Chronic myelogenous leukemia (CML) presents a unique opportunity to develop therapeutic strategies using vaccination against a truly tumor-specific antigen that is also the oncogenic protein required for neoplasia. CML is characterized by the t(9;22) that results in the bcr-abl fusion oncogene and in the expression of a chimeric protein product p210. Previously we have shown that peptides derived from amino acid sequences crossing the b3a2 fusion breakpoint in p210 elicit class I restricted cytotoxic T lymphocytes and class II responses, respectively, in vitro. Such sequences may thus comprise absolutely tumor-specific antigens in a peptide-based vaccine. We evaluated the safety and immunogenicity of a multidose, bcr-abl breakpoint peptide vaccine in 12 adults with chronic-phase CML. Cohorts of 3 patients each received either 50 μg, 150 μg, 500 μg, or 1500 μg total peptide mixed with 100 μg QS-21 as an immunological adjuvant. Delayed-type hypersensitivity (DTH), humoral responses, and unprimed ex vivo autologous proliferation (3H-thymidine incorporation) and cytotoxicity (chromium-51 release) responses were measured. All 68 vaccinations were well tolerated without significant adverse effects. In 3 of the 6 patients treated at the 2 highest dose levels of vaccine, peptide-specific, T-cell proliferative responses (n = 3) and/or DTH responses (n = 2) were generated that lasted up to 5 months after vaccination. Cytotoxic T lymphocytes have not been identified. In conclusion, a tumor-specific, bcr-abl derived peptide vaccine can be safely administered to patients with chronic-phase CML and can elicit a bcr-abl peptide-specific immune response despite the presence of active disease in these patients and approximately 1012 leukemia cells.
Chronic myeloid leukemia (CML) is a pluripotent stem cell disorder characterized by the presence of the Philadelphia chromosome (Ph1). Ph1 is the result of a translocation of the c-abl oncogene from chromosome 9 to the breakpoint cluster region (bcr), within the bcr gene on chromosome 22, forming a chimeric bcr-abl gene.1-3 The fused genes encode an 8.5-kb chimeric mRNA that is translated to a 210-kd protein.4-6 This p210 bcr-abl protein shows tyrosine kinase activity, is present in the leukemia cells of patients with CML, and is necessary and sufficient for transformation.7 In 95% of patients, the breakpoint in the bcr gene occurs either between bcr exon 2 (b2) and 3 (b3) or between bcr exon 3 (b3) and 4 (b4). Hence, 2 alternative chimeric p210 bcr-abl proteins, comprising either a b3a2 or a b2a2 junction, can result from this fusion gene.8
The chimeric fusion protein is a tumor-specific antigen because the junctional regions of p210 contain a sequence of amino acids that is not expressed in a normal cell; in addition, as a result of the codon split on the fused message, a new amino acid (lysine in b3a2 and glutamic acid in b2a2) is present at the exact fusion point in each protein. Despite the intracellular location of the intact p210, cellular processing of the products of the fusion proteins can yield peptides capable of being presented on the cell surface, within the cleft of human leukocyte antigen (HLA) molecules; in this form they can be recognized by T cells.9-11
By screening large numbers of fusion peptides from the junctional sequences of CML, our group and others identified p210/b3a2-derived fusion protein amino acid sequences with appropriate anchor motifs for binding to class 1 and 2 HLA molecules. We identified 4 peptides derived from the b3a2 CML breakpoint that bound with high or intermediate affinity to HLA-A3, A11, B812, and HLA A2.1.9 These peptides can elicit HLA-restricted cytotoxicity in vitro.9,13-15 Furthermore, we and others16 have found peptides that bind to major histocompatibility complex (MHC) class 2 molecules DR3(DRB1*0301), DR4(DRB1*0402), and DR11 (DRB1*1101) (Bocchia M, Sette A, Scheinberg D, unpublished observations). These DR types, as well as DR1 and DR2, can also support HLA-restricted and specific helper responses in vitro.11,14 16-18
The bcr-abl derived fusion proteins represent a reasonable target for an immunologic approach to the treatment of CML; the data in vitro provide the rationale for developing peptide-based vaccines for this disease. Moreover, T-cell infusions into patients who have relapsed after allogenic bone marrow transplantation can re-induce complete molecular remission, further supporting a role for T-cell effectors in the control of this disease.19,20 Therefore, we initiated a phase 1 dose-escalation trial to evaluate the safety and immunogenicity of the b3a2 breakpoint peptides when administered as a vaccine to patients with CML. We included the 4 peptides that we previously described as capable of binding to HLA class 1 molecules and eliciting T-cell responses.12,14 In addition because not all the class 1 and 2 motifs are not known and certain motifs may not be entirely exclusive or inclusive with regard to the peptides that can bind to them, it is possible that other unidentified, antigenic peptides existed within the 25-mer b3a2 junctional peptide we described as a class-2 binding peptide.14 Hence this larger peptide was included to elicit class 2 help and to be processed by cells for class 1 presentation. Moreover, because of the uncertainty regarding HLA restriction described above, all patients, regardless of HLA type, were eligible for this trial to avert missing potential reactivities.
