The process of vasculogenesis was characterized in the 6.5- to 9.5-day mouse embryo and in allantoic culture by analysis of spatial and temporal expression patterns of the endothelial or hematopoietic lineage-associated proteins, TAL1, Flk1, platelet/endothelial cell adhision molecule (PECAM), CD34, VE-cadherin, and Tie2. The study establishes that: (1) TAL1 and Flk1 are coexpressed in isolated mesodermal cells that give rise to endothelial cells and thus can be defined as angioblasts; (2) hematopoietic cells of blood islands express TAL1, but not Flk1; (3) vasculogenesis in the embryo proper is initiated by mesoderm fated to give rise to the endocardium; (4) the maturation/morphogenesis of blood vessels can be defined in terms of a sequential pattern of expression in which TAL1 and Flk1 are expressed first followed by PECAM, CD34, VE-cadherin, and later Tie2; and (5) TAL1 expression is down-regulated in endothelial cells of mature vessels.
The first blood vessels to form in the embryo are generated by vasculogenesis. Essential steps in this process are the establishment of the endothelial cell lineage (angioblasts), the assembly of angioblasts into cord-like vascular structures, the formation of vascular lumens, and the organization of vascular networks.1-3 New insight into vasculogenesis in mammals is emerging from studies of transgenic mice.4-6 However, the potential of these mice to provide insight into vasculogenic processes is impaired by a lack of understanding of the normal morphologic and biochemical aspects of murine vasculogenesis. Here the temporal and spatial expressions of an array of proteins associated with the endothelial and hematopoietic lineages were examined in the developing mouse embryo, TAL1,7-9 Flk1,4,10,11platelet/endothelial cell adhesion molecule (PECAM),12CD34,13,14 VE-cadherin,15,16 and Tie2.6,17 18 The analysis used a novel protocol that renders the normally curved or lordotic mouse embryo into a planar format. This procedure, combined with capability of the confocal microscope that is able to represent all embryonic vessels in a single image, facilitates analysis of vascular patterns and developmental gradients. The data provide a number of new insights into the processes of vasculogenesis and hematopoiesis that include a more detailed understanding of the relationship between TAL1 and Flk1 expression in these lineages.
Materials and methods
Antibodies
Rabbit polyclonal antimouse TAL1/SCL9 was obtained from Stephen J. Brandt (Vanderbilt University and Veterans Affairs Medical Center, Nashville, TN). Rabbit antimouse Flk14 was provided by Andre Schuh (University of Toronto, Toronto, Ontario, Canada). Rabbit antimouse CD3419 was provided by Lawrence Lasky (Genentech, Inc., San Francisco, CA). Rat monoclonal antimouse Tie220 was obtained from Steven Stacker (Ludwig Institute for Cancer Research, Victoria, Australia). Rat monoclonal antibodies to recombinant VE-cadherin (clone 19E6)21 were provided by Elisabetta Dejana and Maria Lampugnani (Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy). Rat antimouse PECAM monoclonal antibodies were purchased from PharMingen (San Diego, CA).
Whole-mount immunolabeling
TAL1, Flk1, CD31, and CD34 embryos at 7.0 to 9.5 days postcoitum (dpc) (0.5 dpc, plug date) were dissected free of the uterine muscle and decidua and placed into embryonic phosphate-buffered saline (EPBS) (4°C). Reichert's membrane and the ectoplacental cone were removed and the embryos flattened by cutting the yolk sac lateral to the embryonic axis and removing the amnionic sac (Figure1). Fixation was by infusion of 3% paraformaldehyde into the EPBS (5 minutes) followed by fixation in 3% paraformaldehyde (10 minutes). Embryos were permeabilized in phosphate-buffered saline/0.01% sodium azide (PBSA) containing 0.02% Triton-X 100 (30 minutes), exposed to a blocking solution, 3% bovine serum albumin (BSA)/PBSA, and then to appropriate primary and secondary antibodies (Jackson Immuno Research Laboratories, Inc., West Grove, PA). Incubations were for a period of 12 to18 hours at 4°C. Embryos were mounted ventral side up using an antiphotobleaching medium.22 Immunolabeling for VE-cadherin and Tie2 was as described above except that embryos were exposed to primary antibodies before fixation (1.5 hours, 4°C).
Reconfiguration of mouse embryos.
Early stage mouse embryos can be reconfigured into a planar format by removing the ectoplacental cone (not shown), then cutting/tearing the extraembryonic tissue along a line perpendicular to the embryonic axis (A, dashed lines) and finally opening the amniotic sac (B, dashed crescent). These procedures allow the embryo to assume a planar orientation. Prominent features of the early embryo provided as points of reference for this protocol include the blood islands (BI), the somites (S), and the allantois (AL).
Reconfiguration of mouse embryos.
Early stage mouse embryos can be reconfigured into a planar format by removing the ectoplacental cone (not shown), then cutting/tearing the extraembryonic tissue along a line perpendicular to the embryonic axis (A, dashed lines) and finally opening the amniotic sac (B, dashed crescent). These procedures allow the embryo to assume a planar orientation. Prominent features of the early embryo provided as points of reference for this protocol include the blood islands (BI), the somites (S), and the allantois (AL).
Allantois culture and immunolabeling
Allantoides of 7.5 dpc embryos were excised, washed in PBSA (4°C) and then pipetted into Nunc 4-chambered culture slides (Fisher Scientific Co., Suwanee, GA) containing 0.4 mL Dulbecco's modified Eagle's medium, 10% fetal bovine serum (FBS), and 1% penicillin streptomycin. Explants were cultured at 37°C in a 5% CO2 incubator for 12 to 20 hours and then fixed and permeabilized as described above. The explants were blocked in 3% BSA/PBS 12 to 18 hours, exposed to PECAM antibodies (1.5 hours, 26°C), washed 3 × 40 minutes in PBS, incubated in appropriate secondary antibodies (1.5 hours, 26°C), washed in PBSA 3 × 30 minutes, and mounted as described above.
