monolayers.
Ionophore-treated RBCs were mixed at varying proportions with untreated RBCs. The percentage of PS-exposing RBCs was measured by FACS analysis of annexin binding. Adherence to HUVECs was by a static assay. The coefficients of the linear regression are r = 0.94,P = .001, n = 4.
Correlation of PS exposure with adherence to EC monolayers.
Ionophore-treated RBCs were mixed at varying proportions with untreated RBCs. The percentage of PS-exposing RBCs was measured by FACS analysis of annexin binding. Adherence to HUVECs was by a static assay. The coefficients of the linear regression are r = 0.94,P = .001, n = 4.
HUVEC monolayers incubated with normal or PS-exposing RBCs.
(A) Normal RBCs. (B) PS-exposing RBCs. EC retraction was caused by an incubation with an RBC population containing 40% PS-exposing cells. Images were obtained at a magnification ×400; the final scale is indicated.
HUVEC monolayers incubated with normal or PS-exposing RBCs.
(A) Normal RBCs. (B) PS-exposing RBCs. EC retraction was caused by an incubation with an RBC population containing 40% PS-exposing cells. Images were obtained at a magnification ×400; the final scale is indicated.
Because ionophore treatment will induce many changes in the red cell membrane, the role of PS needed to be confirmed. Since annexin V binds to PS-exposing RBCs, we incubated mixtures containing 5% to 10% PS-exposing RBCs with annexin V before the static adherence assay. Pretreatment of PS-exposing RBCs with annexin V significantly reduced adherence to ECs by 55% (P = .03, n = 6, Figure3), indicating that annexin V shields PS on the RBCs from interacting with the HUVEC monolayer. We hypothesized that, if PS on the surface of the RBCs served as a recognition site for the HUVEC monolayer, the presence of phospholipid vesicles that contained PS would interfere with this recognition. Figure 3 shows that the presence of phospholipid vesicles that contain 80% PC and 20% PS significantly blocked adherence by 73% (P = .007, n = 5) compared with control experiments with vesicles that contain PC only. We also tested for the effect of these components on the adherence of sickle RBCs to HUVEC monolayers. The sickle cell samples contained up to 5% PS-exposing cells, and adherence was similarly and significantly reduced by the presence of PC/PS vesicles and annexin V. The presence of PC vesicles had no effect on adherence (data not shown).
Effect of blocking agents on PS-exposing RBC adherence to EC monolayers.
PS-exposing RBC suspensions were incubated for 5 minutes with 1 of the following before layering over ECs in a static assay: buffer (Control), annexin V, PS vesicles (20% PS, 80% PC, 5 mmol/L lipid), PC vesicles (100% PC, 5 mmol/L lipid). The mean of the means and standard error of the means of 5 experiments are plotted as percentage of the buffer-treated control adherence, which is set to 100%. * Indicates significant differences from the control.
Effect of blocking agents on PS-exposing RBC adherence to EC monolayers.
PS-exposing RBC suspensions were incubated for 5 minutes with 1 of the following before layering over ECs in a static assay: buffer (Control), annexin V, PS vesicles (20% PS, 80% PC, 5 mmol/L lipid), PC vesicles (100% PC, 5 mmol/L lipid). The mean of the means and standard error of the means of 5 experiments are plotted as percentage of the buffer-treated control adherence, which is set to 100%. * Indicates significant differences from the control.