The 5 peptides were mixed with the immunologic adjuvant QS-21 before injection. Vaccines containing various protein or peptide antigens plus this component have been described to induce proliferative and cytotoxic T cells against target cells expressing these antigens without significant toxicity.21
In the trial presented, cohorts of 3 patients each received 1 of 4 dose levels of peptide. In 3 of the 6 patients treated at the 2 highest dose levels of vaccine, peptide-specific T-cell proliferative responses ex vivo (n = 3) and/or DTH responses (n = 2) were generated that lasted up to 5 months after vaccination.
Material and methods
Trial design
This protocol was approved by the Memorial Hospital Institutional Review Board and was conducted under a United States Food and Drug Administration investigational new drug application held by Sloan Kettering. All patients gave written informed consent before enrolling in the study.
Patients required a primary diagnosis of CML with a 9;22 translocation or a bcr-abl transcript and a b3a2 breakpoint. Patients were in partial or complete remission induced by interferon (IFN)-α or hydroxyurea with white blood counts less than 20 000/μL. Patients were required to have adequate hepatic and renal function and performance status. Therapy within the previous 4 weeks other than with IFN or hydroxyurea was prohibited.
Treatment plan
The trial was designed as a dose escalation with 3 patients per cohort in 4 cohorts. All 12 patients received a combination of 5 peptides in each of 5 vaccinations over a 10-week period, on days 0, 14, 28, 42, and 70. The first group of patients received a total of 50 μg peptide (10 μg per each of 5 peptides) per dose, and the subsequent cohorts received 150, 500, and 1500 μg total peptide per dose, respectively. All vaccinations were administered subcutaneously with vaccination sites rotated between extremities. Delayed-type hypersensitivity (DTH), humoral responses, unprimed ex vivo autologous proliferation (3H-thymidine incorporation), and unprimed cytotoxicity (51Cr release) responses were measured before the first vaccination, after the third vaccination, and 2 weeks after the last vaccination (see details below).
Bone marrow aspirates were examined for morphology, cytogenetics, and reverse transcription–polymerase chain reaction (RT-PCR) for bcr-abl at the beginning and 2 weeks after the end of the study. HLA typing, broad T-cell function tests including response to mitogens, and mixed lymphocyte culture were also performed before entry to the study. A specific HLA type was not required for entry. According to protocol, 3 patients were eligible for 3 additional monthly booster vaccinations because they demonstrated a positive immune response during the initial phase. Immune responses were rechecked 2 weeks after the third booster vaccination.
Peptides
Each of the peptides used in this study was shown to be pure, sterile, and endotoxin free. The 4 CML class 1 peptides, 9 and 10 amino acids long, and the single CML class 2 peptide, 25 amino acids long, were synthesized by Sloan Kettering Microchemistry Core Facility Lab (New York, NY) by F-MOC solid-phase synthesis and purified by high-pressure liquid chromatography. Peptides were sterile, more than 98% pure, and endotoxin free. The amino acid sequences are shown in Figure 1. For in vitro experiments we also used 6 control peptides, including mutated ras peptide mRASp21: TEYKLVVVGARGVGKSALTIQ (a kind gift of Drs A. Houghton and P. Chapman), and 5 other irrelevant peptide sequences from human immunodeficiency virus, influenza, enkephalin, and the pml/rar α breakpoint.
