Abstract
Despite being a well-characterized neurotrophic factor, nerve growth factor (NGF) influences survival, differentiation, and functions of mast cells. We investigated whether NGF was able to induce directional migration of rat peritoneal mast cells (PMCs). NGF clearly induced chemotactic movement of PMCs in a dose-dependent manner with the drastic morphological change and distribution of F-actin, which was completely blocked by pretreatment with Clostridium botulinumC2 toxin, an actin-polymerization inhibitor. Because PMCs constitutively express the NGF high-affinity receptor (TrkA) with a tyrosine kinase domain, we focused on downstream effectors in signaling cascades following the TrkA. NGF rapidly activated both mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K), and the addition of inhibitors specific for MAPK kinase and PI3K suppressed cell migration and these signals. In the coculture system with PMCs and fibroblasts, which produce biologically active NGF, directional migration of PMCs to fibroblasts was observed, and the addition of anti-NGF polyclonal antibodies significantly suppressed the migration of PMCs. These findings suggested that NGF initiated chemotactic movement of PMCs through both MAPK and PI3K signaling pathways following TrkA activation. Thus, locally produced NGF may play an important role in mast cell accumulation in allergic and nonallergic inflammatory conditions.
Numerous mast cells accumulate at local tissues for various conditions such as wound healing, tumors, host defense responses against helminth parasites and ectoparasites, and acute and chronic allergic disorders.1-4 Proliferation and differentiation of mast cells are regulated by some growth factors from at least 2 distinct cell populations: T cells (interleukin 3 [IL-3], IL-4, IL-9, and IL-10)5-8 and fibroblasts (stem cell factor [SCF] and nerve growth factor [NGF]).9-11 In several in vitro studies,12-15 IL-3, SCF, and other factors, such as transforming growth factor-β, monocyte chemotactic protein, and anaphylatoxins C3a and C5a, were found to induce mast cell migration. However, exact mechanisms of mast cell recruitment to the affected sites have been poorly understood. In prior studies, we demonstrated that NGF is able to promote proliferation of murine bone marrow–derived cultured mast cells, induce a phenotypic change to connective tissue–type mast cells,11 and support survival by preventing apoptosis of rat peritoneal mast cells (PMCs).16 Stimulation with NGF leads to histamine release from rat PMCs in the presence of lysophosphatidylserine17,18 as well as human basophils.19 NGF is also produced from newly generated keratinocytes and fibroblasts in cutaneous wounds.20 These experimental findings give rise to possible multifunctional properties of NGF on mast cells as well as neuronal tissues. Although NGF can lead to chemotactic migration of polymorphonuclear leukocytes in vivo21 and in vitro,22 there is no evidence to demonstrate effects of NGF on mast cell migration.
NGF is a target-derived neurotrophic factor that is necessary for the survival, development, and functions of peripheral and central neurons.23-25 Biological actions of NGF are mediated through 2 types of specific receptors with distinct affinities26,27: 75-kd glycoprotein (p75LNGFR) and a 140-kd molecule with a transmembrane tyrosine kinase domain that is coded by the trk proto-oncogene28 (TrkA). Autophosphorylation of TrkA leads to some second messenger cascades including activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K), which eventually induce gene expression in neuronal cells.29,30 Although freshly isolated PMCs express TrkA but not p75LNGFR on the surface of the membrane,16 signal cascades in the postreceptor process have not been clearly defined. In the present study we demonstrate that (1) stimulation of rat PMCs with NGF induced rapid activation of both MAPK and PI3K signals, following tyrosine phosphorylation of the TrkA protein, leading to their chemotactic migration with polymerization of actin filaments and (2) biologically active NGF produced by 3T3 fibroblasts actually induced migration of rat PMCs.
Materials and methods
Isolation of PMCs
Outbred male Sprague-Dawley rats (8-16 weeks of age) (Clea Japan, Tokyo, Japan) were kept in our laboratory more than 1 week before they were sacrificed. PMCs were obtained by using a modification of the technique described previously.16 Briefly, rats were injected intraperitoneally with 50 mL phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA), and the peritoneal lavage fluids were recovered after abdominal massage for 3 minutes. A 10-mL cell suspension was layered in α-MEM (Modified Eagle's Medium) with 1% BSA on 15 mL 75% Percoll solution (Pharmacia Biotech, Uppsala, Sweden) and centrifuged at 600g for 25 minutes at room temperature. Sedimented PMCs (greater than 95% purity and 98% viability) were washed twice and resuspended at a concentration of 1 × 105 cells/mL in α-MEM containing 1% BSA, 50 U/mL penicillin, and 50 μg/mL streptomycin (assay medium).
Cytokines and other reagents
A preparation of 2.5S NGF isolated from murine submaxillary glands (gift from Drs A. M. Stanisz and J. Bienenstock, McMaster University, Hamilton, Ontario, Canada) was further purified by affinity column chromatography with antimouse NGF monoclonal antibody (mAb). The preparation was free of epidermal growth factor activity and endotoxin activity even at a high concentration (10 μg/mL). Neurotrophic activity of the affinity-purified NGF preparation was determined as previously described.11 Rat recombinant SCF (rSCF) was a gift from Amgen (Thousand Oaks, CA). Polyclonal antibodies (pAbs) included rabbit antimouse NGF (Sigma Chemical, St Louis, MO), and rabbit antimouse SCF (Genzyme, Cambridge, MA). Clostridium botulinum C2 toxin (Dr I. Ohishi, Nippon Veterinary and Animal Science University, Tokyo, Japan) consists of 2 dissimilar protein components, components I and II.31 We also used rabbit antirat MAPK/extracellular signal-regulated kinase (ERK)1/2, rabbit antirat PI3K, and p85 subunit pAbs and mouse antimouse phosphotyrosine mAb conjugated with horseradish peroxidase (Upstate Biotechnology, Lake Placid, NY); phosphospecific MAPK pAb (New England Biolabs, Beverly, MA); and goat antirabbit immunoglobulin G (IgG) pAb conjugated with horseradish peroxidase (Sigma Chemical). Unless otherwise indicated, all chemicals were purchased from Sigma Chemical.
