During sepsis, lipopolysaccharide (LPS) triggers the development of disseminated intravascular coagulation (DIC) via the tissue factor-dependent pathway of coagulation resulting in massive thrombin generation and fibrin polymerization. Recently, animal studies demonstrated that hirudin reduced fibrin deposition in liver and kidney and decreased mortality in LPS-induced DIC. Accordingly, the effects of recombinant hirudin (lepirudin) was compared with those caused by placebo on LPS-induced coagulation in humans. Twenty-four healthy male subjects participated in this randomized, double-blind, placebo-controlled, parallel group study. Volunteers received 2 ng/kg LPS intravenously, followed by a bolus-primed continuous infusion of placebo or lepirudin (Refludan, bolus: 0.1 mg/kg, infusion: 0.1 mg/kg/h for 5 hours) to achieve a 2-fold prolongation of the activated partial thromboplastin time (aPTT). LPS infusion enhanced thrombin activity as evidenced by a 20-fold increase of thrombin-antithrombin complexes (TAT), a 6-fold increase of polymerized soluble fibrin, termed thrombus precursor protein (TpP), and a 4-fold increase in D-dimer. In the lepirudin group, TAT increased only 5-fold, TpP increased by only 50%, and D-dimer only slightly exceeded baseline values (P < .01 versus placebo). Concomitantly, lepirudin also blunted thrombin generation evidenced by an attenuated rise in prothrombin fragment levels (F1 + 2,P < .01 versus placebo) and blunted the expression of tissue factor on circulating monocytes. This experimental model proved the anticoagulatory potency of lepirudin in LPS-induced coagulation activation. Results from this trial provide a rationale for a randomized clinical trial on the efficacy of lepirudin in DIC.
Despite a growing understanding of the pathogenesis of sepsis-induced disseminated intravascular coagulation (DIC),1,2 infusion of low doses of heparin remains the only specific therapeutic option available.3 The use of heparins, however, is hampered by functional limitations, which may have precluded their widespread use in DIC.2 These limitations include the necessity of sufficient plasma levels of antithrombin III (AT III) as an endogenous cofactor and the risk of heparin-induced thrombocytopenia. Furthermore, heparin less effectively inactivates clot-bound thrombin4 and its anticoagulant efficacy may be decreased by the release of endogenous inhibitors like platelet factor 4 and lactoferrin, which are increasingly released during systemic inflammation.5-9
In contrast, hirudin is devoid of these limitations. Hirudin, in contrast to heparin, has not been reported to activate platelets, and thus carries a lower risk for drug-induced thrombocytopenia, which is difficult to differentiate from platelet consumption during DIC.10-12 The latter could be particularly important in DIC where platelet counts are frequently low. Furthermore, recombinant hirudin inhibited the expression of tissue factor (TF) on endothelial cells and media at the sites of angioplasty in rabbits and pigs.13
The theoretical advantages of lepirudin have fueled a number of animal studies on the effects of lepirudin in lipopolysaccharide (LPS)-induced DIC, the results of which are promising. Lepirudin prevented fibrin deposition in kidney and liver of rats and decreased mortality of rabbits from 70% to less than 20%.14-16 Furthermore, a small uncontrolled study in 6 patients with leukemia and DIC suggested that lepirudin may interrupt thrombin activity.17
However, to the best of our knowledge, no controlled clinical studies have yet investigated the efficacy of lepirudin in LPS-induced coagulation. The injection of LPS into human volunteers has proven to be a standardized powerful model to explore new treatment strategies for the initial phase of coagulation activation during endotoxemia.18 19
Thus, we hypothesized that infusion of the direct thrombin inhibitor, lepirudin, blocks LPS-induced thrombin activity and fibrin generation. We further aimed to clarify whether lepirudin inhibits thrombin generation by interrupting its positive feedback loop. Finally, we set out to evaluate whether lepirudin blunts LPS-induced TF expression on monocytes.