Microscopy and image processing
Embryos were analyzed using a Bio-Rad MRC 1024 Laser Scanning Confocal Microscope (Bio-Rad Microscopy Division, Cambridge, MA). Optical sectioning along the dorsoventral axis (Z axis) was performed and the images collapsed into a single focal plane using the manufacturer's software. Differential interference contrast images were generated using a research grade Leitz photomicroscope equipped with a Photometrics (Tucson, AZ) Quantix CCD camera. Images were processed using NIH Image 1.62 software (National Institutes of Health, Bethesda, MD) and Adobe Photoshop 5.0 (Adobe Systems, Inc., San Jose, CA).
Results
Throughout this study, temporal and spatial aspects of vasculogenic and hematopoietic processes were immunologically evaluated in mouse embryos rendered into a planar format, a procedure that facilitates the analysis of vascular patterns and gradients of development (see Figure1).
Characterization of the angioblast and the hematopoietic cell phenotype
Initial characterization of the angioblast was conducted in 8.3-dpc embryos, a stage when both established and forming vessels are present. Double immunofluorescence demonstrated that TAL1 and Flk1 colabeled endothelial cells of morphologically identifiable vessels (compare Figure 2, A and B) as well as dispersed mesodermal cells (Figure 2, C and D). To pursue the possibility that the dispersed TAL1+/Flk1+ cells represent the progenitors of endothelial cells, blood vessel development was followed in 6.5- to 7.0-dpc embryos. At 6.5 dpc, dispersed TAL1+/Flk1+ mesodermal cells were detected in extraembryonic regions (Figure 3, A and B, arrowheads). When the corresponding regions of 7.0- to 7.3-dpc embryos were examined, polygonal arrangements of small-caliber vessels (primary vascular networks) were evident in the regions previously populated by the TAL1+/Flk1+ cells (compare Figure 3, A and B, arrowheads, to Figure 4, A and B, braces). These data combined with previous work linking TAL1 expression with endothelial progenitor cells suggest that TAL1+/Flk1+ cells (angioblasts) are the precursors of endothelial cells.8 9
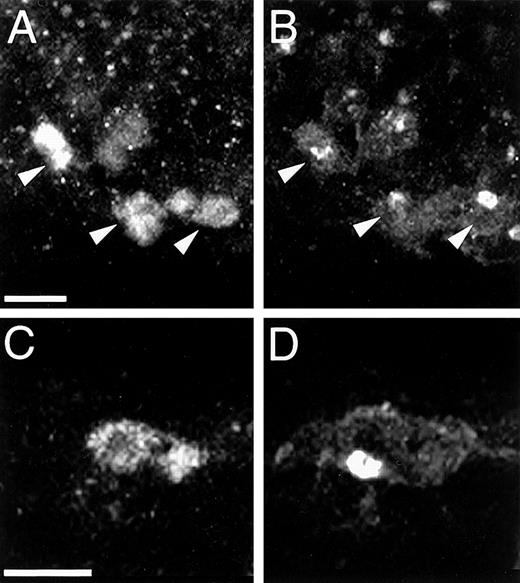
Angioblasts are TAL1+/Flk1+cells.
Double immunolabeling demonstrates that endothelial cells of forming vessels (panels A and B, arrowheads) and isolated mesodermal cells (panels C and D) coexpress TAL1 and Flk1. Note the distinctive immunostaining pattern of each protein. Magnification bar: panels A and B = 20 μm, panels C and D = 10 μm.
Angioblasts are TAL1+/Flk1+cells.
Double immunolabeling demonstrates that endothelial cells of forming vessels (panels A and B, arrowheads) and isolated mesodermal cells (panels C and D) coexpress TAL1 and Flk1. Note the distinctive immunostaining pattern of each protein. Magnification bar: panels A and B = 20 μm, panels C and D = 10 μm.
Angioblasts are the precursors of endothelial cells.
At 6.5 dpc an extraembryonic population of dispersed TAL1 and Flk1 immunopositive cells (panels A and B, arrowheads) are detected in regions where vascular networks form. Extraembryonic hematopoietic stem cells are TAL1+/Flk1−—TAL1 (panel A) and Flk1 (panel B) immunofluorescence associated with the 6.5-dpc blood islands (arrows) differ in immunostaining intensity. The absence of TAL1 (panel A, bracket) and Flk1 (panel B, bracket) immunostaining in intraembryonic regions indicates that vasculogenesis in the embryo proper is not initiated prior to 6.5 dpc. Magnification bar = 100μm.
Angioblasts are the precursors of endothelial cells.
At 6.5 dpc an extraembryonic population of dispersed TAL1 and Flk1 immunopositive cells (panels A and B, arrowheads) are detected in regions where vascular networks form. Extraembryonic hematopoietic stem cells are TAL1+/Flk1−—TAL1 (panel A) and Flk1 (panel B) immunofluorescence associated with the 6.5-dpc blood islands (arrows) differ in immunostaining intensity. The absence of TAL1 (panel A, bracket) and Flk1 (panel B, bracket) immunostaining in intraembryonic regions indicates that vasculogenesis in the embryo proper is not initiated prior to 6.5 dpc. Magnification bar = 100μm.
Vasculogenesis in 7.0- to 7.8-dpc embryos.
TAL1 (panel A) and Flk1 (panel B) immunofluorescences are associated with extraembryonic blood islands (arrows), vascular networks (braces), and the allantois (bracket) of the 7.0- to 7.3-dpc embryo. TAL1 and Flk1 are differentially expressed by cells of the forming blood islands. At 7.0 dpc strong TAL1 (panel A, arrows) and weak Flk1 (panel B, arrows) expressions are associated with the blood islands. Intraembryonic vasculogenesis is initiated at 7.3 dpc in the primordia of the endocardium. In panel B, Flk+ mesodermal cells located cranially define the endocardial primordia (doubleheaded arrow). The dorsal aortae are first discernible at 7.6 dpc. TAL1+ cells arranged along the embryonic axis define the aortic primordia (panel C, arrowheads). PECAM expression is confined to the primordia of “larger” vessels—PECAM+ cells are detected in the 7.8-dpc aortic primordia (arrowheads); note the absence of PECAM expression in regions lateral to the aortae (compare panels C and D). Dashed lines in panel C suggest the boundary between the intraembryonic and extraembryonic regions. Magnification bar = 100 μm.
Vasculogenesis in 7.0- to 7.8-dpc embryos.