Chemotaxis assay
Various concentrations of NGF (500 μL) or the assay medium alone were applied into each well of 24-well culture plates (Nalge Nunc International, Roskilde, Denmark). After 10-mm tissue culture inserts (Nalge Nunc International) were placed into each well, 5 × 104 PMCs (500 μL) were added into each insert. The lower compartment of the well was separated from the cell suspension in the upper compartment with an 8-μm pore-size polycarbonate membrane of the culture inserts. PMCs were incubated for 3 hours at 37°C in a humidified atmosphere flushed with 5% carbon dioxide (CO2) in air. Following aspiration of nonadherent PMCs in the upper compartment, cells adherent to the upper surface of the membrane were removed by scraping with a rubber blade. Migrated cells adherent to the lower surface of the membrane were fixed with methanol for 5 minutes and stained with 0.5% toluidine blue. The membranes were mounted on glass slides by routine histological methods. The total number of mast cells that migrated across the membrane was counted under a light microscope. In some experiments, rabbit anti-NGF (1:500 dilution) or anti-SCF (5 ng/mL) pAb was added to 50 ng/mL NGF or 1 ng/mL rSCF in the lower compartments.
Atomic force microscopy
We applied 50 ng/mL NGF or the assay medium alone into 24-well culture plates, and then culture inserts were placed into each well. A total volume of 500 μL cell suspension was added in each culture insert. After incubation for 3 hours, the polycarbonate membrane was fixed with 3% paraformaldehyde/PBS for 15 minutes. Surface structure of migrating cells on the membrane was examined with an atomic force microscope (SPM-9500; Shimadzu, Kyoto, Japan).
Pretreatment of Clostridium botulinum C2 toxin
PMCs (2 × 106 cells/mL) were preincubated for 2 hours with or without 300 ng/mL C botulinum C2toxin (component I, 100 ng/mL; component II, 200 ng/mL) in α-MEM containing 1% BSA. C botulinum C2toxin has an adenosine 5′-diphosphate–ribosyl (ADP-ribosyl) transferase activity to nonmuscle actin monomer at arginine 177 (Arg-177), which results in the inhibition of actin filament assembly.32 The pretreated cells were resuspended in the assay medium and allowed to demonstrate the chemotactic ability of NGF.
Detection of polymerized actin (F-actin) was determined in PMCs migrating toward the lower side of the membrane according to the method described by Pfeiffer and Oliver.33 Briefly, PMCs were preincubated with or without C botulinum C2 toxin for 2 hours and seeded into each culture insert for chemotaxis assay or into each well of 6-well culture plates (Nalge Nunc International) for flow cytometric analysis. After stimulation with NGF for 1 or 2 hours, PMCs were fixed with 3% paraformaldehyde/PBS for 1 hour at room temperature, washed 3 times with PBS, and permeabilized with 1% Triton X-100/PBS for 15 minutes. The preparations were stained for 30 minutes with 1 U/mL Oregon Green 488-phalloidin (Molecular Probes, Eugene, Oregon) at room temperature. All specimens were examined with a confocal laser scanning microscope (TCS NT; Leica Microscope, Heidelberg, Germany) using an argon ion laser, which is capable of excitation at 488 nm, and analyzed by flow cytometry (EPICS XL; Beckman Coulter, Fullerton, CA).
Pretreatment of PMCs with signal transduction inhibitors
Before being applied to a chemotaxis assay, PMCs were preincubated for 1 hour with the following inhibitors: 50 ng/mL K-252a (Calbiochem-Boehring, La Jolla, CA), a TrkA inhibitor34,35; 100 μmol/L PD98 059 (New England Biolabs, Beverly, MA), a MAPK/ERK kinase 1 inhibitor36; or 50 μmol/L LY294 002 (Calbiochem-Boehring), a PI3K inhibitor.37 The PMCs were pretreated with inhibitors or a diluent solution (0.5% dimethylsulfoxide assay medium), resuspended in the assay medium, and allowed to demonstrate chemotactic ability of NGF.
Western blot analysis of tyrosine phosphorylated protein
Freshly isolated PMCs (1-2 × 106 cells) were incubated for 4 hours in α-MEM with 1% BSA in 60-mm plastic culture dishes (Nalge Nunc International). This was followed by pretreatment with or without the signal transduction inhibitors for 1 hour at 37°C in a humidified atmosphere flushed with 5% CO2 in air. After treatment with 50 ng/mL NGF for 5 minutes or 30 minutes, the cells were washed twice with ice-cold PBS. The cells were lysed in buffer containing 50 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane hydrochloride; pH 7.4), 1% Triton X-100, 150 mmol/L NaCl (sodium chloride), 1% nonidet P-40 (NP-40), 1 mmol/L Na3VO4, 1 mmol/L C8H10FNO2S/HCl, and 1 μg/mL aprotinin, then frozen and thawed 3 times. Lysates were centrifuged at 15 000 rpm for 20 minutes, and supernatants were incubated with anti-MAPK/ERK1/2 or anti-PI3K pAb conjugated with protein-A beads (Sepharose CL-4B, Pharmacia Biotech) at 4°C overnight with gentle rotation. After washing with lysis buffer twice, the immunocomplex was resuspended in 50 μL of electrophoresis buffer containing 250 mmol/L Tris-HCl (pH 6.8), 20% glycerol, 5% SDS (sodium dodecyl sulfate), 5% 2-mercaptoethanol, 12% urea, and 0.1% bromophenol blue and boiled for 5 minutes. Samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and then electrically transferred to a membrane (Immobilon-P; Millipore, Bedford, MA). For detection of phosphorylated MAPK, the membrane was immunoblotted with phosphospecific MAPK pAb (1:1000 dilution) at 4°C overnight and followed to reincubate with horseradish peroxidase–conjugated antirabbit IgG pAb. For detection of phosphorylated PI3K, the membrane was immunoblotted with horseradish peroxidase–conjugated antiphosphotyrosine mAb at a concentration of 1 μg/mL for 1 hour at room temperature. The phosphorylated protein products were visualized with an enhanced chemiluminescent detection reagent (Amersham, Arlington Heights, IL), and the images were analyzed (Gel Print 200i/VGA and Basic Quantifier; Genomic Solutions, Ann Arbor, MI) to determine their relative intensity.