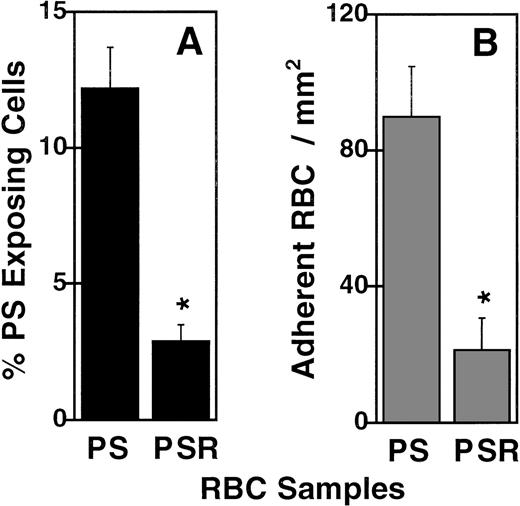
It has been reported that PS exposure in ionophore-treated RBCs can be reversed without changing other effects that the ionophore treatment has on cell size, shape, and surface qualities.31 Following this method, ionophore-treated RBCs were incubated with MgCl2and EGTA before removal of the ionophore with BSA. This resulted in a 76% decrease of PS-exposing cells from an RBC population that originally contained 12% PS-exposing cells (P = .01, n = 3, Figure 4A). This dramatic decrease in PS exposure paralleled a 76% decrease in adherence, from 89.7 ± 15.0 (SEM) to 21.2 ± 9.5 RBCs/mm2, (P = .04, n = 3, Figure 4B), suggesting that the reversal of PS exposure was an important factor in the decrease of adherence. Taken together, these data strongly suggest a role for PS on the surface of the RBCs in the recognition by and adherence to HUVEC monolayers.
Reversing PS exposure on ionophore-treated RBCs reduces RBC adherence to ECs.
Percentage annexin V positive RBC (A) and static RBC adherence to HUVECs (B) were measured using ionophore-treated (PS), or ionophore-treated and reversed RBCs (PSR). * indicates a significant reduction by the Student t test (n = 3). Error bars depict the standard error.
Reversing PS exposure on ionophore-treated RBCs reduces RBC adherence to ECs.
Percentage annexin V positive RBC (A) and static RBC adherence to HUVECs (B) were measured using ionophore-treated (PS), or ionophore-treated and reversed RBCs (PSR). * indicates a significant reduction by the Student t test (n = 3). Error bars depict the standard error.
Other factors may also play a role in adherence of these cells, in part indicated by the inability to completely block adherence under conditions that decrease the availability of PS, as described previously. We investigated potential ionophore treatment–induced exposure of the known cytoadhesive molecules, CD36 and the band 3 peptide 3d. Normal RBCs were used as negative control, and because CD36 is expressed on sickle reticulocytes, we used a sickle RBC sample with a high reticulocyte count as positive control. RBCs were labeled with MAb OKM5 to CD36, to test for the presence of this cell surface molecule. Figure 5 shows that CD36 was present in the sickle RBC samples only. No CD36 could be observed on either control RBCs or ionophore-treated, PS-exposing RBCs. Similarly, 1F4 prepared against peptide 3d (residues 547-553 of band 3) was used to test for the presence of this cryptic band 3 site on ionophore-treated cells. Fluorescent labeling of band 3 occurred only in the ionophore-treated, PS-exposing RBCs, and not on the sickle RBCs or control RBCs (Figure 5). Importantly, no nonspecific fluorescent labeling was observed when cells were labeled with secondary phycoerythrin-conjugated antibody only. These data suggest some relationship between the expression of the band 3 cytoadherence peptide and PS exposure on ionophore-treated RBCs. No indication as such could be found for CD36.
Exposure of PS, CD36, and band 3 peptide 3d on normal-, PS–exposing-, or sickle RBCs.
Normal untreated RBCs (Normal), RBCs treated with 0.5 mmol/L CaCl2, and 2 μmol/L A23187 (PS), or sickle RBCs (SS) were labeled with annexin V-FITC (solid bars), or with MAb OKM5 against CD36 (striped bars) or 1F4 against band 3 peptide 3d (shaded bars) and subsequently with antimouse/PE, and analyzed by FACS. The percentage of cells in the population positive for either of these surface markers are indicated as percentage of gated events. *Indicates significant differences by the Student t test from normal RBC labeling. Error bars depict the standard error; n = 4.
Exposure of PS, CD36, and band 3 peptide 3d on normal-, PS–exposing-, or sickle RBCs.
