Abstract
The anticoagulant factor protein S is a secreted vitamin K-dependent γ-carboxylated protein that is mainly made in the liver. Protein S is homologous to the growth arrest specific protein, Gas6, the expression of which is up-regulated in cultured fibroblasts upon serum withdrawal. We report here the synthesis and secretion of protein S by cultured human vascular smooth muscle cells (HVSMCs). Western blot analysis revealed that similar amounts of protein S are secreted by both growing and growth-arrested HVSMCs. HVSMC-derived protein S was found to be γ-carboxylated as it was precipitated by barium citrate and was shown to possess protein C cofactor activity. Treatment with the vitamin K antagonist warfarin led to the accumulation of intracellular undercarboxylated protein S forms that were rapidly secreted upon the reintroduction of vitamin K. Northern blotting analysis showed that cultured HVSMCs express a protein S transcript. The expression of protein S messenger RNA was unaffected by either warfarin, growth arrest, or various VSMC mitogens, such as platelet-derived growth factor-BB, basic fibroblast growth factor, transforming growth factor-β, or hepatocyte growth factor. Thrombin, however, induced an up-regulation of protein S expression at both messenger RNA and protein levels. The evidence we provide for protein S secretion by cultured HVSMCs and its up-regulation by thrombin, together with earlier reports showing that protein S acts as a mitogen for these cells, suggests that, in addition to its known role in regulating blood clotting, protein S may also be an important autocrine factor in the pathophysiology of the vasculature.
Protein S is a 69 kd single-chain plasma glycoprotein that acts as a cofactor for activated protein C in the inactivation of coagulation factors Va and VIIIa.1 It requires no activation by proteolytic cleavage and circulates at a concentration of about 270 nmol/L in both a free active form (40%) and in an inactive form (60%) bound to C4b-binding protein.2Protein S belongs to the family of mainly secreted vitamin K-dependent proteins that includes a number of zymogens and cofactors of the coagulation cascade in which some glutamyl residues are posttranslationally modified to γ-carboxyglutamic acid (Gla) residues.3 The Gla posttranslational modification is catalyzed by a microsomal γ-glutamyl carboxylase that requires vitamin K in its reduced form as a cofactor.4 The enzymatic reaction generates γ-carboxyglutamate and vitamin K 2,3,-epoxide that is then recycled back to the hydroquinone form by a reductase enzyme.4 Warfarin (3-[α-acetonyl-benzyl-4-hydroxycoumarin]), a vitamin K antagonist, binds and inhibits the activity of the vitamin K epoxide reductase and thus blocks the vitamin K cycle.3,4 This property of warfarin has led to its use in anticoagulant therapy.5 Gla residues allow Ca++-dependent protein-phospholipid complex formation and facilitate conformational changes that are essential for zymogen activation or for cofactor activity.6 Following the Gla region is a domain unique to protein S, which consists of a disulphide loop that is highly susceptible to proteolysis by serine proteinases such as thrombin.7-9 This domain is followed by four epidermal growth factor-like domains. The C-terminus of protein S is unrelated to other coagulation factors and is homologous to the plasma sex hormone binding globulin8 and the basement membrane proteins laminin A and merosin.10 The latter two proteins have been shown to play a role in cell proliferation, migration, and differentiation.10
The major producer of protein S and other factors of the coagulation system is the liver.3 However, protein S is also synthesized at extrahepatic sites, including the brain and spleen11,12 as well as endothelial cells,13,14megakaryocytes,15 and osteoblasts,16 and cells of the nervous system,17 suggesting that it has functions distinct from its anticoagulant activities. The product of a gene specifically expressed during serum starvation in fibroblasts, named growth arrest specific gene 6 (Gas6), represents a new member of the vitamin K-dependent protein family that is homologous to protein S.18 Apart from its lack of a thrombin-cleavage site, Gas6 protein exhibits strong homology with all the other structural domains of protein S.18 Gas6 is synthesized and secreted by cultured vascular smooth muscle cells (VSMCs) and was shown to potentiate thrombin-induced proliferation in these cells.19,20 These effects of Gas6 are dependent on its γ-carboxylation as Gla-deficient Gas6 lacks receptor-binding and growth potentiating activities.20,21 Gas6 was also shown to prevent fibroblast, endothelial, and VSMC death by apoptosis and hence was postulated to be a cell survival factor.22-24
Gasic et al25 and our laboratory26 have previously demonstrated that protein S induces the proliferation of cultured rat and human VSMCs (HVSMCs), respectively, and evidence for the existence of a specific protein S receptor(s) on HVSMCs has been provided.26 The cellular activities of protein S on VSMCs in vitro occur within its circulating range2 (270 nmol/L), thus suggesting that such activities may occur in vivo. In the present report, we show that cultured HVSMCs produce and secrete protein S into their conditioned media and express a protein S transcript. The expression of protein S by serum-starved, growth-arrested, and growing VSMCs as well as the regulation of its synthesis and secretion by vitamin K, warfarin, and various cytokines was investigated.
