Abstract
Deficiency of tristetraprolin (TTP), the prototype of the CCCH zinc finger proteins, results in a complex inflammatory syndrome in mice. Most aspects of the syndrome are secondary to excess circulating tumor necrosis factor (TNF)–, a consequence of increased stability of TNF- messenger RNA (mRNA) in TTP-deficient macrophages. TTP can bind directly to the AU-rich element in TNF- mRNA, increasing its lability. Here we show that TTP deficiency also results in increased cellular production of granulocyte-macrophage colony–stimulating factor (GM-CSF) and increased stability of its mRNA, apparently secondary to decreased deadenylation. Similar findings were observed in mice also lacking both types of TNF- receptors, excluding excess TNF- production as a cause of the increased GM-CSF mRNA levels and stability. TTP appears to be a physiological regulator of GM-CSF mRNA deadenylation and stability.
Tristetraprolin (TTP) is the prototype of a growing family of zinc finger proteins of the CCCH class. Members of this family have been found in a wide variety of organisms, ranging from human to yeast.1-8 TTP is localized to the nucleus of quiescent fibroblasts, but it is rapidly phosphorylated on serine residues and translocated to the cytosol after stimulation of these cells with serum or other mitogens.9,10 In macrophages, it is almost completely cytosolic.10,11 Studies of TTP-deficient mice generated in our laboratory demonstrated that the absence of the protein resulted in a complex phenotype that included invasive polyarticular arthritis, myeloid hyperplasia with extramedullary hematopoiesis, dermatitis, and autoimmunity.12 Since these animals resembled previous mouse models of tumor necrosis factor (TNF)–α excess, we postulated that TNF-α might be involved in the development of the TTP-deficiency phenotype. Treatment of newborn TTP-deficient mice with a neutralizing antibody against TNF-α appeared to completely prevent the development of the TTP-deficiency phenotype at 80 days of age.12
These data raised the possibility that TTP was a regulator of TNF-α synthesis or action. We then found that macrophages derived from TTP-deficient mice secreted approximately fivefold more TNF-α protein into the culture medium than control macrophages after stimulation with lipopolysaccharide (LPS), and that the cells accumulated approximately twice as much TNF-α messenger RNA (mRNA) as controls under the same conditions.13 Further studies on TNF-α mRNA stability revealed that TTP-deficient macrophages exhibited an increase in the half-life of TNF-α mRNA from 39 minutes in the controls to 85 minutes in the TTP-deficient macrophages.11 This increased mRNA stability presumably resulted in increased TNF-α secretion from macrophages and ultimately in the characteristic inflammatory phenotype associated with TTP deficiency.
The mechanism by which TTP destabilizes TNF-α mRNA has not been completely elucidated. However, we have shown that TTP can bind directly to the AUUUA-rich element (ARE) present in the 3′ untranslated region (3′ UTR) of TNF-α mRNA.11 We have also shown that co-transfection of TTP with a construct in which the ARE from TNF-α was placed 3′ of the c-fos promoter and the β-globin coding sequence14,15 in 293 cells led to rapid degradation of the β-globin mRNA.11 More recently, we found that when TTP was co-transfected with a truncated and modified TNF-α mRNA, which contains about 70% of the wild-type ARE and 33 As of an artificial polyA tail, it caused the apparent deadenylation and degradation of this TNF-α mRNA construct.16
In the same co-transfection studies, we also found that when the ARE from granulocyte-macrophage colony–stimulating factor (GM-CSF) mRNA was placed 3′ to the β-globin coding sequence, TTP expression also led to decreased accumulation of this fusion mRNA.11Although these experiments used an artificial transfection system in 293 cells, they raised the possibility that TTP might be involved in the physiological regulation of GM-CSF mRNA stability. We addressed this possibility in the present studies using primary cultures of bone marrow stromal cells (BMSCs) derived from wild-type (WT) and TTP-deficient mice (KO). The absence of TTP resulted in increased accumulation of GM-CSF in the supernatant of BMSCs incubated in the presence of LPS, increased steady-state levels of GM-CSF mRNA after stimulation with LPS or TNF-α, and increased half-life of GM-CSF mRNA after stimulation with LPS. These changes were accompanied by the almost complete absence of the deadenylated form of GM-CSF mRNA in cells from the TTP-deficient mice, whereas the deadenylated form was a prominent species in the control cells. Similar changes in mRNA stability and adenylation status were observed in BMSCs from mice that were deficient in TTP as well as both TNF-α receptors (TNFR) 1 and 2. These studies indicate that TTP is a normal, physiological regulator of GM-CSF mRNA deadenylation and stability. In addition, the presumed excess circulating levels of GM-CSF in the TTP-deficient mice may contribute to the myeloid hyperplasia characteristic of this condition.
Materials and methods
Mice
Mice deficient in TTP were generated in our laboratory by interbreeding heterozygous animals and were genotyped as described.12 Mice deficient in both TNFRs17 18were kindly provided by Dr Mark W. Moore (Genentech, South San Francisco, CA), and were interbred with the TTP-heterozygous animals. Genotyping of the offspring was performed by polymerase chain reaction (PCR) of tail DNA, using primers that span the regions of the WT genes disrupted by the targeting vectors. Genotyping was also performed by means of Southern blotting of tail DNA after digestion withBglII and probing for Neo with a 0.7-kilobase (kb) fragment of the vector PMC1neoPolyA (Stratagene, LaJolla, CA); this technique revealed 3 bands: approximately 3.5 kb (TNFR1), approximately 2.5 kb (TTP), and approximately 2 kb (TNFR2). Triple-heterozygous mice were interbred to yield triple-homozygous offspring. All animals were maintained in autoclaved microisolator cages in a barrier facility. Animal care and all experiments were in accordance with institutional guidelines for animal use.
Culture of bone marrow stromal cells
Primary cultures of BMSCs were established according to the protocol described by Dexter et al19,20 and modified by Van Den Heuvel et al.21 For identification of cell types, cells were trypsinized (0.05% trypsin [wt/vol]/0.53 mmol/L EDTA, GIBCO BRL, Grand Island, NY) and replated in culture medium at 50 000 cells/well in 4-well Lab-Tek tissue-culture chambers (Nunc, Thousand Oaks, CA) and were incubated for another 48 hours before any of the stains or assays were performed. Morphology was assessed by staining the cells with the Diff-Qick Stain Set (Baxter Healthcare Corp, McGaw Park, IL). Nonspecific esterase staining was performed as described,22 with the use of α-naphthyl acetate as a substrate (Sigma Chemical Co, St Louis, MO). Phagocytosis of latex beads was performed for 30 minutes as previously described,23 with the use of 0.8 μm latex beads (Sigma). Oil red O stain was used to identify fat cells. Cells were analyzed and photographed with a Nikon Eclipse 400 microscope (Southern Micro Instruments, Atlanta, GA), equipped with an Olympus PM-C35B camera (Olympus America Inc, Lake Success, NY). Uptake of Dil-acetylated low-density lipoprotein (LDL) (Biomedical Technologies, Inc, Stoughton, MA) was used to identify macrophages and endothelial cells, and was performed as described by Agui et al.24 At least 500 cells per genotype, in duplicate, were counted in each assay.
Northern blotting
When indicated, the cells were stimulated with lipopolysaccharide (LPS) (1 μg/mL) (Sigma) or mouse recombinant TNF-α (10 ng/mL) (R & D Systems, Inc, Minneapolis, MN) for different periods of time, and RNA was extracted with the RNeasy kit from Qiagen, Inc (Valencia, CA), according to the directions provided by the manufacturer. RNA was analyzed by Northern blot as described,25 except that the gels contained 1.5% (wt/vol) agarose. Filters were sequentially probed with complementary DNA (cDNA) probes to mouse GM-CSF (W. S. L. and P. J. B., unpublished data) and rat GAPDH (glyceraldehyde-3-phosphate dehydrogenase).26 The 423–base pair (bp) SalI-EcoRV insert from the GM-CSF and the 1.3-kb EcoRI insert from the GAPDH cDNAs were isolated from low–melting-point agarose gels and random primer labeled with α-32P dCTP (deoxycytidine 5′-triphosphate) for Northern hybridization.