Normal untreated RBCs (Normal), RBCs treated with 0.5 mmol/L CaCl2, and 2 μmol/L A23187 (PS), or sickle RBCs (SS) were labeled with annexin V-FITC (solid bars), or with MAb OKM5 against CD36 (striped bars) or 1F4 against band 3 peptide 3d (shaded bars) and subsequently with antimouse/PE, and analyzed by FACS. The percentage of cells in the population positive for either of these surface markers are indicated as percentage of gated events. *Indicates significant differences by the Student t test from normal RBC labeling. Error bars depict the standard error; n = 4.
To evaluate the role of band 3 in the adherence of ionophore-treated PS-exposing RBCs, we investigated whether the 3d peptide could block adherence of ionophore-treated PS-exposing RBCs to HUVEC monolayers. A similar peptide, 3dS, consisting of the same amino acids as 3d but in scrambled order was used as control. The 3dS peptide had no effect on adherence in any experiment. The potential blocking effect of the 3d peptide differed greatly from experiment to experiment. Of 6 independent experiments, blocking was observed in 3 cases with a maximum in 1 case of 60% decrease in adherence; however, in the other 3 experiments no effect was observed. These data do not indicate a clear role for band 3 in the adherence of ionophore-treated PS-exposing cells to HUVEC monolayers. We repeated these experiments using RBCs from sickle cell patients that contained PS-exposing cells with similar results. The 3dS control peptides had no effect, whereas adherence was slightly but inconsistently reduced in some cases by 3d peptides (data not shown).
These results indicated a role for PS in the adherence of RBCs to HUVEC monolayers, and given the preferential binding to the edges of the cells and gaps between cells, suggested the importance of the endothelial matrix in the binding. We therefore decided to evaluate the adherence of PS-exposing RBCs to purified adhesive molecules of the endothelial matrix, TSP, and fibronectin. Purified proteins were immobilized on glass slides and tested for their ability to support RBC adherence under dynamic flow conditions (1 dyne/cm2). The results shown in Figure 6 were expressed relative to the adherence of normal RBCs to immobilized BSA, set arbitrarily to 1. Normal RBCs adhered only slightly to BSA and fibronectin, and significantly more so to TSP (201 ± 44, 317 ± 128, and 1822 ± 157 RBCs/mm2, respectively). PS-exposing RBCs adherence was significantly greater to BSA (405 ± 61 RBCs/mm2, P = .01) and to TSP (2895 ± 192 RBCs/mm2, P = .005) than normal RBC adherence to these proteins, and binding to fibronectin was inconsistent as indicated by the relatively large standard error. These results suggested that TSP may be an important factor in the adherence of PS-exposing cells to the edges of ECs and in the gaps of HUVEC monolayers.
Adherence by shear flow of normal- or PS-RBCs to immobilized matrix proteins.
Purified BSA, fibronectin, or thrombospondin were immobilized onto glass slides for 1 hour before being exposed to normal (solid bars) or PS-exposing (shaded bars) RBCs at 1 dyne/cm2 in a dynamic flow assay. RBC adherence is reported as a ratio, relative to normal RBC adherence to BSA, which was arbitrarily set to 1. Error bars represent standard error of the means calculated from the absolute values and normalized relative to normal RBC adherence to BSA. *Indicates significant differences between normal- and PS-exposing RBC adherence to BSA (P = .01, n = 5) and between normal- and PS-exposing RBC adherence to TSP (P = .005, n = 5).
Adherence by shear flow of normal- or PS-RBCs to immobilized matrix proteins.
Purified BSA, fibronectin, or thrombospondin were immobilized onto glass slides for 1 hour before being exposed to normal (solid bars) or PS-exposing (shaded bars) RBCs at 1 dyne/cm2 in a dynamic flow assay. RBC adherence is reported as a ratio, relative to normal RBC adherence to BSA, which was arbitrarily set to 1. Error bars represent standard error of the means calculated from the absolute values and normalized relative to normal RBC adherence to BSA. *Indicates significant differences between normal- and PS-exposing RBC adherence to BSA (P = .01, n = 5) and between normal- and PS-exposing RBC adherence to TSP (P = .005, n = 5).
