Abstract
In response to the chemoattractants interleukin 8, C5a,N-formyl-methionyl-leucyl-phenylalanine, and interleukin 15, adhesion molecules P-selectin glycoprotein ligand 1 (PSGL-1), intercellular adhesion molecule 3 (ICAM-3), CD43, and CD44 are redistributed to a newly formed uropod in human neutrophils. The adhesion molecules PSGL-1 and ICAM-3 were found to colocalize with the cytoskeletal protein moesin in the uropod of stimulated neutrophils. Interaction of PSGL-1 with moesin was shown in HL-60 cell lysates by isolating a complex with glutathione S-transferase fusions of the cytoplasmic domain of PSGL-1. Bands of 78- and 81-kd were identified as moesin and ezrin by Western blot analysis. ICAM-3 and moesin also coeluted from neutrophil lysates with an anti-ICAM-3 immunoaffinity assay. Direct interaction of the cytoplasmic domains of ICAM-3 and PSGL-1 with the amino-terminal domain of recombinant moesin was demonstrated by protein-protein binding assays. These results suggest that the redistribution of PSGL-1 and its association with intracellular molecules, including the ezrin-radixin-moesin actin-binding proteins, regulate functions mediated by PSGL-1 in leukocytes stimulated by chemoattractants.
Leukocytes emigrating from the bloodstream toward an inflammatory focus follow different chemoattractant signals that guide their path.1 An early response of these cells to such stimuli is a transition from a spherical to a polarized morphologic conformation, which occurs as soon as the cell begins to move.2 This response also occurs with several chemokines that induce both leukocyte activation and migration.3,4Interleukin 8 (IL-8) belongs to the subfamily of CXC chemokines that mainly attract neutrophils. It is released in response to inflammatory stimuli and induces the transmigration of neutrophils across vascular endothelium.5 CXC chemoattractants induce the full range of neutrophil activation events, including oxygen free-radical production and degranulation. In contrast, other neutrophil-activating agents, such as tumor necrosis factor α (TNF-α) do not act as primary chemotactic factors and induce degranulation only after priming with other agents.6,7 Similar to the classic leukocyte chemoattractants C5a and N-formyl peptides, chemokines bind to 7-trans-membrane spanning receptors coupled to a heterotrimeric G-protein signaling pathway.8-10
On activation, endothelial cells express specific adhesion molecules that are able to bind free-flowing neutrophils. The initial contact and rolling of neutrophils along the endothelium are predominantly mediated by E- and P-selectins, which are expressed by activated endothelium, and by L-selectin, which is constitutively expressed on leukocytes.11 All 3 selectins bind, in a calcium-dependent manner, to sialylated and fucosylated oligosaccharides such as sialyl Lewisa and its isomer sialyl Lewisx.11,12 P-selectin glycoprotein ligand 1 (PSGL-1) has been identified as a ligand for all 3 selectins.13 This molecule is expressed on leukocytes and belongs to the family of heavily O-glycosylated sialomucins rich in serine and threonine.14-16 PSGL-1 requires sulfation for high-affinity binding to both L- and P-selectin.16,17 P-selectin binds to PSGL-1 on all myeloid cells but only in a subset of T lymphocytes corresponding to memory cells.15 16
Chemokines cause polarization of lymphocytes and redistribution of several adhesion molecules to the uropod, a cytoplasmic projection that forms at the rear end of the moving cell.18-20 Similarly, activation of neutrophils with platelet-activating factor or theN-formyl-methionyl-leucyl-phenylalanine (fMLP) causes the redistribution of PSGL-1 to the uropod and diminished adhesion to P-selectin.21 However, adhesion molecules that are concentrated in the uropod facilitate binding to other cells. This has been described for PSGL-1 in interactions between neutrophils and platelets22 and for ICAMs in lymphocyte cell-cell interactions.23
A similar redistribution has been observed for members of the closely related ezrin-moesin-radixin (ERM) family of proteins in locomoting lymphocytes. Moesin is codistributed and interacts with ICAM-3, CD43, and CD44 adhesion molecules in the distal portion of uropods.24,25 The ERM proteins function as linking proteins between the plasma membrane and the actin cytoskeleton26-28and are generally associated with cell-surface protrusions such as microvilli, filopodia, microspikes, retraction fibers, and membrane ruffling.29-32 Suppression of their expression has profound effects on cell functions.33 Ezrin and moesin are also expressed in neutrophils,34 and because activation with different chemoattractants and movement depend on adhesion and on the actin cytoskeleton, we explored the distribution of a variety of adhesion molecules and moesin and possible interactions between them. We found that several receptors were distributed to the uropod of migrating neutrophils, where they were codistributed with moesin. Moreover, we observed that, in vitro, the amino-terminal domain of moesin interacted directly with the cytoplasmic domain of at least 2 of these receptors—ICAM-3 and PSGL-1.