Materials and methods
Study design
The study design was randomized, double-blind, placebo-controlled in parallel groups in 24 healthy male subjects. To warrant adequate blinding, treatments were indistinguishable from each other and prepared by a study nurse otherwise not involved in the trial. The randomization code was broken only after all data had been entered into the database. The study was approved by the Institutional Ethics Committee of the Vienna University Medical School, and written informed consent was obtained from all participants before their enrollment in the study.
Study subjects
The mean age of the study subjects was 29 ± 7 years and mean body mass index was 23.4 ± 2.2 kg/m2. Determination of health status included medical history, physical examination, laboratory parameters, and virologic screening. Drug abuse (ie, benzodiazepine, cannabis, cocaine, and opioids) was excluded in all study subjects by analyzing urinary samples with EMIT (Behring, Marburg, Germany). Additional exclusion criteria were hereditary thrombophilia, that is, antithrombin deficiency and activated protein C resistance, and regular or recent intake of medication including nonprescription medication.
Study protocol
Volunteers were admitted to the study ward at 8:00 amafter an overnight fast. Throughout the entire study period, subjects were confined to bed rest and kept fasting for 8.5 hours following LPS infusion. Vital parameters (electrocardiogram, heart rate and oxygen saturation, blood pressure) were monitored on an automated monitoring system (Care View System, Hewlett Packard, Böblingen, Germany). Participants in the trial received 500 mg acetaminophen (Paracetamol Genericon Pharma, Lannach, Austria) to alleviate subjective LPS-related symptoms without compromising the systemic host response to LPS.20 Thirty minutes thereafter, all subjects received an intravenous bolus of 2 ng/kg LPS (National Reference Endotoxin, Escherichia coli; USP Convention, Rockville, MD). Ten minutes after LPS infusion, study subjects in the lepirudin group received 0.1 mg/kg recombinant hirudin (INN: lepirudin; Refludan, Hoechst Marion Roussel, Austria) followed by a continuous infusion of lepirudin at a rate of 0.1 mg/kg/h for 5 hours. Identical volumes of saline were given in the placebo group.
The dosages of the lepirudin bolus and continuous infusion were based on a large scale trial in 3000 patients with myocardial infarction receiving lepirudin as an adjunctive therapy after thrombolysis. In this trial, patients in the lepirudin-treated group were more likely to achieve the targeted degree of anticoagulation (ie, activated partial thromboplastin time [aPTT] 55-85 seconds) than heparin-treated individuals.21
Sampling
Blood samples were collected by venipunctures into citrated Vacutainer tubes (final concentration 0.13 mmol/L sodium citrate Vacutainer, Becton Dickinson, San Jose, CA) before drug administration and at 1, 2, 3, 4, 6, and 24 hours after LPS administration, applying minimal venostasis. Plasma samples were processed immediately by centrifugation at 2000g at 4°C for 15 minutes and stored at −80°C before analysis.
Analyses
All participants in the study were screened for AT III deficiency (STA Antithrombin III, Diagnostica Stago, Asnieres-Sur-Seine, France), factor V, Leyden mutation (Coatest APC Resistance, Chromogenix, Mölndal, Sweden) and the protein C (Coamatic, Protein C, Chromogenix) and S deficiency (Asserachrom Protein S, Diagnostica Stago) to exclude hereditary thrombophilia.
Commercially available assays were used to measure prothrombin fragment F1 + 2 (Behring; normal values: < 1.9 nmol/L),22 polymers of soluble fibrin, termed thrombus precursor protein23,24 (TpP, American Biogenetic Sciences, Copiague, NY, normal values < 6 μg/mL),25 which are non–cross-reactive with D-dimer up to concentrations of 1000 ng/mL in vitro (data not shown), fibrin-split product D-dimer (Boehringer Mannheim, Mannheim, Germany; normal values: < 400 ng/mL), and thrombin/antithrombin III complexes (Enzygnost TAT micro, Behring; normal range: 1.0-4.1 μg/L).