TAL1 (panel A) and Flk1 (panel B) immunofluorescences are associated with extraembryonic blood islands (arrows), vascular networks (braces), and the allantois (bracket) of the 7.0- to 7.3-dpc embryo. TAL1 and Flk1 are differentially expressed by cells of the forming blood islands. At 7.0 dpc strong TAL1 (panel A, arrows) and weak Flk1 (panel B, arrows) expressions are associated with the blood islands. Intraembryonic vasculogenesis is initiated at 7.3 dpc in the primordia of the endocardium. In panel B, Flk+ mesodermal cells located cranially define the endocardial primordia (doubleheaded arrow). The dorsal aortae are first discernible at 7.6 dpc. TAL1+ cells arranged along the embryonic axis define the aortic primordia (panel C, arrowheads). PECAM expression is confined to the primordia of “larger” vessels—PECAM+ cells are detected in the 7.8-dpc aortic primordia (arrowheads); note the absence of PECAM expression in regions lateral to the aortae (compare panels C and D). Dashed lines in panel C suggest the boundary between the intraembryonic and extraembryonic regions. Magnification bar = 100 μm.
To characterize extraembryonic hematopoietic cells, TAL1 and Flk1 immunofluorescence was followed in 6.5- to 7.0-dpc embryos. At 6.5 dpc blood islands were characterized by intense TAL1 and weak Flk1 immunostaining (see Figure 3, A and B, arrows). A similar pattern of expression was evident in the blood islands at 7.0 to 7.3 dpc (see Figure 4, A and B, arrows). Analysis of optical sections demonstrated that endothelial cells, which comprise the outer component of the blood island, were Flk1+, whereas cells representing the hematopoietic lineage, those forming the “core,” were Flk1− (data not shown). Based on these data, it is concluded that extraembryonic hematopoietic cells are TAL1+/Flk1−.
Intraembryonic vasculogenesis 6.5 to 8.0 dpc: TAL1, Flk1, and PECAM expression
Intraembryonic vasculogenesis is initiated in the cranial region of 7.3-dpc embryos. Evident cranially were 2 populations of Flk1+(see Figure 4B, doubleheaded arrow) and TAL1+(data not shown) cells that were joined across the midline by a “string” of cells forming a crescent. The bilateral distribution of the TAL1+/Flk1+ cells coincides with regions of the embryo that are fated to give rise to the heart,23suggesting that the TAL1+/Flk1+ cells are endocardial progenitors.
The interval between 7.0 and 7.8 dpc is an active period of vasculogenesis (see Figure 4). During this period, TAL1+and Flk1+ cell numbers increase dramatically (compare Figure 4A to Figure 4C) and the aortic primordia first become discernible (Figure 4C, arrowheads). The first intraembryonic PECAM immunofluorescence was localized to the aortic primordia of 7.8-dpc embryos (Figure 4D, arrowheads). Comparison of PECAM immunostaining (see Figure 4D) to that of TAL1 (see Figure 4C) and Flk1 (data not shown) demonstrates that PECAM is not expressed by all TAL1+/Flk1+ cells; note the absence of PECAM expression in the TAL1+/Flk1+ cells located lateral to the aortae. These data establish that TAL1 and Flk1 are expressed earlier than PECAM (compare Figure 4, A-C, with Figure 4D) and suggests that angioblasts, isolated TAL1+/Flk1+ cells, do not express PECAM.
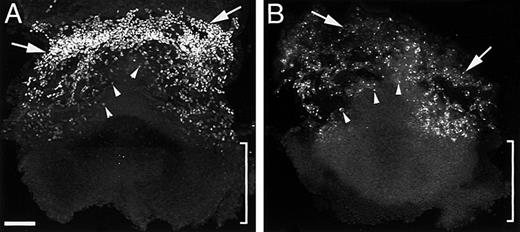
Allantoic vasculogenesis: TAL1, Flk1, and PECAM expression
Initial blood vessel formation in the allantois is indicated by the presence of a small number of dispersed TAL1+/Flk1+cells at 7.0 dpc (see Figure 4A, bracket). By 7.3 to 7.5 dpc, TAL1+/Flk1+ cells are numerous (see Figure 4B and Figure 5A). At this stage, no organized blood vessels or vessel primordia could be detected (data not shown). By 8.3 dpc PECAM immunofluorescence indicated the presence of both vessel primordia and vascular networks in the allantois (Figure 5B).
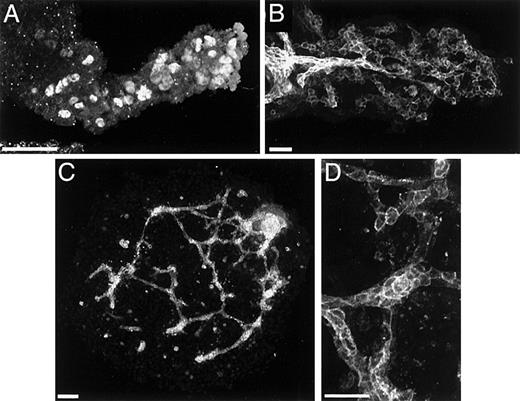
Blood vessels of the allantois arise via vasculogenesis from TAL1+/Flk1+ cells (angioblasts).
In panel A, isolated TAL1+ cells populate the allantoic mesoderm at 7.5 dpc. In panel B, PECAM+ vascular networks are evident in the 8.3-dpc allantois. In panel C, explanted prevascular (7.5 dpc) allantoides cultured for 24 hours establish a PECAM+ vasculature. Panel D is a high magnification image of the vessels depicted in panel C. Magnification bars: panels A, B, and D = 50 μm; panel C = 100 μm.
Blood vessels of the allantois arise via vasculogenesis from TAL1+/Flk1+ cells (angioblasts).
In panel A, isolated TAL1+ cells populate the allantoic mesoderm at 7.5 dpc. In panel B, PECAM+ vascular networks are evident in the 8.3-dpc allantois. In panel C, explanted prevascular (7.5 dpc) allantoides cultured for 24 hours establish a PECAM+ vasculature. Panel D is a high magnification image of the vessels depicted in panel C. Magnification bars: panels A, B, and D = 50 μm; panel C = 100 μm.