Materials and methods
Materials
Purified human α-thrombin (3000 U/mg) and human protein S were from Enzyme Research Laboratories (UK). Recombinant human platelet-derived growth factor-BB (PDGF-BB) and basic fibroblast growth factor (b-FGF) were from Bachem (UK). Human recombinant hepatocyte growth factor, human platelet transforming growth factor beta-1, vitamin K1, and 3-(α-acetonylbenzyl)-4-hydroxy-coumarin (warfarin) were from Sigma (UK). Tritiated thymidine ([3H]-TdR; 120 Ci/mmol/L), [α-32P]-dCTP (>3000 Ci/mmol/L), and [35S]-methionine/[35S]-cysteine (Pro-Mix™) were from Amersham-Pharmacia Biotech (UK). Rabbit antihuman protein S antibody and horseradish peroxidase-conjugated goat antirabbit or antimouse immunoglobulin G were from Dako (UK). Monoclonal antibodies to protein S Gla domain (HSP 21) and epidermal growth factor-like region (HSP 41) were a kind gift from Professor Bjorn Dahlbäck (Department of Clinical Chemistry, University of Lund, Malmö, Sweden).8
Cell culture
Analysis of protein S synthesis and secretion
Protein S production was assessed in conditioned media and cell lysates. Two cell culture conditions were used: growth-arrested and growing HVSMCs. Growth-arrested cells were obtained by transferring confluent cultures (grown at about 90% confluency in their characteristic “hills and valleys” pattern)26,27 into serum-free DMEM for 48 hours prior to the commencement of media conditioning. Growing cells were subconfluent cultures (around 60% confluency) initially grown in serum that were placed in a defined serum-free growth medium (to avoid possible interference because of serum-derived protein S) at the start of media conditioning. The serum-free growth promoting medium consisted of DMEM containing a supplement of insulin (5 μg/mL), transferrin (5 μg/mL), sodium selenite (5 ng/mL) (Sigma, UK), PDGF-BB (2 ng/mL), and b-FGF (2 ng/mL). These conditions were found to support the growth and proliferation of HVSMCs in the absence of any serum supplement for 3 to 5 days. To confirm the growth state of the cells, parallel cultures were set up and used to monitor levels of [3H]-TdR incorporation. Cells were pulse-labeled for 4 hours with 5 μCi/mL [3H]-TdR at 24-hour intervals, and [3H]-TdR incorporation was measured as described previously.26-28 Under these conditions, the levels of [3H]-TdR incorporation for growing cells expressed as average counts per minute from 3 determinations were as follows: 13 460 ± 945 at day 1; 15 890 ± 950 at day 2; and 20 250 ± 765 at day 3. For growth-arrested cells, levels of [3H]-TdR incorporation in counts per minute determined at daily intervals after the 48-hour growth-arrest period were: 3765 ± 408 at day 1; 1659 ± 98 at day 2; and 1275 ± 108 at day 3. Fresh serum-free DMEM (or DMEM with growth supplements described above for growing cells) was added to the cells at a ratio of 5 mL/106 cells for specified periods of time after which conditioned media were collected. The influence of vitamin K1 (1-10 μg/mL), of warfarin (1 μg/mL), or of specific growth factors or cytokines on protein S production was determined by including these agents in serum-free media. Proteins in conditioned media and cell lysates were quantified using a Biorad microassay. To take into account differences in cell numbers between the various cell culture conditions used (growth-arrested vs growing cells or cells treated with various growth factors), for each individual experiment and/or time point reported, the volumes of conditioned media that were analyzed were adjusted to total cellular protein.
Western blotting analysis of protein S synthesis and secretion
Proteins in conditioned media were precipitated with trichloroacetic acid, washed in acetone, and resuspended in Laemmli sample buffer.29 Cell monolayers were lysed directly in sample buffer. Protein S expression was analyzed by Western blotting, using polyclonal and monoclonal antiprotein S antibodies as detailed previously.8 Proteins were visualized by enhanced chemiluminesence ECL (Amersham Pharmacia Biotech). To exclude the possibility that the protein S detected was a contaminant from serum, metabolic labeling and immunoprecipitation experiments using antiprotein S antibodies followed by SDS-PAGE and autoradiography were performed as described by Wu et al.30
Determination of the extent of γ-carboxylation of secreted protein S
The selective precipitation of Gla proteins in conditioned media by barium citrate was performed as described by Berkner.31Conditioned media were treated with sodium citrate (20 mmol/L) and BaCl2 (40 mmol/L) and incubated on ice for 1 hour. The precipitate was collected by centrifugation, washed in 100 mmol/L BaCl2/100 mmol/L NaCl, and resolubilized in phosphate-buffered saline containing 150 mmol/L sodium citrate and 0.1% bovine serum albumin. Supernatants and resolubilized precipitates were further analyzed by Western blotting as described previously.8
Northern blotting
Total RNA was extracted using an Rneasy RNA extraction kit (Qiagen, UK). Hybridization was performed using a human protein S complementary DNA probe, kindly provided by Professor Bono Bouma (University of Utrecht, The Netherlands) that was labeled with [α-32P]-dCTP as we described previously.27Membranes were stripped and reprobed with a G6PD complementary DNA probe to normalize for equal loading, and autoradiograms were densitometrically scanned.