In the RNA stability experiments, BMSCs were cultured in the presence of 1 μg/mL LPS for 2 hours, after which the LPS-containing medium was removed and replaced by fresh medium containing 5 μg/mL of actinomycin D (Sigma). Cells were then harvested for the preparation of RNA at 20-minute intervals, with the use of the Qiagen RNeasy kit as described above. Analysis of Northern blots for TNF-α and GAPDH mRNA was performed by means of PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA); in the case of the GM-CSF mRNA, laser-scanning densitometry was performed with a Zeineh soft laser scanning densitometer (model SL-504-XL, Biomed Instruments Inc, Fullerton, CA). This was attempted only when at least 1 of the peak areas was 20 arbitrary densitometry units or more. RNase H assays were performed as described.16
Measurement of GM-CSF secretion
To assess GM-CSF secretion, BMSCs were cultured in 24-well plates for 6 weeks, then stimulated with LPS (1μg/mL) for 24 hours, after which the supernatants were removed and stored at −80°C until used. GM-CSF secretion was assessed by enzyme-linked imunosorbent assay (ELISA), by means of a specific kit for mouse GM-CSF from Endogen (Woburn, MA), following the specifications of the manufacturer.
Results
Characteristics of bone marrow stromal cells from wild-type and tristetraprolin-deficient mice
Although we were not able to detect GM-CSF mRNA expression in primary macrophages by Northern blotting, our previous data suggested that BMSCs might be involved in the development of the TTP-deficiency phenotype.13 BMSCs are a mixture of fibroblastlike cells, macrophage-like cells, endothelial cells, and adipocytes that provide the microenvironment and growth factors needed for the normal development of the hematopoietic system.19 27
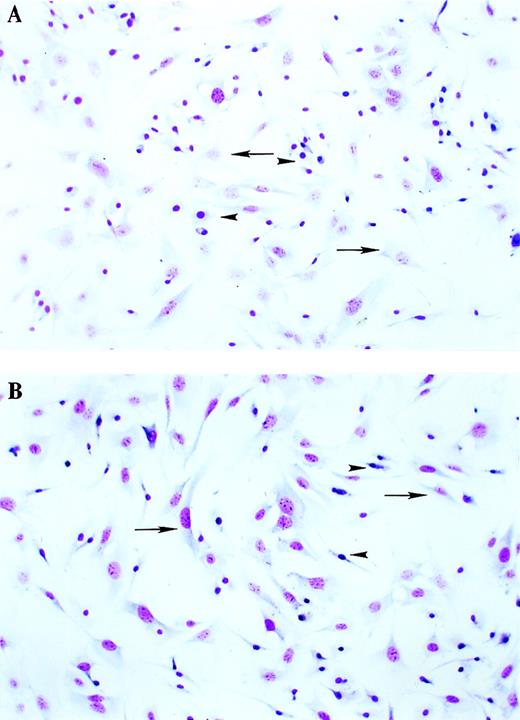
We first examined primary BMSC cultures from WT and TTP-deficient mice after 4 to 6 weeks of culture at 33°C. These conditions maintain the cultures in a nonhematopoietic state; ie, hematopoietic progenitors do not survive the first stages of the culture, leaving behind purely stromal cells that are still capable of producing hematopoietic growth factors.21 This point was confirmed by the absence of hematopoietic precursors or mature polymorphonuclear cells in the stained cultures (Figure 1). To evaluate the relative proportions of each cell type in the cultures, we performed a series of specific assays. Dil-acetylated LDL is avidly taken up by macrophages and endothelial cells, which can be then differentiated by morphology.24 Using this assay, we found that WT cultures contained 64% positive cells, while TTP-deficient cultures contained 60% positive cells. The negative cells in these cultures are considered to be fibroblastlike cells.24 Nonspecific esterase is a selective cytochemical stain for macrophages.22 Using this method, we found that 47% cells in the WT cultures and 48% in the TTP-deficient cultures were positive. Macrophages are also highly phagocytic for latex beads. In these cultures, 41% of the WT cells and 39% of the TTP-deficient cells were phagocytic for latex beads. The use of oil red O stain, specific for fat cells, revealed that fewer than 1% of cells were fat cells in either WT or TTP-deficient cultures.24 28 Taken together, these results indicate that the relative proportions of each cell type in WT and TTP-deficient cultures were comparable. Thus, the differences observed between the 2 genotypes with respect to GM-CSF production were not likely to be due to differences in the proportions of different cell types in the cultures.
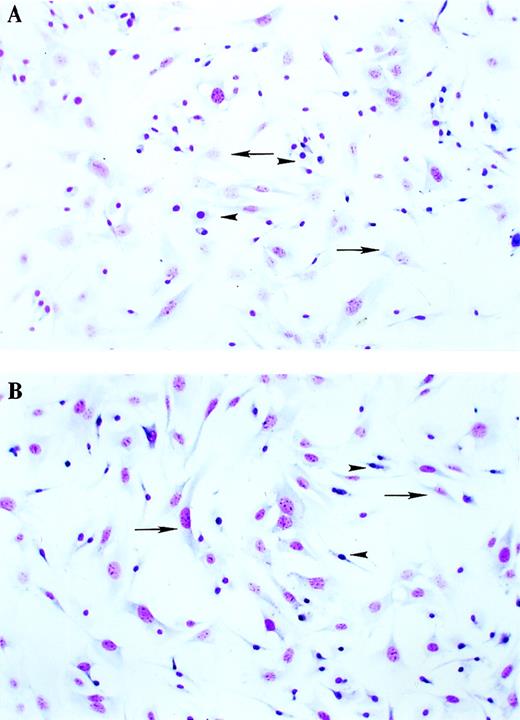
Morphology of BMSCs from WT and TTP-deficient mice.
BMSCs were cultured for 6 weeks as described in “Materials and Methods.” After this period, the cells were trypsinized and replated at 50 000 cells/well in 4-well Lab-Tek tissue-culture chambers. After incubation for 48 hours, the slides were stained with the Diff-Qick Stain Set and photographed under light microscopy (60 × magnification). (A) WT cells. (B) TTP-deficient cells. Arrows indicate fibroblastlike cells, and arrowheads indicate macrophagelike cells.
Morphology of BMSCs from WT and TTP-deficient mice.
BMSCs were cultured for 6 weeks as described in “Materials and Methods.” After this period, the cells were trypsinized and replated at 50 000 cells/well in 4-well Lab-Tek tissue-culture chambers. After incubation for 48 hours, the slides were stained with the Diff-Qick Stain Set and photographed under light microscopy (60 × magnification). (A) WT cells. (B) TTP-deficient cells. Arrows indicate fibroblastlike cells, and arrowheads indicate macrophagelike cells.
