Amegakaryocytic thrombocytopenic purpura (AMTP), first reported by Korn,1 is a rare disease characterized by severe thrombocytopenia associated with a total absence or a selective decrease in bone marrow megakaryocytes. Previous studies suggest a variety of pathogenetic mechanisms for AMTP, such as the intrinsic defect at the megakaryocyte progenitor cell level and humoral and cellular suppression of megakaryocytic differentiation.2Thrombopoietin (TPO), recently isolated, is the hematopoietic factor that potently stimulates megakaryocytopoiesis and platelet production.3 Since TPO is the principal regulator of platelet production, a decreased availability or function of TPO might lead to a form of AMTP. There was a recent report of a patient with pure red-cell aplasia who had a circulating autoantibody against erythropoietin, the primary regulator of red blood cell production.4 These findings suggest that a similar mechanism may be responsible for some cases of AMTP, that is, an autoantibody that blocks the action of endogenous TPO on megakaryocytopoiesis. To explore this possibility, we checked for the presence of antibodies against TPO in a patient (a 70-year-old woman)with AMTP who achieved remission following treatment with cyclosporin A(cyA) with some modifications, according to Hill's report.5
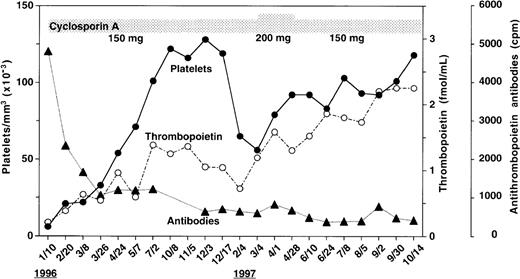
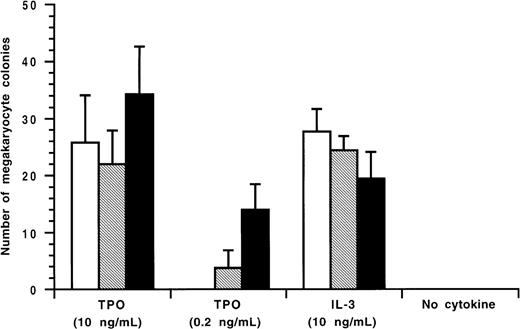
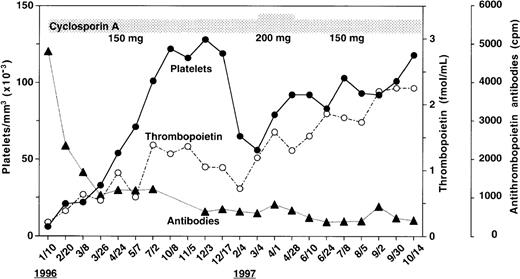
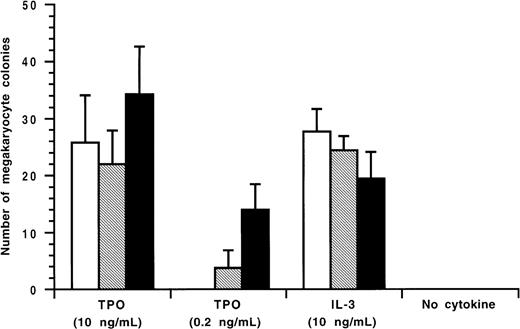
As shown in Figure 1, the patient's serum before therapy had the highest level of anti-TPO IgG antibodies during the scope of the study. With cyA therapy, the antibody levels declined and did not return to the pretreatment value until the end of the study. Endogenous TPO levels appear to be regulated by c-Mpl, the receptor for TPO, expressed on platelets and megakaryocytes, a mechanism of end-cell regulation. Previous studies showed that serum TPO levels were markedly elevated in patients with AMTP before therapy.6 In contrast to these findings, Figure 1 shows that in spite of severe thrombocytopenia, the pretreatment level of TPO(0.21 fmol/mL) was lower than baseline values of normal subjects (0.33 to 1.72 fmol/mL) as previously reported.7 Throughout the study period, circulating platelet counts appeared to increase in response to the elevation in endogenous TPO levels (Figure 1). In an effort to characterize the patient's anti-TPO antibody, we examined the effect of the IgG fractions from the patient's serum on the growth of megakaryocyte colonies from adherent cell-depleted normal human bone marrow mononuclear cells (Figure 2). The IgG fraction before cyA therapy or during therapy (October 14,1997) completely or partially reduced the number of megakaryocyte colonies stimulated with 0.2 ng/mL of glycosylated recombinant human TPO, respectively. This inhibition of megakaryocyte colony formation was able to be overcome by increasing the concentration of human TPO in the cultures to 10 ng/mL. The IgG fractions, however, did not affect human interleukin-3 (IL-3)-induced megakaryocyte colony formation. These data indicate that the IgG autoantibodies can specifically neutralize the in vitro biologic activity of TPO.
