Abstract
Patients with severe congenital neutropenia (SCN) are at increased risk for the development of acute myelogenous leukemia (AML). In the subset of patients with SCN that progresses to AML, acquired mutations in the receptor for granulocyte colony-stimulating factor (G-CSF) have been detected that result in the expression of truncated forms of the G-CSF receptor (G-CSFR) protein. G-CSFR truncation mutants from these patients trans-duce hyperproliferative growth responses. In this paper, we show that the most frequently isolated mutant G-CSFR form from patients with SCN/AML (▵716) confers resistance to apoptosis and prolongs cell survival through a mechanism involving Akt, a downstream target of PI3-kinase. G-CSF stimulation of cells expressing the G-CSFR truncation mutant induces sustained activation of Akt and prolonged phosphorylation of the pro-apoptotic protein Bad, resulting in enhanced cell survival. Extension of cell survival allowing for sufficient time for the acquisition of additional oncogenic events may represent an important mechanism by which G-CSFR mutations contribute to leukemogenesis. These data provide further insight into the pathophysiologic contribution of G-CSFR mutations to AML.
Granulocyte colony-stimulating factor (G-CSF) is the major cytokine that drives granulopoiesis in humans and animals.1 G-CSF supports proliferation and neutrophilic differentiation of hematopoietic progenitor cells, as well as their survival. The signaling molecules activated by the G-CSF receptor (G-CSFR) to promote cell survival remain largely unknown. We previously reported that PI3-kinase is activated in response to G-CSF and that its activation correlates with inhibition of apoptosis.2 The downstream targets of PI3-kinase in the G-CSF signaling cascade that mediate cell survival have not yet been elucidated.
The biologic activities of G-CSF are mediated by specific receptors on the surface of responsive cells.3,4 Recently, mutations in the G-CSFR gene resulting in truncation of the carboxy-terminal region have been detected in a subset of patients with severe congenital neutropenia (SCN) transforming to acute myelogenous leukemia (AML).5 6 Mutations in the G-CSFR in patients with SCN/AML have been found to localize to a critical cytoplasmic region spanning nucleotides 2384-2429 of the G-CSFR. These mutations, which have been detected in cells of the myeloid lineage only, have all been nonsense mutations leading to truncation of the distal cytoplasmic region of the G-CSFR and rendering cells hypersensitive to G-CSF. In the cases studied, the mutations were found to be acquired, suggesting that disruption of the normal signaling functions of the G-CSFR may contribute to leukemogenesis.
Mice carrying a targeted “knock in” mutation in their G-CSFR that reproduces the most commonly found G-CSFR mutation in patients with AML preceded by SCN have been generated as an in vivo model system for studying the role of G-CSFR mutations in the pathogenesis of SCN/AML.7-9 Notably, these mice have not been found to develop AML and do not exhibit the SCN phenotype with severe neutropenia and a block in myeloid maturation. However, as in humans, an increased proliferative response to G-CSF has been observed with cells from the mutant mice.
The observation that mice carrying targeted G-CSFR mutations do not develop AML implicates a requirement for additional oncogenic events. To better understand the mechanisms by which G-CSFR mutations contribute to leukemogenesis, we investigated the signaling pathways activated by the G-CSFR to promote cell growth and survival.
In this article, we have identified Akt and Bad as downstream targets of PI3-kinase that promote cell survival by G-CSF. We show that mutations in the G-CSFR in SCN/AML transduce signals for increased resistance to apoptosis and enhanced cell survival, which are mediated by sustained activation of Akt and Bad. Our data suggest a mechanism whereby extension of cell survival to permit the acquisition of additional oncogenic events could underlie the development of AML in patients with antecedent SCN.
Materials and methods
Reagents
Recombinant human G-CSF was a kind gift from Amgen (Thousand Oaks, CA). Reagents for maintenance of cell lines were purchased from GIBCO-BRL (Grand Island, NY). 32Pγ-ATP (3000 Ci/mmol) was obtained from Amersham (Piscataway, NJ). All other reagents were obtained from Sigma (St. Louis, MO), unless otherwise indicated.
Antibodies
The 4G10 anti-phosphotyrosine antibody was a generous gift from Dr Brian Druker (Oregon Health Sciences University, Portland, OR). The sheep anti-Akt1 and rabbit anti–14-3-3 antibodies were obtained from Upstate Biotechnology, Inc (Lake Placid, NY). Antibodies recognizing the S and T (single-letter amino acid code) phosphorylated forms of Akt were purchased from New England Biolabs (Beverly, MA). Monoclonal antibody to Bad was obtained from Transduction Laboratories (Lexington, KY). Secondary antibodies conjugated to horseradish peroxidase were from Amersham and Kirkegaard & Perry Laboratories (Gaithersburg, MD).
Cells
Parental Ba/F3 cells and Ba/F3 cells transfected with the wild-type (WT) or Δ716 cDNAs were maintained in RPMI 1640 medium with 2 mmol/L glutamine, 10% fetal bovine serum (FBS), and 10% WEHI-3–conditioned media (WEHI-CM) as a source of interleukin-3 (IL-3). Details for the generation of Ba/F3 cells stably expressing the WT or the Δ716 G-CSFR form have been described previously.2
Proliferation studies
Cells were washed in phosphate-buffered saline (PBS) and resuspended at 1 × 105 cells/mL in RPMI 1640 medium containing 10% FBS, 2 mmol/L glutamine, and either 10% WEHI-CM or 1.9 ng/mL G-CSF. The cells were cultured in liquid suspension at 37°C in a humidified incubator with 5% CO2.
Analysis of apoptosis
Cells were grown in RPMI 1640 containing 10% FBS and either 10% WEHI-CM or 1.9 ng/mL G-CSF for 10 days. The cells were washed, resuspended in medium devoid of cytokines, and incubated at 37°C. At varying times (0-72 hours), cells were removed from culture for analysis of apoptosis using the TUNEL assay. For the TUNEL assay, cells (1 × 105) were fixed in 4% formaldehyde, spun onto glass slides, subjected to the TUNEL reaction (Calbiochem, San Diego, CA) according to the manufacturer's instructions, and counterstained with methyl green. The cells were then analyzed by light microscopy. Cells in which the nuclei stained brown were scored as positive for apoptosis. Data were expressed as the percentage of negative apoptotic cells from a 200-cell count.