To investigate whether these vessels arise by vasculogenesis, or by angiogenesis, allantoides similar to that depicted in Figure 5A were isolated and cultured. After 12 hours in culture, PECAM staining revealed both vessel primordia and vascular networks (Figure 5, C and D). Because these vessels arose from tissue containing TAL1+/Flk1+ cells but no organized blood vessels, it can be concluded that neovascularization occurred via vasculogenesis and that the TAL1+/Flk1+ cells are the precursors of endothelial cells (Figure 5C).
TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 expression in intraembryonic vasculogenesis: 8.0 to 8.5 dpc
Between 8.0 and 8.5 dpc, a rudimentary circulatory system is established. Figure 6 depicts the expression patterns of TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 in the vessels of 8.2 to 8.3 dpc (Figure 6, A-F) and 8.5 embryos (Figure 6, G-L). The expression of these proteins in prominent morphologic structures of the circulatory system such as the bilateral aortae, the endocardial primordia, and the primary vascular networks that form lateral to the embryonic axis, which are referred to as lateral vascular networks, are summarized in Table1.
Intraembryonic vasculogenesis 7.0 to 7.5 dpc.
The upper panels (A-F) show the immunofluorescence patterns of TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 in 8.2- to 8.3-dpc embryos; the lower panels (G-L), show expression at 8.35 to 8.5 dpc. At 8.2 to 8.3 dpc TAL1 and Flk1 immunostaining (panels A and B) is associated with the aortic primordia (bracket), the endocardial primordia (arrows), and the lateral vascular networks (asterisk). TAL1 expression is down-regulated as part of endocardiogenesis. Comparison of the immunofluorescence patterns of TAL1 (panel A) and Flk1 (panel B) at 8.2 dpc indicates that these proteins are coexpressed by cells of the endothelial lineage. At 8.5 dpc endocardial expression of TAL1 and Flk1 diverge; although Flk1 (panel H, arrow) continues to be expressed, TAL1 immunostaining (panel G, arrow) is no longer evident. PECAM, CD34, and VE-cadherin expression is restricted to the primordia of larger vessels. Comparison of PECAM, CD34, and VE-cadherin immunofluorescence (panels C-E and I-K) to that of TAL1 and Flk1 (panels A, G and B, H) shows colabeling of the aortic primordia (bracket), the endocardial primordia (arrowsheads), and the endocardium (arrow). Conspicuously absent in these embryos is colabeling in the lateral vascular networks (asterisk). Tie2 is rapidly up-regulated. At 8.2 to 8.3 dpc, Tie2 immunofluorescence is weak as compared to that of the other proteins evaluated in this study (compare panel F to panels A-E); however, by 8.5 dpc (panel L) clear Tie2 expression is associated with the aortic primordia (bracket) and the endocardium (insert). Magnification bar = 100 μm.
Intraembryonic vasculogenesis 7.0 to 7.5 dpc.
The upper panels (A-F) show the immunofluorescence patterns of TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 in 8.2- to 8.3-dpc embryos; the lower panels (G-L), show expression at 8.35 to 8.5 dpc. At 8.2 to 8.3 dpc TAL1 and Flk1 immunostaining (panels A and B) is associated with the aortic primordia (bracket), the endocardial primordia (arrows), and the lateral vascular networks (asterisk). TAL1 expression is down-regulated as part of endocardiogenesis. Comparison of the immunofluorescence patterns of TAL1 (panel A) and Flk1 (panel B) at 8.2 dpc indicates that these proteins are coexpressed by cells of the endothelial lineage. At 8.5 dpc endocardial expression of TAL1 and Flk1 diverge; although Flk1 (panel H, arrow) continues to be expressed, TAL1 immunostaining (panel G, arrow) is no longer evident. PECAM, CD34, and VE-cadherin expression is restricted to the primordia of larger vessels. Comparison of PECAM, CD34, and VE-cadherin immunofluorescence (panels C-E and I-K) to that of TAL1 and Flk1 (panels A, G and B, H) shows colabeling of the aortic primordia (bracket), the endocardial primordia (arrowsheads), and the endocardium (arrow). Conspicuously absent in these embryos is colabeling in the lateral vascular networks (asterisk). Tie2 is rapidly up-regulated. At 8.2 to 8.3 dpc, Tie2 immunofluorescence is weak as compared to that of the other proteins evaluated in this study (compare panel F to panels A-E); however, by 8.5 dpc (panel L) clear Tie2 expression is associated with the aortic primordia (bracket) and the endocardium (insert). Magnification bar = 100 μm.
TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 expression in endocardial development: 8.2 to 8.5 dpc
As described above, endocardiogenesis is initiated at 7.3 dpc (see Figure 4B). Between 8.2 and 8.5 dpc the bilateral heart fields are translocated to the midline forming the definitive endocardium (Figure6—compare panel B, arrowheads, and panel H, arrow). At 8.2 to 8.3 dpc, Flk1 expression was observed throughout the merging heart fields (see Figure 6B, arrowheads). In contrast, although TAL1 expression was associated with the caudal portions of the heart fields, those lying along the anterior intestinal portal (see Figure 6A, more posterior arrowheads), only weak staining was detected in the more cranial portions of the fields (see Figure 6A, more anterior arrowheads). At 8.5 dpc the endocardium is characterized by strong Flk1 immunofluorescence (see Figure 6H, arrow) and the absence of detectable TAL1 immunofluorescence (Figure 6G, arrow). Unlike TAL1, immunofluorescence associated with PECAM, CD34, VE-cadherin, and Tie2 was readily detected on the endocardium (Figure 6, I-L, arrows). It is concluded from these data that the TAL1+/Flk1+cells observed in cranial regions at 7.3 dpc (see Figure 4B) and in heart fields at 8.2 dpc (see Figure 6, A and B) represent the progenitors of the TAL1−/Flk1+endocardial endothelial cells seen at 8.5 dpc (see Figure 6, G and H, arrows).
TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 expression in the dorsal aortae: 8.2 to 8.5 dpc
The dorsal aorta is derived from the fusion of bilateral primordia, the dorsal aortae. At 8.3 dpc both cranial and caudal portions of the dorsal aortae exhibited intense PECAM staining (see Figure 6C and Figure 7C, arrowheads), whereas the more intermediate portion stained less intensely (Figure 7C, bracket, and 7E). This immunostaining pattern coincided with morphogenetic features of the developing aortae. Intense PECAM staining was associated with segments that, based on physical sections, had a defined lumen, whereas less intense staining was detected in segments composed of primary vascular networks. It is concluded that the aortae form in a bidirectional manner and that vascular networks are an essential component of aortic morphogenesis. Similar to PECAM, immunostaining for TAL1, Flk1, CD34, and VE-cadherin was localized to the aortic primordia of 8.2- and 8.5-dpc embryos. In contrast to these proteins, Tie2 immunofluorescence was absent at 8.2 dpc (see Figure 6F); however, expression was detected at 8.5 dpc (see Figure 6L). This observation suggests that Tie2 expression correlates with a discrete step in vessel maturation.
Angioblasts are TAL1+/ Flk1+and PECAM− cells.
PECAM expression (panel B) is associated with the aortic endothelial cells (arrowheads) but is absent in the more lateral mesoderm (brackets), which is populated by TAL1+ cells (panel A, brackets). Aortic morphogenesis is bidirectional. In panel C, a segment of an 8.3-dpc aortae is labeled with PECAM antibodies. Labeling is most intense in the cranial and caudal regions, the more mature segments of the aortae (arrowheads), and diminishes in intensity in the network forming region (bracket). Protein expression in the lateral vascular networks distinguishes this vascular bed from others. Panel D depicts a segment of an aorta and the associated lateral mesoderm immunolabeled using Flk1 antibodies. Vessel primordia (arrows) and angioblasts (asterisks) are evident in the lateral region, a site where no PECAM, CD34, or VE-cadherin immunofluorescence was detected (see Figure 6, I-K, asterisk). Cells of the aortic primordia differ in their temporal and spatial expression of PECAM, CD34, and VE-cadherin. Panels E and F show PECAM immunofluorescence associated with the aortae of 8.2- and 8.5-dpc embryos. Panels G and H show, respectively, CD34 and VE-cadherin immunofluorescence associated with the aortae of 8.5-dpc embryos. Magnification bar = 50 μm.
Angioblasts are TAL1+/ Flk1+and PECAM− cells.
PECAM expression (panel B) is associated with the aortic endothelial cells (arrowheads) but is absent in the more lateral mesoderm (brackets), which is populated by TAL1+ cells (panel A, brackets). Aortic morphogenesis is bidirectional. In panel C, a segment of an 8.3-dpc aortae is labeled with PECAM antibodies. Labeling is most intense in the cranial and caudal regions, the more mature segments of the aortae (arrowheads), and diminishes in intensity in the network forming region (bracket). Protein expression in the lateral vascular networks distinguishes this vascular bed from others. Panel D depicts a segment of an aorta and the associated lateral mesoderm immunolabeled using Flk1 antibodies. Vessel primordia (arrows) and angioblasts (asterisks) are evident in the lateral region, a site where no PECAM, CD34, or VE-cadherin immunofluorescence was detected (see Figure 6, I-K, asterisk). Cells of the aortic primordia differ in their temporal and spatial expression of PECAM, CD34, and VE-cadherin. Panels E and F show PECAM immunofluorescence associated with the aortae of 8.2- and 8.5-dpc embryos. Panels G and H show, respectively, CD34 and VE-cadherin immunofluorescence associated with the aortae of 8.5-dpc embryos. Magnification bar = 50 μm.
TAL1, Flk1, PECAM, CD34, VE-cadherin, and Tie2 expression in the lateral vascular networks: 8.2 to 8.5 dpc
Between 8.2 and 8.5 dpc the lateral vascular networks are formed. These networks extend from a region just lateral to the aortae to an ill-defined boundary where they connect with the extraembryonic vasculature (see Figure 4C, dashed lines). Isolated TAL1+/Flk1+ cells can be detected within the lateral regions as early as 7.6 dpc (see Figure 4C); by 8.2 dpc the first networks are apparent (see Figure 6, A and B, asterisk) and by 8.5 dpc the lateral vascular networks are clearly discernible (see Figure 6, G and H, asterisk). Double immunofluorescence experiments revealed that TAL1 and Flk1 are coexpressed in cells of both the forming and established lateral vascular networks (data not shown). In contrast to the expression of TAL1 and Flk1, PECAM expression was conspicuously absent in these vessels at both 8.2 dpc (compare PECAM in Figure 6C to that of TAL1 in Figure 6A and Flk1 in Figure 6B) and 8.5 dpc (compare PECAM in Figure 6I to that of TAL1 in Figure 6G and Flk1 in Figure 6H). The immunostaining patterns of CD34 (see Figure 6, D and J) and VE-cadherin (see Figure 6, E and K) at 8.2 and 8.5 dpc were similar to that of PECAM, with expression associated with the forming aortae (bracket) but absent in the lateral vascular networks (asterisk).
The absence of PECAM, CD34, and VE-cadherin expression in the lateral vascular networks at 8.5 dpc was unexpected, because each of these proteins was associated with the morphogenesis/maturation of other primary vascular networks (ie, in the developing allantois and aortae [see Figure 5B and Figure 6, C-E, respectively]). This finding was pursued in double immunofluorescence studies. Immunolabeling of 8.5-dpc embryos with TAL1 and PECAM antibodies demonstrated colabeling of the aortic primordium (see Figure 7, A and B, arrowheads) and the absence of PECAM expression in the TAL1+ cells of lateral vascular networks (see Figure 7, A and B, brackets). Double-immunolabeling studies using Flk1 and PECAM antibodies yielded similar results (data not shown). These data established that cells of the aortic primordia (arrowheads) are TAL1+/Flk1+/PECAM+, whereas those of the lateral vascular networks (brackets) are TAL1+/Flk1+/PECAM−. Similar studies comparing TAL1 and Flk1 expression to that of either CD34 or VE-cadherin demonstrated that coexpression of TAL1 and Flk1 was confined to the aortae, whereas laterally, only TAL1+/Flk1+ cells were detected (data not shown).