Materials and methods
Antibodies, chemokines, and reagents
The anti-ICAM-3 HP2/19 and TP1/25, anti-ICAM-1 MEM 111, anti-CD44 HP2/9, anti-CD43 TP1/36, anti-CD45 D3/9, anti-PSGL-1 KPL1, and antimoesin/antiradixin 38/37 monoclonal antibodies (mAbs) were described previously.17,25,35,36,37 Antimoesin 95/2 and the antiezrin 90/3 polyclonal antisera (pAb) were raised in rabbits by immunization with recombinant human moesin and purified human ezrin, respectively.29 38 Antivinculin mAb was purchased from Sigma Chemical Co (St Louis, MO). Goat anti-GRP78 (HSP78) pAb was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Aprotinin, iodoacetamide, C5a, human recombinant TNF-α, and fMLP were purchased from Sigma. IL-8 and interleukin 15 (IL-15) were purchased from Peprotech EC Ltd (London, UK). The 80-kd fibronectin fragment (FN80) was a gift of A. Garcı́a Pardo (Centro de Investigaciones Biológicas, Madrid, Spain). A mixture of protease inhibitors (Complete Protease Inhibitors) was from Boehringer Mannheim Corp (Indianapolis, IN). Sodium orthovanadate was purchased from Sigma.
Cells
Neutrophils were isolated from fresh human blood by Ficoll-Hypaque density gradient centrifugation, which was followed by sedimentation at 1g in dextran (Sigma) at room temperature. The neutrophil-enriched fraction was further purified by hypotonic lysis of erythrocytes, which yielded a purity of greater than 98%. Neutrophils were resuspended in 0.05% heat-denatured bovine serum albumin (BSA) RPMI 1640 medium. Human umbilical vein endothelial cells were obtained as described previously.39 The umbilical vein was cannulated, washed, and incubated with 0.1% collagenase P (Boehringer Mannheim) for 20 minutes at 37°C. Detached cells were then seeded into flasks and cultured in M199 medium (BioWhittaker, Walkersville, MD) supplemented with 20% fetal-calf serum, 50 μg/mL of endothelial cell growth supplement, and 100 μg/mL of heparin (Sigma).
Immunofluorescence microscopy
Purified neutrophils (7 × 105) were incubated in flat-bottomed, 24-well plates (Costar Corp, Cambridge, MA) in a final volume of 400 μL of RPMI 1640 with 0.05% heat-denatured BSA on coverslips coated with FN80 (20 μg/mL). After 10 minutes, 2 nmol/L of a chemoattractant was added, except for IL-15, which was used at 10 nmol/L. The cells were incubated at 37°C in a 5% carbon dioxide atmosphere for 5 minutes, fixed with 1% (wt/vol) paraformaldehyde in phosphate-buffered saline (pH 7.4) at room temperature, and rinsed in triethanolamine-buffered saline (TBS). Cells were then incubated with the selected mAb, washed, and stained with a rabbit F(ab′)2 antimouse IgG (Pierce Montlucon, France) labeled with fluorescein-isothiocyanate, conjugated (FITC). The number of uropod-bearing cells was determined in each experiment by direct counting of the total cells (300-400) and the uropod-bearing cells in 10 randomly selected fields (60 × objective).