Immunoprecipitations and Western blot analyses
Cells were serum- and cytokine-deprived for 4 hours at 37°C in RPMI 1640 medium containing 0.1% bovine serum albumin and 2 mmol/L glutamine, then stimulated with 100 ng/mL G-CSF for the indicated times, and lysed in buffer A containing 1% Nonidet P-40 (Boehringer Mannheim Biochemical, Indianapolis, IN), 1 mmol/L EDTA (pH 8.0), 20 mmol/L Tris (pH 8.0), 150 mmol/L NaCl, 0.15 U/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin A, 1 mmol/L sodium orthovanadate, and 1 μmol/L microcystin. Protein determinations of whole-cell lysates cleared of insoluble material were performed using the BCA assay (Pierce, Rockford, IL). For immunoprecipitations, 1 mg of protein was precleared with protein G–agarose (GIBCO) before immunoprecipitation with the relevant antibody. Samples stimulated with 50 μmol/L peroxyvanadate served as positive controls for activation of Akt. Western blotting was performed as described previously using enhanced chemiluminescence (ECL).2
Akt kinase assay
Akt kinase assays were performed as described previously.10 11 Briefly, whole-cell lysates were immunoprecipitated at 4°C with 4 μg of anti-Akt1 conjugated to protein G–agarose. Immune complexes were washed 4 times in buffer A containing 500 mmol/L NaCl, then washed twice in kinase assay buffer containing 20 mmol/L MOPS (pH 7.2), 25 mmol/L sodium glycerophosphate, 1 mmol/L sodium orthovanadate, and 1 mmol/L dithiothreitol. The immune complexes were resuspended in kinase buffer containing 500 μmol/L ATP, 75 mmol/L MgCl2, 1 μCi32Pγ-ATP, and 10 μg histone H2B (Boehringer Mannheim Biochemical), and incubated for 20 minutes at 30°C. The reactions were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% acrylamide gel. Phosphorylation of histone H2B was quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software.
Results
Growth characteristics of G-CSFR transfectants
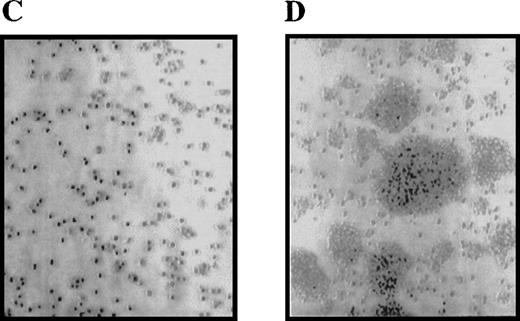
We previously reported that cells expressing the Δ716 G-CSFR were hypersensitive to G-CSF.12 We performed studies to further characterize the abnormal growth response of cells expressing this G-CSFR form, which reproduces the most frequently isolated mutant G-CSFR form from patients with SCN/AML. IL-3–dependent Ba/F3 cells transfected with either the WT or Δ716 G-CSFR form were grown in liquid-suspension cultures in media containing either 10% WEHI-CM (as a source of IL-3) or 1.9 ng/mL G-CSF. Growth of the cells was examined at various times over 1 month. Notably, Δ716 cells cultured in G-CSF–containing media grew as large cell clusters, which were apparent within 4 days after transfer of the cells to G-CSF–containing media (Figure 1). The unusual growth characteristics of Δ716 cells in the presence of G-CSF were observed in more than 10 independent experiments. Although the growth properties of G-CSF–expanded Δ716 cells suggested a transformed phenotype, these cells did not become growth factor–independent and continued to require either IL-3 or G-CSF for long-term growth (data not shown). In contrast, WT transfectants grown in either IL-3 or G-CSF and Δ716 transfectants grown in IL-3 grew as single-cell suspensions without the formation of large cell clusters.
Enhanced proliferation and cluster formation of ▵716 cells grown in G-CSF.
Ba/F3 cells transfected with the wild-type (WT) (A, B) or Δ716 G-CSFR (C, D) were grown for 4 days in RPMI 1640 + 10% FBS containing either 10% WEHI-CM as a source of IL-3 (A, C) or 1.9 ng/mL G-CSF (B, D). Cells were examined by inverted microscopy at 200 × and photographed.
Enhanced proliferation and cluster formation of ▵716 cells grown in G-CSF.
Ba/F3 cells transfected with the wild-type (WT) (A, B) or Δ716 G-CSFR (C, D) were grown for 4 days in RPMI 1640 + 10% FBS containing either 10% WEHI-CM as a source of IL-3 (A, C) or 1.9 ng/mL G-CSF (B, D). Cells were examined by inverted microscopy at 200 × and photographed.