Expression of granulocyte-macrophage colony–stimulating factor messenger RNA in bone marrow stromal cells from wild-type and tristetraprolin-deficient mice
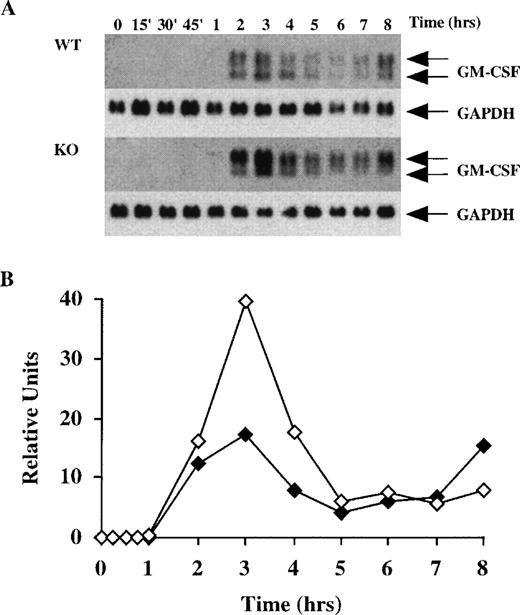
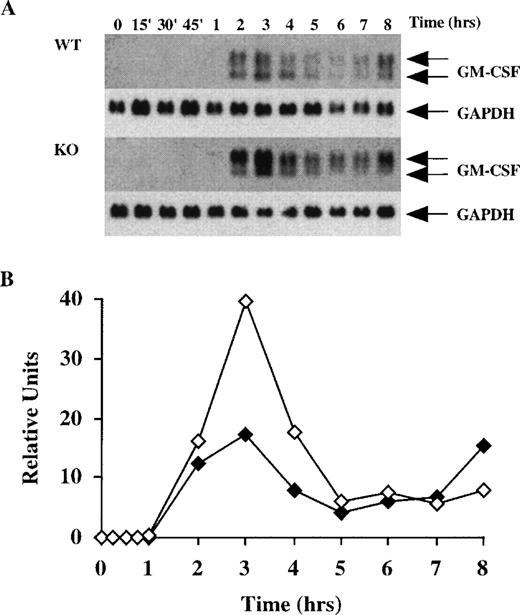
After the BMSCs became confluent, they were stimulated with either LPS (1μg/mL) or TNF-α (10 ng/mL), and the effect of these factors on GM-CSF mRNA accumulation was studied for a period of 8 hours. When comparing WT and KO samples (Figure 2) and heterozygous (Htz) and KO samples (Figure3), we subjected identical amounts of total cellular RNA to electrophoresis in parallel gels. Blotting was performed in parallel, and both blots were hybridized together with the same probe and exposed to film in the same autoradiography cassette. Htz mice were occasionally used as sex-matched littermates if WT controls were not available; previous studies have indicated that results with these cells are indistinguishable from those with WT cells. As shown in Figure 2A, LPS induced detectable levels of GM-CSF mRNA in WT cells within 2 hours; these levels peaked at 3 hours and then slowly decreased over the next several hours, with a slight increase at 8 hours. Note that GM-CSF mRNA does not appear as a single species in these cells, but as 2 major components of approximately 1.0 and 0.8 kb, as previously described.29 When an identical study was performed with cells derived from the TTP-deficient mice, however, there were several differences in the pattern of GM-CSF mRNA expression (Figure 2A). First, the GM-CSF mRNA was detectable earlier in the TTP-deficient cells (after 1 hour of exposure to LPS); second, the overall accumulation of mRNA at the peak time (3 hours) appeared to be approximately twofold greater in the TTP-deficient cells than in the WT cells (after normalization for GAPDH mRNA expression); and third, the distribution of the 2 major mRNA species was different. In the WT cells, the average proportion of the lower band, expressed as a percentage of the total GM-CSF mRNA, was 40 ± 3% (mean ± SEM of 7 values) (Figure 2A); in contrast, the lower band from the TTP-deficient cells contained only 16 ± 1% (mean ± SEM of 8 values) of the total (P < .0001 when compared with WT values by Student t test).
Time course of GM-CSF mRNA accumulation in BMSCs stimulated with LPS.
(A) 60 mm dishes containing confluent BMSCs derived from wild-type (WT) or TTP-deficient (KO) mice were prepared as described in “Materials and Methods.” Cells were then stimulated with LPS (1 μg/mL), and RNA was extracted at different time points for up to 8 hours. Then, 16 μg of total cellular RNA was separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse GM-CSF cDNA probe were performed as described in “Materials and Methods.” Blots were exposed to autoradiographic film in the same cassette for 3 days. The arrows indicate the position of the 2 species of GM-CSF mRNA, of approximately 1.0 and 0.8 kb. Note the clear presence of the 2 species in the WT cells, whereas the 0.8-kb band was decreased in the KO cells. Note also that GM-CSF mRNA was already detectable after 1 hour in the KO cells, but was not detectable until 2 hours in the WT cells. The same blots were then hybridized with a rat GAPDH cDNA probe as a loading control, as indicated. Exposure of the blots to film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid symbols, WT; open symbols, TTP-deficient cells.
Time course of GM-CSF mRNA accumulation in BMSCs stimulated with LPS.
(A) 60 mm dishes containing confluent BMSCs derived from wild-type (WT) or TTP-deficient (KO) mice were prepared as described in “Materials and Methods.” Cells were then stimulated with LPS (1 μg/mL), and RNA was extracted at different time points for up to 8 hours. Then, 16 μg of total cellular RNA was separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse GM-CSF cDNA probe were performed as described in “Materials and Methods.” Blots were exposed to autoradiographic film in the same cassette for 3 days. The arrows indicate the position of the 2 species of GM-CSF mRNA, of approximately 1.0 and 0.8 kb. Note the clear presence of the 2 species in the WT cells, whereas the 0.8-kb band was decreased in the KO cells. Note also that GM-CSF mRNA was already detectable after 1 hour in the KO cells, but was not detectable until 2 hours in the WT cells. The same blots were then hybridized with a rat GAPDH cDNA probe as a loading control, as indicated. Exposure of the blots to film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid symbols, WT; open symbols, TTP-deficient cells.
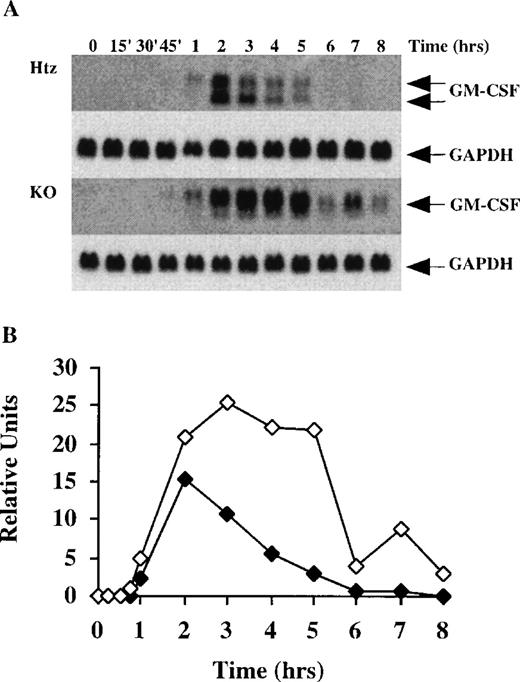
Time course of GM-CSF mRNA accumulation in BMSCs stimulated with TNF-.
(A) This experiment was similar to the one described in the legend to Figure 2, except that the control cells were derived from an Htz mouse, and were stimulated with TNF-α (10 ng/mL) instead of LPS; 20 μg of total cellular RNA was loaded into each gel lane. The blots for GM-CSF mRNA were exposed to autoradiographic film in the same cassette for 7 days. Note the clear presence of the 2 species of GM-CSF mRNA in the Htz cells, whereas the smaller band was essentially absent in the KO cells. Note also that GM-CSF mRNA was already detectable after 45 minutes in the KO cells, but was not detectable until 1 hour in the Htz cells; in addition, GM-CSF mRNA was detectable for up to 8 hours in the KO cells, whereas the mRNA was not detected after 5 hours in the Htz cells. As in Figure 2, the same blots were then hybridized with a rat GAPDH cDNA probe as a loading control; exposure of the blots to film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid symbols, Htz; open symbols, TTP-deficient cells.
Time course of GM-CSF mRNA accumulation in BMSCs stimulated with TNF-.
(A) This experiment was similar to the one described in the legend to Figure 2, except that the control cells were derived from an Htz mouse, and were stimulated with TNF-α (10 ng/mL) instead of LPS; 20 μg of total cellular RNA was loaded into each gel lane. The blots for GM-CSF mRNA were exposed to autoradiographic film in the same cassette for 7 days. Note the clear presence of the 2 species of GM-CSF mRNA in the Htz cells, whereas the smaller band was essentially absent in the KO cells. Note also that GM-CSF mRNA was already detectable after 45 minutes in the KO cells, but was not detectable until 1 hour in the Htz cells; in addition, GM-CSF mRNA was detectable for up to 8 hours in the KO cells, whereas the mRNA was not detected after 5 hours in the Htz cells. As in Figure 2, the same blots were then hybridized with a rat GAPDH cDNA probe as a loading control; exposure of the blots to film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid symbols, Htz; open symbols, TTP-deficient cells.