For double immunofluorescence analysis of neutrophils, fixed cells were incubated with the 38/87 mAb and then with a tetrarhodamine isothiocyanate (TRITC)-labeled rabbit antimouse IgG. Subsequently, coverslips were saturated with normal mouse serum and incubated with FITC-conjugated TP1/24 anti-ICAM-3 mAb. In some experiments, neutrophils were incubated for 5 minutes with confluent monolayers of endothelial cells previously activated with TNF-α (20 ng/mL) for 4 hours. For double staining of endothelial cells and neutrophils, coverslips were incubated with anti-ICAM-1 mAb MEM 111 followed by TRITC-labeled rabbit antimouse IgG. Coverslips were then saturated with normal mouse serum and incubated with FITC-conjugated TP1/24 anti-ICAM-3 mAb. Cells were observed with 60 × and 100 × oil-immersion objectives and a photo-microscope (Nikon Labophot-2; Nikon, Inc, Melville, NY). Images were recorded on Ektachrome 400 film (Eastman Kodak Co, Rochester, NY).
Construction, expression, and purification of glutathione S-transferase (GST) fusion proteins
Complementary DNA fragments for expression of the cytoplasmic regions of ICAM-3 and PSGL-1 were obtained by polymerase chain reaction amplification and cloned as SalI-NotI fragments into pGEX-4T (Pharmacia LKB Biotechnology, Uppsala, Sweden). Primers for amplification of the PSGL-1 cytoplasmic region were SALCYPSGL5′-TCCGCCGTCGACCGCCTCTCCCGCAAGGGCCACA-3′ and NOTCYPSGL 5′-ATAAGAATGCGGCCGCCTAAGGGAGGAAGCTGTGCA-3′; those for ICAM-3 were SALCY50TH 5′-CAACGCGTCGACCAGGGAGCACCAACGG-3′ and NOTCY50TH 5′-ATAAGAATGCGGCCGCTCACTCACTCAGCTCTGGA-3′.
Constructs for expression of GST fusions of full-length moesin (pGhuMo) and its amino-terminal region containing amino acid residues 1 to 310 (pKG-MSNn) were described previously.40
Expression of GST-fusion proteins in DHIOB cells and purification were carried out by following the manufacturer's instructions. Proteins were stored at 4°C on glutathione-Sepharose 4B beads as a 50% slurry in 10 mmol/L of Tris (pH 7.4), 140 mmol/L of sodium chloride (NaCl), 0.5% Triton X-100, 0.02% sodium azide (NaN3), and protease inhibitors (Boehringer Mannheim). The concentration of fusion proteins was estimated by Coomassie-blue staining of polypeptide bands after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). BSA was used as the standard protein for estimating concentrations of recombinant proteins.
Isolation of moesin by ICAM-3 antibody-affinity chromatography and Western blot analysis
ICAM-3 was isolated as described previously.37 Briefly, human neutrophils were lysed in 20 mmol/L of Tris-hydrochloric acid (HCl) (pH 8.0), 0.5% Triton X-100 buffer, 5 mmol/L of iodoacetamide, protease inhibitors, and 0.025% NaN3. Cell lysates were centrifuged at 2500g for 60 minutes, and the supernatants were passed through a Sepharose CL-4B anti-ICAM-3 mAb column. The column was washed with 50 mmol/L of ethanolamine (pH 10), 0.5 mol/L of NaCl, and 1% N-octylglucoside, and bound proteins were eluted in a step-wise manner with the same buffer, adjusted to pH 11, pH 11.5, and pH 12. Eluted fractions were identified by 8% SDS-PAGE, and proteins were visualized with use of silver staining. Fractions containing ICAM-3 were pooled and concentrated 30-fold by using microconcentrators (Centricon-100; Amicon, Danvers, MA).