G-CSF enhances the survival of ▵716 cells
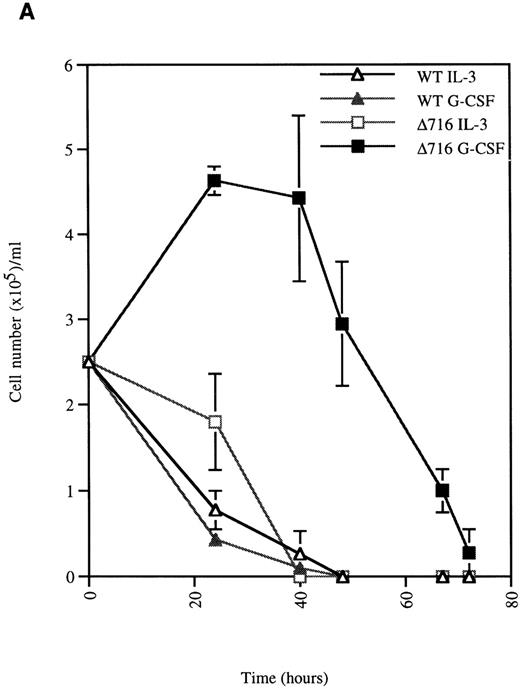
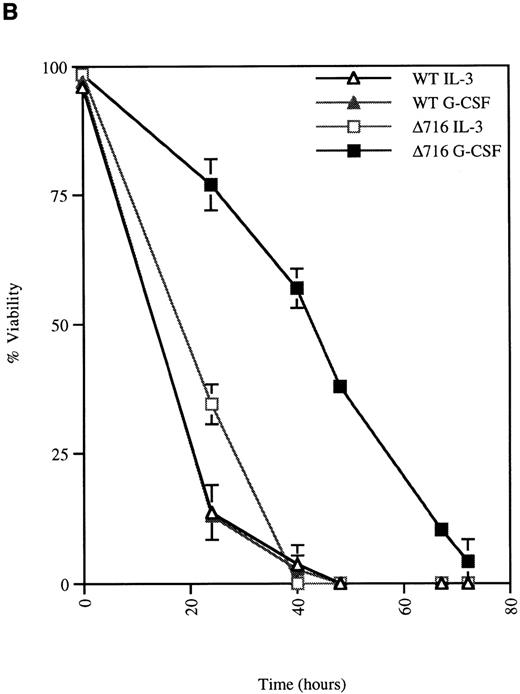
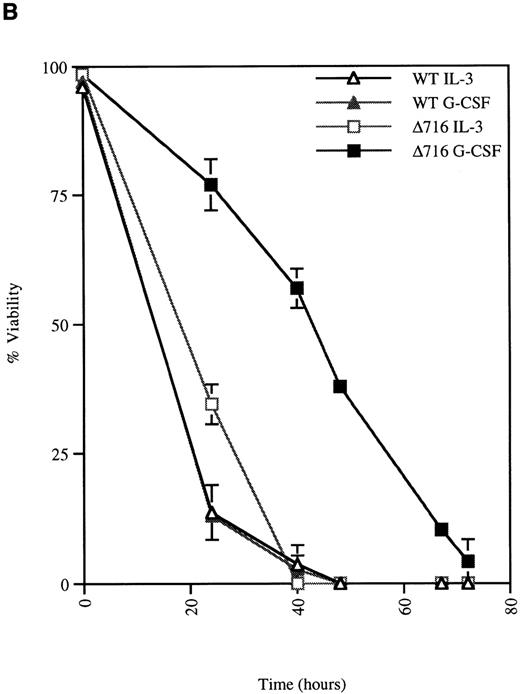
We next examined the survival of WT and Δ716 cells following growth factor deprivation. IL-3–dependent Ba/F3 cells transfected with the WT G-CSFR and grown in either G-CSF– or IL-3–containing media ceased proliferation and died within 48 hours after growth factor withdrawal (Figure 2). In contrast, Ba/F3 cells transfected with the Δ716 G-CSFR and grown in G-CSF–containing media showed prolonged survival and continued to proliferate for 48 hours after G-CSF withdrawal. Similar to WT transfectants grown in IL-3, Δ716 transfectants grown in IL-3 and subsequently deprived of IL-3 failed to proliferate and died within 48 hours. The prolonged survival of G-CSF–cultured Δ716 cells after growth factor deprivation was independent of the presence of serum and only partially abrogated by removal of serum from the culture medium (data not shown).
G-CSF pretreatment enhances the survival and proliferation of ▵716 cells following growth factor withdrawal.
Ba/F3 cells transfected with either the WT or the Δ716 G-CSFR were grown in media containing either 10% WEHI-CM (source of IL-3) or 1.9 ng/mL G-CSF. The cells were washed twice and then resuspended in RPMI 1640 containing 10% FBS and 2 mmol/L glutamine with no added cytokine. At the indicated times, cells were stained with trypan blue and cell number (A) and percent viability (B) were determined. SDs were calculated from 3 independent experiments.
G-CSF pretreatment enhances the survival and proliferation of ▵716 cells following growth factor withdrawal.
Ba/F3 cells transfected with either the WT or the Δ716 G-CSFR were grown in media containing either 10% WEHI-CM (source of IL-3) or 1.9 ng/mL G-CSF. The cells were washed twice and then resuspended in RPMI 1640 containing 10% FBS and 2 mmol/L glutamine with no added cytokine. At the indicated times, cells were stained with trypan blue and cell number (A) and percent viability (B) were determined. SDs were calculated from 3 independent experiments.
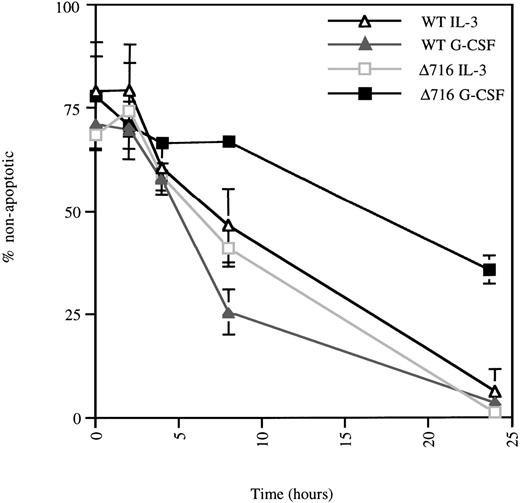
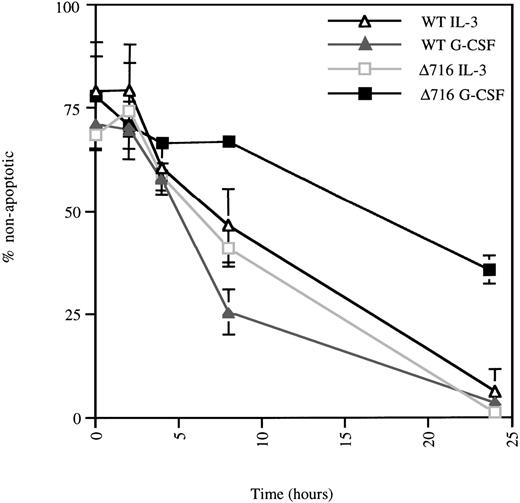
We next investigated the mechanisms responsible for G-CSF–mediated enhanced growth and survival of Δ716 cells. Using the TUNEL assay to assess apoptotic cells, we observed that Δ716 cells were more resistant to apoptosis than WT cells after G-CSF withdrawal. As shown in Figure 3, IL-3 withdrawal induced comparable levels of apoptosis in WT and Δ716 transfectants. However, a striking difference in the numbers of apoptotic cells was observed between WT and Δ716 cells after G-CSF withdrawal. At 24 hours, only 60% of Δ716 cells were apoptotic, compared with approximately 97% of WT cells. At 48 hours after withdrawal from G-CSF, 22% of Δ716 cells still had not undergone apoptosis, but by 72 hours no viable cells were detected (data not shown).