Quantification and cofactor activity assays
Secreted protein S was quantified using an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostica-Stago, France) according to the manufacturer's instructions, except that purified protein S was used as a calibrator in place of the supplied serum standard. Conditioned media were treated with a cocktail of protease inhibitors (Complete™, EDTA-free, Roche Diagnostics, UK) and concentrated 10-fold using 3000 Da molecular weight cut-off Microcon 3 microconcentrators (Amicon, USA) prior to being assessed for their protein S content. The functional activity of cell-secreted protein S was determined using a “Statclot” clotting assay kit (Diagnostica-Stago) according to the manufacturer's instructions, except that purified protein S was used for the construction of the calibration curve. Concentrated conditioned media were buffer-exchanged in phosphate-buffered saline, and aliquots were assessed for their cofactor activity expressed as a prolongation in clotting time. Average values from four determinations expressed as prolongation in clotting times obtained with ng quantities of purified protein S in the calibration curve were as follows: 25 ng, 12 ± 4 seconds; 50 ng, 22 ± 3 seconds; 100 ng, 37 ± 4 seconds; and 200 ng, 53 ± 2 seconds).
Results
Synthesis and secretion of protein S by cultured HVSMCs
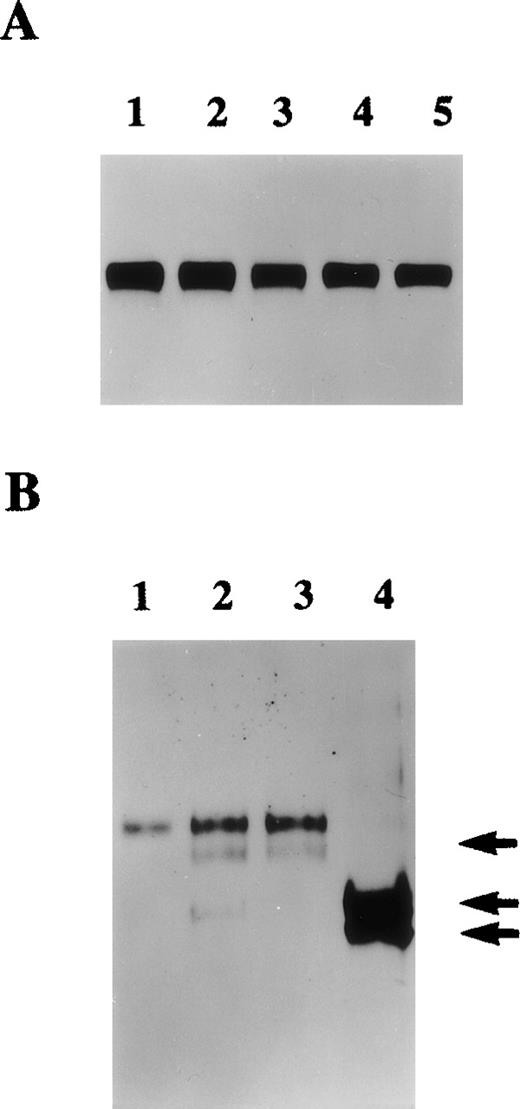
The presence of protein S in cultured HVSMC-conditioned media was first analyzed by Western blotting. Figure1 shows that both growth-arrested and growing cells secrete protein S that is detected as a single band of 70 kd. This 70-kd protein was specifically recognized by both anti-protein S monoclonal (HSP41 and HSP21)8 and a rabbit polyclonal antibody. The results shown in Figure 1 were obtained with the polyclonal antibody that was used subsequently in our study. The above antibodies did not detect purified human Gas6 that is also known to be produced by VSMCs in culture. The possibility that protein S detected in HVSMC-conditioned media could be a serum contaminant was ruled out. Metabolic labeling experiments were performed in which HVSMCs were labeled with a mixture of [35S]-methionine and [35S]-cysteine for 24 hours after which conditioned media were collected and subjected to immunoprecipitation followed by SDS-PAGE and autoradiography (data not shown). These experiments confirmed the data obtained by Western blotting, as similar amounts of a single 70-kd [35S]-labeled protein were detected in both growing and growth-arrested HVSMC conditioned media.
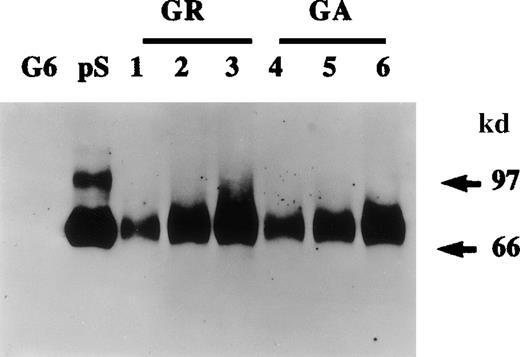
Protein S production by growing and growth-arrested human vascular smooth muscle cells (HVSMCs).