Concentrated fractions were subjected to 8% SDS-PAGE under reducing conditions and transferred onto a nitrocellulose membrane (Millipore Corp, Bedford, MA) in Tris-glycine-methanol buffer for 30 minutes at 17 V by using a transfer cell (Transfer-Blot SD Semi-Dry Transfer Cell; Bio-Rad Laboratories, Hercules, CA). To detect moesin or ezrin, membranes were blocked overnight in TBS containing 3% BSA, washed 3 times with TBS and 0.1% Tween 20 for 15 minutes, and incubated for 1 hour with a 1:1000 dilution of the rabbit antimoesin pAb 95/2 or with a 1:4000 dilution of the rabbit antiezrin pAb 90/3. After 3 washes, blots were incubated with a peroxidase-conjugated goat antirabbit IgG (Pierce Montlucon), and proteins were visualized with use of an enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK).
Purification of recombinant moesin and in vitro binding assay
Recombinant full-length moesin and the N-terminal domain of moesin containing amino acid residues 1 to 310 were expressed inEscherichia coli and isolated and purified as GST-fusion proteins as described previously.40 41 Cleavage of the fusion protein bound to glutathione-Sepharose 4B beads (Pharmacia LVB Biotech) preequilibrated with 50 U/mL of thrombin (Pharmacia LVB Biotech) in Tris buffer (50 mmol/L of Tris-HCl [pH 7.5] and 150 mmol/L of NaCl) occurred after shaking at room temperature for 12 hours. The supernatant was loaded onto a 0.8 × 4-cm column (Pharmacia LVB Biotech) preequilibrated with washing buffer (50 mmol/L of Tris-HCl [pH 7.5], 1 mmol/L of phenylmethylsulfonyl fluoride, and 4 μg of leupeptin); the column included 6 mL of heparin and Sepharose. Proteins were eluted by using a step gradient of 0.2 mol/L, 0.4 mol/L, 0.5 mol/L, and 1 mol/L of NaCl. All purification procedures were performed at 4°C. Fractions containing full-length and N-terminal moesin were pooled and dialyzed against Tris buffer (50 mmol/L of Tris-HCl [pH 7.5] and 150 mmol/L of NaCl). The amount of recombinant moesin obtained was estimated by SDS-PAGE with use of known amounts of BSA.
Recombinant moesin was assayed for binding activity by incubation with glutathione- Sepharose–linked GST-ICAM-3 and GST-PSGL-1 fusion proteins in binding buffer (150 mmol/L of NaCl, 20 mmol/L of Tris-HCl [pH 8.3] and 0.2% Triton X-100) at 4°C. After 1.5 hours, the beads were washed 6 times in binding buffer. GST fusion proteins and their associated proteins were eluted by using 50 mmol/L of Tris buffer containing 20 mmol/L of glutathione. Bound moesin was assessed by SDS-PAGE and immunoblotting using moesin 95/2 pAb.
In vitro translation and binding assay
The pCR3 plasmids carrying the N-terminal domain of moesin containing inserts of amino acid residues 1 to 310 (MSNn/pCR3) were described previously.40 These plasmids were transcribed and translated in vitro by using a TNT-coupled rabbit reticulocyte lysate system (Promega, Madison, WI) in the presence of T7 RNA polymerase and methionine labeled with sulfur 35 (35S), according to the manufacturer's instructions. Isotope-labeled moesin was incubated in binding buffer (20 mmol/L of Tris-HCl [pH 8.3], 150 mmol/L of NaCl, and 0.2% Triton X-100) at 4°C with GST-ICAM-3 and PSGL-1 fusion proteins that had been immobilized on glutathione-Sepharose beads. After 1.5 hours, the beads were washed 6 times in binding buffer, resolved on SDS-PAGE, and subjected to autoradiography.
