Alterations in hematopoiesis are common in experimental infectious disease. However, few studies have addressed the mechanisms underlying changes in hematopoietic function or assessed the direct impact of infectious agents on the cells that regulate these processes. In experimental visceral leishmaniasis, caused by infection with the protozoan parasite Leishmania donovani, parasites persist in the spleen and bone marrow, and their expansion in these sites is associated with increases in local hematopoietic activity. The results of this study show that L donovani targets bone marrow stromal macrophages in vivo and can infect and multiply in stromal cell lines of macrophage, but not other lineages in vitro. Infection of stromal macrophages increases their capacity to support myelopoiesis in vitro, an effect mediated mainly through the induction of granulocyte macrophage-colony stimulating factor and tumor necrosis factor-. These data are the first to directly demonstrate that intracellular parasitism of a stromal cell population may modify its capacity to regulate hematopoiesis during infectious disease.
Within the extravascular spaces of the bone marrow, hematopoietic stem cells and progenitor cells are found in association with a network of hematopoietic and nonhematopoietic cells termed the stroma. Stromal elements may regulate the discrete spatial organization of progenitor cells in vivo,1-3 and the regulation of progenitor activity also depends on cytokines and extracellular matrix components secreted by the stroma.4-6 Many of these events can be recapitulated in long-term bone marrow cultures (LTBMC).7 LTBMC support ex vivo hematopoiesis in the absence of exogenous growth factors, dependent on the generation of an adherent layer of stromal cells. LTBMC produce many cytokines with recognized hematopoietic activity, including granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), interleukin (IL)-3, IL-1, IL-7, Flt-3 ligand, stem cell factor (SCF), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and macrophage inflammatory protein-1α (MIP-1α), as do stromal cell lines isolated from these cultures.8-11 Long-term stroma-derived cell lines also display a variety of phenotypes representative of macrophage, endothelial, fibroblast, adipocyte, osteoclast, and reticular cell origin.3,10 12
Alterations in hematopoiesis are commonly associated with infection by viral, bacterial, and protozoan pathogens. For example, hematopoiesis is suppressed during experimental infection with murine cytomegalovirus,13-16 LP-BM5 murine leukemia virus,17 and Salmonella typhimurium.18In the case of LP-BM5 infection, inhibition of hematopoiesis is associated with altered cytokine production, specifically the induction of TGF-β and IL-4, and the inhibition of GM-CSF.17,19,20In addition, increased hematopoiesis has also been noted in experimental malaria,21,22 schistosomiasis23,24and leishmaniasis.25 Increased myelopoiesis during the late stages of visceralizing infection with L major in BALB/c mice has been suggested to provide “safe targets” for parasite replication and to be predominantly a consequence of increased IL-3 production by Th2 cells.25 26 Yet, despite these numerous observations, few studies have specifically addressed whether pathogens directly influence the function of either hematopoietic stem cells and progenitor cells or the stromal elements themselves.
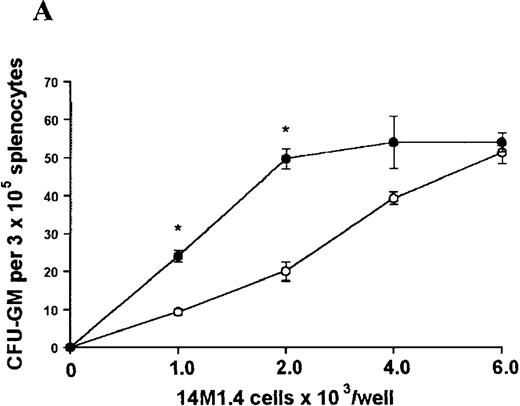
We have recently analyzed the alterations in hematopoietic activity seen in BALB/c mice infected with L donovani72, an obligate intracellular parasite of macrophages. In this model of visceral leishmaniasis (VL), parasites replicate within Kupffer cells in the liver over the first 28 days of infection and are then cleared by a T-cell-dependent granulomatous response.27-29 Bone marrow-derived monocytes are also essential for effective clearance of parasites from this organ30 and are likely to be activated to a leishmanicidal state by the predominantly Th1 cytokine environment of the granuloma.30-34 In contrast to events in the liver, parasites are initially contained within the spleen and bone marrow, with limited replication over the first 28 days of infection. However, after this time parasites expand in numbers and thereafter maintain a persistent infection.35 36 Analysis of hematopoietic progenitor cell activity in both the spleen and bone marrow at this critical time of infection indicates a marked increase in progenitor cell numbers and proliferative activity. In the spleen, this was selective for myelopoiesis, in that the numbers of colony-forming unit-granulocyte, monocyte (CFU-GM) increased 20- to 30-fold, compared to 5- to 10-fold increases in the numbers of burst forming unit-erythrocyte (BFU-E) and colony-forming unit-granulocyte, erythrocyte, monocyte, megakaryocyte (CFU-GEMM). The onset of hematopoietic activity and parasite expansion in the spleen and bone marrow suggests that the 2 events may be related.
In the present study, we sought to identify the factors associated withL donovani infection, which regulate hematopoiesis, by studying the interaction between this intracellular pathogen and stromal cells responsible for regulating hematopoietic colony formation. Our results indicate that stromal macrophages are a target for L donovaniinfection in vivo and in vitro, and that as a consequence of the selective induction of GM-CSF and TNF-α production, infected stromal macrophages preferentially support increased levels of myelopoiesis. This is the first demonstration of an intracellular pathogen directly modifying stromal macrophage function.
Materials and methods
Animals and parasites
Female BALB/c mice, 6 to 8 weeks of age, were obtained from Tuck and Co. (Battlesbridge, UK) and housed under conventional conditions, with food and water provided ad libitum. Parasites of the Ethiopian strain of L donovani (LV9) were maintained by passage in Syrian hamsters. Amastigotes were isolated from the spleen of an infected hamster by homogenization and saponin lysis as described elsewhere.35 Amastigotes were counted using a Thoma bacteriologic counting chamber (Weber Scientific International, Middlesex, UK) and resuspended in complete RPMI (RPMI1640, supplemented with 1 mM sodium pyruvate, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin (all Gibco, Paisley, UK).
Primary cells and cell lines
The RAW .264 cell line (American Type Culture Collection, Rockville, MD) was maintained in complete RPMI containing 10% heat-inactivated (HI) fetal calf serum (FCS). Bone marrow stromal cell lines were derived via limiting dilution culture of stromal components of LTBMC and characterized according to morphology, cytochemistry and their ability to produce extracellular matrix components.12,37,38The bone marrow stroma-derived adventitial reticular cell line +/+-1.LDA11 (a gift from Dr Scott Boswell, Indiana University School of Medicine, Indianapolis, IN37), was maintained in complete RPMI containing 10% HI FCS. The bone marrow stroma-derived cell lines MBA1 and MBA1.1 (both fibroblast), MBA 13 (fibroendothelial), and 14M1.4 (macrophage; all gifts from Dr D. Zipori, Weizmann Institute of Science, Rehovot, Israel12 38) were maintained in complete Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% HI FCS. In the case of the 14M1.4 line, 15% (v/v) supernatant from confluent L929 cells (European Collection of Animal Cell Cultures, Salisbury, UK), was added when the cells were at low density, as a source of M-CSF.
The progenitor cell line LyD9 (a gift from Dr T. Honjo, Kyoto University Faculty of Medicine, Kyoto, Japan39) was maintained in complete RPMI with 10% HI FCS and 150 U/mL rmIL-3 (R&D Systems, Oxford, UK). The progenitor cell line FDCP-Mix (a gift from Dr E. Spooncer, Paterson Institute for Cancer Research, Manchester, UK40) was maintained in Fischer's medium (Gibco) supplemented with 20% (v/v) horse serum (Stem Cell Technology, Metachem Diagnostics, Northampton, UK) and 150 U/mL rmIL-3.
Immunohistology of bone marrow
Femurs were prepared for cryosectioning as detailed elsewhere.41 42 They were fixed by overnight incubation at 4°C in periodate-lysine-paraformaldehyde (0.1 mol/L sodium periodate dissolved in 3 parts 0.1 mol/L lysine-HCl, 0.05 mol/L Na2PO4, pH 7.4, and 1 part 4% [w/v] paraformaldehyde), 5.4% (w/v) glucose (both BDH, Leicestershire, UK) in deionized water). Femurs were then transferred to 10% (w/v) EDTA, 0.1 mol/L Tris (both BDH), pH 6.95 for 5 days at 4°C to allow decalcification. Following a final overnight incubation in 15% (w/v) sucrose (BDH) in phosphate-buffered saline (PBS) at 4°C, femurs were embedded in OCT compound (Raymond Lamb, London, UK) on a cork block. Blocks were snap frozen in isopentane (BDH) cooled in liquid nitrogen, then stored at −70°C until required. Then 4-μm cryosections were cut onto polylysine glass slides (BDH) using a cryostat (Bright Instrument Co. Ltd., Huntingdon, UK) and air dried prior to fixation with ice-cold acetone for 10 minutes at room temperature. Sections were blocked with 1.5% (v/v) serum in PBS for 30 minutes and then incubated with mAb SER4 (rat IgG2a antimouse sialoadhesin; a gift from Dr P. Crocker, University of Dundee, UK) or RAM34 (rat IgG2a anti-mouse CD34; Pharmingen, San Diego, CA). After 30 minutes, sections were washed in PBS, and then incubated with 5μg/mL biotinylated rabbit antirat IgG (Vector Laboratories, Peterborough, UK). After 30 minutes, sections were washed, endogenous peroxidase activity quenched (0.3% [v/v] H2O2 in methanol) and avidin biotinylated-HRP complexes (Vector Laboratories) were added. Sections were developed using 3,3-diaminobenzidine tetrahydrochloride developing substrate (Vector Laboratories), which was terminated in tap water. Sections were counterstained with Harris hematoxylin (Sigma, Poole, UK), dehydrated through ethanol and xylene and mounted in DePeX (BDH). At least 2 sections were examined from 3 individual mice per time course and from 2 independent experimental infections.