▵716 cells are resistant to apoptosis induction following culture in G-CSF.
Ba/F3 cells transfected with the WT or Δ716 G-CSFR were grown in RPMI 1640 medium containing 10% FBS and 2 mmol/L glutamine, supplemented with either 10% WEHI-CM (IL-3 source) or 1.9 ng/mL G-CSF. At time 0, the cells were washed, resuspended in media without added cytokine, and incubated at 37°C. At the indicated times, cells (1 × 105) were subjected to the TUNEL reaction and analyzed by light microscopy, as described in “Materials and methods.” Data are expressed as the percentage of non-apoptotic cells (negative for TUNEL reaction) in a 200-cell count from 2 independent experiments.
▵716 cells are resistant to apoptosis induction following culture in G-CSF.
Ba/F3 cells transfected with the WT or Δ716 G-CSFR were grown in RPMI 1640 medium containing 10% FBS and 2 mmol/L glutamine, supplemented with either 10% WEHI-CM (IL-3 source) or 1.9 ng/mL G-CSF. At time 0, the cells were washed, resuspended in media without added cytokine, and incubated at 37°C. At the indicated times, cells (1 × 105) were subjected to the TUNEL reaction and analyzed by light microscopy, as described in “Materials and methods.” Data are expressed as the percentage of non-apoptotic cells (negative for TUNEL reaction) in a 200-cell count from 2 independent experiments.
G-CSF induces sustained cellular activation in cells expressing the ▵716 G-CSFR mutant
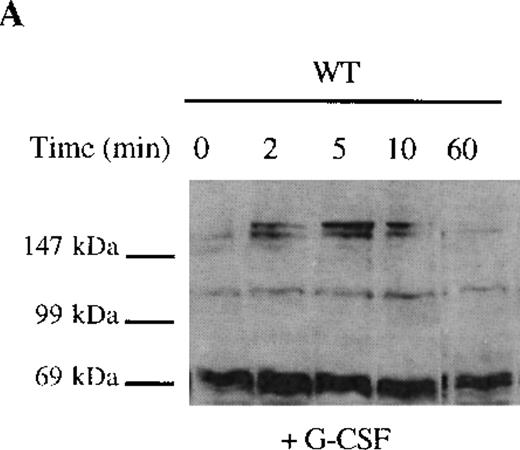
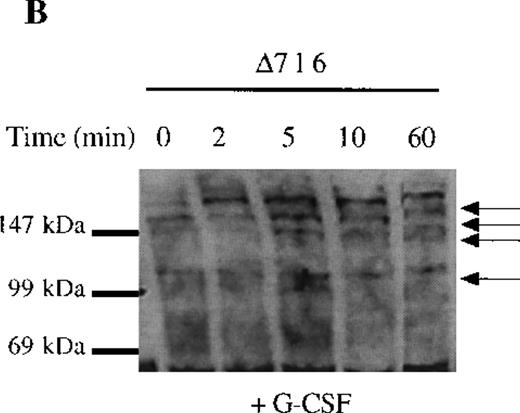
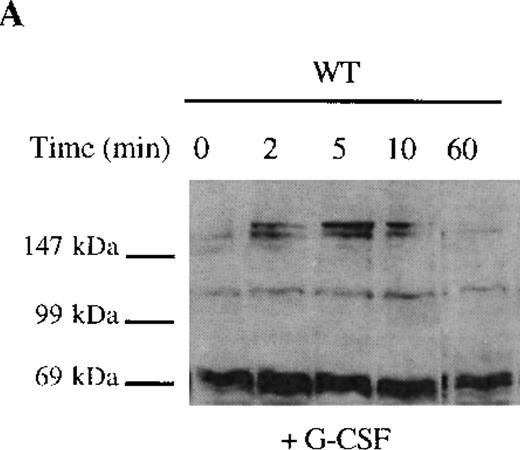
The selective growth and survival advantage imparted by G-CSF to Δ716 transfectants upon withdrawal of G-CSF and the failure to observe this in Δ716 transfectants following culture in IL-3 excluded an autocrine-mediated mechanism for enhanced cell survival and proliferation and supported a mechanism involving sustained activation of signaling pathways triggered by G-CSF in Δ716 cells. Studies were therefore done to examine intracellular signaling in G-CSF–stimulated WT and Δ716 cells. G-CSF rapidly induced tyrosine phosphorylation of multiple cellular proteins in both WT and Δ716 cells (Figure4). Tyrosine phosphorylation was transient and by 60 minutes was no longer apparent in WT cells stimulated with G-CSF (Figure 4A). In contrast, G-CSF–induced tyrosine phosphorylation of the majority of substrates in Δ716 cells was still apparent at 60 minutes (Figure 4B).
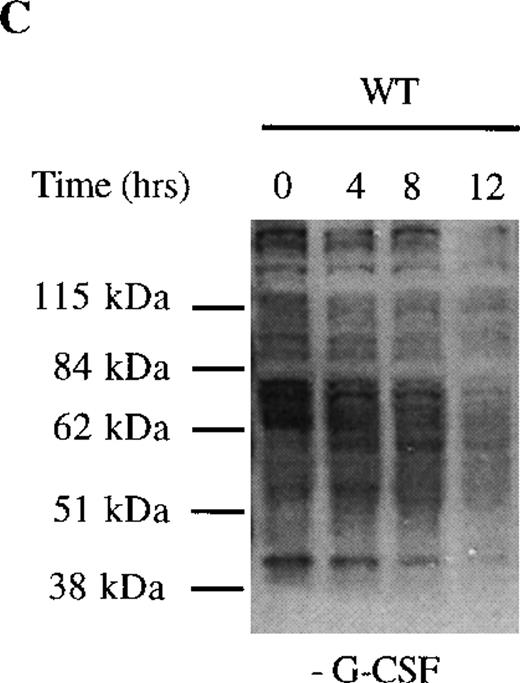
Sustained activation of ▵716 cells in response to G-CSF.