Protein S production was assessed in conditioned media. Two cell culture conditions were used: growth-arrested (GA) and growing (GR) cells. Growth-arrested cells were obtained by transferring 90% confluent cultures into serum-free Dulbecco's modified Eagle's medium (DMEM) for 48 hours prior to the commencement of media conditioning. Growing cells, were subconfluent cultures (60% confluency) that were placed in a defined growth promoting serum-free medium at the start of media conditioning. Conditioned media were collected after 24 hours (lanes 1, 4), 48 hours (lanes 2, 5), and 72 hours (lanes 3, 6) and were analyzed by Western blotting using a rabbit anti-protein S antibody. Purified 1 ng protein S (pS) and 50 ng recombinant human growth arrest specific protein 6 (G6) were included as controls.
Protein S production by growing and growth-arrested human vascular smooth muscle cells (HVSMCs).
Protein S production was assessed in conditioned media. Two cell culture conditions were used: growth-arrested (GA) and growing (GR) cells. Growth-arrested cells were obtained by transferring 90% confluent cultures into serum-free Dulbecco's modified Eagle's medium (DMEM) for 48 hours prior to the commencement of media conditioning. Growing cells, were subconfluent cultures (60% confluency) that were placed in a defined growth promoting serum-free medium at the start of media conditioning. Conditioned media were collected after 24 hours (lanes 1, 4), 48 hours (lanes 2, 5), and 72 hours (lanes 3, 6) and were analyzed by Western blotting using a rabbit anti-protein S antibody. Purified 1 ng protein S (pS) and 50 ng recombinant human growth arrest specific protein 6 (G6) were included as controls.
The quantities of protein S made by HVSMCs in culture were estimated by both ELISA and Western blotting. From such experiments, it was estimated that HVSMC cultures secreted on average 40 ± 0.3 ng of protein S/106 cells/24 h. The functional cofactor activity of HVSMC-secreted protein S was investigated using the “Statclot” protein S clotting assay and 10-fold concentrated HVSMC-conditioned media obtained after a 72-hour incubation in serum-free DMEM. A prolongation of 18 ± 3 seconds in clotting activity was obtained with three separate concentrated conditioned media samples each containing 50 ng of HVSMC-derived protein S as estimated by ELISA. As a comparison, in this assay, the prolongation in clotting observed with 50 ng purified human protein S was 22 ± 3 seconds (n = 4). Because the γ-carboxylation of protein S is required for its cofactor activity,1 it may be deduced that HVSMC-secreted protein S had been fully processed.
Relationship between protein S synthesis and secretion and its γ-carboxylation state
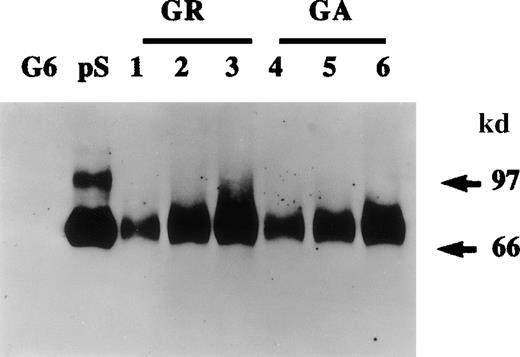
Growth-arrested HVSMCs in serum-free medium were treated with either vitamin K (1 μg/mL), warfarin (1 μg/mL), or diluent for 48 hours after which the presence of protein S in conditioned media and cell lysates was analyzed by Western blotting. Figure2A shows that warfarin treatment resulted in a drastic decrease in the levels of protein S detected in HVSMC-conditioned media that was paralleled by its intracellular accumulation, hence implying that warfarin prevents protein S secretion. It should be noted here that several cell types in culture take up vitamin K from serum and have the capacity to recycle and reuse it.31 32 Nevertheless, as an additional control, we have added exogenous vitamin K to cells kept in serum-free medium. As expected, the addition of extra exogenous vitamin K had no significant effect on the secretion or intracellular expression of protein S as compared with control cells.
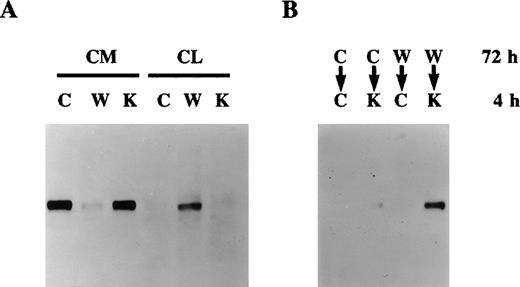
Effect of warfarin on protein S secretion.