3T3 fibroblast-derived chemotactic activity
A 3T3-Swiss albino fibroblast line (Japanese Cancer Research Resources Bank, Tokyo, Japan) was maintained in DMEM supplemented with 10% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin at 37°C in a humidified atmosphere flushed with 5% CO2 in air. A total of 5 × 105 3T3 fibroblasts was seeded in 1 mL of culture medium in 24-well culture plates. The culture medium was removed 2 days later, and a confluent monolayer of 3T3 fibroblasts was washed gently 3 times. After 500 μL of the assay medium supplemented with anti-NGF and/or anti-SCF pAb or control pAb was applied onto the monolayer, culture inserts were placed into each well. PMCs were added into each culture insert, and then a chemotaxis assay was carried out as described previously. In another experimental group, the confluent 3T3 fibroblasts were fixed with 1% paraformaldehyde before a chemotaxis assay.
Statistical analysis
Student's t test was performed for statistical analysis of the data, and P < .05 was taken as the level of significance.
Results
Migration of PMCs induced by NGF
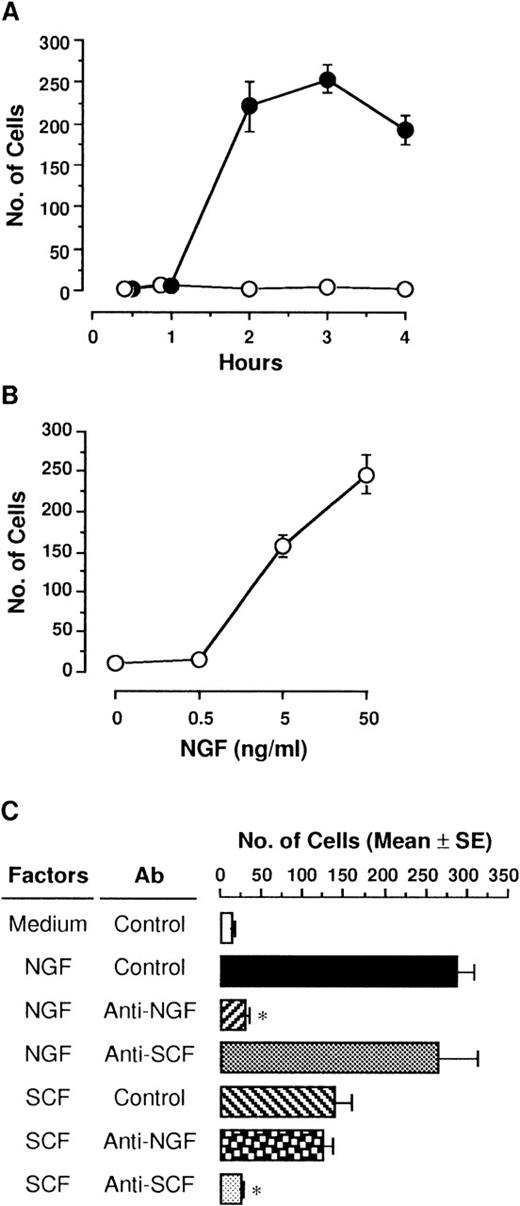
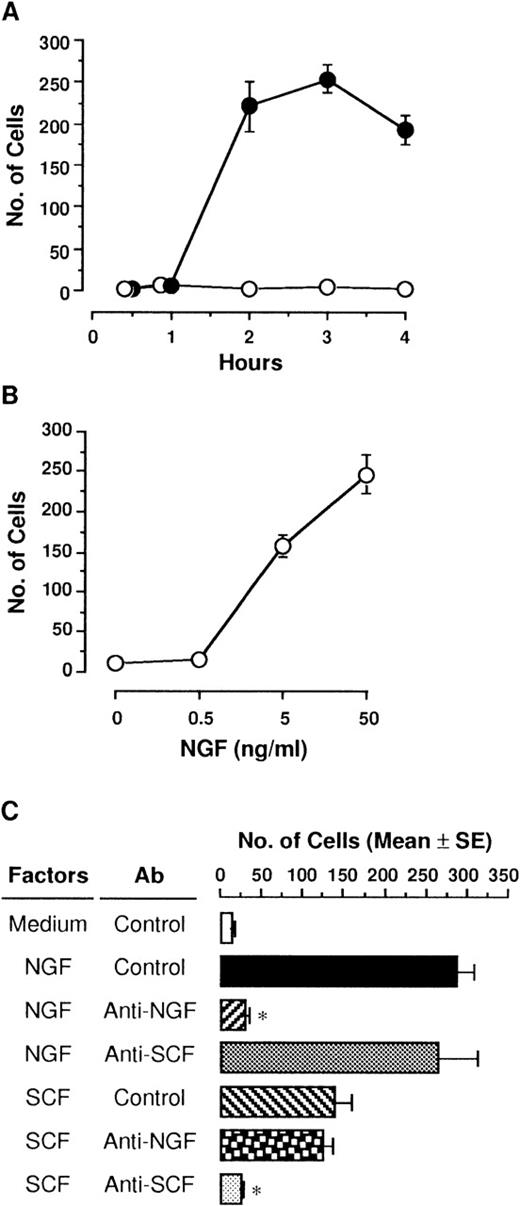
NGF (50 ng/mL) was placed in the lower compartment, and then PMCs were incubated for various hours in the upper compartment. PMCs that migrated toward the lower surface of the polycarbonate membrane through 8-μm pores were markedly increased at 2 hours, and 3 hours later, the maximum number of 254 cells was reached (Figure1A). PMCs were still migrating 4 hours later, but cells detached from the membrane toward the lower compartment were detected. In contrast, medium alone without NGF had no effect on migration of PMCs in culture for up to 4 hours. Various concentrations of NGF (0.5, 5, and 50 ng/mL) were applied in the lower compartment, and then PMCs were incubated for 3 hours in the upper compartment (Figure 1B). The addition of NGF resulted in a significant increase in the number of migrated PMCs in a dose-dependent manner; the minimum effective dose of NGF was 5 ng/mL.