When TNF-α was used as the stimulus, these differences were even more pronounced. As shown in Figure 3, the total amount of GM-CSF mRNA accumulation in the KO cells was much greater (approximately fivefold at the time of the greatest difference, ie, 5 hours) than that seen in the control cells (in this case, derived from an Htz mouse). In addition, GM-CSF mRNA expression was detected earlier (45 minutes) and remained detectable at identical autoradiographic exposures for a much longer period (up to 8 hours in the KO versus 5 hours in the Htz cells) (Figure 3A). Finally, almost no smaller form of GM-CSF mRNA was detectable in the TTP-deficient cells (46 ± 4% in the Htz, n = 7,versus 11 ± 2% in the KO cells, n = 9; P < .0001).
Granulocyte-macrophage colony–stimulating factor messenger RNA adenylation state in bone marrow stromal cells from wild-type and tristetraprolin-deficient mice
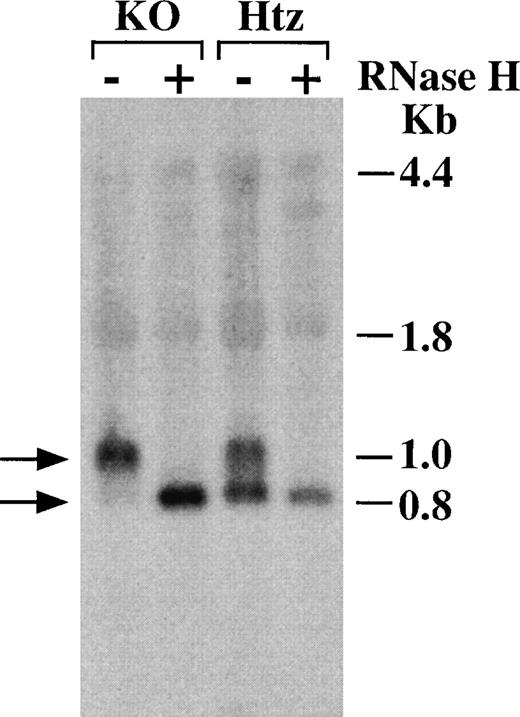
Because TTP has been shown to promote the deadenylation of a synthetic modified TNF-α mRNA in a co-transfection system,16 we determined whether the smaller GM-CSF mRNA species that was prominent in the WT and Htz cells but nearly absent in the TTP-deficient cells was the deadenylated form of the GM-CSF mRNA. Figure 4 shows the effect of RNase H treatment on selected samples of GM-CSF mRNA from the experiment shown in Figure 3. After hybridization with oligo (dT) and digestion with RNase H to remove any remaining polyA tails, the RNA was analyzed by Northern blot. The addition of RNase H completely eliminated the larger species of GM-CSF mRNA, leaving only a single form that comigrated exactly with the smaller band present in the Htz cells but almost absent in the TTP-deficient cells (Figure 4). This result establishes that the 2 major forms of GM-CSF mRNA observed in the WT and Htz cells, as well as in earlier studies,29 correspond to the polyadenylated and deadenylated forms of GM-CSF mRNA, respectively, and that the absence of TTP results in a marked increase in the proportion of the polyadenylated form relative to the deadenylated form. By comparing the migration position of the 2 bands with RNAs of known size, we estimate that the polyA tail of the fully polyadenylated form was approximately 220 residues long in these cells.
Evidence that the smaller species of mouse GM-CSF mRNA represents deadenylated mRNA.
BMSCs were stimulated with TNF-α (10 ng/mL) for 2 or 3 hours, and total cellular RNA was harvested as described in “Materials and Methods.” As indicated, the RNA samples were treated with 1 unit of RNase H for 40 minutes at 37°C, then loaded onto a gel and used for Northern blotting. −, no RNase H was used, and 16 μg of total cellular RNA from BMSCs treated with TNF-α for 2 hours (samples 2Htz and 2KO in Figure 3) was loaded into each lane. +, oligonucleotide poly(dT)12-18 (1 μg) and RNase H were added to 10 μg of RNA from BMSCs treated with TNF-α for 3 hours (samples 3Htz and 3KO in Figure 3). The Northern blot was probed with a 32P-labeled mouse GM-CSF cDNA. The positions of the 18S and 28S ribosomal RNA are indicated as 1.8 kb and 4.4 kb, respectively. The position of the fully deadenylated mouse GM-CSF mRNA is indicated as 0.8 kb. (The mouse GM-CSF mRNA without its polyA tail contains 775 bases.) The position of the polyA-containing mouse GM-CSF mRNA is indicated as 1.0 kb; this approximate size was obtained from a semi-logarithmic plot derived from the migration distances of the 18S and 28S ribosomal RNA and deadenylated mouse GM-CSF mRNA.
Evidence that the smaller species of mouse GM-CSF mRNA represents deadenylated mRNA.
BMSCs were stimulated with TNF-α (10 ng/mL) for 2 or 3 hours, and total cellular RNA was harvested as described in “Materials and Methods.” As indicated, the RNA samples were treated with 1 unit of RNase H for 40 minutes at 37°C, then loaded onto a gel and used for Northern blotting. −, no RNase H was used, and 16 μg of total cellular RNA from BMSCs treated with TNF-α for 2 hours (samples 2Htz and 2KO in Figure 3) was loaded into each lane. +, oligonucleotide poly(dT)12-18 (1 μg) and RNase H were added to 10 μg of RNA from BMSCs treated with TNF-α for 3 hours (samples 3Htz and 3KO in Figure 3). The Northern blot was probed with a 32P-labeled mouse GM-CSF cDNA. The positions of the 18S and 28S ribosomal RNA are indicated as 1.8 kb and 4.4 kb, respectively. The position of the fully deadenylated mouse GM-CSF mRNA is indicated as 0.8 kb. (The mouse GM-CSF mRNA without its polyA tail contains 775 bases.) The position of the polyA-containing mouse GM-CSF mRNA is indicated as 1.0 kb; this approximate size was obtained from a semi-logarithmic plot derived from the migration distances of the 18S and 28S ribosomal RNA and deadenylated mouse GM-CSF mRNA.
Half-life of granulocyte-macrophage colony–stimulating factor messenger RNA in bone marrow stromal cells from wild-type and tristetraprolin-deficient mice
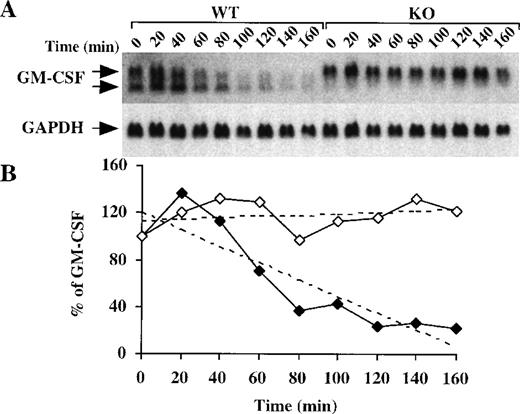
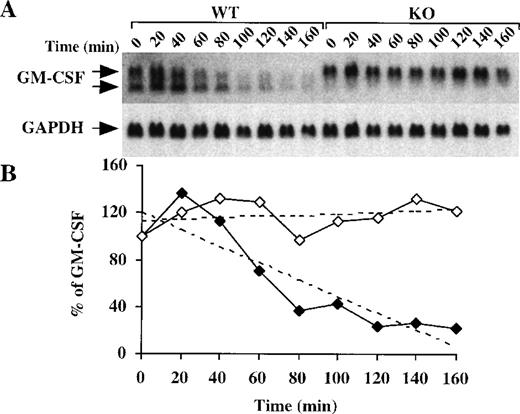
Because deadenylation is a process associated with the initiation of mRNA degradation,30 we next evaluated the half-life of GM-CSF mRNA in BMSCs from WT and TTP-deficient mice. After incubation of BMSCs with LPS for 2 hours followed by the addition of actinomycin D (5 μg/mL), there was a gradual disappearance of GM-CSF mRNA from the WT cells (Figure 5A), with an estimated half-life of 99 minutes (Figure 5B). In contrast, in the TTP-deficient cells, there was essentially no disappearance of the GM-CSF mRNA over the 160-minute period of actinomycin D treatment (Figure 5A), with no calculable half-life (Figure 5B). These data confirm that the absence of TTP results in an increase in the stability of GM-CSF mRNA. As in the previous experiments, the proportion of GM-CSF mRNA in the smaller, deadenylated form was much greater in the WT (51 ± 7%, n = 9) than in the TTP-deficient cells (6 ± 3%, n = 9;P < .0001). Although we cannot exclude effects of TTP deficiency on transcription of the GM-CSF gene, these results demonstrate that both GM-CSF mRNA stability and the proportion of the message in the fully polyadenylated form are increased in the BMSCs from the TTP-deficient mice.