To determine if the absence of PECAM, CD34, and VE-cadherin expression had morphologic consequences, vasculogenesis in the lateral regions was evaluated using Flk1 antibodies. Analysis of Flk1 immunostaining indicated that vascular morphogenesis, including those events requiring endothelial cell–cell adhesion, had proceeded normally (see Figure7D). As part of this analysis, a population of Flk1+ and TAL1+ (data not shown) cells located along the lateral margin of the aortae were detected. The position of these TAL1+/Flk1+ cells is consistent with the possibility that such cells are angioblasts (see Figure 7D, arrowheads and asterisks), some of which seem to be in the process of “joining” the developing aortae (see Figure 7D, arrowheads).
Although PECAM, CD34, and VE-cadherin were each expressed by cells of the aortic primordia, differences in their temporal and spatial immunofluorescence patterns were observed. For instance, PECAM expression on the aortic primordia was initially associated with the entire cell surface (see Figure 7E); later expression was localized to sites of cell–cell contact (see Figure 7F). In contrast, VE-cadherin expression, when observed, was always present at sites of cell–cell contact (see Figure 6, I and J, and Figure 7H).
TAL1 is down-regulated as part of endothelial cell maturation
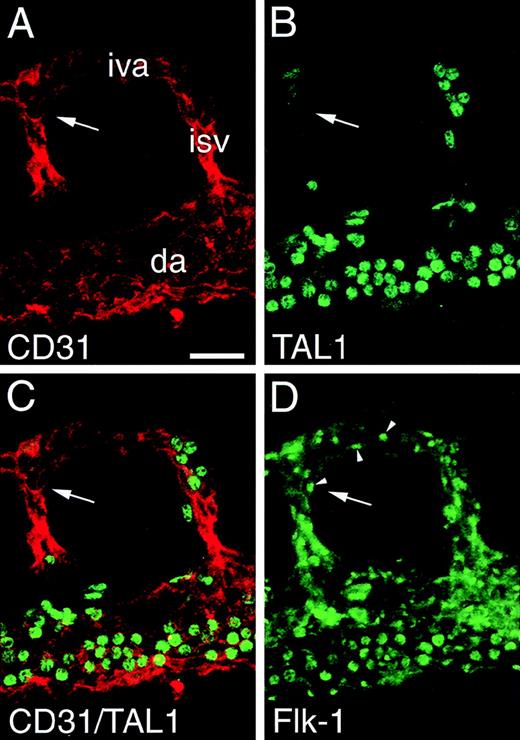
The diminution of TAL1 expression associated with endocardial development (see Figure 6, A and B) suggested a relationship between the level of TAL1 expression and the state of endothelial cell maturation. To investigate this possibility, TAL1 expression was followed during aortic development. Although strong TAL1 immunofluorescence was associated with the aortae of 8.2- and 8.4-dpc embryos (see Figure 6, A and B), by 9.0 dpc no expression was detected. In Figure 8, expression of TAL1, Flk1, and PECAM in the aortae of 9.0-dpc embryos was examined in triple immunofluorescence studies. Figure 8A shows a segment of aortae and the associated intersomitic and intervertebral vessels immunostained with PECAM antibodies. In contrast to the uniform expression of PECAM on endothelial cells, TAL1 immunofluorescence was confined to a population of uniformly round cells (see Figure 8B). Analysis of optical sections demonstrated that these cells were confined to the vascular lumen, suggesting that they are associated with the hematopoietic rather than the endothelial lineage. When the TAL1 and PECAM immunostaining patterns are superimposed (see Figure 8C), the lack of detectable TAL1 expression in endothelial cells was evident; this is most apparent in the vessel segment indicated by the arrow. Flk1 expression was examined to determine if a correlation exists between the level of TAL1 expression and that of Flk1 (see Figure 8D). Clear Flk1 immunofluorescence was associated with endothelial cells (compare Figure 8A and D); this expression (arrowheads) was most evident in the vessel segment denoted by the arrow. Comparison of TAL1 and Flk1 expression (Figure 8, B and D) establishes that mature endothelial cells are TAL1−/Flk1+. The ability to detect Flk1 protein in endothelial cells lacking TAL1 expression suggests that the expressions of these proteins are independently regulated.
TAL1 expression is down-regulated as part of vascular morphogenesis/maturation.
Panels A through D depict a segment of a 9.0-dpc aorta triple immunolabeled with TAL1, Flk1, and PECAM antibodies. In panel A, PECAM immunofluorescence is associated with endothelial cells of the dorsal aortae (da), the intersomitic vessels (isv), and the intervertebral arteries (iva). In panel B, TAL1 immunofluorescence is associated with uniformly round cells within the vessel lumen (blood cells). In panel C, a superimposition of the PECAM and TAL1 immunostaining patterns shows that endothelial cells lack detectable TAL1 expression; this is most apparent in the vessel segment indicated by the arrow. In panel D, Flk1 immunofluorescence (arrowheads) is associated with endothelial cells including those in the vessel segment indicated by the arrow. Magnification bar = 25 μm.
TAL1 expression is down-regulated as part of vascular morphogenesis/maturation.
Panels A through D depict a segment of a 9.0-dpc aorta triple immunolabeled with TAL1, Flk1, and PECAM antibodies. In panel A, PECAM immunofluorescence is associated with endothelial cells of the dorsal aortae (da), the intersomitic vessels (isv), and the intervertebral arteries (iva). In panel B, TAL1 immunofluorescence is associated with uniformly round cells within the vessel lumen (blood cells). In panel C, a superimposition of the PECAM and TAL1 immunostaining patterns shows that endothelial cells lack detectable TAL1 expression; this is most apparent in the vessel segment indicated by the arrow. In panel D, Flk1 immunofluorescence (arrowheads) is associated with endothelial cells including those in the vessel segment indicated by the arrow. Magnification bar = 25 μm.