Migration of PMCs in response to NGF.
(A) PMCs (5 × 104 cells/500 μL) placed in culture inserts (upper compartment) were allowed to migrate toward 50 ng/mL NGF (closed) or medium alone (open) in each well of 24-well culture plates (lower compartment). Mast cell migration was assessed by counting the number of PMCs through the polycarbonate membrane. Each point represents the mean ± SE (standard error) of 4 separate experiments. (B) Various concentrations of NGF were applied into the lower compartment. After incubation for 3 hours, migratory cells were counted. Each point represents the mean ± SE of 4-11 separate experiments. (C) Anti-NGF (1:500 dilution), anti-SCF (5 μg/mL), or control pAb was added into 50 ng/mL NGF or 1 ng/mL rSCF in the lower compartment. After incubation for 3 hours, migratory PMCs were counted. Data were obtained from 5-9 separate experiments. *P < .001, when compared with NGF or rSCF pAb and control pAb.
Migration of PMCs in response to NGF.
(A) PMCs (5 × 104 cells/500 μL) placed in culture inserts (upper compartment) were allowed to migrate toward 50 ng/mL NGF (closed) or medium alone (open) in each well of 24-well culture plates (lower compartment). Mast cell migration was assessed by counting the number of PMCs through the polycarbonate membrane. Each point represents the mean ± SE (standard error) of 4 separate experiments. (B) Various concentrations of NGF were applied into the lower compartment. After incubation for 3 hours, migratory cells were counted. Each point represents the mean ± SE of 4-11 separate experiments. (C) Anti-NGF (1:500 dilution), anti-SCF (5 μg/mL), or control pAb was added into 50 ng/mL NGF or 1 ng/mL rSCF in the lower compartment. After incubation for 3 hours, migratory PMCs were counted. Data were obtained from 5-9 separate experiments. *P < .001, when compared with NGF or rSCF pAb and control pAb.
Checkerboard analysis of NGF-induced migration
We conducted experiments to determine whether the mast cell migratory response induced by NGF was due to directional (chemotaxis) or random (chemokinesis) activation. As shown in Table1, checkerboard analyses with various concentrations of NGF in the upper and lower compartments demonstrated the gradient-dependent migration of PMCs. Moreover, the addition of increasing concentrations of NGF in the upper compartment led to slight dose-dependent migration to the lower compartment without NGF. Thus, we concluded that predominant chemotaxis and slight chemokinesis were induced by NGF.
Specificity of NGF-induced chemotaxis
To determine the specificity of NGF on chemotactic response of PMCs, rabbit anti-NGF pAb (1:500 dilution) or anti-SCF pAb (5 μg/mL) was added to the assay medium containing the optimal dose of NGF (50 ng/mL) or the optimal dose of rSCF (1 ng/mL)38 in the lower compartment (Figure 1C). When PMCs were cultured in the upper compartment for 3 hours, both NGF and rSCF led to chemotactic migration of PMCs; the chemotactic ability of NGF was about 2-fold more potent than that of rSCF. The addition of anti-NGF pAb completely abolished the NGF-induced chemotaxis of PMCs, whereas rabbit control pAb or anti-SCF pAb had no effect on it. Neither anti-NGF pAb nor control pAb neutralized the rSCF-induced chemotaxis.
Shape of PMCs stimulated with NGF
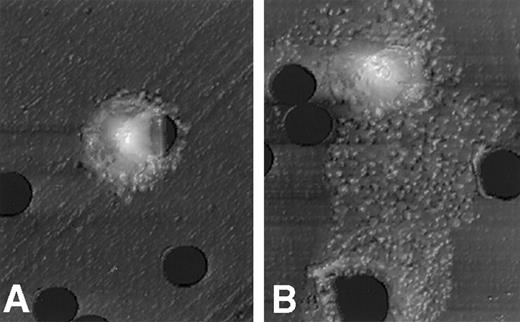
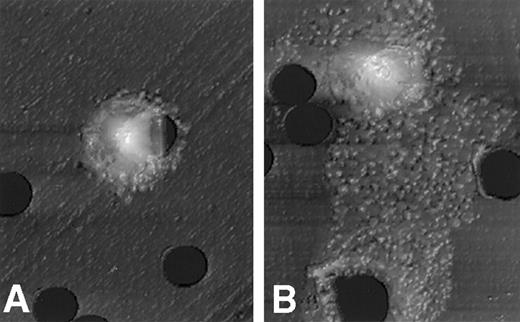
After stimulation with 50 ng/mL NGF for 3 hours, PMCs on the polycarbonate membrane were fixed with 3% paraformaldehyde/PBS. The surface structure of PMCs migrating on the lower surface of the membrane through pores was scanned with an atomic force microscope. Figure 2 clearly shows that PMCs exposed to the optimal dose of NGF resulted in a drastic shape change with a polarized morphology. On the other hand, PMCs exposed to control medium alone remained resting, with little or no shape alteration.
Atomic force microscopic features of PMCs stimulated with NGF.
A morphologic change of PMCs was examined after incubation for 3 hours with assay medium (A) or 50 ng/mL NGF (B). The mast cell stimulated with NGF reveals a polarized morphology, with membrane extension assuming lamellipodia. Scale: 1 cm = 7.7 μm.
Atomic force microscopic features of PMCs stimulated with NGF.