To test whether PS-exposing RBC adherence would be further enhanced by increasing the TSP exposure, we treated ECs with agents to cause the cells to retract, as indicated in “Materials and Methods.” Panels A and B of Figure 7 show buffer- or histamine-treated EC monolayers immunolabeled with polyclonal antibodies to TSP. Panel A shows that in confluent monolayers, TSP is entirely covered with ECs and unavailable to the antibody. In contrast, histamine treatment resulted in abundant matrix TSP exposure (panel B). Similar results are found after treating ECs with thrombin or EDTA, agents that also lead to retraction of endothelial cells (data not shown). When ECs were pretreated with thrombin, histamine, or EDTA, adherence of PS-exposing RBCs increased significantly, approximately 2-fold (Figure 7, panel C).
Effect of EC retraction on matrix TSP exposure and PS-exposing RBC adherence.
EC monolayers were pretreated with buffer (A) or histamine (B), labeled with antibodies to TSP, and viewed by fluorescent microscopy. Histamine-induced EC contraction exposed TSP (B), which is normally cryptic (A). Pretreatment with thrombin (white bar), histamine (striped bar), or EDTA (shaded bar) causes enhanced adherence of PS-exposing RBCs (C). Adherence is reported relative to the adherence of the untreated control (solid bar), which was arbitrarily set to 1. Error bars represent standard error of the means calculated from the absolute values from 4 experiments and normalized relative to the adherence of the buffer-treated control. *Indicates significant differences between PS-exposing RBC adherence to ECs treated with buffer, and thrombin, histamine, or EDTA.
Effect of EC retraction on matrix TSP exposure and PS-exposing RBC adherence.
EC monolayers were pretreated with buffer (A) or histamine (B), labeled with antibodies to TSP, and viewed by fluorescent microscopy. Histamine-induced EC contraction exposed TSP (B), which is normally cryptic (A). Pretreatment with thrombin (white bar), histamine (striped bar), or EDTA (shaded bar) causes enhanced adherence of PS-exposing RBCs (C). Adherence is reported relative to the adherence of the untreated control (solid bar), which was arbitrarily set to 1. Error bars represent standard error of the means calculated from the absolute values from 4 experiments and normalized relative to the adherence of the buffer-treated control. *Indicates significant differences between PS-exposing RBC adherence to ECs treated with buffer, and thrombin, histamine, or EDTA.
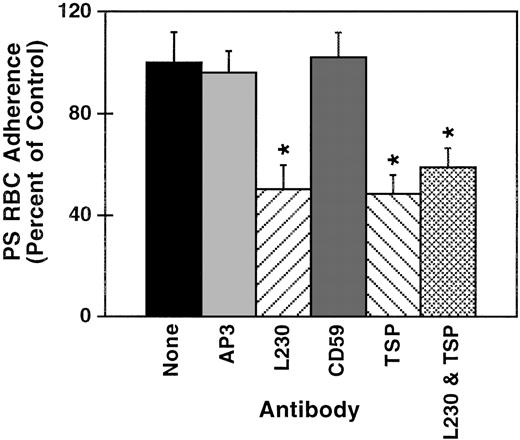
When ECs were layered with PS-exposing RBCs in the presence of polyclonal antibodies to TSP, the adherence was reduced significantly by 52% (P = .04, n = 4, Figure8). Because anti-TSP did not completely block adherence, we also tested for an inhibiting effect of antibodies to its receptor, αvβ3. PS-exposing RBC adherence to ECs was reduced 50% (P = .05, n = 4) by L230 a monoclonal antibody to αvβ3. Both antibodies together did not additionally block adherence, suggesting a synergistic rather than an additive effect, and that the receptor-bound TSP conformer might be necessary to mediate PS-exposing RBC adherence to EC monolayers. Control antibodies had no effect on adherence.