A. Human vascular smooth muscle cell (HVSMC) cultures at 90% confluency were incubated for 72 hours in control serum-free medium (C) or medium containing warfarin (1 μg/mL) (W) or vitamin K (1 μg/mL) (K). Conditioned media (CM) and cell lysates (CL) were analyzed by Western blotting using a rabbit anti-protein S antibody. B. HVSMC cultures at 90% confluency were incubated first for 72 hours with either control medium (C) or medium containing warfarin (1 μg/mL) (W); cells were then placed in new control medium (C) or medium containing vitamin K (10 μg/mL) (K), which was collected after 4 hours and analyzed by Western blotting using a rabbit anti-protein S antibody.
Effect of warfarin on protein S secretion.
A. Human vascular smooth muscle cell (HVSMC) cultures at 90% confluency were incubated for 72 hours in control serum-free medium (C) or medium containing warfarin (1 μg/mL) (W) or vitamin K (1 μg/mL) (K). Conditioned media (CM) and cell lysates (CL) were analyzed by Western blotting using a rabbit anti-protein S antibody. B. HVSMC cultures at 90% confluency were incubated first for 72 hours with either control medium (C) or medium containing warfarin (1 μg/mL) (W); cells were then placed in new control medium (C) or medium containing vitamin K (10 μg/mL) (K), which was collected after 4 hours and analyzed by Western blotting using a rabbit anti-protein S antibody.
The inhibition of protein S secretion by warfarin is a reversible process. Indeed, Figure 2B shows that, if cells that had been treated with warfarin for 72 hours were switched to a medium containing a 10-fold excess of vitamin K, intracellularly accumulated protein S was released within 4 hours. No detectable protein S was found in conditioned media collected over a 4-hour period from either control cells or cells previously treated with warfarin and then placed in control medium, thus excluding the possibility that the protein S detected on the reintroduction of vitamin K was due to new synthesis and secretion. The possibility that the observed inhibition of protein S secretion by warfarin was due to an overall nonspecific inhibition of total protein secretion was ruled out. Indeed, HVSMC cultures were metabolically labeled with [35S]-methionine and [35S]-cysteine in the presence of either vitamin K, warfarin, or control for 48 hours. No differences in the total protein secretion were observed between control, vitamin K-, or warfarin-treated cells (data not shown). Taken together, data presented in Figures 2A and 2B demonstrate that undercarboxylated protein S accumulates intracellularly in HVSMCs, implying that its γ-carboxylation is a prerequisite for its secretion.
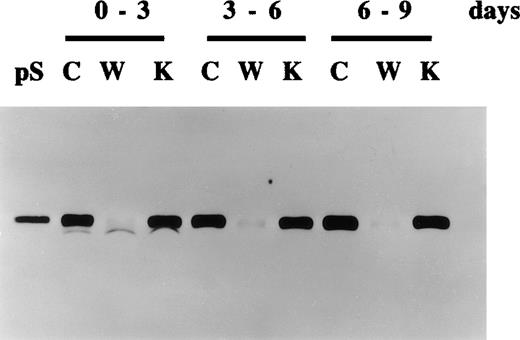
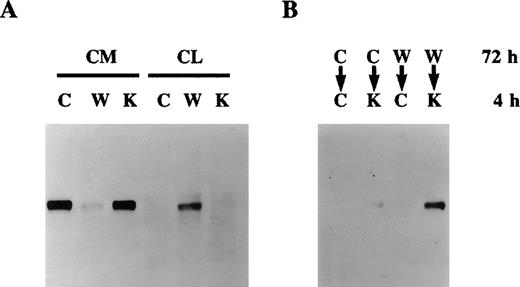
Further experiments were performed to study the secretion of protein S and its inhibition by warfarin in growth-arrested HVSMC over longer periods of time. Figure 3 shows that HVSMCs continue to secrete protein S for up to at least 9 days following their growth arrest in serum-free medium. As expected, vitamin K addition did not significantly change the levels of protein S secretion, confirming that vitamin K was recycled by the cells and hence is not a rate-limiting factor for protein S γ-carboxylation. The inhibition of protein S secretion by warfarin was also consistently observed over this period.
Continued production of protein S by human vascular smooth muscle cells (HVSMCs) following extended periods of growth arrest.
HVSMC cultures at 90% confluency were placed in either control serum-free medium (C), medium containing vitamin K (1 μg/mL) (K), or warfarin (1 μg/mL) (W). Conditioned media were replaced every 3 days and were analyzed by Western blotting using a rabbit anti-protein S polyclonal antibody. pS represents 1 ng purified protein S.
Continued production of protein S by human vascular smooth muscle cells (HVSMCs) following extended periods of growth arrest.
HVSMC cultures at 90% confluency were placed in either control serum-free medium (C), medium containing vitamin K (1 μg/mL) (K), or warfarin (1 μg/mL) (W). Conditioned media were replaced every 3 days and were analyzed by Western blotting using a rabbit anti-protein S polyclonal antibody. pS represents 1 ng purified protein S.