Ba/F3 cells were transfected with the WT (A, C) or Δ716 G-CSFR (B, D). In (A) and (B), cells were serum- and cytokine-deprived and then stimulated with 100 ng/mL G-CSF for the indicated times. In (C) and (D), cells continuously growing in G-CSF–containing media were subsequently transferred to media devoid of all cytokines and analyzed at the indicated times. Whole-cell lysates were immunoblotted with the 4G10 anti-phosphotyrosine antibody. Arrows indicate proteins with prolonged tyrosine phosphorylation in Δ716 cells.
Sustained activation of ▵716 cells in response to G-CSF.
Ba/F3 cells were transfected with the WT (A, C) or Δ716 G-CSFR (B, D). In (A) and (B), cells were serum- and cytokine-deprived and then stimulated with 100 ng/mL G-CSF for the indicated times. In (C) and (D), cells continuously growing in G-CSF–containing media were subsequently transferred to media devoid of all cytokines and analyzed at the indicated times. Whole-cell lysates were immunoblotted with the 4G10 anti-phosphotyrosine antibody. Arrows indicate proteins with prolonged tyrosine phosphorylation in Δ716 cells.
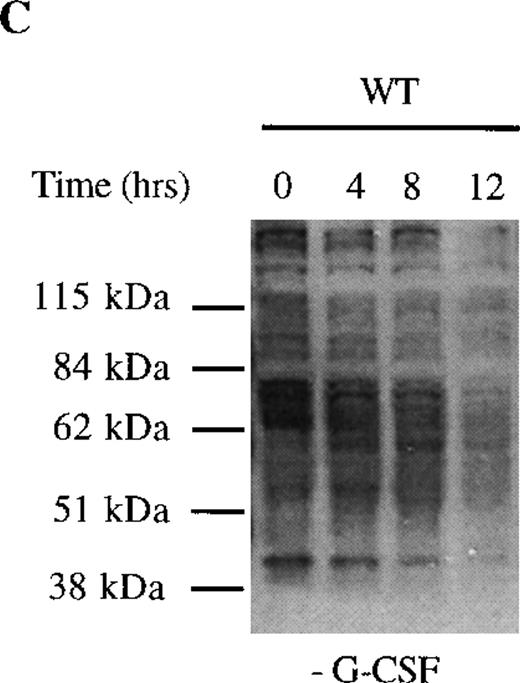
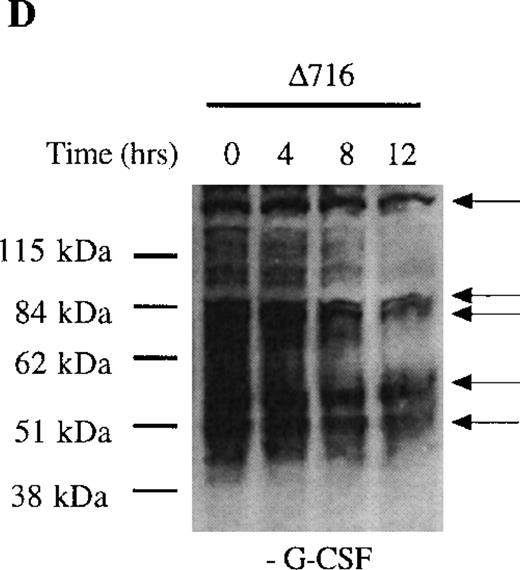
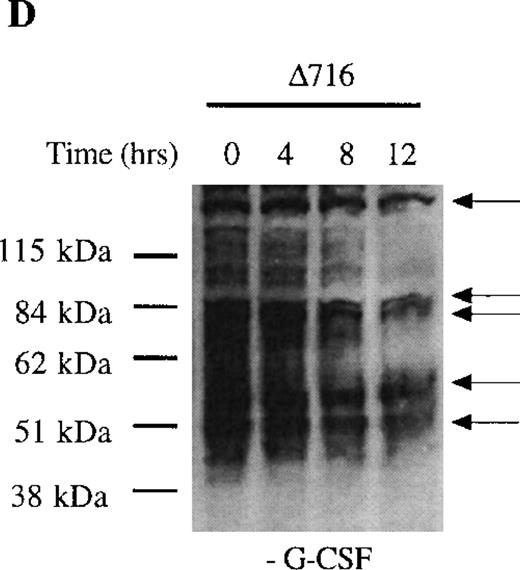
We next examined dephosphorylation of tyrosine phosphorylated proteins in WT and Δ716 cells. Following removal from G-CSF–containing media, a significant reduction in the state of tyrosine phosphorylation of all proteins in WT cells was apparent within 4 hours, with a return to basal levels of phosphorylation in all proteins by 12 hours (Figure4C). In contrast, the majority of tyrosine phosphorylated proteins activated in response to G-CSF in Δ716 cells could still be strongly detected at 12 hours (Figure 4D).
Sustained activation of Akt in ▵716 cells
Because we previously reported that activation of PI3-kinase by G-CSF correlates with inhibition of apoptosis,2 we investigated whether G-CSF also induced activation of the S/T anti-apoptotic kinase Akt, which is a downstream target of PI3-kinase.13-15 As shown in Figure5, G-CSF induced phosphorylation of Akt at T308 and S473 in both WT and Δ716 cells. Phosphorylation of Akt was found to correlate with activation of its enzymatic activity (Figure6). Notably, activation of the enzymatic activity of Akt in response to G-CSF was prolonged in Δ716 cells compared with WT cells. In 4 independent experiments, we consistently observed prolonged activation of Akt in response to G-CSF in Δ716 cells. Activation of Akt in WT cells was rapid and transient, with maximal activation apparent within 5 minutes and inactivation by 30 minutes. In contrast, a markedly increased level of activation of Akt was observed in response to G-CSF stimulation in Δ716 cells compared with WT cells, with maximal activation at 30 minutes and persistent kinase activity still apparent at 3 hours.