Effect of blocking antibodies on PS-exposing RBC adherence to EC monolayers.
Confluent EC monolayers were washed and layered in a static assay with PS-exposing RBC suspensions in the presence of 1 of the following: polyclonal antibodies to CD59 or TSP, monoclonal antibodies to αv (L230), or a nonblocking monoclonal antibody to β3 (AP3), or in buffer alone (control). Results are reported as percentage of the control adherence, which is set to 100%. Error bars represent standard error of the means calculated from the absolute values from 4 experiments and normalized relative to the adherence of the buffer-treated control. *Indicates significant differences from the control by the Student t test.
Effect of blocking antibodies on PS-exposing RBC adherence to EC monolayers.
Confluent EC monolayers were washed and layered in a static assay with PS-exposing RBC suspensions in the presence of 1 of the following: polyclonal antibodies to CD59 or TSP, monoclonal antibodies to αv (L230), or a nonblocking monoclonal antibody to β3 (AP3), or in buffer alone (control). Results are reported as percentage of the control adherence, which is set to 100%. Error bars represent standard error of the means calculated from the absolute values from 4 experiments and normalized relative to the adherence of the buffer-treated control. *Indicates significant differences from the control by the Student t test.
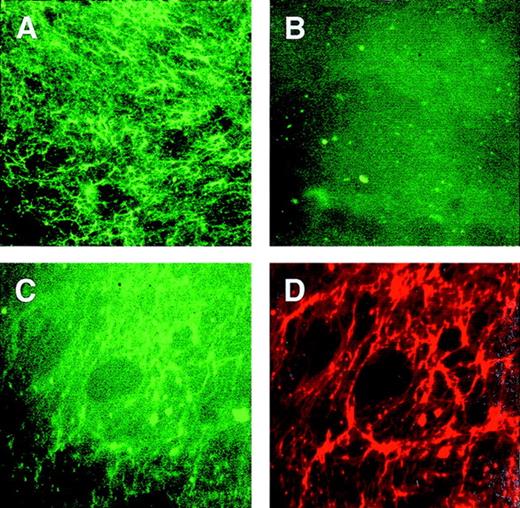
To test for the interaction between PS and TSP in the matrix, we prepared fluorescent phospholipid vesicles that contained 50% PS. Pure PC vesicles served as control. The ECs were gently removed from the matrix and the presence of TSP in the remaining surface was confirmed by using TSP antibody and fluorescence microscopy. The EC-depleted matrix was then incubated with lipid vesicles in the presence or absence of calcium, and subsequently labeled for immunofluorescence by polyclonal antibodies to TSP. Figure 9shows that PS vesicles (A), but not PC vesicles (B), bound to matrix proteins. Panels C and D are micrographs of the matrix incubated first with PS vesicles and then with polyclonal antibodies to TSP. These 2 micrographs are of the same field showing that the PS vesicles (green fluorescence, panel C) bound to TSP (red fluorescence, panel D). Although much of the lipid vesicles in panel C had been displaced by the antibody labeling (note the difference between panels A and C), this data shows the interaction between PS vesicles and TSP, which was not detected between PC vesicles and TSP. No difference in PS vesicle binding to TSP was found due to the presence or absence of calcium.
PS vesicles adhere to matrix TSP.
EC-depleted matrix was incubated with lipid vesicles and subsequently labeled for immunofluorescence by polyclonal antibodies to TSP and streptavidin-Texas Red. Panel A was labeled with PS vesicles; panel B was labeled with PC vesicles. Panels C and D are of the same fields labeled with both PS vesicles and TSP; C was photographed with excitation for green fluorescence to detect the phospholipid labeling, and D was photographed with excitation for red fluorescence to detect TSP.
PS vesicles adhere to matrix TSP.
EC-depleted matrix was incubated with lipid vesicles and subsequently labeled for immunofluorescence by polyclonal antibodies to TSP and streptavidin-Texas Red. Panel A was labeled with PS vesicles; panel B was labeled with PC vesicles. Panels C and D are of the same fields labeled with both PS vesicles and TSP; C was photographed with excitation for green fluorescence to detect the phospholipid labeling, and D was photographed with excitation for red fluorescence to detect TSP.