Preparation of splenocyte and bone marrow single cell suspensions
Mice were killed by cervical dislocation and the spleen and femurs removed. Spleen cell suspensions were made using a 20-μm nylon sieve, and cells were then washed by centrifugation (1200 rpm, 10 minutes, room temperature). Erythrocytes were lysed with Tris ammonium chloride (140 mM NH4Cl; 17 mM Tris, pH 7.5; 5 minutes at room temperature) and cells subsequently were counted using a hemocytometer (Weber Scientific International). Femurs were flushed with ice-cold complete RPMI using a 23-gauge needle, and the resulting cell suspension was treated as above.
In vitro infection of cell lines
Cells were harvested and resuspended (5 × 106 in 1 mL complete RPMI) in 14 mL polypropylene tubes (Falcon, Marathon Laboratory Supplies, London, UK). Freshly isolated L donovaniamastigotes were added to the cells at various multiplicities of infection, ranging from 5 to 50:1, to give a total volume of 2 mL/sample. After gentle mixing, tubes were incubated at 37°C in 5% (v/v) CO2 for 1 hour. Unphagocytosed parasites were removed by 3 washes in complete RPMI (1200 rpm, 10 minutes at room temperature). The efficiency of infection (intracellular parasites per 100 cell nuclei) was determined from triplicate samples of Geimsa-stained cytospin preparations either immediately after washing or after incubation in complete RPMI plus 10% HI FCS for up to 72 hours at 37°C in 5% (v/v) CO2. Infected cell samples of 5 × 106 cells were prepared for RNA extraction by resuspension of the washed cell pellet in 1 mL TRI-reagent (Sigma) or for coculture in hematologic assays by resuspension in Iscove's modified Dulbecco's medium (IMDM; Gibco).
In vitro colony assays
Control uninfected or L donovani infected 14M1.4 cells were added to the wells of a 24-well plate (Falcon) and allowed to adhere for 2 hours at 37°C. Bone marrow and spleen cell suspensions were washed and resuspended in complete IMDM (Gibco). SCF (25 μg/mL; R&D Systems) and hemin (bovine hemin chloride, 100 μM; Sigma) were added to each sample to give a final volume of 100 μL, followed by 900 μL Methocult 3430 (comprising 0.1% [w/v] methylcellulose, 30% [v/v] FCS, 1% [w/v] bovine serum albumin [BSA], 100 μM 2-ME, 2mMl-glutamine, 2% [v/v] pokeweed mitogen-stimulated murine spleen cell conditioned medium [PWM-SCCM], 3 U/mL rh erythropoietin [Epo]; Stem Cell Technology). Alternatively, bone marrow or spleen cell suspensions were resuspended in IMDM plus hemin only, followed by 900 μL Methocult 3230 (comprising 0.1% [w/v] methylcellulose, 30% [v/v] FCS, 1% [w/v] BSA, 100 μM 2-ME, 2mMl-glutamine; Stem Cell Technology), in the absence of growth factors. Cells plus Methocult were mixed thoroughly and then triplicate samples of 250 μL were dispensed into the wells of a 24-well tissue culture plate containing adhered 14M1.4 cells or medium alone. The final number of cells plated per well was 2.0 × 104 bone marrow cells or 3.0 × 105 spleen cells. In some experiments, neutralizing antibodies to TNF-α (hamster mAb TN319.12, a gift from Professor R. D. Shreiber, University of Washington, St. Louis, MO); GM-CSF (hamster mAb MP1-22E9.11, a gift from Dr G. Bancroft, LSHTM); and MIP-1α (goat IgG; R&D Systems) or control hamster mAb (GL117.41, a gift from Dr G. Bancroft) and goat IgG (R&D Systems) were added to 14M1.4/splenocyte coculture assays. Antibodies were diluted in IMDM to 3 to 30 μg/mL, and added to the 14M1.4 cells prior to addition of Methocult 3230 (Stem Cell Technology). For transwell experiments, additional 14M1.4 cells diluted in 200 μL complete IMDM plus 30% FCS (v/v) were added to cell culture inserts (0.45-μm polycarbonate membrane; Falcon).
In all experiments, plates were examined microscopically for colony growth after 7 days incubation at 37°C in 5% (v/v) O2and 5% (v/v) CO2. Colonies (> 50 cells) were scored as either CFU-GEMM, visible as circular colonies with characteristic brown/pink coloring; CFU-GM, spherical or dispersed clear/gray colonies; or BFU-E, multicentred colonies with characteristic brown/pink coloring. In transwell experiments, inserts were gently lifted out of culture wells, to enable examination of colonies that had developed underneath. To confirm CFU identification, colonies were picked and cytospun onto glass microscope slides. They were then fixed with methanol and stained for hemoglobin content with a 5:1:1 mixture of 0.2% (w/v) O-dianisidine in methanol/ 3% (v/v) hydrogen peroxide solution/1% (w/v) sodium nitroferricyanide solution (all Sigma), for 10 minutes at room temperature in the dark. Slides were rinsed under tap water and then counterstained with Giemsa (BDH). Erythroid cell types could be identified microscopically as those exhibiting a positive brown/yellow cytoplasmic reaction for hemoglobin, in contrast to myeloid cells showing a characteristic blue/gray cytoplasmic staining.43
Measurement of cytokine and chemokine mRNA accumulation
Messenger RNA (mRNA) was isolated and analyzed using a semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) assay as previously described.44 All PCR primers and probes were the same, with the addition of GM-CSF,45G-CSF,46 M-CSF,46 MIP-1α47 and SCF.46 All PCR cycles consisted of a denaturation step of 1 minute at 95°C, an annealing step of 1 minute at 55°C, and an extension step of 2 min at 72°C. PCR products were Southern blotted, and visualized using horseradish-peroxidase conjugated cytokine-specific oligonucleotide probes (R&D Systems) reacted with an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK). The intensity of signal generated by mRNA encoding the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) was used to ensure approximately even loading of target cDNA into PCRs. Densitometric analysis was subsequently performed such that levels of cytokine mRNA accumulation were expressed in arbitrary densitometry units normalized for the expression of HPRT. Graphic results are presented as the mean cytokine/HPRT ratio of 4 PCR samples analyzed individually at each time point.
Statistical analysis
Statistically significant differences between groups were determined using the unpaired Student t test, using Fig. P. software (Biosoft, Cambridge, UK).