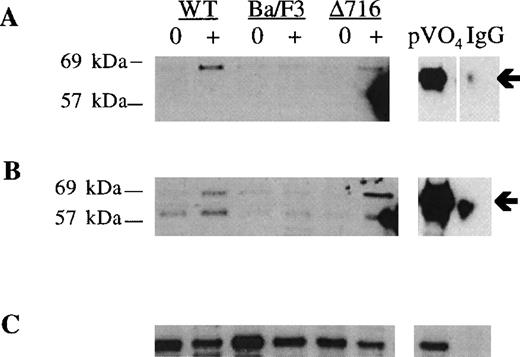
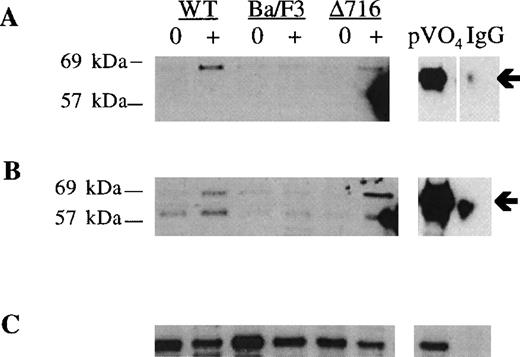
Induction of S and T phosphorylation of Akt by G-CSF.
Parental Ba/F3 cells or Ba/F3 cells transfected with the WT G-CSFR or the Δ716 G-CSFR were untreated (0) or stimulated with 100 ng/mL G-CSF (+) for 10 minutes, then lysed and immunoprecipitated with anti-Akt antibody. (A) Immunoblotting with anti–phospho-Akt (S473). (B) The blot in (A) was stripped and reblotted with antibody recognizing Akt phosphorylated at T308. (C) The blot in (B) was stripped and reblotted with anti-Akt to assess for equivalent protein loading. Arrows indicate the phosphorylated forms of Akt. Peroxyvanadate (pVO4)-stimulated Δ716 cells are shown as a positive control. Δ716 cells immunoprecipitated with normal sheep serum (IgG) instead of anti-Akt antibody represent a negative control.
Induction of S and T phosphorylation of Akt by G-CSF.
Parental Ba/F3 cells or Ba/F3 cells transfected with the WT G-CSFR or the Δ716 G-CSFR were untreated (0) or stimulated with 100 ng/mL G-CSF (+) for 10 minutes, then lysed and immunoprecipitated with anti-Akt antibody. (A) Immunoblotting with anti–phospho-Akt (S473). (B) The blot in (A) was stripped and reblotted with antibody recognizing Akt phosphorylated at T308. (C) The blot in (B) was stripped and reblotted with anti-Akt to assess for equivalent protein loading. Arrows indicate the phosphorylated forms of Akt. Peroxyvanadate (pVO4)-stimulated Δ716 cells are shown as a positive control. Δ716 cells immunoprecipitated with normal sheep serum (IgG) instead of anti-Akt antibody represent a negative control.
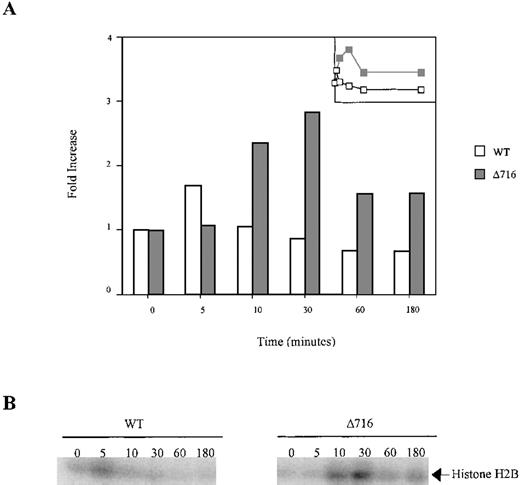
Sustained activation of Akt in ▵716 cells in response to G-CSF.
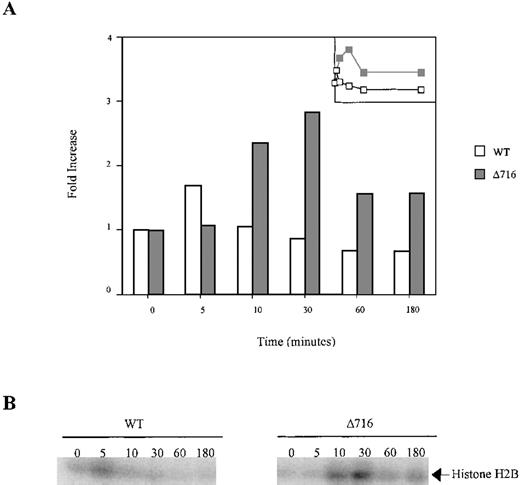
Ba/F3 cells transfected with the WT or Δ716 G-CSFR were serum- and cytokine-deprived for 4 hours. The cells were stimulated with 100 ng/mL G-CSF for the indicated times, then lysed and immunoprecipitated with anti-Akt1. The immune complexes were washed and incubated in kinase buffer containing 500 μmol/L ATP, 75 mmol/L MgCl2, 1 μCi of 32PγATP, and 10 μg histone H2B for 20 minutes at 30°C. The reactions were subjected to SDS-PAGE. Phosphorylation of histone H2B was quantified with a PhosphorImager using ImageQuant software. (A) Akt activity was measured as the fold-increase in histone H2B phosphorylation over unstimulated samples. Inset is a linear representation of the data. (B) Detection of histone H2B phosphorylation from samples in (A).
Sustained activation of Akt in ▵716 cells in response to G-CSF.
Ba/F3 cells transfected with the WT or Δ716 G-CSFR were serum- and cytokine-deprived for 4 hours. The cells were stimulated with 100 ng/mL G-CSF for the indicated times, then lysed and immunoprecipitated with anti-Akt1. The immune complexes were washed and incubated in kinase buffer containing 500 μmol/L ATP, 75 mmol/L MgCl2, 1 μCi of 32PγATP, and 10 μg histone H2B for 20 minutes at 30°C. The reactions were subjected to SDS-PAGE. Phosphorylation of histone H2B was quantified with a PhosphorImager using ImageQuant software. (A) Akt activity was measured as the fold-increase in histone H2B phosphorylation over unstimulated samples. Inset is a linear representation of the data. (B) Detection of histone H2B phosphorylation from samples in (A).