Results
Induction by chemoattractants of redistribution of adhesion receptors and moesin to the uropod in neutrophils
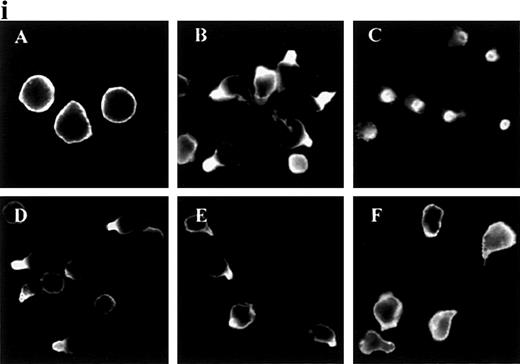
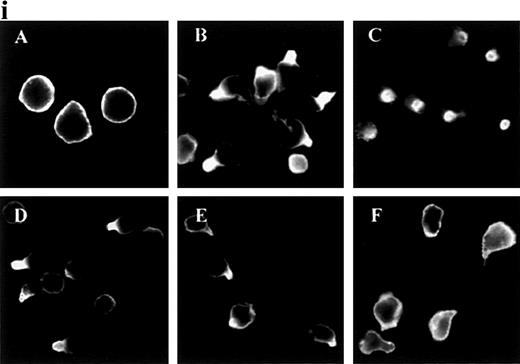
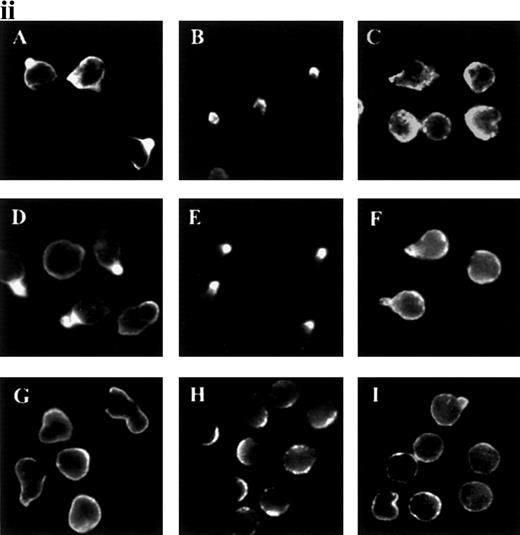
In response to chemoattractants, neutrophils begin to migrate and to form uropods, and we decided to analyze this response in detail. ICAM-3 and other adhesion molecules were evenly distributed in the plasma membrane in unstimulated neutrophils (Figure1i and data not shown). When treated with the chemokine IL-8, the adhesion molecules ICAM-3, PSGL-1, CD43, and CD44, but not CD45, redistributed to uropods. Other chemoattractants (fMLP and C5a) and the chemotactic cytokine IL-15 also triggered this response for ICAM-3 and PSGL-1 (Figure 1ii and data not shown). IL-8 and C5a were the most potent chemoattractants in inducing receptor polarization. In contrast, TNF-α did not act as a primary chemotactic factor, and it caused neither uropod formation nor receptor redistribution (Figure 1ii).
Redistribution of adhesion receptors to the uropod of neutrophils stimulated by chemoattractants.
(i) Neutrophils were allowed to bind to coverslips coated with 20 μg/mL of an 80-kd fibronectin (FN80) fragment for 10 minutes at 37°C and stimulated with interleukin 8 (IL-8) for an additional 5 minutes. Neutrophils were then fixed and stained with the following monoclonal antibodies (mAb): anti-intercellular adhesion molecule 3 (ICAM-3) TP1/25 (B), anti-P-selectin glycoprotein ligand 1 (PSGL-1) KPL-1 (C), anti-CD43 TP1/36 (D), anti-CD44 HP2/9 (E), and anti-CD45 D3/9 (F). The control was unstimulated neutrophils stained with the anti-ICAM-3 TP1/25 mAb (A). (ii) Neutrophils were stimulated in the presence of 2 nmol/L of C5a (A, B, and C),N-formyl-methionyl-leucyl-phenylalanine (D, E, and F), or tumor necrosis factor (TNF-α) (G, H, and I) for 5 minutes at 37°C. Cells were then fixed and stained with the following mAb: anti-ICAM-3 TP1/25 (A, D, and G), anti-PSGL-1 KPL1 (B, E, and H), and anti-CD45 D3/9 (C, F, and I).
Redistribution of adhesion receptors to the uropod of neutrophils stimulated by chemoattractants.