GM-CSF mRNA stability in BMSCs after addition of actinomycin D.
(A) Confluent BMSCs were stimulated for 2 hours with LPS (1 μg/mL). The culture medium was then removed and replaced with fresh medium containing 5 μg/mL actinomycin D. Cells were harvested, and total cellular RNA was extracted at 20-minute intervals for 160 minutes. Then, 15 μg of total cellular RNA from each time point were separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse GM-CSF cDNA probe were performed. The blots were exposed to autoradiographic film in the same cassette for 8 days. Arrows indicate the positions of the 2 species of GM-CSF mRNA. Note that the smaller band is essentially absent in the KO cells; note also that the GM-CSF mRNA dramatically decreased after 1 hour in the WT cells, whereas its level remained largely unaffected in the KO cells. The same blot was then hybridized with a rat GAPDH cDNA probe as a loading control; the exposure of this blot for GAPDH was 4 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of the GM-CSF mRNA decay values. With the use of these regressions, the estimated half-lives for GM-CSF mRNA were found to be 99 minutes in the WT cells but were impossible to determine in the TTP-deficient cells.
GM-CSF mRNA stability in BMSCs after addition of actinomycin D.
(A) Confluent BMSCs were stimulated for 2 hours with LPS (1 μg/mL). The culture medium was then removed and replaced with fresh medium containing 5 μg/mL actinomycin D. Cells were harvested, and total cellular RNA was extracted at 20-minute intervals for 160 minutes. Then, 15 μg of total cellular RNA from each time point were separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse GM-CSF cDNA probe were performed. The blots were exposed to autoradiographic film in the same cassette for 8 days. Arrows indicate the positions of the 2 species of GM-CSF mRNA. Note that the smaller band is essentially absent in the KO cells; note also that the GM-CSF mRNA dramatically decreased after 1 hour in the WT cells, whereas its level remained largely unaffected in the KO cells. The same blot was then hybridized with a rat GAPDH cDNA probe as a loading control; the exposure of this blot for GAPDH was 4 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of the GM-CSF mRNA decay values. With the use of these regressions, the estimated half-lives for GM-CSF mRNA were found to be 99 minutes in the WT cells but were impossible to determine in the TTP-deficient cells.
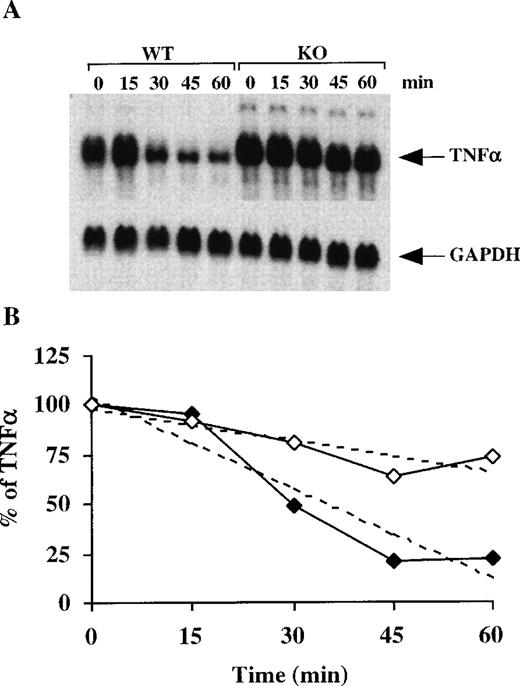
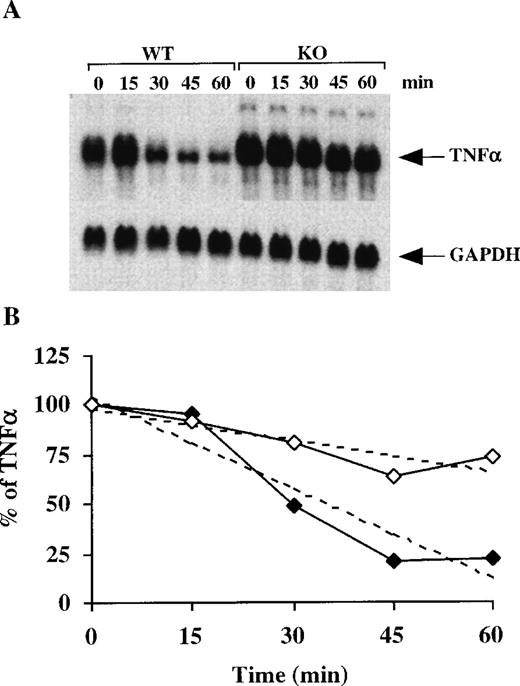
To test for the specificity of this effect, we probed the same filters with cDNAs for both TNF-α and c-fos. Figure6 shows the increased stability of TNF-α mRNA in the TTP-deficient BMSCs, confirming our previous results in macrophages11; in the BMSCs, the calculated half-life for TNF-α mRNA in the WT cells was 35 minutes versus 90 minutes in the TTP-deficient cells. Note also the apparent absence of a stable, deadenylated intermediate form of the TNF-α mRNA, in contrast to the results with GM-CSF mRNA (Figure 6). When similar studies were performed with c-fos mRNA, another short-lived mRNA that contains a so-called class I ARE,31 the estimated half-life of the mRNA was 43 minutes in the WT cells versus 41 minutes in the TTP-deficient cells (data not shown). These results suggest, but do not prove, that the TTP effect is specific to a particular set of mRNAs: those containing class II AREs.
TNF- mRNA stability in BMSCs after the addition of actinomycin D.
(A) Confluent BMSCs were stimulated for 2 hours with LPS (1 μg/mL). The culture medium was then removed and replaced with fresh medium containing 5 μg/mL actinomycin D. Cells were harvested, and total cellular RNA was extracted at 15-minute intervals for 60 minutes. Then, 20 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse TNF-α cDNA probe were performed. The blots were exposed to autoradiographic film in the same cassette for 2 days. The arrow indicates the position of TNF-α mRNA. The same blot was then hybridized with a rat GAPDH cDNA probe as a loading control; the exposure of this blot for GAPDH was 12 hours. (B) Relative amounts of TNF-α mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of TNF-α mRNA decay. With the use of these regressions, the estimated half-lives for TNF-α mRNA were found to be 35 minutes in the WT cells and 90 minutes in the TTP-deficient cells.
TNF- mRNA stability in BMSCs after the addition of actinomycin D.
(A) Confluent BMSCs were stimulated for 2 hours with LPS (1 μg/mL). The culture medium was then removed and replaced with fresh medium containing 5 μg/mL actinomycin D. Cells were harvested, and total cellular RNA was extracted at 15-minute intervals for 60 minutes. Then, 20 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel, and Northern blotting and hybridization with a mouse TNF-α cDNA probe were performed. The blots were exposed to autoradiographic film in the same cassette for 2 days. The arrow indicates the position of TNF-α mRNA. The same blot was then hybridized with a rat GAPDH cDNA probe as a loading control; the exposure of this blot for GAPDH was 12 hours. (B) Relative amounts of TNF-α mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of TNF-α mRNA decay. With the use of these regressions, the estimated half-lives for TNF-α mRNA were found to be 35 minutes in the WT cells and 90 minutes in the TTP-deficient cells.