Results
Stromal cells rather than progenitor cells are infected with L donovani
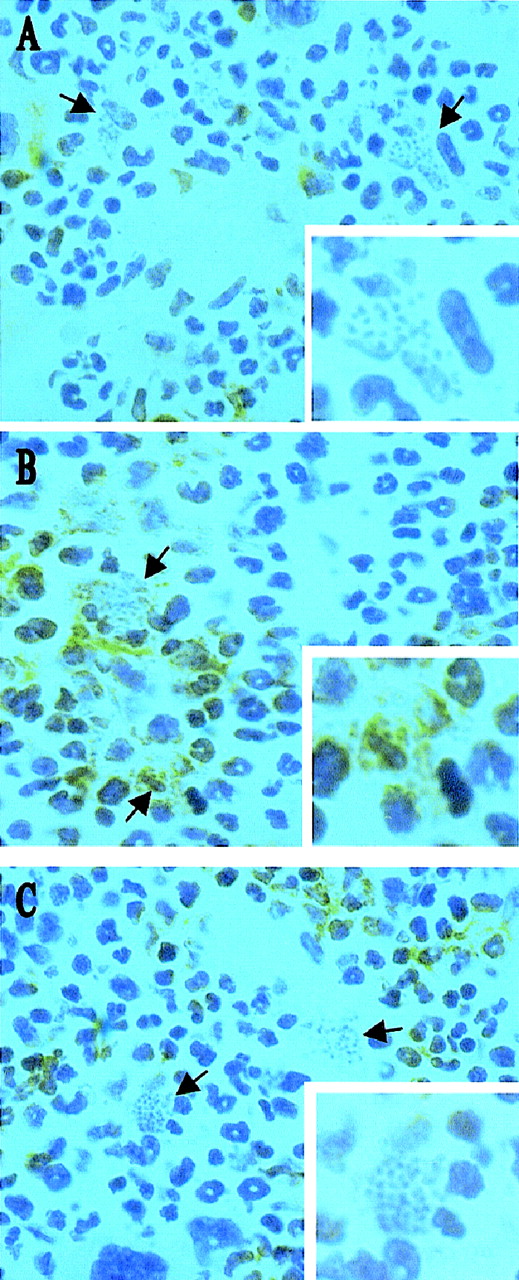
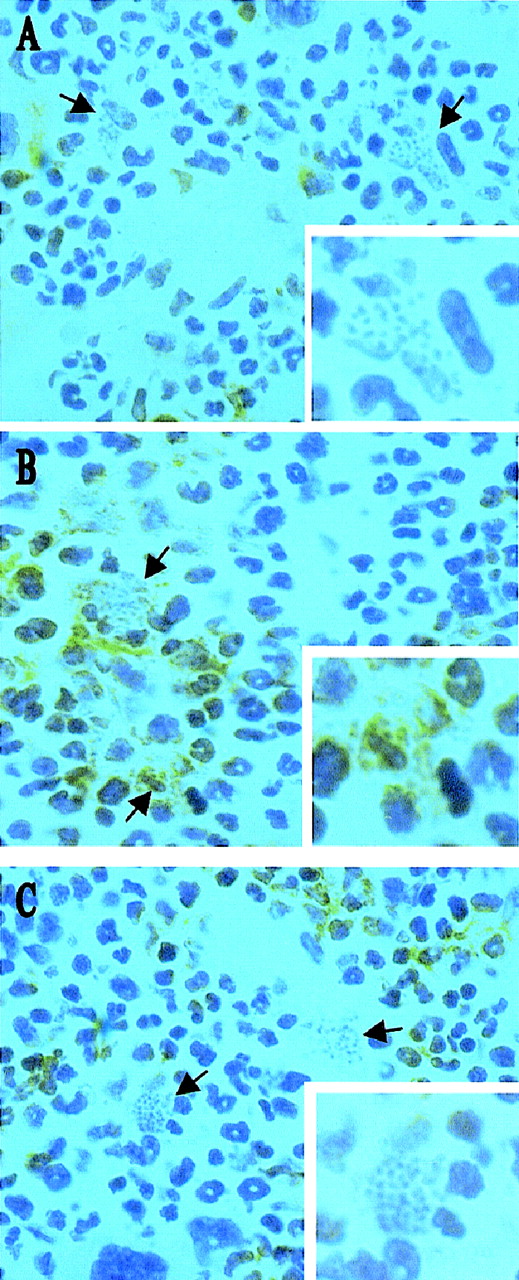
Infection with L donovani may regulate hematopoietic activity via direct infection of progenitor cells or via infection of an hematopoietic stromal cell population. To distinguish between these possibilities, we initially used immunohistochemistry to identify the targets of L donovani infection in the bone marrow of infected BALB/c mice. Cryosections of decalcified femur were stained for expression of CD34 (a marker of hematopoietic stem cells, progenitor cells, vascular endothelia, and embryonic fibroblasts48,49) and SER-4 (a sialoadhesin expressed on stromal macrophage populations of lymphohematopoietic tissue, but not on developing mononuclear phagocytes50). Intracellular amastigotes were readily detected by their morphologic appearance after routine hematoxylin counterstaining. CD34+ cells in the femur of BALB/c mice displayed various morphologies and intensities of staining, consistent with the expression of this antigen on a range of hematopoietic precursor cells in adult mice (Figure1A). Amastigote-containing cells were often in close proximity to CD34+ cells, but the latter rarely contained parasites. In contrast, SER-4+ cells had morphology typical of resident stromal macrophages (Figures 1B and 1C; also see reference 50) and amastigotes were clearly visible within both SER-4+ (see Figure 1B) and SER-4− (see Figure 1C) cells in the bone marrow of infected mice. Hence, stromal macrophages are a subset of the infected mononuclear phagocyte population of the infected bone marrow, whereas CD34+progenitor cells do not appear to be targets for infection.
L donovani infects stromal macrophages but not progenitor cells in the bone marrow of BALB/c mice.
Whole femurs were removed from BALB/c mice at day 28 after infection, decalcified, and stained for CD34 (A) and SER 4 (B and C). Sections were counterstained with Harris hematoxylin. Arrows indicate cells containing L donovani amastigotes (magnification × 100). Insert shows a single L donovani-infected cell (magnification × 200).
L donovani infects stromal macrophages but not progenitor cells in the bone marrow of BALB/c mice.
Whole femurs were removed from BALB/c mice at day 28 after infection, decalcified, and stained for CD34 (A) and SER 4 (B and C). Sections were counterstained with Harris hematoxylin. Arrows indicate cells containing L donovani amastigotes (magnification × 100). Insert shows a single L donovani-infected cell (magnification × 200).
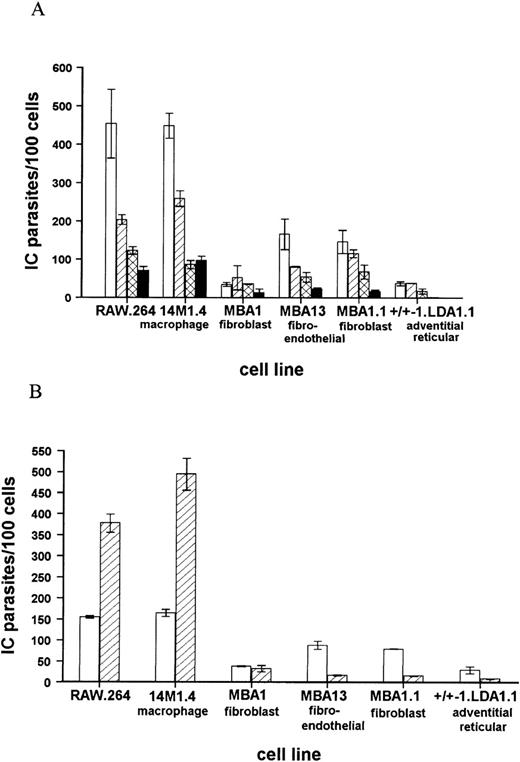
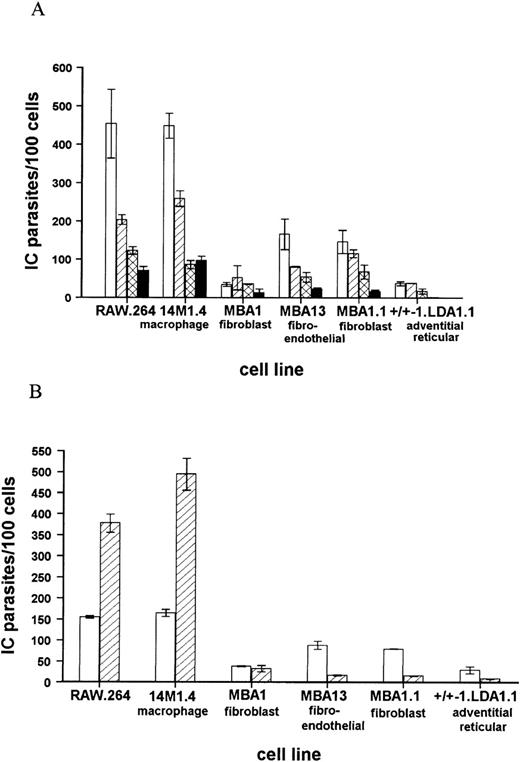
These conclusions were confirmed and extended by in vitro coculture of amastigotes with a panel of progenitor and stromal cell lines. In each case, comparison was made with the levels of infection seen in the macrophage tumor line RAW .264. The progenitor cell lines LyD939,51-53 and FDCP-Mix40 were either not infected or minimally infected by amastigotes. For example, at a multiplicity of infection of 50 amastigotes per cell, LyD9 cells did not phagocytose amastigotes, and the infection level in FDCP-mix was only 6.5 ± 0.5 amastigotes per 100 cells. In comparison, RAW .264 macrophages were heavily infected under the same conditions (425.0 ± 71.2 amastigotes per 100 cells). In contrast to the failure of progenitor cells to phagocytose significant numbers of amastigotes, a panel of stromal cell lines derived from long-term bone marrow cultures12,37 were readily infected with amastigotes (Figure 2). The macrophage-like line 14M1.412 showed comparable levels of infection to RAW .264 cells at each multiplicity of infection examined, whereas the fibroblast, fibroendothelial, and adventitial reticular cell lines contained 3- to 9-fold lower numbers of amastigotes, compared to 14M1.4 cells. Importantly, 14M1.4 cells were also able to support a 3-fold increase in parasite numbers over a 3-day culture period, whereas none of the nonmacrophage stromal cell lines supported amastigote growth (Figure 2B). Thus, although a number of bone marrow stroma-derived cell lines could be infected with L donovani amastigotes in vitro, significant levels of infection and parasite growth were supported only by the macrophage-like cell line, 14M1.4.
Bone marrow stroma-derived cell lines show differing levels of infectivity with L donovani amastigotes.
(A) Bone marrow stroma-derived cell lines were infected with amastigotes at a multiplicity of infection of 50:1 (open bars), 25:1 (hatched bars), 10:1 (cross-hatched bars), or 5:1 (solid bars). At 1 hour after infection, cells were washed and cytospun onto glass slides. (B) Bone marrow stroma-derived cell lines were infected with amastigotes at a ratio of 25:1. At 1 hour after infection, cells were washed and cytospun onto slides (open bars) or incubated for a further 72 hours (hatched bars). The number of intracellular (IC) parasites per 100 cell nuclei was determined following fixation and Giemsa staining. Data represent the mean ± SEM for triplicate samples at each infection ratio and time point and are representative of 2 independent experiments.
Bone marrow stroma-derived cell lines show differing levels of infectivity with L donovani amastigotes.