Amino acid sequences of the 5 peptides in the vaccine.
The asterisk denotes the fusion point in bcr-abl. HLA-binding capability in vitro is shown on the right. N/A, not applicable.
Amino acid sequences of the 5 peptides in the vaccine.
The asterisk denotes the fusion point in bcr-abl. HLA-binding capability in vitro is shown on the right. N/A, not applicable.
Vaccine preparation
Ten micrograms, 30 μg, 100 μg, or 300 μg of each peptide (50, 150, 500, 1500 μg total peptide) was mixed with 100 μg QS-21 (a complex amphiphilic lipid extracted from the bark of the South American tree Quillaja Saponaria Molina), the immunological adjuvant, and placed in a vial of phosphate-buffered saline (PBS; pH 7.4). For the 10- and 30-μg levels, 0.5 mL PBS was used, and for the 100- and 300-μg levels, 1 μL PBS was used. The vaccine was stored frozen at −80°C. The QS-21 was provided by Aquilla Biopharmaceuticals (Worcester, MA) and was used under their investigational new drug application.
Enzyme-linked immunosorbent assay
Sera were obtained before vaccination and at days 42 and 84. In 3 patients we obtained sera after 3 additional vaccinations. The presence of anti-CML peptide antibodies in these sera was measured by ELISA on Covalink ELISA plates (Nalgene-Nunc, Naperville, IL) coated with 100 ng antigen solution in 50μL per well of carbonate buffer, pH 9.6 overnight at 4°C. Test and control serum in 1% human serum albumin–PBS at different dilutions were detected with alkaline phosphatase-conjugated goat antihuman IgG and IgM (H+L) plus substrate (Jackson ImmunoResearch Laboratories, West Grove, PA).
Unprimed 51Cr release–cytotoxicity assay
Cells were prepared by Ficoll sedimentation from peripheral blood of the patients. Fresh peripheral blood mononuclear cells (PBMC; 4 × 106) were labeled with 300 μCi of Na2CrO4 (NEN Life Science Products, Boston, MA) for 1 hour at 37°C. After washing, cells at 2 × 106/mL were incubated with CML bulk peptides (the 5 peptides used in the vaccine) or control bulk peptides or were nonpulsed at a concentration of 20 μg/mL for 3 hours at 20°C in the presence of β2-microglobulin at 3 μg/mL. After washing by centrifugation, targets cells were resuspended in complete media at 5 × 104 cells/mL and plated in a 96-well U-bottom plate (Becton Dickinson, NY) at 5 × 103 cells per well with effector cells at effector-to-target ratios ranging from 50:1 to 25:1. Plates were incubated for 5 hours at 37°C in 5% CO2. Supernatant fluids were harvested, and radioactivity was measured in a gamma counter. Percentage specific lysis was determined from the following formula: 100 × [(experimental release−spontaneous release)/(maximum release−spontaneous release)]. Maximum release was determined by lysis of targets in 2.5% Triton X-100. Alternatively, HLA-matched established cell lines were used as a targets.
Unprimed proliferation assay
Proliferation tests were performed using a standard [3H]thymidine incorporation assay. Briefly, cells were prepared by Ficoll sedimentation of peripheral blood of the patients and resuspended at 3 × 106 cells/mL and were incubated with the 5 mixed bulk CML peptides, bulk mixed control peptides, b3a2 long peptide alone, or ras peptide alone at concentrations of 20 μg/mL or 50 μg/mL for 2.5 hours at 37°C. After washing, cells were irradiated (70 Gy), resuspended at 1 × 106 cells/mL, and plated in a 96-well U-bottom plate (Becton Dickinson) at 1 × 105 cells per well as APC with autologous PBMC as responders at ratios of 2:1 to 4:1 (0.5 × 105 to 0.25 × 105cells per well) in 200 μL culture media with 5% heat-inactivated ABO serum and were incubated at 37°C in 5% CO2 for 72 hours. Then cells were incubated for 6 hours with 2μCi/mL of [3H]thymidine (NEN Life Science Products, Boston, MA). Plates were harvested using a Skatron cell harvester, and proliferative responses were assessed as a function of [3H]thymidine incorporation measured in a β counter versus controls.