Discussion
TAL1/Flk1
Based on this study, we propose a working definition of the phenotype of angioblasts, embryonic endothelial cells, and extraembryonic hematopoietic stem cells. Angioblasts are dispersed (not associated with an organized vessel) TAL1+/Flk1+ mesodermal cells. That such cells represent angioblasts and not hemangioblasts is indicated by the fact that blood vessels lacking blood cells (see Figure 4, A and B, braces) form in extraembryonic regions where dispersed TAL1+/Flk1+ cells were detected earlier in development (see Figure 3B, arrowheads) and the fact that blood vessels in the embryo proper, which are known to form without associated hematopoietic development, also arise from TAL1+/Flk1+ cells (see Figure 4 and Figure 6, A-F).2,3 Additionally, that TAL1+/Flk1+ cells represent angioblasts, which are defined as the progenitors of endothelial cells, is supported by the observation that endothelial cells of early embryonic blood vessel are TAL1+/Flk1+. Our definition of endothelial cells is conditional because the data indicate that the endothelial cells of more mature blood vessels are TAL1−/Flk1+ (see Figure 8).That mature endothelial cells do not express TAL1 is also indicated by work demonstrating that embryonic endothelial cells of zebra fish express messenger RNA (mRNA) for Flk1 but not TAL1.7 Based on these data we define endothelial cells comprising the most primitive vessels of 7.0- to 8.5-dpc embryos as TAL1+/Flk1+ cells, whereas those of more mature vessels are TAL1−/Flk1+ cells. Based on our analysis of protein expression in the 6.5-dpc embryo, we have defined extraembryonic hematopoietic stem cells as TAL1+/Flk1−. This definition is supported by in situ RNA hybridization studies that show a population of blood island cells in zebra fish embryos that are TAL1+/Flk1−,7 and the finding that Flk−/−embryonic stem cells implanted into normal mouse embryos can generate at least primitive components of the hematopoietic lineage.24
The analysis of TAL1 and Flk1 expression in the early mouse embryo raises questions as to the relationship of the endothelial and hematopoietic lineages. Work by a number of groups has provided in vitro evidence for the existence of a cell, the hemangioblast, that has the potential to give rise to both the endothelial and hematopoietic lineages.4,25-27 Whether these 2 lineages share a common precursor in vivo or are derived from separate mesodermal populations is yet to be established. Despite this uncertainty, efforts have been made to characterize the in vivo phenotype of cells that contribute to each lineage. For example, Gering and colleagues have reported that TAL1+/Flk1+ expressing cells observed early in development represent hemangioblasts.7 In contrast, our work as discussed above indicates that such cells more likely represent angioblasts. To reconcile our data with the hemangioblast as a TAL1+/Flk1+ cell would require that mechanisms exist whereby: (1) Flk1 expression is rapidly down-regulated in the progenitors of extraembryonic hematopoietic stem cells as they commit to the hematopoietic lineage and (2) the hematopoietic program is blocked in TAL1+/Flk1+ cells that form blood vessels without associated hematopoiesis. Because we cannot rule out the existence of such mechanisms, it is possible that TAL1+/Flk1+ cells represent hemangioblasts. The long-standing debate over the existence of hemangioblasts is testament to the inherit problems of proving the existence of such cells in vivo. Clearly, more research is need before the mystery of the hemangioblast is resolved.
Several concepts have emerged from our analysis of TAL1 and Flk1 expression. First, our studies indicate that TAL1 expression is more closely associated with vasculogenesis than with angiogenesis. This conclusion is based on our data demonstrating that high levels of TAL1 expression are associated with the earliest stages of vascular development (prior to 8.5 dpc, see Figures 3, 4, and 6) and the finding that at stages more closely associated with angiogenesis (8.5-9.0 dpc), TAL1 expression is diminished or absent in endothelial cells (see Figure 8). Although our data suggest a role for TAL1 in vasculogenesis, work by others showing that vasculogenesis proceeds in TAL1−/− mice argue against this view.28,29 Even though the role of TAL1 in vasculogenesis remains open, studies in TAL1−/− mice where TAL1 expression is driven by the GATA-1 promoter have demonstrated a role for TAL1 later in vascular development.30 An explanation of the TAL1−/− and “GATA-1/TAL1” phenotypes that is compatible with TAL1 having a role in vasculogenesis is that vasculogenesis in the TAL1−/− mice is not normal. In this scenario the abnormal yolk sac vessel development observed in the “GATA-1/TAL1” mice is a consequence of faulty vasculogenesis that becomes apparent due to the extended survival time of these mice.
Second, our data demonstrate that extraembryonic blood vessels arise by 2 morphologically distinct processes: directly from dispersed angioblasts and from angioblasts associated with mesodermal aggregates (blood islands). This finding was unexpected because studies in avians suggested that the assembly of blood vessels from dispersed angioblasts was confined to the intraembryonic regions and that vessels in the extraembryonic regions formed exclusively in association with blood islands.2,3 Because this does not appear to be the case in mice, the mechanisms regulating these processes are not likely to rest solely in the gross differences between the intraembryonic and extraembryonic regions, in particular differences in the intra- and extraembryonic endoderm.31-33
PECAM, CD34, and VE-cadherin
The immunolocalization of PECAM, CD34, and VE-cadherin observed in this study demonstrated similar temporal and spatial expression patterns. At 8.0 dpc expression of each was intense on aortic endothelial cells, moderate on primordial endothelial cells, and absent on angioblasts. These patterns suggest that there is a correlation between protein expression and the state of endothelial cell maturation/vessel morphogenesis. Previous studies examining the expression patterns of these proteins in vivo are limited to CD34.13,14 19 Our observations of CD34 expression were similar to those previously reported but differ as regards expression by angioblasts.
It is important to note that although PECAM, CD34, and VE-cadherin were all expressed by cells of the aortic primordia, clear differences were observed in both the temporal and spatial expression patterns. For example, PECAM immunostaining was initially detected on the entire cell surface (see Figure 7E), whereas later it was localized at sites of cell–cell contact (see Figure 7F). In contrast, VE-cadherin immunostaining was always localized at sites of cell–cell contact (compare Figure 7H to Figure 7, F and G).