Changes in anti-TPO antibody levels, TPO concentrations,and platelet counts during cyA treatment.
Anti-TPO IgG antibodies were measured with a solid-phase radioimmunoassay. Briefly, microtiter plates were incubated with 4μg/mL of recombinant human TPO at 4°C overnight and washed. Test sera diluted at 1:10 in phosphate-buffered saline were added to the wells and incubated at 37°C for 1 hour. After washing, the wells were incubated with radioiodinated protein A at room temperature for 2 hours. Finally, the radioactivity bound to the wells was measured with a gamma counter. Data from the assay validation using rabbit polyclonal IgG antibodies against human TPO showed that the intra- and interassay coefficients of variation ranged from 6.3% to 10.3% and from 7.9% to 13.4%, respectively. Serum TPO levels were measured as described previously.7
Changes in anti-TPO antibody levels, TPO concentrations,and platelet counts during cyA treatment.
Anti-TPO IgG antibodies were measured with a solid-phase radioimmunoassay. Briefly, microtiter plates were incubated with 4μg/mL of recombinant human TPO at 4°C overnight and washed. Test sera diluted at 1:10 in phosphate-buffered saline were added to the wells and incubated at 37°C for 1 hour. After washing, the wells were incubated with radioiodinated protein A at room temperature for 2 hours. Finally, the radioactivity bound to the wells was measured with a gamma counter. Data from the assay validation using rabbit polyclonal IgG antibodies against human TPO showed that the intra- and interassay coefficients of variation ranged from 6.3% to 10.3% and from 7.9% to 13.4%, respectively. Serum TPO levels were measured as described previously.7
Inhibitory effect of the patient's IgG on TPO-induced human megakaryocyte colony formation.
The megakaryocyte progenitor assay was performed in a soft agar medium containing recombinant human TPO or recombinant human IL-3 as described previously.8 Glycosylated recombinant human TPO or recombinant human interleukin-3 was preincubated with the patient's IgG fractions before therapy (January 10, 1996) or during therapy(October 14, 1997), and added to a soft agar culture containing nonadherent human bone marrow mononuclear cells. After 14 days of culture, megakaryocyte colonies were immunohistochemically stained and counted. IgG fraction (96/1/10) is indicated by □; IgG fraction (97/10/14) is indicated by ▧; and 0.1% bovine serum albumin is indicated by ▪.
Inhibitory effect of the patient's IgG on TPO-induced human megakaryocyte colony formation.
The megakaryocyte progenitor assay was performed in a soft agar medium containing recombinant human TPO or recombinant human IL-3 as described previously.8 Glycosylated recombinant human TPO or recombinant human interleukin-3 was preincubated with the patient's IgG fractions before therapy (January 10, 1996) or during therapy(October 14, 1997), and added to a soft agar culture containing nonadherent human bone marrow mononuclear cells. After 14 days of culture, megakaryocyte colonies were immunohistochemically stained and counted. IgG fraction (96/1/10) is indicated by □; IgG fraction (97/10/14) is indicated by ▧; and 0.1% bovine serum albumin is indicated by ▪.
The effectiveness of cyA in improving thrombocytopenia strongly suggests an immune-mediated pathogenetic mechanism in the patient. Although it is unclear that anti-TPO autoantibody is the sole cause of the thrombocytopenia, the recovery of serum TPO levels and peripheral platelet counts appeared to be closely related to the decrease in the antibody levels. Low levels of measurable TPO before cyA therapy could be due to the antibody-mediated increased clearance of TPO or the interference by the antibody in the detection of serum TPO. In either case, the autoantibodies might result in a decrease in the effective TPO concentrations for stimulating megakaryocytopoiesis, leading to thrombocytopenia. In recent clinical trials of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF), a very small proportion of healthy volunteers who had received repeated subcutaneous injections of PEG-rHuMGDF-developed antibodies against endogenous TPO and eventually became thrombocytopenic (unpublished data). The present results, together with these observations, suggest that an autoantibody against TPO should be included in the pathogenetic mechanisms underlying AMTP.