A morphologic change of PMCs was examined after incubation for 3 hours with assay medium (A) or 50 ng/mL NGF (B). The mast cell stimulated with NGF reveals a polarized morphology, with membrane extension assuming lamellipodia. Scale: 1 cm = 7.7 μm.
F-actin formation in PMCs treated with NGF
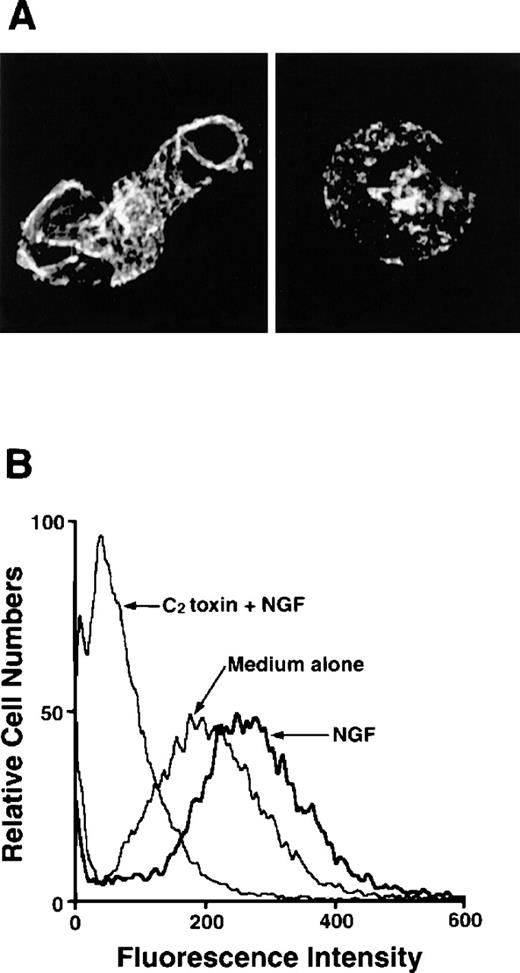
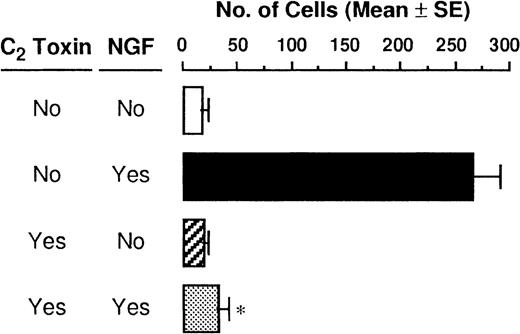
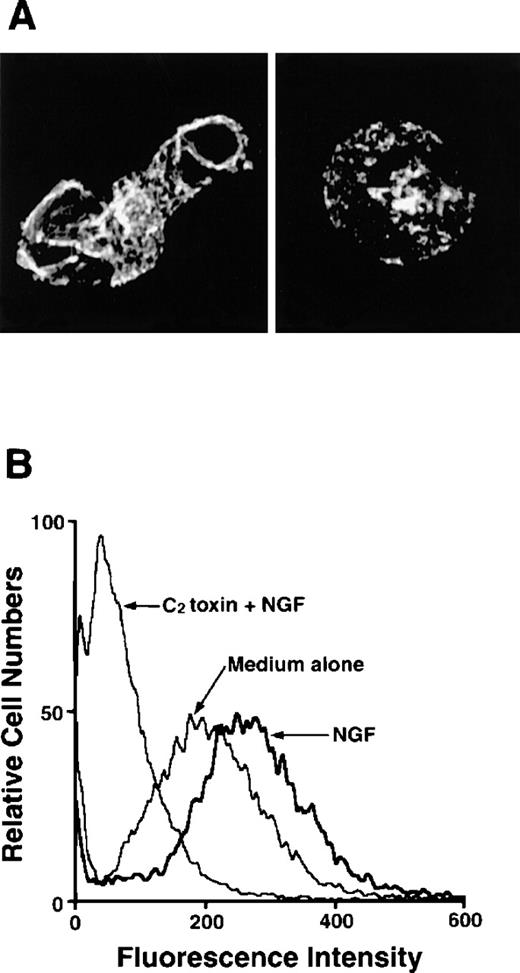
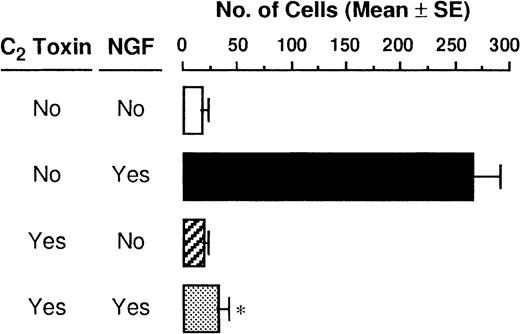
As F-actin formation is well known to be associated with cell motility, we next examined migratory PMCs on the distribution of F-actin. F-actin taken from PMCs that were passing through the pore toward 50 ng/mL NGF was stained with Oregon Green 488-phalloidin, and a strong positive reaction was detected at the plasma membrane, particularly at the protrusion site of the polarized cell (Figure3A). To determine whether the polymerization of actin filaments was caused by stimulation with NGF, PMCs were pretreated with C botulinum C2 toxin, an actin-polymerization inhibitor, for 2 hours before the chemotaxis assay; this pretreatment inhibited the NGF-mediated F-actin formation (Figure 3A). In addition to confocal laser scanning microscopic analysis, flow cytometric analysis clearly demonstrated that enhanced formation of F-actin was induced by treatment with 50 ng/mL NGF, but it was completely inhibited by pretreatment with C botulinumC2 toxin (Figure 3B). Next, we attempted to determine the possible inhibitory effect of C botulinum C2 toxin on NGF-mediated chemotactic movement; the pretreatment with C botulinum C2 toxin resulted in complete neutralization of the chemotactic response of PMCs to NGF (Figure4).
Detection of F-actin in NGF-stimulated PMCs with or without pretreatment with C botulinum C2 toxin.