(A) Bone marrow stroma-derived cell lines were infected with amastigotes at a multiplicity of infection of 50:1 (open bars), 25:1 (hatched bars), 10:1 (cross-hatched bars), or 5:1 (solid bars). At 1 hour after infection, cells were washed and cytospun onto glass slides. (B) Bone marrow stroma-derived cell lines were infected with amastigotes at a ratio of 25:1. At 1 hour after infection, cells were washed and cytospun onto slides (open bars) or incubated for a further 72 hours (hatched bars). The number of intracellular (IC) parasites per 100 cell nuclei was determined following fixation and Giemsa staining. Data represent the mean ± SEM for triplicate samples at each infection ratio and time point and are representative of 2 independent experiments.
L donovani infection enhances the capacity of 14M1.4 cells to support hematopoietic colony formation
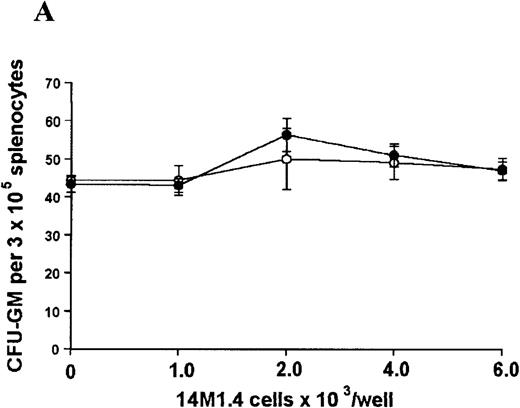
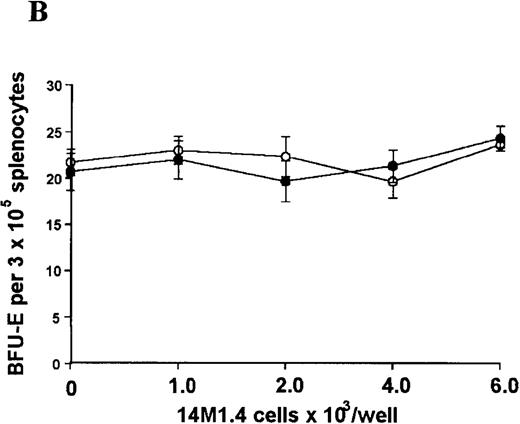
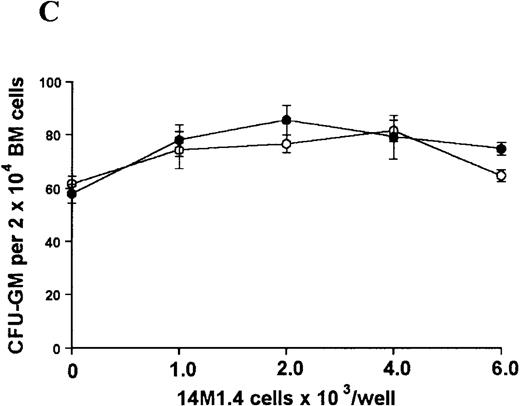
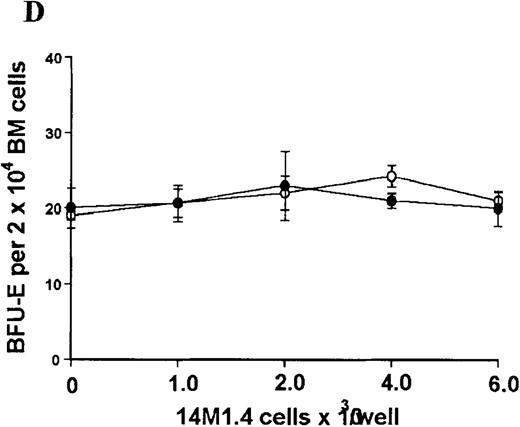
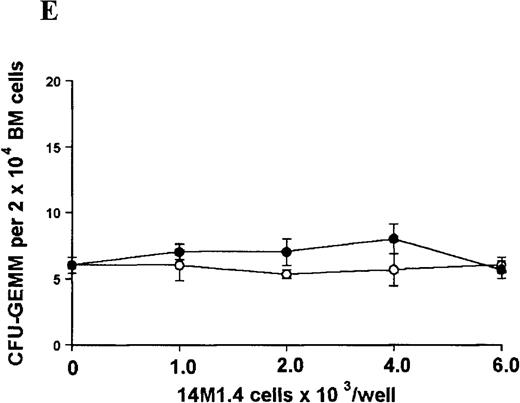
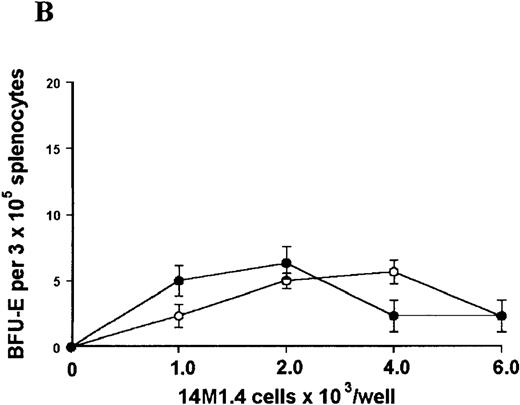
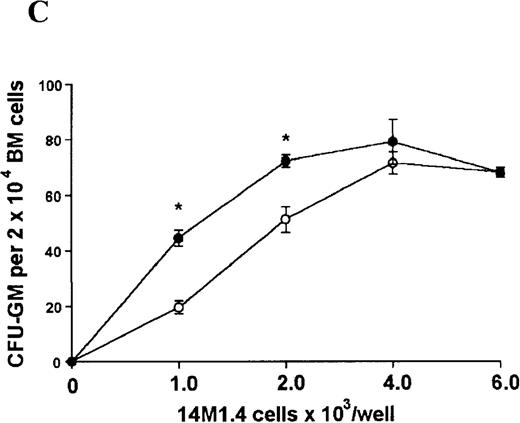
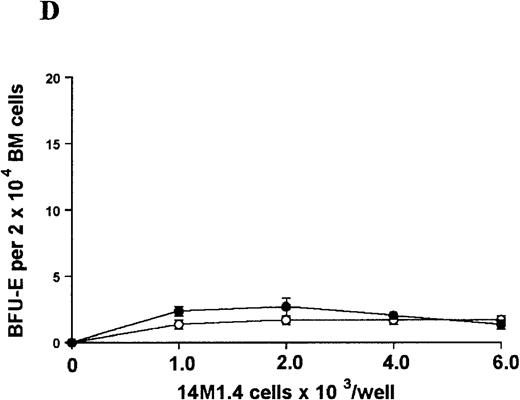
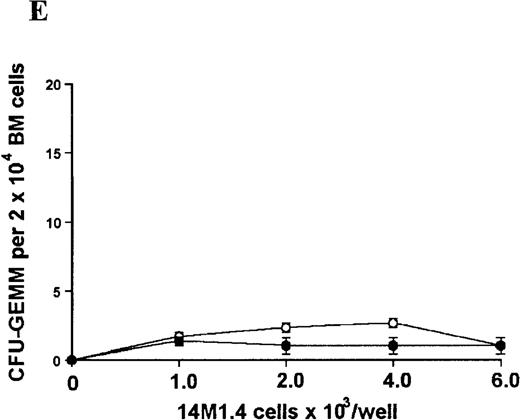
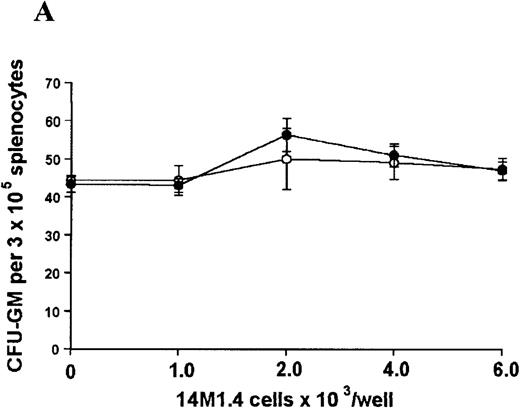
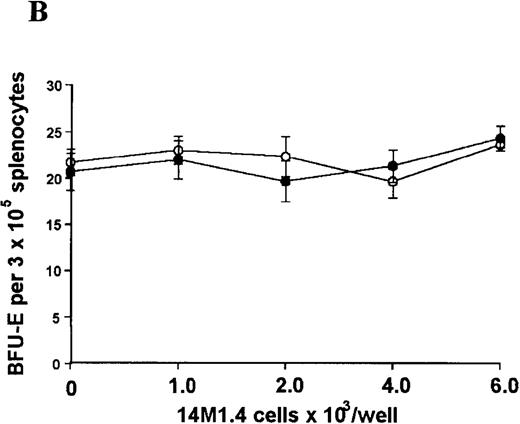
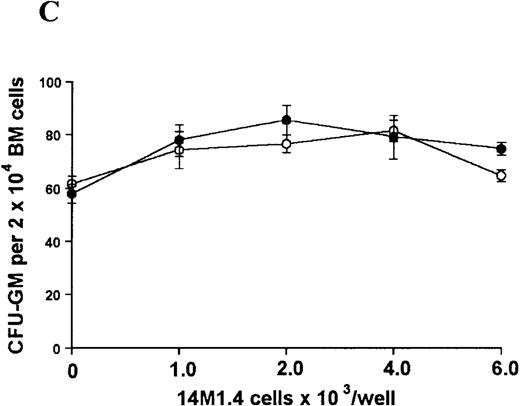
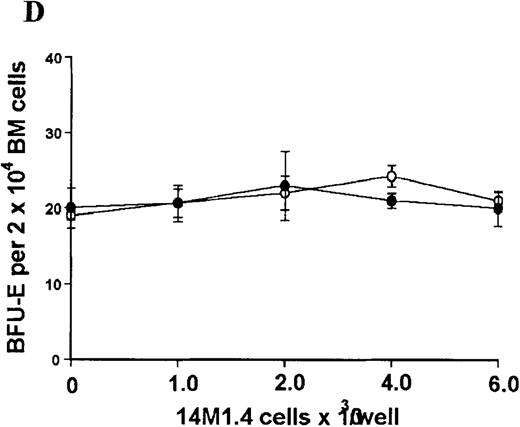
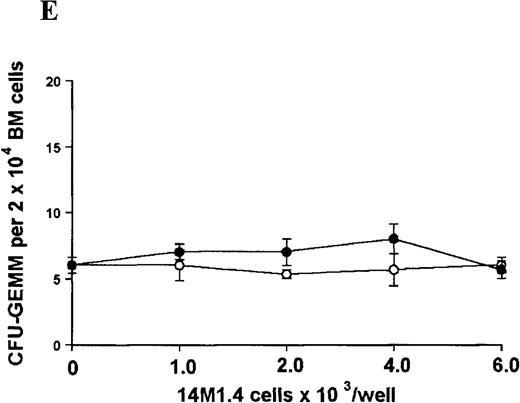
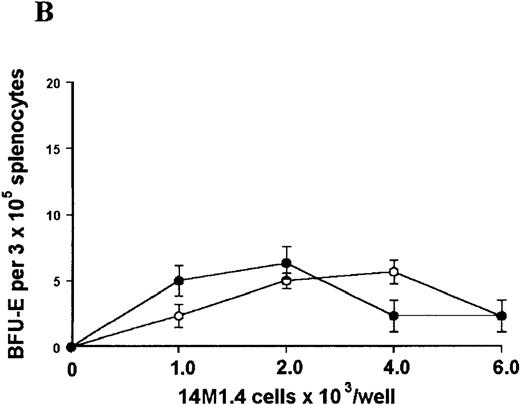
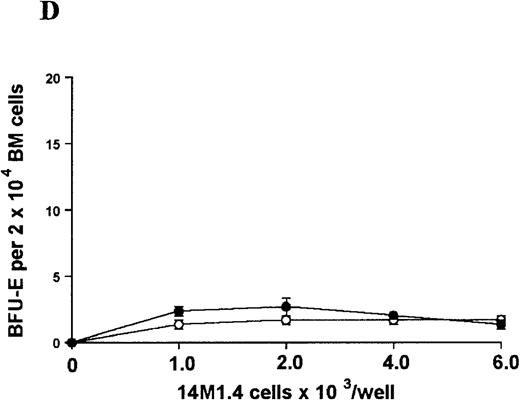
The studies above indicate that stromal macrophages can be infected with L donovani both in vitro and in vivo. We therefore sought to determine whether infection had any influence on the functional capacity of these cells to support hematopoiesis. To address this question, colony assays were performed in which adherent 14M1.4 cells were overlaid with syngeneic bone marrow or spleen cells as a source of progenitor cells. Using conventional methylcellulose assays, in the presence of exogenous Epo, SCF, and PWM-SCCM (see “Materials and methods”), we could readily detect CFU-GM and BFU-E when spleen cells were used as a source of progenitor cells. In addition, when bone marrow cells were used, we could also detect measurable numbers of CFU-GEMM under the same culture conditions (Figure3). The addition of 14M1.4 cells to these growth factor-supplemented cultures had limited effect on colony formation (see Figure 3). Furthermore, the addition of L donovani-infected 14M1.4 cells was also without significant effect, indicating that L donovani-infected stromal cells neither promote nor interfere with colony formation in the presence of optimal levels of exogenous growth factors.
Stromal macrophages do not affect hematopoietic colony formation in the presence of exogenous growth factors.
14M1.4 cells were either untreated (open circles) or infected with amastigotes at a ratio of 25:1 (closed circles), and were overlaid with spleen (A and B) or bone marrow (C-E) cells suspended in methylcellulose containing PWM-SCCM, Epo, and SCF. After 7 days, mature colonies were scored as CFU-GM (A and C), BFU-E (B and D) and CFU-GEMM (E). Data represent the mean ± SEM for triplicate wells and are representative of 2 independent experiments.