Secretion of granulocyte-macrophage colony–stimulating factor from bone marrow stromal cells from wild-type and tristetraprolin-deficient mice
Our previous results with TNF-α showed that the increased stability of TNF-α mRNA was accompanied by increased secretion of the protein.13 To determine whether a similar situation occurred with GM-CSF, we incubated confluent layers of BMSCs in the presence of LPS (1 μg/mL) for 24 hours and measured the amount of GM-CSF secreted into the culture medium using a specific ELISA. There were no statistically significant differences between the levels of GM-CSF secreted by WT and TTP-deficient BMSCs in basal, unstimulated conditions, but the levels were barely detectable in this assay (data not shown). On the other hand, after 24 hours of stimulation with LPS, the medium from the WT cells contained 2.5 ± 0.6 pg of GM-CSF/mL/μg of DNA (mean ± SEM of 5 samples), versus 14.6 ± 4.5 pg of GM-CSF/mL/μg of DNA in the medium from the TTP-deficient cells (5 samples). This 5.8-fold difference was statistically significant (P < .05 with the use of Student t test). This result suggested that, as in the case of TNF-α,11 13 the absence of TTP resulted not only in an increase in the stability of GM-CSF mRNA, but also in the increased production of GM-CSF from these cells.
Effect of tumor necrosis factor– receptor deficiency on granulocyte-macrophage colony–stimulating factor messenger RNA stability in tristetraprolin-deficient mice
TNF-α is well known as an inducer of GM-CSF synthesis32-35; it has also been shown to increase the stability of GM-CSF mRNA.33 Therefore, there was a theoretical possibility that in the TTP-deficient cells, the increased stability of GM-CSF mRNA could be secondary to the excess circulating TNF-α levels that characterize these animals. To test this possibility, we generated mice that were deficient in TTP as well as in the 2 TNF-α receptors (triple-KO mice). In these mice, any phenotypic changes observed should be due to factor(s) other than TNF-α. A complete description of the phenotype of these mice will appear elsewhere.
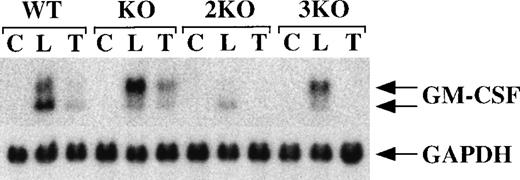
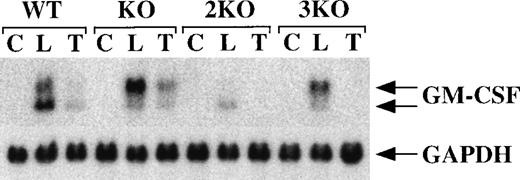
BMSCs from WT mice, TTP-deficient mice, mice deficient in both TNF-α receptors (but WT for TTP; double KO), and mice deficient in both receptors and TTP (triple KO) were stimulated for 3 hours with either LPS (1 μg/mL) or recombinant murine TNF-α (10 ng/mL). As shown in Figure 7, both WT and TTP-deficient cells exhibited a striking induction of GM-CSF mRNA in response to either LPS or TNF-α. As before (Figures 2 and 3), a stronger response was observed in the TTP-deficient cells, in which the larger of the 2 mRNA species again predominated (Figure 7). In contrast, cells deficient in both TNF-α receptors responded to LPS but not to TNF-α (Figure 7); no GM-CSF mRNA was detectable in the samples from the double- and triple-KO mice, even after longer exposure of the autoradiograph. These results confirm the lack of any response to TNF-α both in the double-KO and in the triple-KO mice.
Lack of response to TNF- in cells from double- and triple-KO mice.
BMSCs were stimulated with LPS (1 μg/mL) or TNF-α (10 ng/mL) for 3 hours. Cells were then harvested; RNA was extracted; and 20 μg of total cellular RNA was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blots were exposed to autoradiographic film for 6 days. WT indicates wild-type mice; KO indicates TTP-deficient mice; 2KO indicates mice deficient in both TNF-α receptors, but WT for TTP; 3KO indicates mice deficient in TTP and both TNF-α receptors. C, control; L, LPS (1 μg/mL); T, TNF-α (10 ng/mL). The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the absence of response to TNF-α in the cells derived from double- and triple-KO mice. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 4 hours.
Lack of response to TNF- in cells from double- and triple-KO mice.
BMSCs were stimulated with LPS (1 μg/mL) or TNF-α (10 ng/mL) for 3 hours. Cells were then harvested; RNA was extracted; and 20 μg of total cellular RNA was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blots were exposed to autoradiographic film for 6 days. WT indicates wild-type mice; KO indicates TTP-deficient mice; 2KO indicates mice deficient in both TNF-α receptors, but WT for TTP; 3KO indicates mice deficient in TTP and both TNF-α receptors. C, control; L, LPS (1 μg/mL); T, TNF-α (10 ng/mL). The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the absence of response to TNF-α in the cells derived from double- and triple-KO mice. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 4 hours.
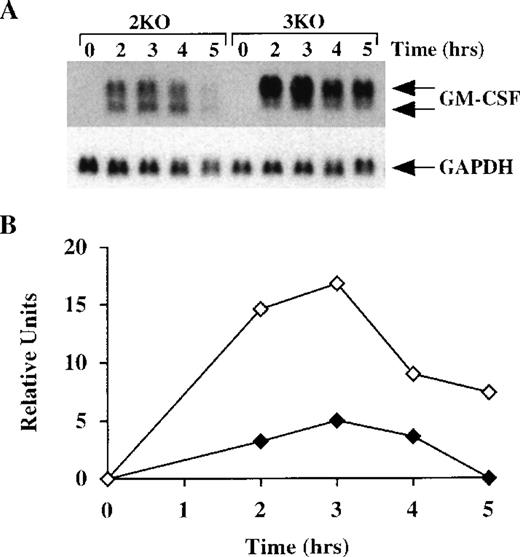
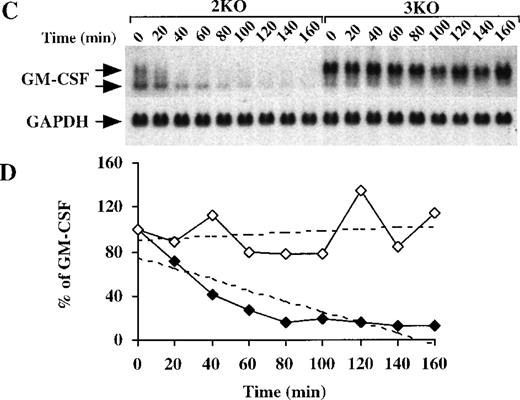
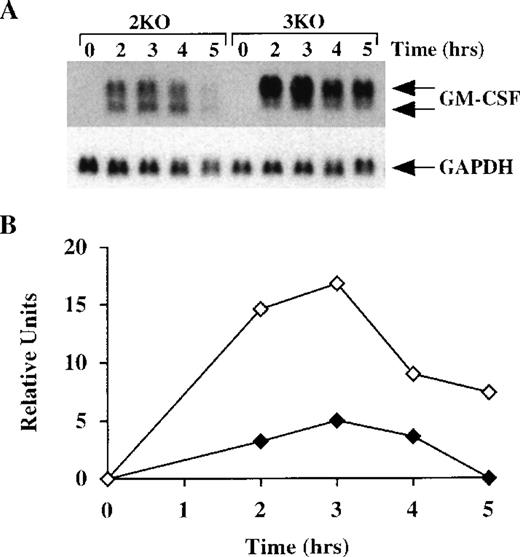
We then determined the half-life of GM-CSF mRNA in BMSCs derived from both double-KO and triple-KO mice. As described above, BMSCs from these mice were stimulated with LPS, with and without actinomycin D. In the absence of both TNF-α receptors, GM-CSF mRNA levels in the BMSCs changed in response to LPS in the same way as in the WT cells, appearing as 2 species of similar intensity of approximately 1.0 kb and 0.8 kb (Figure 8A). However, in the triple-KO cells, when TTP was also absent, a pattern identical to that observed in the TTP-deficient mice was seen; ie, essentially the only form of GM-CSF mRNA present in these cells was the larger, polyadenylated species. In the double-KO cells, the deadenylated form represented an average of 41 ± 6% (n = 3) of the total GM-CSF mRNA versus 7.5 ± 0.4% (n = 4) in the triple-KO cells (P < .0005) (Figure 8A). There was also marked accumulation of total hybridizable mRNA in the triple-KO cells compared with the double-KO cells, with an approximately fourfold increase observed at 3 hours (Figure 8B). Studies of GM-CSF mRNA stability after actinomycin D (Figure 8C) confirmed that the absence of TTP resulted in a prolonged half-life of GM-CSF mRNA, particularly the polyadenylated form, even in the absence of both TNF-α receptor subtypes. In this experiment, the smaller, deadenylated form represented 62 ± 7% (n = 9) of the total GM-CSF mRNA in the double-KO cells versus 8 ± 4% (n = 9) in the triple-KO cells (P < .0001). The calculated half-life of GM-CSF mRNA in the double-KO cells was 49 minutes, but no measurable decay was observed in the triple-KO cells (Figure 8D). These results establish that the absence of TTP per se is sufficient to inhibit deadenylation and increase the stability of GM-CSF mRNA, even in the absence of TNF-α signaling.