Discussion
Phospholipid asymmetry is well maintained in normal plasma membranes, and the loss of this asymmetry with the exposure of PS at the outer surface of the cell has significant physiologic consequences.1 The importance of PS availability at the cell surface as a docking site for factors in the hemostatic system has been very well characterized.2 In addition, it has become apparent that exposure of PS in the early stages of programmed cell death is seminal for the recognition and removal of the apoptotic cell.35 Hence, the presence of PS on the surface may be a trigger for cell-cell interaction, and in this case, RBC-endothelial interaction.
This interaction seems of particular physiologic consequence in those disorders where PS exposure on RBCs as well as vascular damage or blood flow complications are indicated. These conditions include sickle cell disease, thalassemia, diabetes, and malaria. Subpopulations of PS-exposing RBCs have been reported in patients with sickle cell and thalassemia4-6 and a correlation was found between the risk for stroke and PS exposure in sickle disease.8 Before the presence of PS-exposing sickle cells in vivo was established,4,5,36 it was suggested that the increased propensity of sickle RBCs to adhere to the vascular wall was related to the abnormal exposure of aminophospholipids in the external leaflet of the RBC membrane.9 This was based on the loss of normal membrane structure of sickle cells incubated under low oxygen tension in vitro. A similar loss of phospholipid asymmetry under low oxygen tension in vivo could in turn increase the interaction with endothelial cells and play a role in vaso-occlusive crisis. We argued that sickling was not necessary for this interaction between RBCs and endothelium, given the presence of a subpopulation of PS exposing RBCs under normal oxygen tension. To test this hypothesis we evaluated the in vitro adherence of PS-exposing RBCs (obtained by calcium loading normal RBCs) to endothelial monolayers.
Our data indicate not only a role for PS but also show the involvement of factors in the matrix, in particular TSP, in the binding of PS-exposing RBCs. The strong correlation between adherence and the number of exposing PS RBCs supports the hypothesis made previously by others9-11,37 that loss of membrane asymmetry may lead to RBC-EC binding. A role for PS in this binding process is indicated by the reduction of adherence when PS on the RBCs is blocked with annexin V. In addition, a significant reduction in adherence was assessed by incubation in a magnesium/EGTA environment, which reversed the PS exposure in ionophore-treated RBCs.31 Moreover, the presence of PS-containing vesicles competed with PS-exposing RBCs for the apparent binding sites on the HUVEC monolayer. In these experiments, it also became apparent that the adherence of RBCs occurred preferentially at the edges of the endothelial cells in the gaps between the cells. This localized interaction was confirmed by the increase of adherence in these areas when endothelial cells were retracted by other factors such as histamine, thrombin, or EDTA before addition of PS-exposing RBCs. Taken together, these data strongly suggest a role for PS in the adherence of PS-exposing RBCs to (damaged) endothelium.
Our data do not exclude that other factors may be additionally important for binding of RBCs to EC monolayers. In particular, RBC binding to sites of exposed TSP may point at factors such as CD36 or band 3 peptide 3d. The thrombospondin receptor CD36, found predominantly on reticulocytes, has been shown to mediate sickle RBC adherence to ECs.18 However, we did not find the appearance of CD36 on ionophore-treated RBCs. In Plasmodium falciparum–infected RBCs, the principal integral protein of the membrane, band 3, aggregates in the membrane bilayer. Structural modifications occur and normally cryptic residues become exposed. Peptides designed to the exposed epitopes of band 3 block adherence of malaria infected RBCs to ECs.38 Clustering of band 3 also occurs in sickle RBCs and the same synthetic 3d peptides were reported to block adherence of sickle- and calcium-loaded RBCs.11 25Antibody binding to ionophore-treated RBCs indeed confirmed the presence of this site on PS-exposing RBCs. Under our conditions, however, the addition of the synthetic 3d peptide seemed to reduce adherence of ionophore-treated cells only in some cases, with rather inconsistent results. Although our data do not exclude a role for other factors such as band 3, they indicate a major role for PS in the adherence of PS-exposing cells to HUVEC monolayers, and in particular to factors in the matrix.