Stromal macrophages do not affect hematopoietic colony formation in the presence of exogenous growth factors.
14M1.4 cells were either untreated (open circles) or infected with amastigotes at a ratio of 25:1 (closed circles), and were overlaid with spleen (A and B) or bone marrow (C-E) cells suspended in methylcellulose containing PWM-SCCM, Epo, and SCF. After 7 days, mature colonies were scored as CFU-GM (A and C), BFU-E (B and D) and CFU-GEMM (E). Data represent the mean ± SEM for triplicate wells and are representative of 2 independent experiments.
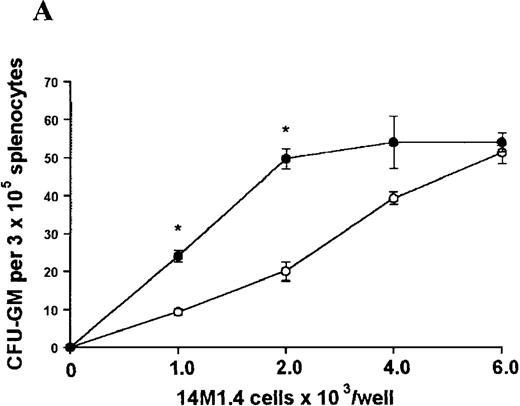
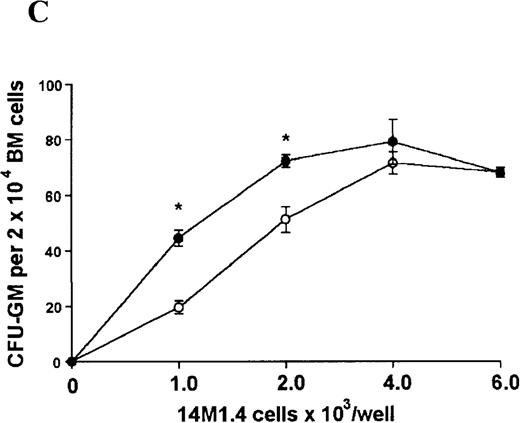
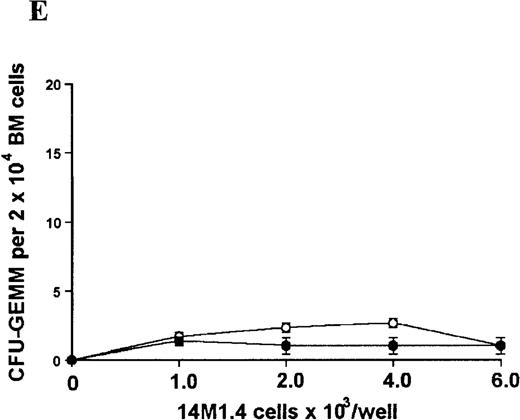
In contrast, when bone marrow or spleen cells were plated in methylcellulose in the absence of exogenous growth factors, the addition of 14M1.4 cells promoted formation of CFU-GM, BFU-E, and additionally in bone marrow, CFU-GEMM (Figure4). As few as 103 14M1.4 cells were required to support colony formation, and the response reached at plateau at higher numbers of 14M1.4 cells. This plateau, at least in the case of GFU-GM, is likely to reflect the input number of progenitor cells in the splenocyte and bone marrow cell samples plated, because the maximum number of colonies was not exceeded even in the presence of optimal exogenous growth factors (see Figures 3 and 4). CFU-GEMM and BFU-E were slightly underrepresented in cultures supported by 14M1.4 cells compared to exogenous growth factors, indicating an underlying selectivity of 14M1.4 cells to the support of myelopoiesis. WhenL donovani-infected rather than uninfected 14M1.4 cells were added to these assays, there was a significant increase in CFU-GM formation. Examination of the dose-response curves for these 2 populations indicates that infected 14M1.4 cells had approximately doubled the capacity for hematopoiesis, compared to uninfected 14M1.4 cells. This enhanced ability to support colony formation was specific for GM-CFU, because no significant increases in BFU-E or CFU-GEMM were observed. Thus, infection with L donovani has the potential to selectively enhance myelopoiesis, via effects on stromal macrophage function.
L donovani infection of 14M1.4 cells promotes CFU-GM formation in the absence of exogenous growth factors.
14M1.4 cells were untreated (open circles) or infected with amastigotes at a ratio of 25:1 (closed circles) and allowed to adhere before addition of either spleen (A and B) or bone marrow (C-E) cells suspended in methylcellulose, in the absence of growth factors. After 7 days, mature colonies were scored as CFU-GM (A and C), BFU-E (B and D), or CFU-GEMM (E). Data represent the mean ± SEM for triplicate wells and are representative of 2 independent experiments. Significant statistical differences between naı̈ve and infected 14M1.4 cells of P < 0.02 (*) are indicated.