GM-CSF mRNA expression in BMSCs from triple-KO mice.
(A) BMSCs were stimulated with LPS (1 μg/mL) for up to 5 hours; cells were then harvested; RNA was extracted; and 14 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blots were exposed to autoradiographic film for 7 days. 2KO indicates mice deficient in both TNF-α receptors, but WT for TTP; 3KO indicates mice deficient in TTP and both TNF-α receptors. The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the greater levels of total GM-CSF mRNA in the 3KO samples, compared with the 2KO, as well as the virtually complete absence of the smaller species of GM-CSF mRNA in the 3KO samples. Note also that the GM-CSF mRNA was almost undetectable by 5 hours in the 2KO cells, but was still present in readily detectable amounts in the 3KO cells. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, 2KO; open diamonds, 3KO cells. (C) BMSCs were stimulated with LPS (1 μg/mL) for 2 hours; the culture medium was then removed and replaced by fresh medium containing 5 μg/mL actinomycin D. Cells were harvested and RNA was extracted at 20-minute intervals for 160 minutes. Then, 15 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blot was exposed to film for 8 days. The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the rapid disappearance of both species of GM-CSF mRNA in the samples from the 2KO cells, whereas GM-CSF mRNA levels remained essentially unchanged in the 3KO cells. Note also the virtually complete absence of the lower species of GM-CSF mRNA in the samples from the 3KO cells. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 4 hours. (D) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of GM-CSF mRNA decay. With the use of these regressions, the estimated half-lives for GM-CSF mRNA were found to be 49 minutes in the 2KO cells but were impossible to determine in the 3KO cells.
GM-CSF mRNA expression in BMSCs from triple-KO mice.
(A) BMSCs were stimulated with LPS (1 μg/mL) for up to 5 hours; cells were then harvested; RNA was extracted; and 14 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blots were exposed to autoradiographic film for 7 days. 2KO indicates mice deficient in both TNF-α receptors, but WT for TTP; 3KO indicates mice deficient in TTP and both TNF-α receptors. The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the greater levels of total GM-CSF mRNA in the 3KO samples, compared with the 2KO, as well as the virtually complete absence of the smaller species of GM-CSF mRNA in the 3KO samples. Note also that the GM-CSF mRNA was almost undetectable by 5 hours in the 2KO cells, but was still present in readily detectable amounts in the 3KO cells. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 2 hours. (B) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, 2KO; open diamonds, 3KO cells. (C) BMSCs were stimulated with LPS (1 μg/mL) for 2 hours; the culture medium was then removed and replaced by fresh medium containing 5 μg/mL actinomycin D. Cells were harvested and RNA was extracted at 20-minute intervals for 160 minutes. Then, 15 μg of total cellular RNA from each time point was separated on a 1.5% agarose gel and used for Northern blotting with a mouse GM-CSF cDNA probe. The blot was exposed to film for 8 days. The arrows indicate the positions of the 2 GM-CSF mRNA species. Note the rapid disappearance of both species of GM-CSF mRNA in the samples from the 2KO cells, whereas GM-CSF mRNA levels remained essentially unchanged in the 3KO cells. Note also the virtually complete absence of the lower species of GM-CSF mRNA in the samples from the 3KO cells. The same blots were hybridized with a rat GAPDH cDNA probe as a loading control. Exposure of the blots to autoradiographic film for GAPDH mRNA was 4 hours. (D) Relative amounts of GM-CSF mRNA after normalization to GAPDH mRNA. Solid diamonds, WT; open diamonds, TTP-deficient cells. The dotted lines represent the linear regression of GM-CSF mRNA decay. With the use of these regressions, the estimated half-lives for GM-CSF mRNA were found to be 49 minutes in the 2KO cells but were impossible to determine in the 3KO cells.
Discussion
The most important finding of the present study is that TTP appears to be a normal, physiological regulator of GM-CSF mRNA stability in the mouse. Using BMSCs derived from TTP-deficient mice, we showed that GM-CSF mRNA accumulation was markedly enhanced in cells lacking TTP relative to control cells after stimulation with either LPS or TNF-α. Using the transcription inhibitor actinomycin D, we demonstrated that this increased mRNA accumulation was due, at least in part, to an increase in GM-CSF mRNA stability in the TTP-deficient cells relative to control cells. Taken together with our earlier results,11,13 these data indicate that cellular levels of both TNF-α and GM-CSF mRNA are controlled to some extent by TTP. As in the case of TNF-α,11 this leads to increased expression of GM-CSF protein from the TTP-deficient cells compared with control cells.
Concerning the mechanism of this effect, the TNF-α, GM-CSF, and interleukin-3 (IL-3) AREs are all so-called class II AREs,15,36 which contain several, usually tandem, AUUUA repeats. Co-transfection experiments in 293 cells, using artificial constructs in which the c-fos promoter was used to drive expression of the β-globin protein coding sequence linked to a 3′-ARE derived from either the TNF-α or GM-CSF mRNA, revealed that TTP expression led to decreased accumulation of these mRNAs, implicating the AREs in this process. In addition, when we estimated the half-life of c-fos mRNA, which contains a class I ARE, in the WT and TTP-deficient BMSCs, there was no difference between the 2 genotypes, suggesting possible specificity of TTP for class II AREs. Direct-binding studies demonstrated that TTP could bind directly to the TNF-α ARE.11 More recently, we showed in co-transfection studies that TTP expression led to the destabilization of a somewhat truncated TNF-α mRNA, in which the ARE was shortened from 7 AUUUA repeats to 3.5, and the spacing between the ARE and a synthetic polyA tail of 33 residues was decreased to 0 bases (b) from the normal 300 b in the mouse (GenBank accession number X02611).16 This TTP-induced mRNA destabilization was accompanied by the formation of a deadenylated intermediate.16 Deadenylation has long been known as an early step in the degradation of mRNA,30particularly in the case of mRNAs containing class II AREs,15,36 and we concluded that a primary role of TTP was to stimulate the process of deadenylation initially and, ultimately, the overall degradation of the mRNA. Nonetheless, we have never seen the accumulation of a deadenylated form of TNF-α mRNA in either normal or TTP-deficient macrophages11 or in BMSCs in the present study; this raises some questions as to the physiological importance of the phenomenon observed in the co-transfection experiments, in which an artificial TNF-α construct was used in cells that do not normally express either TNF-α or TTP.16
However, the data from the present study firmly establish a physiological role for TTP as a promoter of GM-CSF mRNA deadenylation. Supporting this conclusion are several types of data. First, in every experiment (n = 7) in which BMSCs from normal mice were used, Northern blotting of GM-CSF mRNA revealed that a substantial proportion(40% to 62%) of the total GM-CSF mRNA was in a smaller form (of approximately 0.8 kb), with the remainder in a larger form (of approximately 1 kb). The existence of these 2 hybridizing forms of GM-CSF mRNA in mouse cells has long been noted in the literature29 37 and has been assumed to be due to use of alternative promoters or alternative transcription start sites. We demonstrated directly, using RNase H with oligo (dT), that the smaller of the 2 species represented completely deadenylated GM-CSF mRNA. Thus, the larger of the 2 species is likely to contain the full polyA tail, calculated to be approximately 220 residues long in these cells. Although the 2 predominant species were routinely detected on Northern blots, we noted between them on most blots a “smear” of intermediate-sized species, presumably representing partially deadenylated mRNA.