The role of matrix TSP was suggested by the binding of PS-exposing RBCs to immobilized purified TSP. The presence of antibodies to TSP and its receptor αvβ3, decreased PS-exposing RBC adherence to HUVEC monolayers. And the presence of PS in pure lipid vesicles led to the binding of these vesicles to EC denuded matrix. These data indicate a direct interaction between PS-containing lipid surfaces and TSP. Such an interaction of TSP and PS-exposing cells had not been previously demonstrated. On the contrary, PS- and TSP-mediated recognition of apoptotic neutrophils by macrophages has been shown to be mutually exclusive.14 But, in the studies so far undertaken on the recognition of PS-exposing cells, a bridging mechanism of soluble TSP was sought. Because the functional properties of TSP depend on its conformation, dictated by the particular environment in which it is found,21 the interaction between PS and TSP apparently necessitates binding properties characteristic of immobilized TSP, either on glass or in the extracellular matrix. In addition, the reduction in adhesion by both polyclonal antibodies against TSP and a monoclonal antibody against the active binding site of αvβ3 suggest that the interaction of both components is important in the binding of PS-exposing RBCs to matrix TSP. This may explain the preferential binding of the RBCs at the edges of the retracted ECs.
This finding seems relevant in pathologies in which vascular injury is prevalent and in which a subpopulation of PS-exposing RBCs is a characteristic, such as sickle cell disease. A number of studies indicate endothelial damage in this disease. Circulating ECs have been observed,39 suggesting vascular injury and matrix exposure. Abnormal levels of inflammatory agents, interleukin-1 (IL-1), TNF, thrombin, or histamine are often found in patients with sickle cell disease.22,40 In vitro, these agents cause EC activation, barrier dysfunction due to EC retraction, and increased RBC adherence,17,41-43 presumably to the exposed matrix. Sickle RBC adherence to matrix components has been studied by dynamic flow of RBCs over immobilized purified proteins. A role for von Willebrand factor, laminin, and collagen I is supported by these works.28,29,44-47 Moreover, sickle RBCs adhere unequivocally to immobilized TSP,29,30,48 an interaction influenced by fibronectin, von Willebrand factor and anionic polysaccharides,24 and recently, a site that binds sickle RBCs has been mapped within the C-terminal cell-binding domain of TSP.48
Our studies suggest that the PS-exposing subpopulation of cells may play an additional important role in adherence of RBCs by causing EC retraction. The mechanism by which PS-exposing RBCs cause barrier dysfunction can only be speculated. One option is the fact that ionophore treatment results in the release of lipid breakdown products that modulate EC barrier properties. RBCs contain a phospholipase D, that is activated under conditions in which PS is exposed on the surface of the cell generating phosphatidic acid,49 and it has recently been reported that phosphatidic acid disrupts barrier integrity.50 Although it may be tempting to suggest a role of phosphatidic acid in EC activation, this remains to be proven.
In conclusion, our data indicate a role for PS on the surface of the RBCs in adherence to endothelial cell monolayers. This interaction preferentially occurs in areas where the normal confluent cell monolayer is disrupted, involves matrix proteins such as thrombospondin, and is relevant in pathologies in which vascular injury occurs in the presence of subpopulations of PSexposing RBCs.
Acknowledgments
We would like to thank Dr Narla Mohandas, Dr Dan Callahan, and Kevin Benson for their assistance with fluorescence microscopy, and Eileen Finnegan for technical assistance.
Sponsored by grants no. HL55213, DK32094, HL20985, and M01RR01271 from the National Institute of Health.
Reprints:Annamaria Manodori, Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr Way, Oakland, CA 94609.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.