L donovani infection of 14M1.4 cells promotes CFU-GM formation in the absence of exogenous growth factors.
14M1.4 cells were untreated (open circles) or infected with amastigotes at a ratio of 25:1 (closed circles) and allowed to adhere before addition of either spleen (A and B) or bone marrow (C-E) cells suspended in methylcellulose, in the absence of growth factors. After 7 days, mature colonies were scored as CFU-GM (A and C), BFU-E (B and D), or CFU-GEMM (E). Data represent the mean ± SEM for triplicate wells and are representative of 2 independent experiments. Significant statistical differences between naı̈ve and infected 14M1.4 cells of P < 0.02 (*) are indicated.
Soluble factors produced by 14M1.4 cells support CFU-GM development
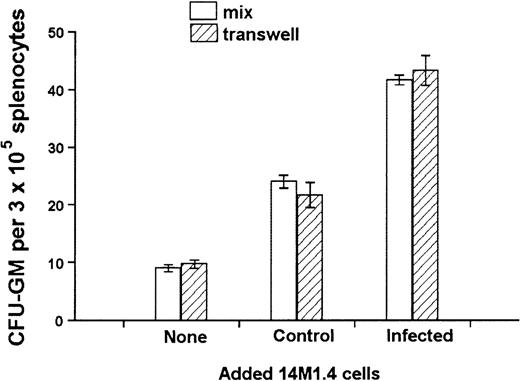
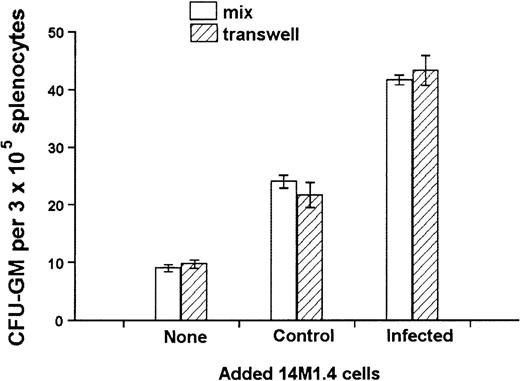
The culture of progenitor cells in semisolid methylcellulose allows for their clonal expansion even in the absence of stromal support, providing an environment that favors cytokine/chemokine regulation of progenitor cell function.54 To determine whether the activity of 14M1.4 cells indeed reflected altered growth factor production, transwell chambers were used to separate 14M1.4 cells from the source of progenitor cells. To enable us to observe either positive or negative effects, a suboptimal level of CFU-GM formation was ensured by coculture of 1 × 103 14M1.4 cells with 3 × 105 spleen cells, the source of colony-forming cells (CFC) in these experiments. Additional 14M1.4 cells were then added either directly to the adherent layer or placed in a transwell above the methylcellulose. As shown in Figure5, comparable increases in CFU-GM number were observed irrespective of whether an additional 103uninfected 14M1.4 cells were added directly to the culture or into the transwell. As expected from the data shown in Figure 4, addition of 103 infected 14M1.4 cells increased the number of CFU-GM above that seen with uninfected 14M1.4 cells. This enhanced activity of infected 14M1.4 cells was also observed irrespective of whether the infected cells were added directly or were in the transwell. These data confirm that soluble factors produced by 14M1.4 cells are sufficient for the support of CFU-GM formation in the absence of exogenous growth factors. Furthermore, the impact of L donovaniinfection on the functional activity of 14M1.4 cells is likely to result from changes in the production of these soluble factors.
Soluble factors produced by L donovani-infected 14M1.4 cells are sufficient to support increased hematopoietic colony formation.
14M1.4 cells (103) were overlaid with spleen cells. Additional 14M1.4 cells were added to the cocultures, either directly to the adherent layer (mix) or into transwells. After 7 days, mature CFU-GM colonies were counted. Data represent the mean ± SEM for duplicate wells and are representative of 2 independent experiments.
Soluble factors produced by L donovani-infected 14M1.4 cells are sufficient to support increased hematopoietic colony formation.
14M1.4 cells (103) were overlaid with spleen cells. Additional 14M1.4 cells were added to the cocultures, either directly to the adherent layer (mix) or into transwells. After 7 days, mature CFU-GM colonies were counted. Data represent the mean ± SEM for duplicate wells and are representative of 2 independent experiments.
L donovani infection of 14M1.4 cells induces expression of GM-CSF, TNF-, and MIP-1
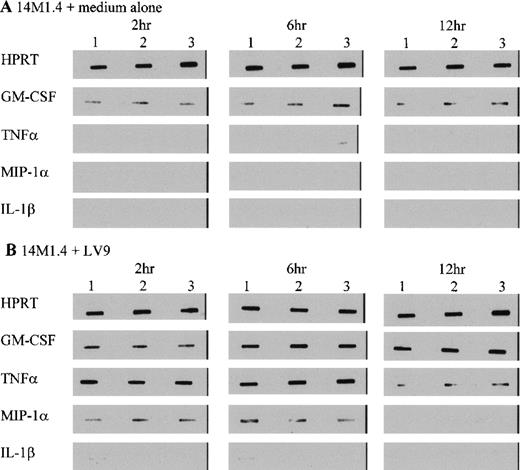
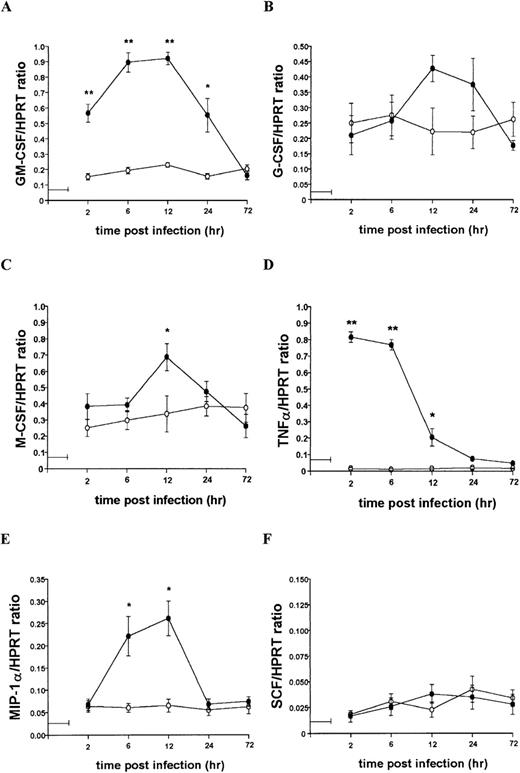
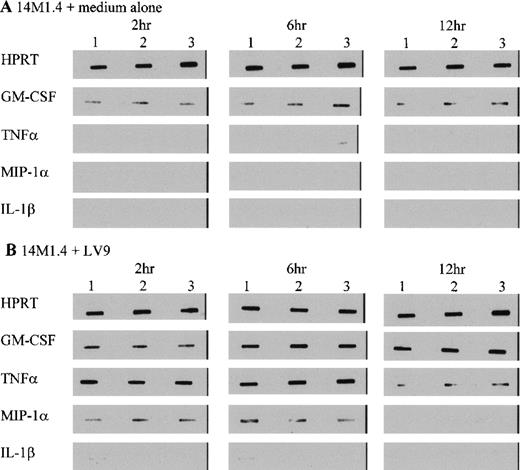
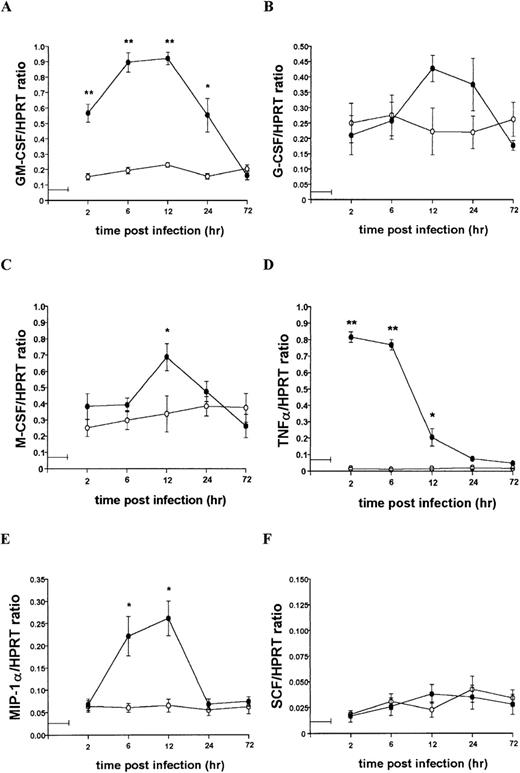
To further characterize the role of soluble factors in the responses observed above, uninfected and infected 14M1.4 cells were analyzed by RT-PCR for the accumulation of mRNA encoding a broad panel of factors with known colony-stimulating activity. Data were analyzed by probing with specific oligonucleotide probes, and representative data are shown in Figure 6. Accumulation of mRNA was further analyzed by densitometry and is expressed as arbitrary units, normalized against the housekeeping gene HPRT (Figure7). Uninfected 14M1.4 cells constitutively expressed mRNA for GM-CSF, G-CSF, and M-CSF, but not MIP-1α, TNF-α, IL-1β, IL-10, SCF, monocyte chemoattractant protein-1 (MCP-1), or interferon-γ inducible protein-10 (γIP-10) (Figures 6 and 7, and data not shown). After infection with L donovani, accumulation of mRNA for GM-CSF, TNF-α, and MIP-1α was significantly and reproducibly induced in 14M1.4 cells. In contrast, the accumulation of mRNA for IL-1β, IL-10, SCF, MCP-1, and γIP-10 was not induced by L donovani infection (Figure 6 and data not shown). mRNA accumulation for GM-CSF increased 4- to 5-fold, reaching a peak at 12 hours after infection, before declining to baseline levels by 72 hours after infection. The induction of TNF-α mRNA peaked earlier and was relatively more dramatic due to the negligible levels of expression in uninfected cells (Figure 7D). As with GM-CSF, TNF-α mRNA accumulation was transient. MIP-1α expression was intermediate in terms of both kinetics and magnitude. Finally, accumulation of mRNA for G-CSF and M-CSF was induced to a much lesser extent by L donovani infection, and this only reached significant values at 12 hours after infection for M-CSF. Hence, L donovani induces a limited range of cytokines/chemokines from stromal macrophages in vitro.