Delayed-type hypersensitivity in patients
Delayed-type hypersensitivity (DTH) tests were performed with the CML peptides in a mixture in PBS without QS-21 at a dose of 2 to 3 μg/peptide. DTH was tested before the first vaccination and at 2 weeks after the 3rd and 5th vaccinations. Positive-control DTH test results included mumps and Candida. A positive skin test reaction was defined as greater than 5 mm-diameter erythema and induration 48 hours after intradermal injection.
Results
Patient characteristics
Twelve patients were enrolled in the study between 1996 and 1998. There were 9 men and 3 women ranging in age from 29 to 73 years, with a median of 56 years (Table 1). All patients received at least the initial series of 5 vaccinations except 1 patient who received 4 vaccinations. This patient dropped out of the study after an unrelated cardiac event. Three patients received 3 additional booster vaccinations (total vaccinations, 8) after we documented a positive response in either DTH or proliferative assays after the 5th vaccination.
T-cell function test results were abnormal in 9 patients before study entry (Table 2). In 7 of 7 patients tested, there was a decrease in the mixed lymphocyte culture response. In 5 of 9 patients, there was an increase in the response to unstimulated mitogen; in 3 of 7 there was an increase in autostimulated response. We did not find an association of these data with the concurrent administration of IFN-α or hydroxyurea.
In 11 of 12 patients, at least 1 HLA molecule was associated with peptide binding, proliferation, or cytotoxicity in vitro according to previously published data. Five patients expressed HLA A2.1, 4 patients expressed HLA A3, and 3 patients expressed HLA A24; 2 patients expressed HLA DR1, 2 expressed DR3, 2 expressed DR4, and 1 expressed DR11. Seven patients expressed both HLA class 1 and class 2 molecules previously reported to bind to or react with CML peptides.
All patients had the b3a2 breakpoint confirmed as a condition to entry into this trial. One patient expressed b3a2 and b2a2 breakpoints. All patients were in the chronic phase of CML morphologically; all had been treated previously with hydroxyurea (3 patients), IFN-α (3 patients), or both (6 patients). Patients remained on their conventional therapy during the vaccinations. The time between diagnosis of CML and entry to this protocol ranged from 1 to 129 months, with a median of 21months.
Anergy tests
An anergy screen was performed against the recall antigens, mumps, and Candida. Seven patients were anergic to these antigens; 4 patients had a positive response to either mumps or Candida antigens (Table 1). In addition, none of the patients had a positive skin test response to the 5 CML peptides before vaccination was begun.
Safety and toxicity
Toxicities were graded according the Common Toxicity Criteria from the National Cancer Institute. The vaccine was administered subcutaneously, and all patients were treated as outpatients. Toxicities were minimal and, if present, generally consisted of local irritation at the site of vaccine administration (10 patients, 71%). Mild, transient erythema and induration at the injection site for 1 to 3 days was seen in 8 patients (67%). Toxicity was grade 1 or grade 2 only. Seven patients (58%) had grade 1 systemic effects, and 8 patients (67%) had grade 1 nonsystemic adverse reactions. Patient 2 had a myocardial infarction unrelated to the vaccinations. Five patients had mild thrombocytopenia or leukopenia (resulting from IFN-α treatment) before enrolling in the protocol; there was no evidence of significant changes in these blood cell counts caused by vaccinations during the trial. Effects on hemoglobin levels or on blood chemistry were not seen during the course of the protocol.
Induction of DTH responses
Tests for DTH reactions against the administered peptides were used for detecting the induction of antigen-specific CD4+ T-cell immunity (Tables 3 and4). The administered peptides would have to have been presented in the context of MHC molecules to have been recognized by effector T cells during DTH reactions. Whereas before vaccination there were no positive DTH reactions to the 5 CML peptides in any of the patients, significant DTH reactivity was observed in 2 patients (patients 7 and 11). Patient 7 had a DTH response after 3 vaccinations and maintained his DTH response for 33 weeks after treatment was finished (his total number of vaccinations was 8). These 2 patients were treated at 1 of the 2 highest dose levels of vaccine (1 each at the 100 μg and 300 μg dose level).