Second, in every experiment (n = 7) performed with BMSCs derived from TTP-deficient mice, there was marked accumulation of the larger, fully polyadenylated form of the GM-CSF mRNA relative to the smaller form, which represented only 6% to 16% of the total GM-CSF mRNA. This is strong evidence that the normal control of GM-CSF deadenylation is regulated in some manner by TTP. As expected from our previous experiments with the TNF-α ARE,11 16 TTP binds and can be cross-linked to the normal, but not ARE-deleted, GM-CSF 3′ UTR probes in cell-free studies (W. S. L., E. C., and P. J. B., unpublished data). Co-transfection of plasmids expressing TTP with those expressing GM-CSF in human 293 cells also led to increased deadenylation of the mRNA and destruction of the mRNA body (W. S. L., E. C., and P. J. B., unpublished data). These results demonstrate that TTP binds to the GM-CSF mRNA ARE and causes its deadenylation and destabilization, by mechanisms that remain to be elucidated.
We also found approximately sixfold greater levels of GM-CSF secretion from the TTP-deficient cells than from the WT cells. This could simply be a reflection of the increased accumulation of GM-CSF mRNA in the cells from the TTP-deficient mice. However, these data also raise an interesting question concerning the translatability of the deadenylated GM-CSF mRNA that composes a large fraction of the hybridizing species on Northern blots of RNA from WT animals. Direct comparisons of translation rates of polyadenylated and deadenylated mRNAs have indicated that the polyA tail is necessary for normal rates of translation of some mRNAs.38-41 Therefore, in the present studies, not only is total hybridizable GM-CSF mRNA increased in cells from the TTP-deficient animals, but the 40% to 62% of the total represented by the deadenylated mRNA species in the cells from the WT animals may not be normally translated.
Finally, the experiments with the triple-KO mice lacking TTP as well as both types of TNF-α receptors indicate that the effect of TTP deficiency to promote accumulation of the fully polyadenylated form of the GM-CSF mRNA was not a consequence of chronic exposure of the cells to high ambient TNF-α concentrations. TNF-α can stimulate the accumulation of GM-CSF mRNA, apparently at least in part by stabilizing it.33 However, we have shown that the cells derived from the TNF-α receptor–deficient mice do not respond to TNF-α (Figure7), and since identical results were obtained in the triple-KO mice as in the TTP-deficient mice in terms of stabilization of GM-CSF mRNA, it is very unlikely that TNF-α excess was involved in the increased GM-CSF mRNA stability noted when TTP was absent. On the other hand, the difference in half-life observed between wild-type and double-KO cells suggests that in normal circumstances, when TTP is expressed, the levels of TNF-α produced by cells stimulated with LPS can contribute to the stabilization of their GM-CSF mRNA.
These studies support a model for the severe inflammatory syndrome that characterizes the TTP-deficient mice,12 in which the absence of TTP leads to elevations in the steady-state levels of mRNA for both TNF-α and GM-CSF, as well as the increased secretion of their encoded proteins. Since each cytokine is known to stimulate the secretion of the other,32-35 42 the initial hypersecretion of each could lead to an interacting pathogenetic spiral in which the hypersecretion of each becomes greater with time.
One of the most striking characteristics of the TTP-deficiency syndrome in mice was the exuberant myeloid hyperplasia noted in bone marrow and in extramedullary sites. Since this was noted in a previous study involving chronic TNF-α administration to rats,43 we concluded that its presence in the TTP KO mice probably reflected the chronically elevated systemic levels of TNF-α that characterized the syndrome. However, our present finding that TTP deficiency also resulted in elevated levels of GM-CSF mRNA in BMSCs and increased secretion of GM-CSF from these cells suggested that these and perhaps other cells might oversecrete this growth factor in the TTP KO mice, contributing in turn to the myeloid hyperplasia. In support of this possibility, mice deficient in the 2 types of TNF-α receptor as well as in TTP displayed myeloid hyperplasia, both medullary and extramedullary (E.C. and P.J.B., unpublished data). This observation might seem difficult to reconcile with our previous results,13 in which animals were analyzed at 80 days of age after 70 days of treatment with anti–TNF-α neutralizing antibodies. The TTP-deficient mice not treated with antibody developed myeloid hyperplasia, whereas those treated with antibody did not.13However, triple-KO mice at that age did not exhibit myeloid hyperplasia; this did not develop until later in life (at about 6 months of age). Therefore, the myeloid hyperplasia characteristic of the TTP-deficient mice may well be contributed to by chronic overstimulation with both GM-CSF and TNF-α. In the absence of TNF-α receptors, the development of the myeloid hyperplasia is delayed but not prevented.
Stabilization of GM-CSF mRNA has been demonstrated previously in several experimental circumstances. In addition to TNF-α stimulation, as described above, activation of protein kinase C has been shown to stabilize this mRNA, presumably through the phosphorylation or changes in synthesis of trans-acting factors.44-46 Certain monocytic tumor cell lines have also been reported to exhibit stabilized GM-CSF mRNA; this increase in stability was attributed to unknown trans-acting factors that might be differentially expressed in these cells.47 Although the present studies implicate TTP in the regulation of GM-CSF mRNA deadenylation and stability, TTP itself is subject to many modes of regulation that might influence its ability to promote ARE binding and/or mRNA degradation. These include regulation at the levels of transcription, subcellular localization, and phosphorylation, all of which are affected by activation of protein kinase C, at least in certain cell types.2,9,10 TTP mRNA is itself very labile,2suggesting that it might be susceptible to similar types of regulation by other proteins. It seems likely that the effect of TTP on mRNA deadenylation and degradation will be subject to regulation by some of these mechanisms, as well as by cell-specific and developmentally regulated expression. In addition, since the 2 known mammalian relatives of TTP also exhibit similar ARE binding and mRNA deadenylating activities, at least in some experimental systems (W.S.L. and P.J.B., unpublished data), the potential exists for extremely complex regulation of mRNAs containing similar class II ARE motifs.
Recombinant GM-CSF has been administered to human patients for a variety of indications, including the bone marrow suppression that accompanies certain forms of chemotherapy,48,49 autologous bone marrow transplantation,50 aplastic anemia,51 and other neutropenic conditions. Although this recombinant protein has represented a major advance in treatment for these conditions, its administration is not without problems. These include the expense of making and purifying the recombinant protein, the requirement for parenteral administration, and the possibility of antibody formation. A small, inexpensive nonprotein molecule that could be administered orally to achieve the same effects as parenteral GM-CSF would represent a significant improvement. The present studies have defined a new target for the development of such drugs, ie, compounds that would stimulate endogenous GM-CSF production by increasing the stability of its mRNA. This would be accomplished by blocking TTP binding to the ARE of the GM-CSF mRNA. TTP binding to ARE probes can be readily demonstrated in cell-free assays,11 16 making possible screens for potential inhibitors of this interaction.
Acknowledgments
We are grateful to Dr Mark W. Moore and Genentech for providing the TNF-α-receptor–deficient mice and to Drs A. Jetten and D. Germolec for critical reading of the manuscript.
Reprints:Perry J. Blackshear, National Institute of Environmental Health Sciences, MD A2-05, PO Box 12233, Research Triangle Park, NC 27709; e-mail: black009@niehs.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.