Plasma levels of tissue factor pathway inhibitor (TFPI) were determined using a 2-stage chromogenic substrate assay.26 27 Values were compared against pooled plasma obtained from 48 normal individuals and TFPI activity was calculated as a percentage of this reference value. Antithrombin levels (AT III, STA Antithrombin; normal range: 75-125%) were determined on the analyzer STA (Stago).
Fibrinolysis was assessed with the following assays: enzyme immunoassay (EIA) plasmin-α2-antiplasmin (PAP) complexes (Enzygnost PAP micro, Behring; normal range: 120-700 μg/L), measuring plasmin activity; EIA tissue plasminogen activator (t-PA), measuring total t-PA antigen, that is, free molecules and molecules complexed to plasminogen activator inhibitor (PAI) (t-PA, Chromogenix AB, normal range 1-12 ng/mL, CV < 7%), and EIA PAI, measuring free active molecules not complexed with t-PA (PAI-1, Technoclone, Vienna, Austria, normal range: 10-30 ng/mL).
Blood cell counts
As described earlier,20 monocyte counts were calculated from scatter histograms obtained with the flow cytometer (Becton Dickinson). Because all samples required immediate processing to avoid artificial activation of monocytes, and because no monocytes could be detected up to 5 hours after endotoxin infusion, cells were stained only before and 6 and 24 hours after endotoxin administration. Staining of TF was assessed using fluorescein-isothiocyanate-coupled antibodies (American Diagnostica, Greenwich, CT) as previously described and isotype-specific control antibodies.28 Flow cytometry was performed by analyzing 30 000 gated events as previously described.29
Data analysis
Data are expressed as mean ± SD unless otherwise indicated or the range. Because data were nonnormally distributed, all comparisons were made by nonparametric statistics. For statistical comparisons within groups, the Friedman ANOVA and the Wilcoxon signed ranks test for post hoc comparisons were applied. For comparisons between the groups, the Mann-Whitney U test was used. Because most measured parameters are interdependent and to limit statistical comparisons to a reasonable amount, F1 + 2 generation was determined a priori as the main outcome variable. Post hoc comparisons were restricted to times of peak values, whereas all other data are presented in a descriptive manner (95% CI).