Induction of CML peptide-specific T-cell proliferation
To test the ability of fresh T cells from patients with CML undergoing vaccination to proliferate in the presence of b3a2 class 2 long peptide or a mix of short class 1 and long peptides, we designed an unprimed proliferation assay that would detect any response directly from the blood without additional expansion in vitro. Before vaccination, no patients exhibited positive responses in the presence of b3a2 peptides.
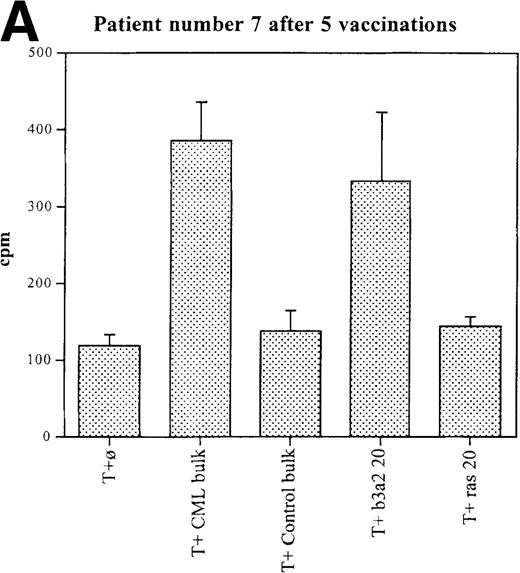
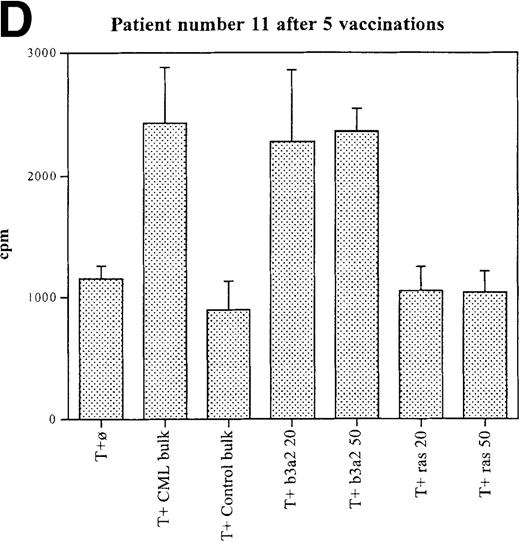
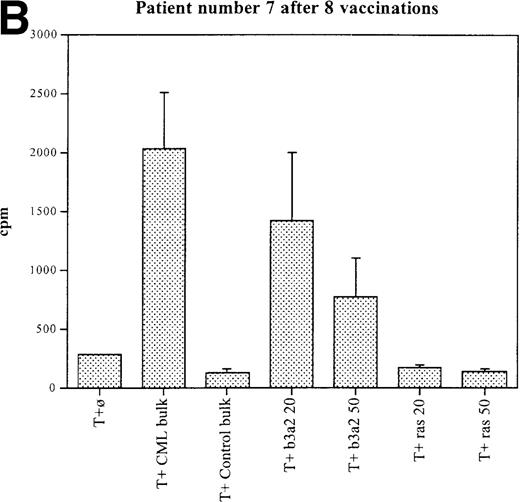
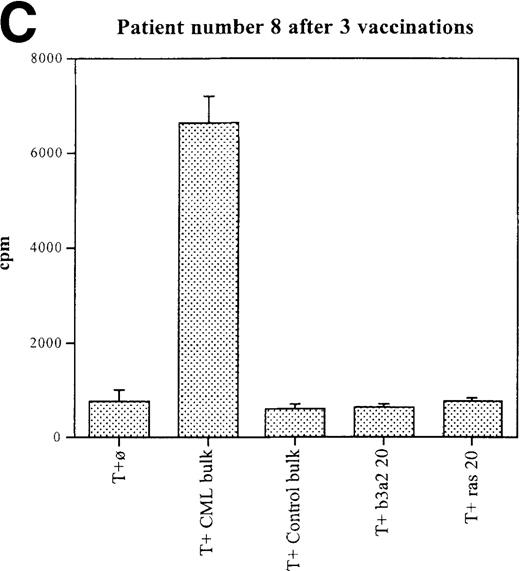
Three patients (each of whom was treated at 1 of the 2 higher dose levels) became positive after at least 3 vaccinations (Tables 3, 4). Patient 7 become positive after 5 vaccinations. The reactivity was with the 5 CML peptides in a mixture and against the b3a2 long 25-mer peptide used alone (Figures 2A, 2B). This patient received 3 additional vaccinations at 1-month intervals (until week 24), and he continued to show positive results at 23, 27, 33, and 44 weeks after the start of treatment. Patient 8 displayed a positive proliferation assay response after 3 vaccinations with the CML peptide mixture (Figure 2C); patient 11 showed positive results after 5 vaccinations (Figure 2D) and again after re-treatment with 3 additional vaccinations. These patients were in the chronic phase of CML; therefore, autologous p210-positive blasts were unavailable to test for stimulation. Patients 7 and 8 expressed HLA A3, and patient 11 expressed HLA A2. In these patients, the control peptide mixture and the ras peptide control did not elicit any significant responses.