(A) PMCs were allowed to migrate toward 50 ng/mL NGF for 1 hour following pretreatment with (right panel) or without (left panel) 300 ng/mL C botulinum C2 toxin for 2 hours. PMCs were fixed, permeabilized, and stained with Oregon Green 488-phalloidin. F-actin was visualized using a confocal laser scanning microscope (objective × 2000). The mast cell stimulated with NGF shows increased fluorescent intensity that is concentrated in lamellipodia and other submembranous sites (left panel). In contrast, the mast cell pretreated with C botulinum C2 toxin before the addition of NGF is rounded and shows a very weak, positive reaction for F-actin (right panel). (B) PMCs were treated with 50 ng/mL NGF for 2 hours following pretreatment with or without 300 ng/mL C botulinum C2 toxin for 2 hours and then analyzed by flow cytometric assay.
Detection of F-actin in NGF-stimulated PMCs with or without pretreatment with C botulinum C2 toxin.
(A) PMCs were allowed to migrate toward 50 ng/mL NGF for 1 hour following pretreatment with (right panel) or without (left panel) 300 ng/mL C botulinum C2 toxin for 2 hours. PMCs were fixed, permeabilized, and stained with Oregon Green 488-phalloidin. F-actin was visualized using a confocal laser scanning microscope (objective × 2000). The mast cell stimulated with NGF shows increased fluorescent intensity that is concentrated in lamellipodia and other submembranous sites (left panel). In contrast, the mast cell pretreated with C botulinum C2 toxin before the addition of NGF is rounded and shows a very weak, positive reaction for F-actin (right panel). (B) PMCs were treated with 50 ng/mL NGF for 2 hours following pretreatment with or without 300 ng/mL C botulinum C2 toxin for 2 hours and then analyzed by flow cytometric assay.
Neutralization of NGF-induced chemotaxis by C botulinum C2 toxin.
PMCs were allowed to migrate toward 50 ng/mL NGF for 3 hours following pretreatment with or without 300 ng/mL C botulinumC2 toxin for 2 hours. Data were obtained from 4-5 separate experiments. *P < .001, when compared with NGF alone.
Neutralization of NGF-induced chemotaxis by C botulinum C2 toxin.
PMCs were allowed to migrate toward 50 ng/mL NGF for 3 hours following pretreatment with or without 300 ng/mL C botulinumC2 toxin for 2 hours. Data were obtained from 4-5 separate experiments. *P < .001, when compared with NGF alone.
Signal transduction pathways triggered by NGF
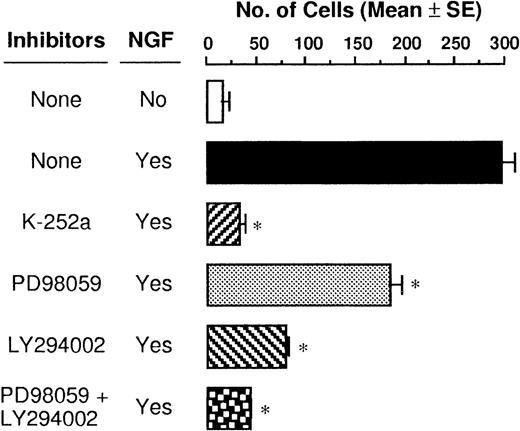
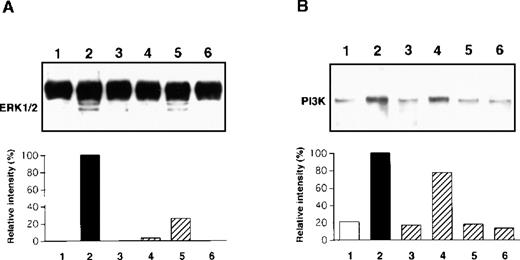
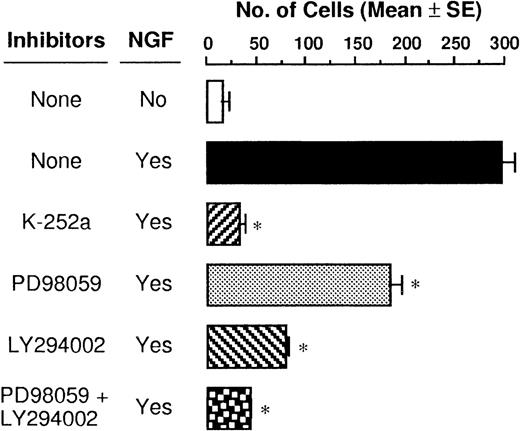
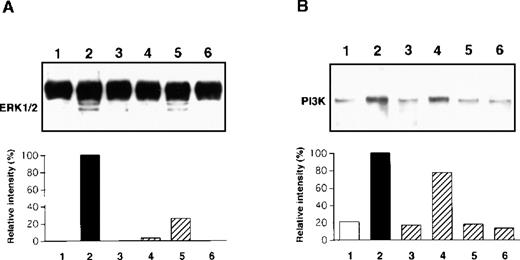
The TrkA high-affinity receptor has a tyrosine kinase domain, and binding of NGF to TrkA immediately induces its autophosphorylation and initiates several second messenger signal pathways such as activation of the MAPK and PI3K cascades in neuronal tissues.29,30 We have recently demonstrated that rat PMCs express only TrkA and that NGF tyrosine phosphorylates the TrkA receptor, thereby resulting in the rescue of PMCs from apoptosis.16 Therefore, we attempted to determine whether the NGF-induced chemotactic response of PMCs was influenced by blockage of phosphorylation of TrkA, MAPK/ERK kinase 1, and PI3K by using specific inhibitors K-252a, PD98 059, and LY294 002, respectively. When PMCs were pretreated with 50 ng/mL K-252a inhibitor for 1 hour, the NGF-induced chemotaxis was completely inhibited (Figure5). Pretreatment with 100 μmol/L PD98 059 and 50 μmol/L LY294 002 reduced the NGF-induced chemotactic response by 38% and 73%, respectively, and pretreatment with both the inhibitors led to an additive blocking effect that was comparable to the inhibitory effect induced by the pretreatment with the K-252a inhibitor. To define rapid activation of MAPK/ERK1/2 and PI3K, PMCs were treated with 50 ng/mL NGF for 30 minutes or 5 minutes, respectively. As shown in Figure 6, phosphorylation of the tyrosine residues was detected for both the signal molecules after the NGF treatment; pretreatment with inhibitor PD98 059 or LY294 002 completely eliminated the NGF-mediated phosphorylation of the individual signal molecules.