L donovani infection of 14M1.4 cells induces selective accumulation of cytokine/chemokine mRNA.
14M1.4 cells were untreated (A) or infected with amastigotes (B) for 1 hour at 37°C. Infected and naive samples were then washed and resuspended in TRI-reagent in preparation for RNA extraction, or incubated for up to 12 hours before resuspension in TRI-reagent. mRNA accumulation was measured by RT-PCR. Data illustrate PCR products visualized by Southern blotting and enhanced chemiluminescence detection. Bands represent PCR products from 3 individual samples at 2 hours, 6 hours, and 12 hours after infection.
L donovani infection of 14M1.4 cells induces selective accumulation of cytokine/chemokine mRNA.
14M1.4 cells were untreated (A) or infected with amastigotes (B) for 1 hour at 37°C. Infected and naive samples were then washed and resuspended in TRI-reagent in preparation for RNA extraction, or incubated for up to 12 hours before resuspension in TRI-reagent. mRNA accumulation was measured by RT-PCR. Data illustrate PCR products visualized by Southern blotting and enhanced chemiluminescence detection. Bands represent PCR products from 3 individual samples at 2 hours, 6 hours, and 12 hours after infection.
Kinetics of mRNA accumulation following L donovani infection of 14M1.4 cells.
14M1.4 cells were untreated (open circles) or infected with amastigotes (closed circles) at 25:1 for 1 hour at 37°C (as indicated by the horizontal line). mRNA accumulation was measured by RT-PCR at various times after infection and results are expressed in arbitrary densitometry units, normalized for levels of expression of HPRT. Data represent the mean ± SEM for 4 samples per group, from 2 independent experiments. Statistically significant differences between naive and L donovani-infected groups ofP < 0.05 (*) and P < 0.005(**) are indicated.
Kinetics of mRNA accumulation following L donovani infection of 14M1.4 cells.
14M1.4 cells were untreated (open circles) or infected with amastigotes (closed circles) at 25:1 for 1 hour at 37°C (as indicated by the horizontal line). mRNA accumulation was measured by RT-PCR at various times after infection and results are expressed in arbitrary densitometry units, normalized for levels of expression of HPRT. Data represent the mean ± SEM for 4 samples per group, from 2 independent experiments. Statistically significant differences between naive and L donovani-infected groups ofP < 0.05 (*) and P < 0.005(**) are indicated.
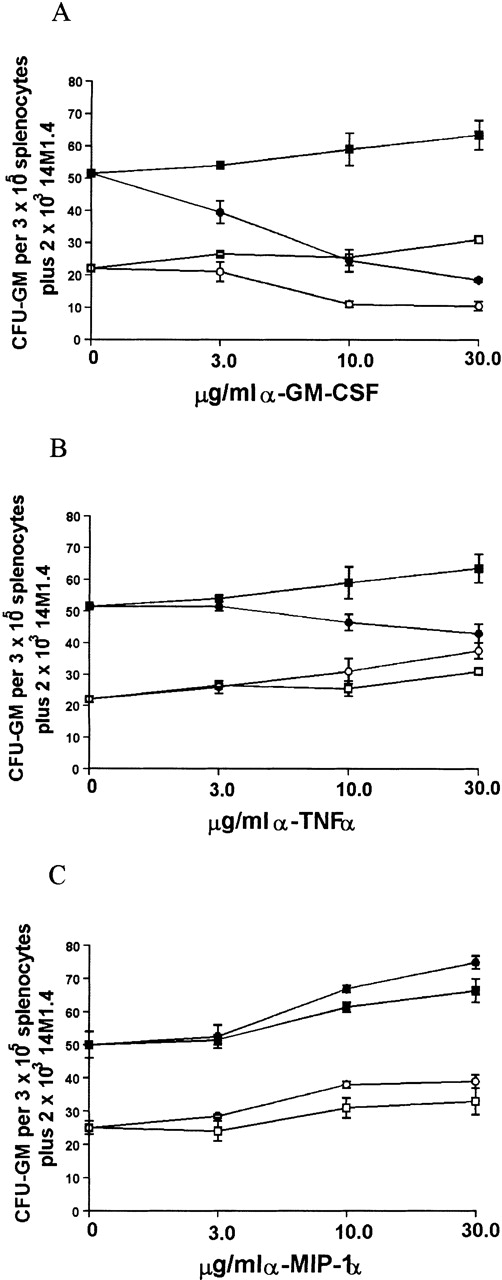
Enhanced colony formation involves contributions by GM-CSF and TNF-
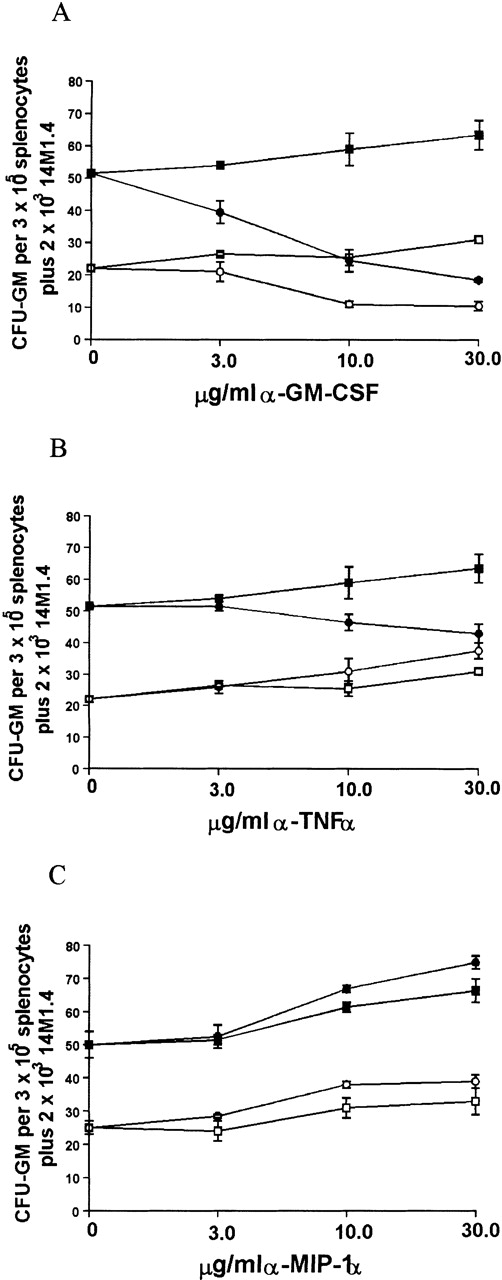
Because infection of 14M1.4 cells with L donovaniresulted in a significant increase in the accumulation of mRNA for GM-CSF, MIP-1α, and TNF-α, we tested the contribution that these cytokines made to the support of myleopoiesis by 14M1.4 cells. As shown in Figure 8A, addition of neutralizing antibody to GM-CSF caused a dose-dependent reduction in the generation of CFU-GM supported by either uninfected or infected 14M1.4 cells. CFU-GM formation could not be totally ablated by treatment with anti-GM-CSF, however, even when antibody concentration was increased to 100 μg/mL (data not shown). Thus, GM-CSF production is not solely responsible for the support of colony formation by 14M1.4 cells. In contrast to the effect of GM-CSF neutralization on cultures supported by uninfected or infected 14M1.4 cells, anti-TNF-α had a selective effect on cultures supported by infected 14M1.4 cells (Figure 8B). This result is consistent with the lack of TNF-α mRNA accumulation in uninfected 14M1.4 cells and induction of this cytokine by L donovani infection (see Figures 6 and 7). The effect of anti-TNF-α was, nevertheless, moderate compared to that of anti-GM-CSF. Although MIP-1α was induced by infection, and has been shown to have a role in hematopoiesis,55-59 neutralization of this chemokine had no significant effect on the generation of CFU-GM supported by either uninfected or L donovani-infected 14M1.4 cells (Figure 8C).
Support of splenocyte hematopoiesis by coculture with infected 14M1.4 cells is dependent on GM-CSF and TNF-.