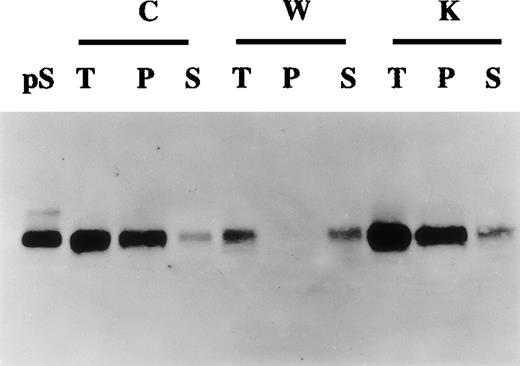
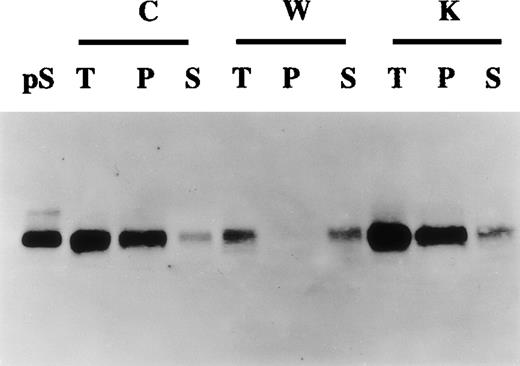
Gamma-carboxylated proteins are selectively precipitated by barium citrate.31 To study further the relation between protein S secretion and its state of γ-carboxylation, barium citrate precipitation of conditioned media experiments was undertaken. Figure4 shows that in control and vitamin K-treated cells most of the secreted protein S was recovered in the barium citrate precipitate fraction, inferring that this protein S was γ-carboxylated. In contrast, in warfarin-treated cells, the small amount of secreted protein S was not precipitated by barium citrate, confirming that, in the presence of warfarin, protein S remained undercarboxylated. Accordingly, when barium citrate precipitation was performed on cell lysates of warfarin-treated cells, protein S was exclusively found in the nonprecipitable fraction (data not shown). Altogether the data presented above strongly suggest that HVSMCs synthesize and secrete fully γ-carboxylated and functional protein S.
Production of γ-carboxylated protein S by human vascular smooth muscle cells (HVSMCs).
Conditioned media collected from 90% confluent HVSMC cultures maintained in either control (C), warfarin (1 μg/mL) (W), or vitamin K (1 μg/mL) (K) for 72 hours were subjected to barium citrate precipitation as described in “Materials and methods.” Aliquots from total unprecipitated (T), barium citrate precipitated (P), and nonprecipitable supernatant fractions (S) were analyzed by Western blotting using an anti-protein S polyclonal antibody. Protein S (1 ng) (pS) was used as a control.
Production of γ-carboxylated protein S by human vascular smooth muscle cells (HVSMCs).
Conditioned media collected from 90% confluent HVSMC cultures maintained in either control (C), warfarin (1 μg/mL) (W), or vitamin K (1 μg/mL) (K) for 72 hours were subjected to barium citrate precipitation as described in “Materials and methods.” Aliquots from total unprecipitated (T), barium citrate precipitated (P), and nonprecipitable supernatant fractions (S) were analyzed by Western blotting using an anti-protein S polyclonal antibody. Protein S (1 ng) (pS) was used as a control.
Regulation of protein S expression
In Northern blotting experiments, using a protein S complementary DNA probe, the expression of protein S transcript in HVSMCs was analyzed, and a single transcript of around 4 kB was detected. In Table1, the ratio of protein S to the housekeeping gene G6PD messenger RNA (mRNA) is represented. HVSMC cultures were either placed in 10% FCS containing medium (growing) or 0.5% FCS (growth arrested). Thymidine incorporation experiments confirmed that under these conditions, whereas cells in the presence of 10% FCS continued to grow and undergo cycles of DNA synthesis, those placed in 0.5% FCS become growth arrested (data not shown) that is in agreement with previous studies.27,28 33 The level of expression of the protein S transcript did not show any significant variation between growing as compared with growth-arrested cells, confirming the observations made at the protein level seen in Figure 1. Neither treatment of cells for 24 or 48 hours with warfarin or vitamin K resulted in any significant changes in protein S transcript levels. These data indicate that protein S mRNA levels are unaltered by the growth state of the cells or their vitamin K status.
The possible regulation of protein S expression by some VSMC mitogens (at concentrations known to induce maximal biological activity in HVSMCs)27,28,33-35 was investigated. Growth-arrested HVSMCs in serum-free medium were treated with either PDGF-BB (10 ng/mL), b-FGF (5 ng/mL), transforming growth factor beta-1 (5 ng/mL), hepatocyte growth factor (20 ng/ml), or α-thrombin (0.5-5 U/mL) for 24 or 48 hours. In agreement with previous studies under these conditions, cells responded mitogenically to the factors above27,28,33-35(data not shown). The strongest mitogenic effect was observed with PDGF-BB that induced a 7.8-fold increase in the level of DNA synthesis as estimated by [3H]-TdR incorporation. Treatment of HVSMCs with either PDGF-BB, b-FGF, hepatocyte growth factor, or transforming growth factor beta-1 led to no significant changes in the level of protein S secreted, shown after a 48-hour incubation (Figure5A). However, as depicted in Figure 5B, the serine proteinase, α-thrombin, which is also a potent HVSMC mitogen,27 led to both a significant increase in the level of protein S secretion and its cleavage that was more pronounced when 5 U/mL thrombin was used.