Our localization of VE-cadherin immunofluorescence to the aortae of 8.2-dpc embryos (see Figure 6E) establishes that this protein is expressed at stages relevant to vasculogenesis. Further, the expression of VE-cadherin as well as that of PECAM and CD34 (see Figure 6, C and D) at 8.2 dpc coincides with a narrow developmental window when cell–cell interactions are likely to be required in vasculogenesis. A role(s) for these proteins in vasculogenesis is indicated by in vitro studies that suggest that PECAM and VE-cadherin function in the assembly and development of blood vessels.15,34-37 In contrast to these works and our study, analysis of PECAM, CD34, and VE-cadherin function in mice using targeted gene deletion do not support a role in vasculogenesis.38-42 In fact, only the VE-cadherin null mice exhibited an abnormal vascular phenotype and even in this case the abrogation of function did not compromise blood vessel formation mediated by vasculogenesis.41 42 Therefore, none of these targeted deletions alone was sufficient to disrupt endothelial cell–cell recognition and adhesion events that are necessary for vasculogenesis. When these data are considered in the context of the protein expression patterns observed in this study, it is reasonable to speculate that these genes share, in some as yet undefined manner, overlapping functions that “mask” their individual role(s) in vasculogenesis.
Tie2
A striking aspect of Tie2 expression was the rapid up-regulation in immunofluorescence intensity seen between 8.3 and 8.5 dpc. In contrast to the other proteins examined, virtually no Tie2 immunostaining was detected on the aortae of 8.2-dpc embryos (compare Figure 6F with Figure 6, A-E). However, by 8.5 dpc distinct Tie2 expression was detected along the entire length of the aortae (see Figure 6L). The “late” expression of Tie2 is in accord with the findings of others that suggest that Tie2 functions in later events of vascular morphogenesis.17 The immunolocalization of Tie2 to the developing aortae and endocardium represents, to our knowledge, the first time that Tie2 expression has been described at the protein level.
Allantoic vasculogenesis
Our in vitro data established that blood vessel formation in the allantois occurs by vasculogenesis and that TAL1+/Flk1+ cells represent the precursors of endothelial cells. When allantoides containing TAL1+/Flk1+ cells but no morphologically distinct vessels were placed in tissue culture (see Figure 5A), networks of PECAM+ vessels were observed after 12 to 24 hours (see Figure 5, C and D). This observation and the work of Downs and colleagues clearly establish that initial neovascularization of the allantois occurs by vasculogenesis.43 Further, we speculate that the TAL1+/Flk1+ cells present at the initiation of culture, or similar cells generated during the culture period, represent the precursors of allantoic endothelial cells.
Endocardiogenesis
The first vasculogenic activity in the embryo proper is associated with the development of the endocardium. At presomite stages (7.3 dpc) 2 bilateral fields of TAL1+/Flk1+ mesodermal cells were evident in cranial regions of the embryo (see Figure 4B). That these TAL1+/Flk1+ cells represent the progenitors of endocardium is supported by fate mapping studies23 and the fact that the expression patterns of TAL1 and Flk1 are virtually superimposable with the pattern of Csx/Nkx-2.5 mRNA, a putative marker of myocardial progenitors.44,45Taken together these data define the primordial heart fields in terms of each of the constituent cell populations (endocardial and myocardial). A second and potentially important observation regarding endocardiogenesis is the down-regulation TAL1 expression in more mature endocardial endothelial cells (compare Figure 6A and Figure 6G). The significance of this observation will only become clear when the function of TAL1 is more clearly defined. Our immunolocalization of Tie2 to the developing endocardium at 8.5 dpc (Figure 6L, insert) links the temporal expression of this receptor with the abnormal cardiac phenotype observed in Tie2 null mice.6
Vascular morphogenesis in the dorsal aortae and lateral vascular networks
An unexpected finding of this study was the restricted pattern of protein expression observed in the lateral vascular networks (compare Figure 6, A and B, to Figure 6, C-F, and Figure 6, G and H, to Figure6, I-L). Although the absence of PECAM, CD34, and VE-cadherin expression (Figure 6, C-E) at 8.2 dpc could be attributed to angioblasts being the predominant cell type (Figure 6, A and B), the lack of staining at 8.5 dpc is not as easily explained. Specifically endothelial cells of vascular structures such as those depicted in Figure 7D (arrows) would “normally” express these proteins. Although the basis for this difference is unknown, data from avians suggests that vascular morphogenesis in this region may be unique. In quail embryos, this region progressively becomes avascular despite the initial presence of endothelial cells.46-48 Although we have no definitive evidence as to the fate of cells in this region, we did observe angioblasts that appeared to be in the process of integrating into existing vessels (see Figure 7D, arrowheads).
Our observation of mouse aortic morphogenesis suggests that the aortae arise from primary vascular networks and that development is bidirectional (see Figure 7C). Although the bidirectionality of aortic development in mice was not anticipated, the role of primary vascular networks in the morphogenesis of a relatively large vessel such as the aorta is compatible with our previous work in avians.46Examining the effects of increased levels of vascular endothelial growth factor on vascular morphogenesis, we note that primary vascular networks can undergo fusion to form large vascular structures. Based on this work we speculate that the aortae are formed through the fusion of primary vascular networks.49
The aims of this study were to establish a morphogenetic basis for mouse vasculogenesis. The data integrate the temporal expression of proteins related to the endothelial lineage with vessel morphogenesis in the mouse embryo. Embryonic vasculogenesis is considered both in its own right and for its potential to provide insights into the process as it occurs in adults.50 51
Acknowledgments
The authors express thanks to the following for their generous gift of antibodies: Dr Stephen J. Brandt (Vanderbilt University and Veterans Affairs Medical Center, Nashville, TN) for TAL1, Drs Elisabetta Dejana and Maria Lampugnani (Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy) for VE-cadherin, Dr Lawrence A. Lasky (Genentech, Inc, San Francisco, CA) for CD34, Dr Andre Schuh (University of Toronto, Toronto, Ontario, Canada) for Flk1, and Dr Steven Stacker (Ludwig Institute for Cancer Research, Victoria, Australia) for Tie2. Additionally, the authors would like to thank Drs Charles Little, Scott Argraves, and Vladimir Mironov (Medical University of South Carolina, Charleston SC) for their insights and critical comments.
Supported by NIH R01 HL 57375 grant to C.J.D.
Reprints:Christopher Drake, Department of Cell Biology, Medical University of South Carolina, PO Box 250508, 173 Ashley Avenue, Charleston, SC 29425; email: drakec@musc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.