Unprimed ex vivo proliferation assays of fresh T cells from vaccinated patients.
The assay was conducted as described in “Materials and Methods.” (A) Patient 7 after 5 vaccinations. (B) Patient 7 after 8 vaccinations. (C) Patient 8 after 3 vaccinations. (D) Patient 11 after 5 vaccinations. The stimulus is shown along the x-axis. T+ø, T cells plus APC without peptide; CML bulk is 5 peptides in the vaccine (see Figure 1); control bulk is control peptides (see “Materials and Methods”); b3a2 20 or b3a2 50 is 20μg or 50μg of the long b3a2 peptide (Figure 1); ras 20 or ras 50 is 20μg or 50μg of the control ras peptide (see “Materials and methods”). Standard deviations are shown.
Unprimed ex vivo proliferation assays of fresh T cells from vaccinated patients.
The assay was conducted as described in “Materials and Methods.” (A) Patient 7 after 5 vaccinations. (B) Patient 7 after 8 vaccinations. (C) Patient 8 after 3 vaccinations. (D) Patient 11 after 5 vaccinations. The stimulus is shown along the x-axis. T+ø, T cells plus APC without peptide; CML bulk is 5 peptides in the vaccine (see Figure 1); control bulk is control peptides (see “Materials and Methods”); b3a2 20 or b3a2 50 is 20μg or 50μg of the long b3a2 peptide (Figure 1); ras 20 or ras 50 is 20μg or 50μg of the control ras peptide (see “Materials and methods”). Standard deviations are shown.
CML peptide-specific T-cell cytotoxicity
An autologous cytotoxicity assay based on 51Cr release was used for detecting cytotoxic T cells in the peripheral blood of the vaccinated patients. Patients were tested before the start of the protocol and at 2 weeks after the 3rd and 5th vaccinations. Peripheral blood cells were tested immediately after removal from the patientswithout any additional priming ex vivo. Target cells (autologous PBMC) were loaded with the CML peptide mixture, control peptides, or no peptides and were used in the assay at different effector-to-target ratios. We were unable to detect any cytotoxic response against autologous peptide-pulsed PBMC or with peptide-pulsed, allogenic-matched leukemic cell lines, when available.
Serologic response to CML peptides
An ELISA was used to detect the serologic response elicited by the peptide vaccinations. Serum samples were tested before starting the vaccinations and after 3 and 5 vaccinations. Samples from healthy donors and from patients with leukemias other than CML were used as a control. We did not detect any antibody responses against b3a2 peptides at baseline. After immunization, only patients 7 and 11 showed some minimal serologic response (less than 2 times control) after 8 vaccinations (not shown). Both patients had shown proliferative and DTH responses as well.
Clinical outcomes
This trial was not designed to assess clinical outcomes because all patients remained on their current therapy while receiving the vaccine. Because this included IFN-α in 9 patients, any clinical benefits of the vaccine would be difficult to distinguish from the effects of interferon. Moreover, other than patient 1, these patients had large (1 or 2 kg) tumors, and, hence, significant therapeutic effects would have been difficult to observe during the short period of study.
Patient 1, who was in cytogenetic remission at the start of study, experienced a transient disappearance of positivity for the b3a2 mRNA by RT-PCR after the vaccination schedule. Patient 7 experienced a transient and partial cytogenetic response during the vaccination schedule. Patients 5 and 10 progressed to an accelerated phase of disease during the study period; patient 5 died 3 months after the end of the treatment.