Regulation of protein S synthesis by growth factors and thrombin.
A. Conditioned media collected from 90% confluent human vascular smooth muscle cell (HVSMC) cultures maintained in serum-free medium for 48 hours in the presence of either control (lane 1), 5 ng/mL basic fibroblast growth factor (b-FGF) (lane 2), 2 ng/mL transforming growth factor beta-1 (TGF-β)1 (lane 3), 5 ng/mL hepatocyte growth factor (HGF) (lane 4), or 5 ng/mL platelet-derived growth factor-BB (PDGF-BB) (lane 5) were analyzed by Western blotting using an anti-protein S polyclonal antibody. B. Conditioned media were collected from 90% confluent cultures maintained in serum-free medium for 24 hours in the presence of either control (lane 1), α-thrombin at either 0.5 U/mL (lane 2), 1 U/mL (lane 3), or 5 U/mL (lane 4). Conditioned media were analyzed by Western blotting using an anti-protein S polyclonal antibody. Arrows indicate cleaved forms of protein S.
Regulation of protein S synthesis by growth factors and thrombin.
A. Conditioned media collected from 90% confluent human vascular smooth muscle cell (HVSMC) cultures maintained in serum-free medium for 48 hours in the presence of either control (lane 1), 5 ng/mL basic fibroblast growth factor (b-FGF) (lane 2), 2 ng/mL transforming growth factor beta-1 (TGF-β)1 (lane 3), 5 ng/mL hepatocyte growth factor (HGF) (lane 4), or 5 ng/mL platelet-derived growth factor-BB (PDGF-BB) (lane 5) were analyzed by Western blotting using an anti-protein S polyclonal antibody. B. Conditioned media were collected from 90% confluent cultures maintained in serum-free medium for 24 hours in the presence of either control (lane 1), α-thrombin at either 0.5 U/mL (lane 2), 1 U/mL (lane 3), or 5 U/mL (lane 4). Conditioned media were analyzed by Western blotting using an anti-protein S polyclonal antibody. Arrows indicate cleaved forms of protein S.
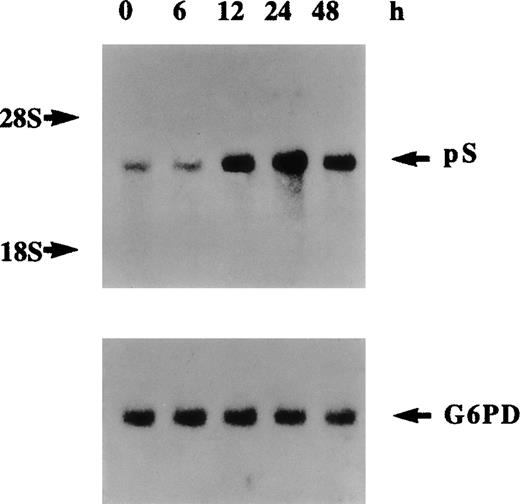
The cleavage of various forms of plasma-derived and recombinant protein S by thrombin has been extensively studied previously.9,36Three potential thrombin-sensitive sites have been identified within the thrombin-sensitive region of human protein S at Arg49, Arg60, and Arg70. Cleavage at one or more of these sites results in the formation of a lower molecular weight protein that corresponds to the band indicated by the top arrow in Figure 5B at about 68 kd. More extensive proteolytic cleavage of protein S by higher concentrations of thrombin was previously9 demonstrated to result in the formation of a protein band of 54 kd that corresponds to the protein indicated by the middle arrow in Figure 5B. A third band of about 50 kd was also detected, indicated by the lower arrow in Figure 5B, that could have resulted from further proteolytic cleavage after exposure of other thrombin-sensitive sites or alternatively via cleavage by other cell secreted thrombin-induced proteases. Figure 6 represents Northern blotting analysis of thrombin effects on protein S transcript expression. A marked increase in protein S mRNA levels was evident at 12 hours, peaked at 24 hours, and then declined at 48 hours after thrombin addition (5 U/mL) to growth-arrested HVSMCs. Therefore, it can be concluded that the observed increase in the level of protein S secretion by thrombin is accompanied by an up-regulation of protein S gene expression and of mRNA stabilization.
Northern blot analysis of regulation of protein S messenger RNA expression by thrombin.
Human vascular smooth muscle cells (HVSMCs) at 90% confluency were incubated with α-thrombin (5 U/mL) for the indicated times. Total RNA was electrophoresed on 1.2% agarose gel, transferred to a nylon membrane, and sequentially hybridized with 32P-labeled protein S and G6PD complementary DNA probes.