Results
Anticoagulation achieved with lepirudin
Baseline values for all parameters were similar in both groups (Table 1). The aPTT was unaffected by LPS infusion in the placebo group (data not shown). In contrast, lepirudin infusion doubled aPTT values from 34 ± 3 seconds to 71 ± 13 seconds at 50 minutes, and aPTT stayed at this level during the rest of the lepirudin infusion (P < .002 versus baseline or placebo).
Lepirudin blunts generation of thrombin, TAT complexes, and fibrin formation
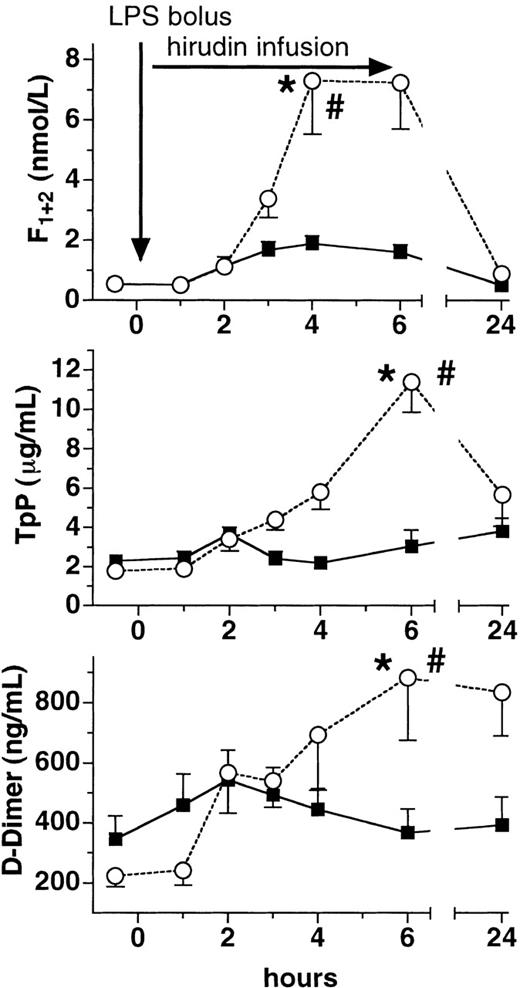
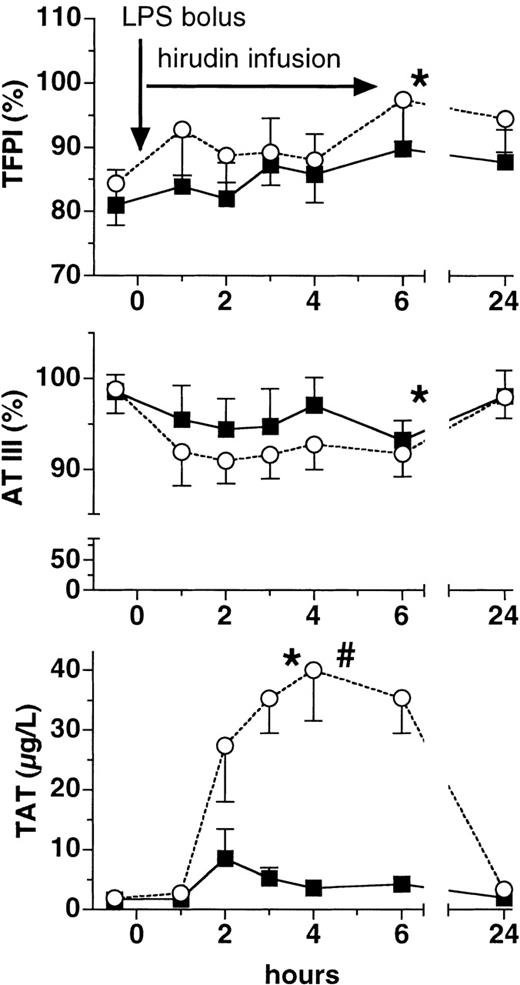
Lepirudin attenuated LPS-induced thrombin generation. Plasma levels of F1 + 2 increased almost 15-fold in the placebo group at 4 hours (P < .005 versus baseline, Figure1). In contrast, peak levels of F1 + 2 increased only approximately 3-fold in the lepirudin group (P < .001 versus placebo), thereby reaching the upper limit of the normal range (ie, 1.9 nmol/L). Likewise, TAT complexes increased more than 20-fold in the placebo group, whereas they increased only 5-fold in the lepirudin group (P < .001 versus placebo, Figure 2). Concomitantly, lepirudin prevented formation of soluble fibrin as evidenced by the changes in TpP levels: LPS enhanced TpP by 600% at 6 hours (P < .01, Figure 1). At the same time, TpP levels rose by only 50% in the lepirudin group (P < .001 versus placebo,P > .05 versus baseline at 6 hours), and reached maximum levels 19 hours after cessation of lepirudin (P < .05). In the placebo group, D-dimer levels peaked 4-fold over baseline at 6 hours (P < .005, Figure 1). In contrast, D-dimer increased by only 50% at 6 hours in the lepirudin group (P < .018 versus placebo, Figure 1).
Plasma levels of prothrombin fragment, TpP, and D-dimer.
Plasma levels (mean ± SEM) of prothrombin fragment (F1 + 2, top panel), thrombus precursor protein (TpP, middle panel), and D-dimer (bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or recombinant lepirudin (▪), (n = 12 per group). *P < .05 versus baseline, # < .05 for comparisons between the groups.
Plasma levels of prothrombin fragment, TpP, and D-dimer.