Discussion
CML is an incurable disease without allogenic bone marrow transplantation (BMT). Although a large part of the antileukemia effects of allogenic BMT arise as part of an alloreactive graft-versus-host reaction caused by major and minor histocompatibility differences between the donor and the recipient, it is possible that additional leukemia eradication is mediated through effector cells directed at specific leukemia antigens, perhaps derived directly or indirectly from the leukemia-specific translocation oncogene product.22,23 In addition, donor lymphocyte infusions have been clearly shown to be active in inducing complete remission in patients who undergo relapse after BMT.19,20 Moreover, the immunogenicity of leukemia cells in the autologous setting has also been supported by the demonstration that the repertoire of TCR Vβ gene usage by T cells associated with CML was restricted when compared to that of normal healthy persons. Furthermore, leukemia-reactive T cells can be isolated from patients with CML, either de novo or after autologous peripheral blood stem cell transplantation.24-26The rare cases of spontaneous molecular remissions described in patients with CML and the recent findings of very low numbers of bcr-abl transcripts in some healthy persons are also consistent with bcr-abl peptide-specific immune surveillance.27 28
For all these reasons, a therapeutic approach to CML based on T-cell immunity is particularly attractive. We and others9-11,14,29 30 have shown that oncogenic fusion proteins uniquely found in CML and selected other leukemias may represent tumor-specific products that can be recognized in a class 1 or a class 2-restricted fashion and can act as target antigens for the recognition of the native tumor cells.
To test whether the CML-derived peptides could be immunogenic in vivo, we conducted a phase 1 dose-escalation trial. We showed that these vaccines can be safely administered at a variety of dose levels without major toxicities and that we can generate specific proliferative and DTH responses at the higher doses of peptide. Vaccinations were administered on an outpatient basis, no significant toxicity was observed, and the treatment was well tolerated in all patients. These data do not, however, provide evidence that p210 can be processed and recognized by these peptide-reactive cells. Moreover, we did not test for technical reasons (lack of p210-positive blasts in these patients in chronic phase) the autologous killing of CML cells. Processing and recognition of p210 by T cells stimulated with these peptide sequences in vitro has been demonstrated by others.9,11 18
Peptides require presentation in the context of cell-surface MHC molecules to be recognized by effector T cells during DTH reactions. We demonstrated evidence of the induction of DTH in 2 of the 6 patients treated at the highest dose levels. In both, DTH reactions were correlated with positive results in ex vivo proliferative assays as well. Nestle et al31 have shown correlation between DTH responses and clinical responses to dendritic cell pulsed melanoma peptide vaccines. In that study in vitro test results for peptide-specific cytotoxicity from T cells derived from the DTH-positive site, which was characterized by a CD8+ infiltration, showed specific cytotoxicity for melanoma peptides after expansion in vitro.
We detected specific proliferative responses in 3 patients after vaccination with b3a2-derived peptides. The vaccine included a 25-mer peptide that might be processed potentially and expressed in either class 1 or class 2 molecules.