Suppressive effects of signal transduction inhibitors on NGF-induced chemotaxis.
PMCs were preincubated at a density of 5 × 104cells/mL with either 50 ng/mL K-252a, 100 μmol/L PD98 059, or 50 μmol/L LY294 002 inhibitor or assay medium for 1 hour before stimulation with 50 ng/mL NGF for 3 hours. Data were obtained from 5 separate experiments. *P < .001, when compared with NGF alone.
Suppressive effects of signal transduction inhibitors on NGF-induced chemotaxis.
PMCs were preincubated at a density of 5 × 104cells/mL with either 50 ng/mL K-252a, 100 μmol/L PD98 059, or 50 μmol/L LY294 002 inhibitor or assay medium for 1 hour before stimulation with 50 ng/mL NGF for 3 hours. Data were obtained from 5 separate experiments. *P < .001, when compared with NGF alone.
Immunoblot analysis of MAPK/ERK 1/2 (A) and PI3K (B) phosphorylation.
PMCs were treated with 50 ng/mL NGF (lanes 2, 3, 4, 5, and 6) or medium alone (lane 1) for 30 minutes (A) or 5 minutes (B) after pretreatment with 50 ng/mL K-252a (lane 3), 100 μmol/L PD98 059 (lane 4), 50 μmol/L LY294 002 (lane 5), or 100 μmol/L PD98 059 and 50 μmol/L LY294 002 (lane 6) inhibitors or assay medium (lane 2) for 1 hour. The phosphorylated protein products were visualized, and their relative intensity was determined. Data are representative of 5 separate experiments.
Immunoblot analysis of MAPK/ERK 1/2 (A) and PI3K (B) phosphorylation.
PMCs were treated with 50 ng/mL NGF (lanes 2, 3, 4, 5, and 6) or medium alone (lane 1) for 30 minutes (A) or 5 minutes (B) after pretreatment with 50 ng/mL K-252a (lane 3), 100 μmol/L PD98 059 (lane 4), 50 μmol/L LY294 002 (lane 5), or 100 μmol/L PD98 059 and 50 μmol/L LY294 002 (lane 6) inhibitors or assay medium (lane 2) for 1 hour. The phosphorylated protein products were visualized, and their relative intensity was determined. Data are representative of 5 separate experiments.
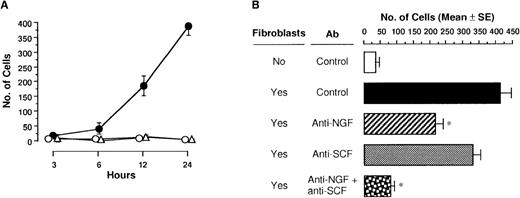
Migration of PMCs to NGF produced by 3T3 fibroblasts
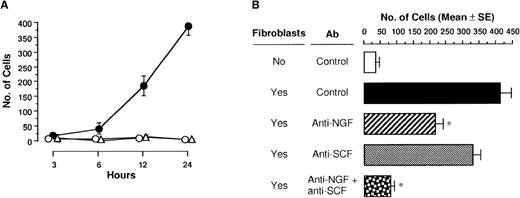
We next examined whether NGF produced by fibroblasts actually induced migration of PMCs. When a confluent monolayer of 3T3-Swiss albino fibroblasts was seeded on the bottom of the lower compartment and PMCs were applied into the upper compartment, the number of migratory PMCs was markedly increased (Figure7A). Neither control medium alone nor paraformaldehyde-fixed 3T3 fibroblasts induced chemotactic migration of PMCs. To define chemoattractants of PMCs produced by 3T3 fibroblasts, anti-NGF pAb and/or anti-SCF pAb was added to the lower compartment onto a monolayer of 3T3 fibroblasts (Figure 7B). The addition of anti-NGF or anti-SCF pAb decreased migration response of PMCs by 47% and 20%, respectively, as compared with 3T3 fibroblasts with control pAb; the application of both anti-NGF and anti-SCF pAbs more significantly inhibited cell migration toward 3T3 fibroblasts in the lower compartment.
3T3 fibroblast-derived chemotactic activity.
(A) PMCs placed in culture inserts were allowed to migrate toward the monolayer of 3T3 fibroblasts unfixed (closed circle) or fixed with paraformaldehyde (open triangle) or medium alone (open circle) in each well of 24-well culture plates. After incubation for various hours, migratory cells were counted. Each point represents the mean ± SE of 4-11 separate experiments. (B) Anti-NGF (1:500 dilution) and/or anti-SCF pAb (40 μg/mL) or control pAb was added into the monolayer of 3T3 fibroblasts in each well of 24-well culture plates. After incubation for 24 hours, migratory cells were counted. Data were obtained from 5-9 separate experiments. *P < .001, when compared with fibroblasts and control pAb.
3T3 fibroblast-derived chemotactic activity.
(A) PMCs placed in culture inserts were allowed to migrate toward the monolayer of 3T3 fibroblasts unfixed (closed circle) or fixed with paraformaldehyde (open triangle) or medium alone (open circle) in each well of 24-well culture plates. After incubation for various hours, migratory cells were counted. Each point represents the mean ± SE of 4-11 separate experiments. (B) Anti-NGF (1:500 dilution) and/or anti-SCF pAb (40 μg/mL) or control pAb was added into the monolayer of 3T3 fibroblasts in each well of 24-well culture plates. After incubation for 24 hours, migratory cells were counted. Data were obtained from 5-9 separate experiments. *P < .001, when compared with fibroblasts and control pAb.