Plasma levels (mean ± SEM) of prothrombin fragment (F1 + 2, top panel), thrombus precursor protein (TpP, middle panel), and D-dimer (bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or recombinant lepirudin (▪), (n = 12 per group). *P < .05 versus baseline, # < .05 for comparisons between the groups.
Plasma levels of TFPI, AT III, and TAT.
Plasma levels (mean ± SEM) of tissue factor pathway inhibitor (TFPI, top panel), antithrombin III (AT III, middle panel), and thrombin-antithrombin III complexes (TAT, bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or lepirudin (▪), (n = 12 in both groups). *P < .05 versus baseline, #P < .05 for comparisons between the groups.
Plasma levels of TFPI, AT III, and TAT.
Plasma levels (mean ± SEM) of tissue factor pathway inhibitor (TFPI, top panel), antithrombin III (AT III, middle panel), and thrombin-antithrombin III complexes (TAT, bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or lepirudin (▪), (n = 12 in both groups). *P < .05 versus baseline, #P < .05 for comparisons between the groups.
Lepirudin does not affect the increases of PAP complexes, t-PA, and PAI-1
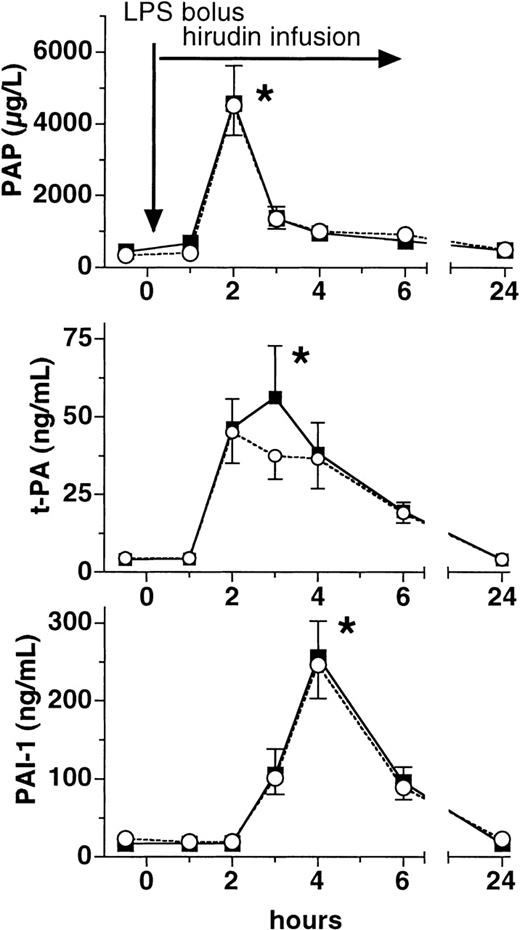
Plasma levels of PAP complexes rose approximately 10-fold in both groups at 2 hours after LPS infusion (P > .05 between groups, Figure 3). In a similar fashion, total t-PA as well as active free PAI-1 increased more than 10-fold at 2 and 4 hours, respectively, after LPS infusion in both groups (P < .01 versus baseline, P > .05 between groups, Figure 3).
Plasma levels of PAP, t-PA, and PAI-1.
Plasma levels (mean ± SEM) of plasmin-α2-antiplasmin complexes (PAP, top panel), tissue plasminogen activator (t-PA, middle panel), and plasminogen activator inhibitor (PAI-1, bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or lepirudin (▪), (n = 12 in both groups). *P < .05 versus baseline.
Plasma levels of PAP, t-PA, and PAI-1.
Plasma levels (mean ± SEM) of plasmin-α2-antiplasmin complexes (PAP, top panel), tissue plasminogen activator (t-PA, middle panel), and plasminogen activator inhibitor (PAI-1, bottom panel) are shown before and after LPS infusion (2 ng/kg) in human volunteers receiving either placebo (○) or lepirudin (▪), (n = 12 in both groups). *P < .05 versus baseline.