14M1.4 cells were untreated (open symbols) or infected with LV9 amastigotes at a ratio of 25:1 (closed symbols). Adherent 14M1.4 cells were preincubated with (A) control HIgG (squares) or anti-GM-CSF mAb (circles), (B) control HIgG (squares) or anti-TNF-α mAb (circles) or (C) control GIgG (circles) or anti-MIP-1α Ab (squares) for 30 minutes before overlaying with naı̈ve splenocytes suspended in methylcellulose, in the absence of growth factors (Methocult 3230), but in the presence of hemin.
Support of splenocyte hematopoiesis by coculture with infected 14M1.4 cells is dependent on GM-CSF and TNF-.
14M1.4 cells were untreated (open symbols) or infected with LV9 amastigotes at a ratio of 25:1 (closed symbols). Adherent 14M1.4 cells were preincubated with (A) control HIgG (squares) or anti-GM-CSF mAb (circles), (B) control HIgG (squares) or anti-TNF-α mAb (circles) or (C) control GIgG (circles) or anti-MIP-1α Ab (squares) for 30 minutes before overlaying with naı̈ve splenocytes suspended in methylcellulose, in the absence of growth factors (Methocult 3230), but in the presence of hemin.
Because both GM-CSF and, to a lesser extent, TNF-α appeared to be involved in the support of progenitor activity by L donovani-infected 14M1.4 cells, the effect of neutralizing both cytokines simultaneously was examined (Table1). As anticipated, the effect of anti-GM-CSF on CFU-GM formation supported by uninfected 14M1.4 cells was not affected by coaddition of anti-TNF-α. Unexpectedly, when anti-GM-CSF and anti-TNF-α were added together in cultures supported by infected 14M1.4 cells, the degree of inhibition of CFU-GM was not significantly different from that achieved by neutralization of GM-CSF alone. Thus, although the presence of TNF-α may augment the response to GM-CSF in cultures containing infected 14M1.4 cells, TNF-α has no independent effect on colony formation under these conditions.
Discussion
This study is the first to functionally address how infection of bone marrow stromal cells with a protozoan pathogen affects regulation of hematopoiesis. We have shown that stromal macrophages can be infected in vivo and in vitro with L donovani. In addition, increased GM-CSF and TNF-α production following infection allow enhanced support of myelopoiesis.
The observation that progenitor cell lines in vitro and CD34+ cells in the bone marrow of infected mice are almost totally refractory to infection suggested that any abnormal hematopoiesis seen in VL was unlikely to be a direct result of infection of progenitor cells. In contrast, in situ identification of infected SER-4+ bone marrow stromal macrophages indicated that these cells were capable of acting as hosts for L donovani amastigotes. This observation is in accord with a previous immunohistologic analysis of a related parasite, L infantum, which identified amastigotes in both SER-4+ and SER-4−, FA11+macrophages in the bone marrow of infected mice.60 However, the functional consequences of this observation were not addressed.L donovani also infected and replicated in the macrophage stromal line 14M1.4, which was derived from a long-term bone marrow culture.12 Although we have not addressed the question of which receptors are used for entry of amastigotes into 14M1.4 cells, it is of interest to note that this line was previously characterized as having low phagocytic activity toward latex beads, compared to M-CSF-derived bone marrow macrophages.12Fibroblast (MBA1, MBA1.1), fibroendothelial (MBA13), and adventitial reticular (+/+-1.LDA1.1) cells could also be infected with L donovani, albeit to a lesser extent than 14M1.4 cells, but these lines failed to support long-term growth of amastigotes. Within the stromal cell populations examined, therefore, only those with macrophage characteristics are able to support long-term infection byL donovani.
The ability to infect a stromal macrophage line allowed us to examine the functional consequences of infection using an in vitro colony assay. Coculture of 14M1.4 cells with spleen cells or bone marrow cells suspended in methylcellulose was sufficient to support the generation of CFU-GM, and low levels of CFU-GEMM and BFU-E formation, in the absence of exogenous growth factors. In addition, the use of transwell cultures indicated that cell-cell contact between progenitor cells and stromal cells was not required for CFU-GM formation and that soluble factors constitutively produced by 14M1.4 cells were capable of supporting myelopoiesis. Constitutive accumulation of mRNA for M-CSF, GM-CSF, and G-CSF was observed by RT-PCR analysis, and the secretion of these cytokines into the coculture milieu may be sufficient to support colony formation. Each of these factors has been shown to support the proliferation of CFU-GM,61,62 and GM-CSF may also act to support proliferation of CFU-GEMM and BFU-E.63-65 However, the relatively low levels of CFU-GEMM and BFU-E supported by 14M1.4, compared to those achieved following culture with exogenous growth factors, may reflect the lack of SCF production by 14M1.4 cells (see Figure 7), an important regulator of CFU-GEMM and BFU-E formation.66 In contrast to the results reported here, confluent monolayers of the parent clone 14M1 were reported to be unable to support CFU-GM formation.12 However, we have noted that when 14M1.4 cells are seeded to confluency in the coculture system described here, CFU-GM formation was also not observed (data not shown). Hence, high densities of stromal cells may prevent colony formation, possibly as a result of contact-inhibition of growth factor production67 or by consumption of myelopoietic cytokines by the stromal cells themselves.68 In this regard, both 14M1 and 14M1.4 cells require M-CSF for growth at low cell densities, and the addition of neutralizing antibodies to M-CSF reduces the growth of 14M1 cells seeded at higher density.12
The support of CFU-GM formation achieved at low densities of 14M1.4 cells was significantly increased if 14M1.4 cells were infected with L donovani amastigotes prior to coculture with spleen or bone marrow cells. In contrast, the production of BFU-E and CFU-GEMM by 14M1.4 was unaffected by infection of these cells. RT-PCR analysis indicated that L donovani infection of 14M1.4 cells stimulates increased accumulation of mRNA for GM-CSF, TNF-α and MIP-1α, but not other cytokines/chemokines with colony-stimulating activity. Interestingly, the stromal macrophages analyzed here are distinct from other macrophage populations studied. For example, resident monocytes and macrophages in the spleen do not produce TNF-α after infection in vivo or in vitro, though they may do so after interferon-γ priming.69,70 Of these cytokines, only GM-CSF and TNF-α were necessary for CFU-GM formation and these cytokines cooperate to provide maximal support of myelopoiesis. Thus, although the stimulatory effects of GM-CSF on myelopoiesis were maximal in the presence of TNF-α, TNF-α had no effect in the absence of GM-CSF. These data demonstrate that L donovani-induced production of TNF-α is not sufficient to independently stimulate myelopoiesis, but this cytokine can augment CFU-GM formation induced by GM-CSF. Costimulation of GM-CSF-induced myelopoiesis by TNF-α has also been observed by others.71 TNF-α expression during the later stages of VL has been well documented by both RT-PCR and by immunohistochemistry.28 31 Interestingly, this cytokine is well regulated within the liver granuloma, whereas its production in the spleen is widespread and exceedingly high. The in vivo contribution that this cytokine makes to the local regulation of hematopoiesis is currently under investigation.
Although antibody neutralization studies indicated a dominant role for GM-CSF and TNF-α, colony formation was nevertheless only reduced by approximately 75% by these antibodies either alone or in combination. Moreover, in the presence of excess neutralizing antibody to GM-CSF, the number of CFU-GM supported by infected 14M1.4 cells remained significantly higher than that supported by uninfected 14M1.4 cells. Although M-CSF and G-CSF are constitutively expressed by 14M1.4 cells, and thus may be responsible for some baseline CFU-GM formation in the absence of GM-CSF, L donovani infection resulted in minimal changes in mRNA accumulation for these cytokines. Further studies will therefore be required to determine which other factors are produced preferentially by infected 14M1.4 cells and which have colony-stimulating activity.
Finally, the results presented here indicate a number of similarities between the regulation of hematopoiesis in vitro and in vivo during chronic VL. We have recently shown that infected BALB/c mice have a selective enhancement of myelopoiesis, notably in the spleen.72 The results presented here, in which infection of stromal macrophages serves to potentiate myelopoiesis driven by these cells, suggests one mechanism underlying this effect in vivo. GM-CSF mRNA and TNF-α, the principal mediators defined in this study, are also elevated during L donovani infection in vivo.31 In contrast, in the later stages of VL in BALB/c mice, there are also increased numbers of CFC-GEMM and BFC-E in cell cycle,72 whereas direct infection of 14M1.4 cells had no effect on the proliferation of these progenitors in vitro. Furthermore, increased G-CSF and M-CSF mRNA was observed following L donovani infection in vivo, although this was again not a major effect of infection of stromal macrophages in vitro. The latter comparisons illustrate the fact that additional sources of cytokines with colony-stimulating activity, as well as changes in microenvironmental arrangements, will also play a role in modulating hematopoiesis in vivo. Nevertheless, our data suggest a plausible mechanism by which infection of stromal cells directly contributes to the enhanced myelopoiesis seen in murine VL. Identifying factors that contribute to this process may allow for selective interventions aimed at blocking this feature of infection and assessment of their impact both experimentally and clinically.
Acknowledgments
The authors thank Drs Zipori, Spooncer, Honjo, and Boswell for the gifts of cell lines used in this study and Genevieve Cleere for assistance in preparation of the manuscript.
Supported by grants from the Wellcome Trust and the Medical Research Council. S.E.J.C. was a recipient of a Wellcome Trust Prize Studentship.
Reprints:Paul Kaye, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT UK; e-mail: paul.kaye@lshtm.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.