Northern blot analysis of regulation of protein S messenger RNA expression by thrombin.
Human vascular smooth muscle cells (HVSMCs) at 90% confluency were incubated with α-thrombin (5 U/mL) for the indicated times. Total RNA was electrophoresed on 1.2% agarose gel, transferred to a nylon membrane, and sequentially hybridized with 32P-labeled protein S and G6PD complementary DNA probes.
Discussion
In this report, it is demonstrated that cultured HVSMCs synthesize and secrete the anticoagulant factor protein S. Warfarin inhibition of the γ-carboxylation process attenuates protein S secretion resulting in its intracellular accumulation. On the reestablishment of a normal vitamin K status, this intracellular pool of protein S is rapidly γ-carboxylated and secreted. Barium citrate precipitation experiments together with the assessment of protein S cofactor activity in conditioned media provided further confirmation that protein S secreted by HVSMCs is effectively γ-carboxylated. In contrast to Gas6, the expression of which was shown to be up-regulated by serum withdrawal in fibroblasts,18 the secretion of protein S in HVSMCs was similar in both growing and growth-arrested cells. Additionally, Nakano et al20 have shown that Gas6 continues to be secreted by rat VSMCs treated with warfarin. Therefore, the mechanisms that regulate the secretion of Gas6 and protein S appear to differ. Cellular, structural, as well as species differences have been shown to mediate the secretory or degradation responses of Gla proteins to warfarin.30,37 For example, in rat H-35 cells, warfarin treatment causes an almost complete cessation of prothrombin secretion with enhanced degradation of the intracellular pools,37whereas in human HepG2 cells undercarboxylated prothrombin is secreted in the presence of warfarin.30 When rat prothrombin was transfected into warfarin-treated HepG2 cells, it was found to accumulate intracellularly, indicating that the retention and degradation of prothrombin by human hepatocytes is related to structural determinants within the rat protein.30
The production of protein S by some fully differentiated cells11-17 and as shown here by HVSMCs taken together with evidence previously provided for its mitogenic activity25,26 raise the possibility that locally produced protein S may have local anticoagulant activity or other as yet unknown functions. Several investigators22-24,38 have demonstrated that Gas6 prevents cell death by apoptosis. Melaragno et al39 showed in a rat model that both Gas6 and its tyrosine kinase receptor Axl are strongly up-regulated in injured blood vessels, suggesting an important role for this homologue of protein S in vascular injury and repair. The sustained production of protein S by cultured HVSMCs even several days after growth arrest may suggest a role for this protein in cell survival. Protein S deficiency was reported to be associated with osteopenia, osteonecrosis, and vascular calcification.40-42 Locally produced protein S may also be involved in vascular tissue calcification, evident in advanced atherosclerotic lesions.43
The up-regulation of protein S expression by thrombin in HVSMCs may be of crucial importance for both hemostasis and vascular repair processes. Previous reports have indicated that thrombin is generated during blood clotting at concentrations of up to 130 nmol/L, is actively incorporated into clots, and is released during clot retraction and fibrinolysis.44 Therefore, the concentration of thrombin (40 nmol/L) found here to result in optimal up-regulation of protein S expression would be expected to be present at sites of vascular injury. Thrombin also causes protein S cleavage, thus rendering it inactive as an anticoagulant.9 Because putative cellular functions for thrombin-cleaved protein S have not been determined as yet, the significance of the observed protein S cleavage by thrombin remains to be unveiled. It will be of particular interest to investigate whether the up-regulation of protein S by thrombin observed in VSMCs also occurs in hepatocytes that are the main known producer cells of factors of the coagulation system, including protein S.3 Scarpati and DiCorletto45 reported the presence of a thrombin responsive element within the PDGF-B gene promoter that consists of a repeat of CCACCC in an ABBA configuration. Our computer search has revealed the existence of this putative thrombin responsive consensus sequence within the protein S gene promoter region. Functional studies of these putative thrombin responsive elements should lead to the identification of the transcription factors specifically activated by thrombin in VSMCs and that might mediate the observed up-regulation of protein S gene expression.
Acknowledgments
The authors are grateful to Professor Bjorn Dahlbäck, Professor Bono Bouma, and Dr Brian Varnum for providing monoclonal antibodies to protein S, a protein S complementary DNA probe, and human recombinant Gas6 protein, respectively. The authors are also grateful to Professor Fedor Bachmann and Dr Vincent Ellis for critical reading of the manuscript.
Supported by the British Heart Foundation PG95/138 and the Thrombosis Research Trust. Salary for O.B. was supported in part by the Gary Weston Foundation.
C.K.'s present address is Tumour Microcirculation Group, PO Box 100, Mount Vernon Hospital, Northwood, Middlesex HA6 2JR, UK; e-mail: kanthou@graylab.ac.uk.
Reprints:Chryso Kanthou, Tumour Microcirculation Group, PO Box 100, Mount Vernon Hospital, Northwood, Middlesex HA6 2JR UK; e-mail:kanthou@graylab.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.