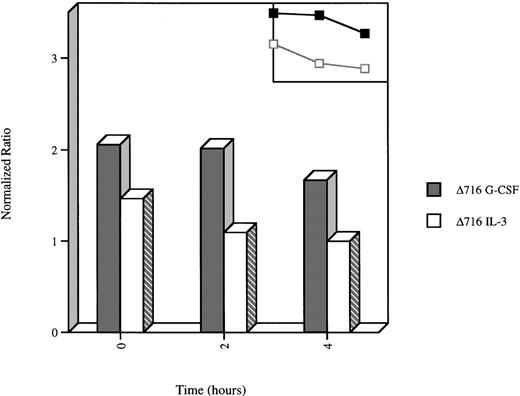
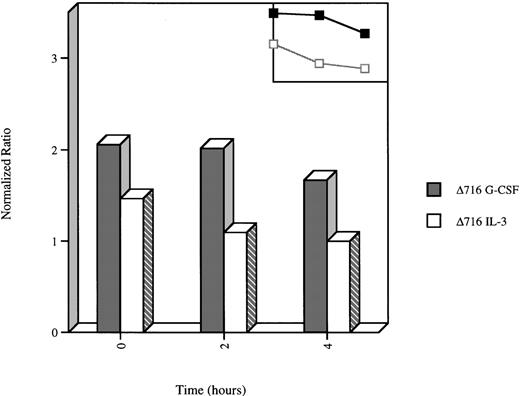
We next examined the time course for extinction of G-CSF–induced Akt activation in WT and Δ716 cells upon withdrawal of the cells from G-CSF–containing media and transfer to media devoid of growth factors. Extinction of G-CSF–induced Akt activation was observed to be delayed in Δ716 cells. As shown in Figure 7, a decrease in the enzymatic activity of Akt was readily apparent by 2 hours after growth factor withdrawal in WT cells grown in G-CSF or IL-3 (data not shown) or in Δ716 cells grown in IL-3 and subsequently deprived of IL-3. In contrast, no reduction in the kinase activity of Akt was apparent in G-CSF–cultured Δ716 cells at 2 hours after transfer to media devoid of all cytokines, and only a minimal reduction in activity was observed at 4 hours. These results suggest that sustained activation of Akt contributes to enhanced survival of Δ716 cells.
Delayed extinction of G-CSF–induced Akt activation in ▵716 cells.
Ba/F3 cells transfected with the Δ716 G-CSFR were grown in RPMI 1640 medium containing 10% FBS and 2 mmol/L glutamine, supplemented with either 10% WEHI-CM (source of IL-3) or 1.9 ng/mL G-CSF for 10 days. The cells were washed twice, then transferred to media devoid of serum and cytokines. At the indicated time points, the cells were lysed, immunoprecipitated with anti-Akt, and subjected to Akt in vitro kinase assays. Data are expressed as the ratio of histone H2B phosphorylation in Akt immunoprecipitates compared with control lysates immunoprecipitated with normal sheep IgG. A linear representation of the data is shown in the inset.
Delayed extinction of G-CSF–induced Akt activation in ▵716 cells.
Ba/F3 cells transfected with the Δ716 G-CSFR were grown in RPMI 1640 medium containing 10% FBS and 2 mmol/L glutamine, supplemented with either 10% WEHI-CM (source of IL-3) or 1.9 ng/mL G-CSF for 10 days. The cells were washed twice, then transferred to media devoid of serum and cytokines. At the indicated time points, the cells were lysed, immunoprecipitated with anti-Akt, and subjected to Akt in vitro kinase assays. Data are expressed as the ratio of histone H2B phosphorylation in Akt immunoprecipitates compared with control lysates immunoprecipitated with normal sheep IgG. A linear representation of the data is shown in the inset.
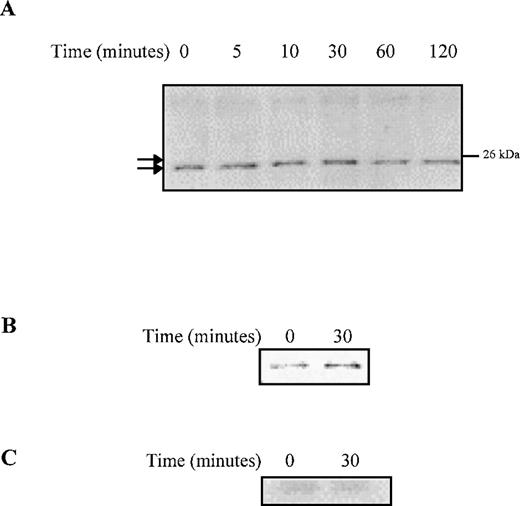
Phosphorylation and cytosolic sequestration of Bad are prolonged in response to G-CSF stimulation in ▵716 cells
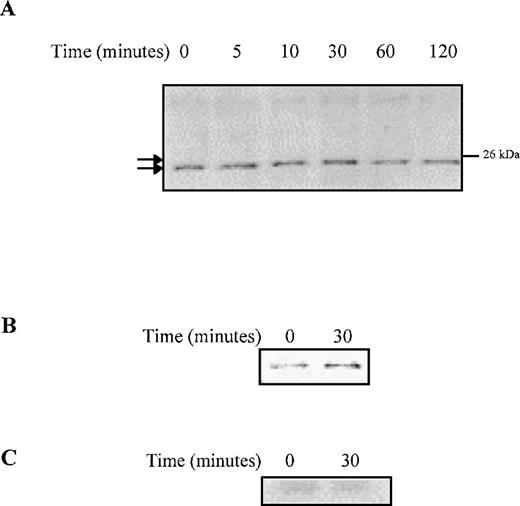
We investigated the downstream targets of Akt that are activated by G-CSF to induce prolonged anti-apoptotic signaling and enhanced survival of Δ716 cells. In response to G-CSF, Bad was phosphorylated in Δ716 cells, as indicated by the upward shift in its migration after G-CSF stimulation (Figure 8). Phosphorylation of Bad correlated with the time course for induction of Akt activation in Δ716 cells in response to G-CSF (Figure 6). Maximal phosphorylation of Bad was observed at 30 minutes, at a time when Akt activation was also maximal in Δ716 cells. Similar to Akt activation, the phosphorylation of Bad was also prolonged in Δ716 cells, with phosphorylated Bad protein still detectable several hours after G-CSF stimulation. Phosphorylated Bad was also found to associate with 14-3-3 in Δ716 cells after G-CSF stimulation, suggesting that Bad was sequestered in the cytosol by 14-3-3 (Figure 8B). Sequestration of Bad in the cytosol should inhibit its binding to Bcl-2 and Bcl-xL, hence promoting cell survival.