Discussion
Directed migration of a variety of inflammatory cells toward a chemical gradient of specific chemoattractants locally produced in inflamed tissues is the first integrated event in the process of allergic and nonallergic inflammatory responses.39,40Chemoattractant ligands stimulate specific receptors on the cell surface that initiate several second messenger cascades; this action results in a change in F-actin distribution from azimuthal symmetry around the cell rim to concentration at a particular region involved in migratory behavior.41 In this study, we provided novel evidence that NGF enabled rat PMCs, which are classified as connective tissue–type mast cells, to rapidly migrate toward a concentration gradient of NGF in vitro. The PMCs treated with NGF manifested rapid distribution of F-actin and a polarized shape change with cytoplasm spread and lamellipodia protrusion. These manifestations were completely inhibited by the pretreatment with C botulinum C2 toxin. These cytoskeletal events following exposure to NGF appear to be consistent with the results reported in human B lymphocytes.42 Furthermore, the consequence of checkerboard analyses with various concentrations of NGF demonstrated that predominant chemotactic movement and slight random activation (chemokinesis) were induced by NGF.
Hartmann et al15 examined the possible activity of many kinds of growth factors and chemokines to induce migration of human mast cells (HMCs) in vitro and reported that NGF failed to promote HMC-1 migration. Immature rodent mast cells and HMCs, such as murine bone marrow–derived cultured mast cells, human umbilical cord blood–derived cultured mast cells, and HMC-1, bear the functional TrkA receptor on the cell surface.43,44 Although NGF incited murine cultured mast cells to promote colony development and induced a phenotypic change,11,43 murine cultured mast cells showed no migration response to NGF through the matrix-uncoated polycarbonate membrane (data not shown). Because NGF influenced development and maturation of human cultured mast cells as well,44 we speculated that to immature mast cells, NGF might promote development and differentiation rather than chemotactic migration.
Ligation of NGF to the TrkA receptor rapidly elicits autophosphorylation of their tyrosine residues, which leads to neurotrophic signaling that implicates multiple signal transduction pathways including MAPK and PI3K.29,30 Stimulation with NGF has been reported to increase expression of the early response genesc-fos and NGF1-A and to activate MAPK in rat PMCs and HMC-1.44,45 On the other hand, PI3K is strongly involved in chemotactic responses and modulation of integrin affinity in several cell types.46-49 Recent studies have reported that in murine mast cells stimulated with SCF or platelet-derived growth factor, PI3K may regulate adhesion to fibronectin, membrane ruffling, actin polymerization, and enhancement of FcεRI-mediated degranulation.50 51 Treatment of PMCs with either inhibitor PD98 059 (MAPK/ERK kinase 1) or LY294 002 (PI3K) partially suppressed NGF-induced chemotaxis, and treatment of PMCs with both inhibitors completely blocked it. This suggests that the shape change with distribution of F-actin and the chemotactic response may be mediated through both signal transduction cascades. In fact, the addition of NGF to PMCs elicited rapid activation of both MAPK/ERK1/2 and PI3K inhibitors, which were blocked by the addition of PD98 059 and LY294 002.
Rac, Cdc42, and Rho, classified as the Rho subfamily of small guanosine 5′-triphosphate–binding (GTP-binding) proteins, regulate formation of actin stress fibers and focal adhesion.52 Recently, oncogenic Ras activation has been reported to drive both MAPK signal pathways and the regulatory proteins,53,54 which interact with PI3K.55,56Because C botulinum C3 toxin, which is able to induce ADP-ribosylation on a Rho protein,57 inhibited NGF-induced PMC migration (data not shown), distribution of F-actin by stimulation with NGF appears to be regulated by small GTP-binding proteins in the Rho subfamily in mast cells.
NGF is released from the submaxillary gland into the blood stream shortly after fighting stress in mice58 and is detected in the peripheral blood of humans after skydiving.59Furthermore, we recently demonstrated that full-thickness skin wounds resulted in the rapid increase of NGF levels in the peripheral blood. NGF production was originated in the submaxillary gland; subsequent NGF production of newly generated epithelial cells occurred at the edge of the wound, and fibroblasts developed in the granulation tissue produced in the wound space.20 These experimental findings support the possibility that in addition to regeneration of the injured neurons, an increased amount of NGF in the blood and/or local tissues may function as an immunomodulator to leukocytes expressing NGFR in the sequence of events in inflammatory responses. In the fibroblast model presented here, we demonstrated that 3T3 fibroblasts led to chemotactic migration of PMCs. Because 3T3 fibroblasts are able to produce and release biologically active NGF and SCF spontaneously,11,20 60 we attempted to demonstrate the possible ability of these growth factors as a chemoattractant for mast cells. The simultaneous treatment with pAbs against these growth factors completely inhibited migration of PMCs. Anti-NGF pAb was more effective than anti-SCF pAb, which suggests that NGF produced by fibroblasts and other cell types may act as one of the major chemoattractants for connective tissue–type mast cells.
Acknowledgments
We wish to thank Drs J. Bienenstock and A. M. Stanisz (McMaster University, Hamilton, Ontario, Canada) for supplying ultrapurified NGF; Dr I. Ohishi (Nippon Veterinary and Animal Science University, Tokyo, Japan) for providing C botulinum C2 toxin; and Amgen (Thousand Oaks, CA) for providing rSCF.
Supported by grant 09460137 from the Ministry of Education, Science, Sports, and Culture, Tokyo, Japan; and grants 3-2-4 and RCP 1999-4120 from the Pioneering Research Project in Biotechnology and Recombinant Cytokine's Project provided by the Ministry of Agriculture, Forestry, and Fisheries, Tokyo, Japan.
Reprints:Hiroshi Matsuda, Department of Veterinary Clinic, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan; e-mail:hiro@cc.tuat.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.