Although it is established that CD8+ cytotoxic T lymphocyte (CTL) may play an important role in resistance to tumor cell growth,32 CD4+ immunosurveillance of tumors is also important.33 Some CD4+ T lymphocytes can exert antigen-specific cytotoxicity in an HLA class 2-restricted manner possibly through indirect mechanisms.34-36 Furthermore, the role of CD4+ T cells in priming of CTL is well documented, which explains why activated CTL but not naive CTL, can mediate potent antitumor effects in the absence of CD4+ T cells. In an experimental tumor model, it was shown that vaccination with a class 2-restricted epitope induced protection against tumor challenge and was synergistic when combined with a class 1 epitope.33
There is considerable support for MHC class 2-based recognition of synthetic bcr-abl fusion peptides. Peptides corresponding to the b3a2 fusion sequences were shown to bind DR3(DRB1*0301), DR4(DRB1*0402), and DR11 (DRB1*1101); b3a2 peptides have been shown to induce HLA-DR1(DRB1*0101)-, DR2(DRB1*1501)-, DR4 (DRB1*0401)-, DR9(DRB1*0901)-, and DR11 (DRB1*1101)-restricted proliferative responses from CD4+ T lymphocytes and cytotoxic T-cell responses associated with DRB1*0901.11,14,16-18,36 Indirect evidence for processing of p210-b3a2 has been described.18,36,37Recent reports also showed that a b3a2 CD4+ cell line proliferated in response to stimulation with b3a2-positive CML blasts in an HLA-DR-restricted manner, which suggests that CD4+ T lymphocytes can directly recognize a bcr-abl fusion peptide that is naturally processed and expressed on CML cells.11
It is likely that effective vaccination strategies will target patients with minimal tumor burdens, not the massive disease seen in this trial. Interestingly, in 2 of the 3 patients who responded to the vaccine, the time between the diagnosis and the start of trial was only 5 months. This short time period correlated with less advanced disease, perhaps allowing an immune response.13 On the other hand, in the early phase of disease, it may be that complete tolerance to the tumor antigens has not yet been achieved, thereby allowing an effective immune response to occur.38 Moreover, these patients had undergone less chemotherapy or IFN treatment. A larger population of patients, treated at a fixed dose level, would make possible a study of the relationship between the disease stage, HLA type, treatment duration, and capacity to mount a specific response after a vaccination. Future approaches of this kind of treatment may include vaccinations in the early phase of disease, after cytoreduction, or after autologous or allogenic bone marrow transplantation.
Van Denderen et al39 40 have shown the generation of a rabbit antiserum to 3 junction-specific peptides from CML (b3a2, b2a2, and e1a2). Their findings indicate that the b3a2 junction is exposed on the chimeric protein. There are no reports in the literature detecting serum immunoreactivity of patients with CML against junction peptides, and we did not detect significant responses in patients before vaccination. After immunization, 2 patients displayed a minimal serologic response to the peptides. These 2 patients also showed a proliferative response to vaccination and DTH responses. Because serologic responses require CD4+ T cells to stimulate the production of immunoglobulin by B cells, these results are not surprising and are internally consistent.
Class 1 restricted and unrestricted b3a2 peptide-specific cytotoxic T cells have been reported.9,10,13-15,23,41,42 We were unable to demonstrate cytotoxic responses in our patients when using fresh, unstimulated PBMC in a 51Cr release assay. This method has not been demonstrated to be sensitive enough to detect low frequencies of cytotoxic precursors; hence, we cannot rule out the possibility of detecting these precursors by newer techniques using the ELISPOT or HLA tetramers.43,44 The lack of effective lysis, if true, could be the result of ineffective processing, weak antigen expression, insufficient dosing, low TCR avidity, or CD8 T-cell tolerance in patients with massive amounts of disease.9,10,41 44-46
Assessing the competence of an immunogen, though yielding an accurate view of the systemic immune response to a vaccine, may not provide sufficient information regarding target–host interactions at the site at which they are likely occur. For example, clinical responses, including complete responses, have been reported with MAGE-3 peptide vaccinations without the detection of CTL activity at the systemic level.47,48 Solutions to these possible obstacles could include improving the efficacy of peptide vaccines by modifying amino acid residues to increase their immunogenicity and CTL responses,49 changing adjuvants,50 or administering in vitro-generated dendritic cells pulsed with specific antigen.31 51-53
In conclusion, this is the first reported trial of vaccination with peptides derived from tumor-specific fusion oncoprotein. Repeated vaccination with bcr-abl-derived peptides is feasible, safe, and capable of eliciting specific immune responses to the fusion peptides. The clinical efficacy of these vaccinations awaits evaluation in a phase II trial.
Acknowledgments
We thank Wendy Roberts, Jim Young, Richard O'Reilly, Friedhelm Helling, Diane George, Phil Livingston, and Alan Houghton for helpful advice, Paul Tempst for help with the production of the peptides, and Oscar Kashala at Aquilla Biopharmaceuticals for the QS-21.
Supported by The Leukemia Society of America (DAS is a Translational Investigator of the Leukemia Society of America), by National Institutes of Health grants PO1 CA23766 and CA08748, by a NATO Fellowship, by a Berlex Scholarship, by the Hairy Cell Leukemia Foundation, by the Cure 2000 Leukemia Fund.
Reprints:D. A. Scheinberg, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10021; e-mail: d-scheinberg@ski.mskcc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.