G-CSF stimulation induces phosphorylation and cytosolic sequestration of Bad in ▵716 cells.
Ba/F3 cells transfected with the Δ716 G-CSFR were serum- and cytokine-deprived for 4 hours, then stimulated with 100 ng/mL G-CSF for the indicated times and lysed. (A) Whole-cell lysates were resolved by SDS-PAGE and transferred to nitrocellulose for blotting with mouse anti-Bad. Phosphorylated Bad is indicated by an upward shift in mobility (upper tick mark) compared with unphosphorylated Bad (lower tick mark). (B) Whole-cell lysates were immunoprecipitated with anti-Bad monoclonal antibody or (C) an irrelevant antibody (anti–G-CSFR) and blotted with a rabbit polyclonal antibody to 14-3-3.
G-CSF stimulation induces phosphorylation and cytosolic sequestration of Bad in ▵716 cells.
Ba/F3 cells transfected with the Δ716 G-CSFR were serum- and cytokine-deprived for 4 hours, then stimulated with 100 ng/mL G-CSF for the indicated times and lysed. (A) Whole-cell lysates were resolved by SDS-PAGE and transferred to nitrocellulose for blotting with mouse anti-Bad. Phosphorylated Bad is indicated by an upward shift in mobility (upper tick mark) compared with unphosphorylated Bad (lower tick mark). (B) Whole-cell lysates were immunoprecipitated with anti-Bad monoclonal antibody or (C) an irrelevant antibody (anti–G-CSFR) and blotted with a rabbit polyclonal antibody to 14-3-3.
Discussion
Patients with SCN are at increased risk for the development of AML. In recent studies of 78 SCN patients, 8 of 9 patients who developed AML were found to have acquired mutations in the G-CSFR gene.9Ectopic expression of G-CSFR truncation mutants from these patients in both myeloid and lymphoid cell lines has been shown to render cells hyperproliferative to G-CSF.6,12 Notably, mice carrying targeted “knock-in” mutations in the G-CSFR similar to patients with SCN/AML do not develop AML but have been found to have hyperproliferative myeloid progenitors.7-9 In response to G-CSF treatment in vivo, the mutant mice developed peripheral neutrophil counts that significantly exceeded those in mice not carrying the G-CSFR mutation. The absolute numbers of G-CSF–responsive progenitors in the bone marrow of the mutant mice were increased, and these progenitors exhibited increased proliferative responses to G-CSF. CFU-G colonies from the mutant mice were also found to be larger and showed fewer signs of degeneration. The observation that CFU-G colonies from the mutant mice showed less degeneration than those from wild-type mice suggests a defect in the induction of apoptosis in progenitor cells from the mutant mice.
We previously reported that PI3-kinase is activated in response to G-CSF and that its activation promotes cell survival.2PI3-kinase is a heterodimeric lipid kinase composed of an 85-kd regulatory subunit and a 110-kd catalytic subunit.16,17PI3-kinase phosphorylates the 3′-position of the inositol ring on phosphoinositides and generates phosphatidylinositol 3,4-bisphosphate (PtdIns3,4P2) as well as phosphatidylinositol 3,4,5-trisphosphate (PtdIns3,4,5P3). Recently, the S/T kinase Akt was identified as a downstream target of PI3-kinase.13-15,18 The phospholipid product PtdIns3,4,5P3 generated from PI3-kinase activation was shown to bind to the pleckstrin homology domain of Akt and to activate Akt.18 19 Full activation has been shown to require phosphorylation of Akt on S473 and T308 and to be mediated in part by the PtdIns3,4,5P3-dependent protein kinase (PDK1).
Activation of Akt has been shown to protect a variety of cell types from apoptosis.20-24 In the case of IL-3, IL-3 stimulation was shown to induce phosphorylation of Bad, a pro-apoptotic molecule that promotes cell death in part through heterodimerization with the anti-apoptotic proteins Bcl-2 and Bcl-xL.25Phosphorylation of Bad was shown to be mediated by Akt and to result in cytosolic sequestration of Bad by 14-3-3 proteins and inhibition of its binding to Bcl-2 and Bcl-xL.25-28
In this article, we show that anti-apoptotic signaling by the G-CSFR is mediated by the PI3-kinase/Akt pathway and that the introduction of a mutant G-CSFR, as observed in patients with SCN/AML, into transfected cell lines disrupts this pathway to prolong cell survival. Our data with G-CSF support a model similar to that previously reported for IL-3 as well as for IL-2, platelet-derived growth factor, insulin-like growth factor-1, and fibroblast growth factor,20-25 whereby growth factor stimulation activates the PI3-kinase/Akt pathway and induces phosphorylation of Bad. In the case of SCN/AML, activation of Akt and phosphorylation of Bad in response to G-CSF are prolonged, leading to an extension in cell survival.
Aberrations in apoptotic signaling pathways have been shown previously to contribute to myeloid transformation. Targeted disruption of Fas in mice was shown to result in marked increases in granulocyte progenitors despite normal steady-state granulopoiesis. However, Fas-deficient mice generated to constitutively express Bcl-2 that was targeted specifically to myeloid cells were found to develop a fatal disease analogous to human AML-M2.29
As in the case of Fas-deficient mice, which have been shown to require constitutive expression of Bcl-2 as a second “hit” for myeloid transformation,29 studies in mice with targeted G-CSFR mutations also suggest a requirement for a second “hit” for leukemic progression. The data presented here support a model whereby G-CSFR mutations in SCN/AML prolong cell survival to permit sufficient time for the acquisition of additional oncogenic events, which together induce myeloid transformation.
Acknowledgment
We thank Norma Haas for her assistance in the preparation of the manuscript.
Supported by National Cancer Institute grant CA75226.
Reprints:Belinda R. Avalos, The Ohio State University Bone Marrow Transplantation Program, A437A Starling-Loving Hall, 320 W Tenth Ave, Columbus, OH 43210; e-mail: avalos-1@medctr.osu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.