The endogenous serine proteases TFPI and AT III are unaffected by lepirudin
Following LPS infusion, TFPI plasma levels increased by about 17% and 12% in the placebo and lepirudin groups, respectively, at 6 hours (P < .03 versus baseline, P > .05 between groups, Figure 2). AT III values declined by 7 ± 7% in the placebo group and by 4 ± 14% in the lepirudin group 3 hours after LPS infusion (P < .05 versus baseline,P > .05 between groups, Figure 2).
Lepirudin abrogates LPS-induced up-regulation of tissue factor on circulating monoyctes
After LPS infusion, monocyte counts fell to undetectable values after 2 hours in both groups. At 6 hours, monocyte counts averaged 0.18 × 109/L (range: 0.05-0.40) in the placebo group and 0.21 × 109/L (range: 0.09-0.34) in the lepirudin group (P > .05 between groups). Twenty-four hours after LPS infusion, monocyte counts were not different from baseline values. Similar to a previous trial,20 expression of TF on monocytes could not be evaluated at 2 hours due to monocytopenia. In the placebo group, the absolute number of TF+ monocytes doubled to 0.025 ± 0.028 × 109/L at 6 hours (Table 2). However, no increase of TF+ monocytes occurred in the lepirudin group at 6 hours (0.006 ± 0.006 × 109/L, P = .024 versus placebo, Table 2). Twenty-four hours after LPS infusion, monocyte counts returned to baseline and TF expression was no longer elevated.
Discussion
This study was designed to investigate the anticoagulant potency of lepirudin during the initial phase of LPS-induced activation of coagulation in human volunteers and to further characterize its mechanism of action.
First, lepirudin effectively blocked the conversion of prothrombin to thrombin (Figure 1). Several authors have recently stated that lepirudin in contrast to heparin predominantly inhibits thrombin activity instead of thrombin generation.10,30 A previous uncontrolled phase I trial in patients with ischemic heart disease found no evidence for inhibited thrombin generation as measured by plasma levels of F1 + 2.30 As admitted by the authors of this study, the uncontrolled study design or repeated blood sampling from an indwelling catheter over more than 24 hours may have presented a limitation of this trial. Along these lines, Rao et al found no decrease of elevated F1 + 2 plasma levels in patients with unstable angina pectoris even after a 3-fold higher dose of lepirudin than in our trial.31
Therefore, a goal of our study was to assess whether this concept holds true under the conditions of systemic coagulation activation. However, in our model, lepirudin effectively blunted both F1 + 2generation and the increase of TAT complexes, representing thrombin activity after LPS infusion (Figures 1 and 2). Still, it must be emphasized that LPS infusion causes a short-lived and self-terminating coagulation activation that may account for the divergent findings from Zoldheyi and Rao. Thus, we postulate that increased thrombin generation during endotoxemia is largely dependent on the presence of thrombin's autofeedback loop and that a selective thrombin inhibitor like lepirudin impedes this mechanism to a great extent.
Secondly, our data show that lepirudin blunted the LPS-induced fibrin polymerization, which was demonstrated at 2 levels. Lepirudin, in contrast to placebo, blunted the increase of TpP, representing polymerized but soluble fibrin (Figure 2). The increase of TpP in the placebo group is in good agreement with 2 of our own previous studies on the effects of LPS on TpP.20,32 Furthermore, lepirudin abrogated the LPS-induced increase in the fibrin split-product D-dimer (Figure 1). The decreased generation of D-dimer was not caused by different fibrinolytic activity between treatment groups, because the well-known LPS-induced increases in PAP, t-PA, and PAI-1 plasma levels33,34 were not affected by lepirudin treatment. In good agreement with a recently published animal study from Biemond et al, our study confirms for humans that lepirudin selectively blocks coagulation without affecting the fibrinolytic system35(Figure 3). Accordingly, infusion of LPS caused profound plasmin generation, evidenced by peak levels of PAP and t-PA at 2 hours, which was followed by the subsequent release of PAI-1.
Thus, the attenuated rise in D-dimer plasma levels can only be attributed to the smaller amount of fibrin that is generated. Taken together, our findings are in line with a number of studies showing that activation of coagulation and fibrinolysis after LPS infusion are regulated by entirely different mechanisms.34,36 37
Interestingly, lepirudin also abolished the LPS-induced increase of the number of TF-expressing circulating monocytes, which has not been reported previously (Table 2). Thus, lepirudin even blunts the induction of the most upstream coagulation factor of LPS-induced coagulation activation, which could represent a novel mechanism of action. It remains to be determined whether thrombin inhibition by lepirudin affects increased TF expression directly or by some other indirect mechanism. Yet, the small increase in thrombin generation in the lepirudin group indicates that cells other than circulating cells (marginated monocytes or endothelial cells) express enough TF to trigger coagulation. Alternatively, it might be that lepirudin only inhibits de novo synthesis of TF without affecting preformed TF. This would be consistent with the observation that lepirudin infusion during balloon angioplasty in animals only attenuated TF expression after 28 days but not at 7 days.13
Whereas heparin enhances TFPI release and potentiates AT III activity,38,39 lepirudin does not increase TFPI plasma levels and acts independently of AT III.40 We were therefore interested to exclude a possible influence of endogenous inhibitors of coagulation like TFPI or AT III. Accordingly, we only found a small though significant increase of TFPI plasma levels without differences between the groups. This is in striking contrast to the enhancing effect of heparin on TFPI plasma levels in a similarly designed study.32 This finding and the modest decrease in AT III plasma levels (ie, 10%) after LPS infusion in both groups of our study make it unlikely that the differences in TF expression, thrombin generation, or thrombin activity could be related to differences in plasma levels of these endogenous inhibitors.
Compared to the aforementioned study with heparin in the same setting,32 lepirudin appears slightly less effective in terms of thrombin generation and thrombin activity. In the former trial, no increase in plasma levels of F1 + 2 was seen in the heparin group, whereas F1 + 2 increased 3-fold in the lepirudin-treated subjects of this study (Figure 1). In the same way, plasma levels of TpP and D-dimer slightly increased in the lepirudin group compared to heparin therapy where no increase was seen. Yet, the aim of the current study was not to compare these 2 drugs, and we cannot exclude that higher doses of lepirudin would demonstrate a more pronounced anticoagulant effect.
Taken together, our data underscore that thrombin exerts a powerful feedback loop on its own generation via the TF-dependent pathway of coagulation. A limitation of the low-dose endotoxin model is that it is representative only for the very early coagulation phase during endotoxemia, but does not mirror the pathophysiologic changes of decompensated DIC during full-blown sepsis. Hence, our results may be difficult to extrapolate to the clinical condition of septic shock. As reviewed recently, it is noteworthy that thrombocytopenia and a considerable depletion of coagulation factors are frequently seen in DIC in the clinical setting.41 For any clinical trial it will therefore be necessary to carefully choose the dosing regimen and to carefully monitor anticoagulation to prevent the occurrence of bleeding in these patients. Our model provides substantial evidence, in a randomized, placebo-controlled setting, that early treatment of endotoxemic subjects with lepirudin prevents potentially detrimental LPS-induced coagulation.
In conclusion, our data show for the first time that lepirudin inhibits thrombin formation and thrombin action as well as fibrin generation in human endotoxemia. Furthermore, lepirudin abrogates TF up-regulation on circulating monocytes. We propose that the findings of our experimental setting provide a rationale for a clinical trial on lepirudin's potential in DIC.
Supported by grant No. 7455 from the Austrian National Bank.
Reprints:Thomas Pernerstorfer, Department of Clinical Pharmacology for The Adhesion Group Elaborating Therapeutics (TARGET), Vienna General Hospital, Währinger Gürtel 18-20, A-1090 Wien, Austria; e-mail: thomas.pernerstorfer@univie.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.