(i) Neutrophils were allowed to bind to coverslips coated with 20 μg/mL of an 80-kd fibronectin (FN80) fragment for 10 minutes at 37°C and stimulated with interleukin 8 (IL-8) for an additional 5 minutes. Neutrophils were then fixed and stained with the following monoclonal antibodies (mAb): anti-intercellular adhesion molecule 3 (ICAM-3) TP1/25 (B), anti-P-selectin glycoprotein ligand 1 (PSGL-1) KPL-1 (C), anti-CD43 TP1/36 (D), anti-CD44 HP2/9 (E), and anti-CD45 D3/9 (F). The control was unstimulated neutrophils stained with the anti-ICAM-3 TP1/25 mAb (A). (ii) Neutrophils were stimulated in the presence of 2 nmol/L of C5a (A, B, and C),N-formyl-methionyl-leucyl-phenylalanine (D, E, and F), or tumor necrosis factor (TNF-α) (G, H, and I) for 5 minutes at 37°C. Cells were then fixed and stained with the following mAb: anti-ICAM-3 TP1/25 (A, D, and G), anti-PSGL-1 KPL1 (B, E, and H), and anti-CD45 D3/9 (C, F, and I).
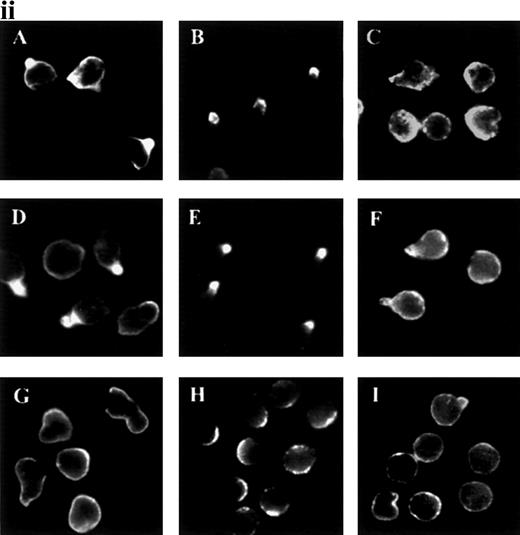
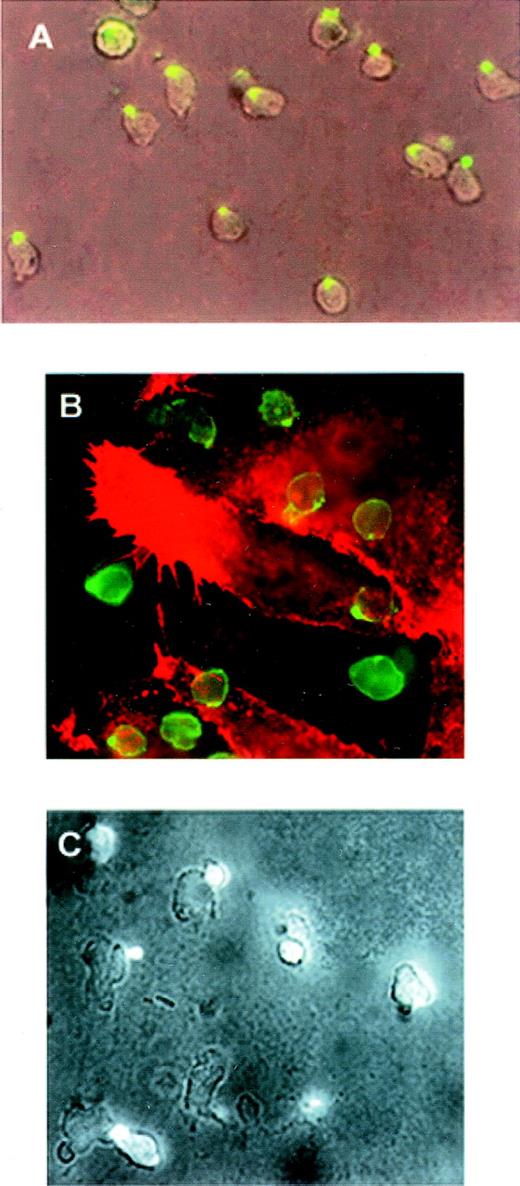
Polarization and redistribution of PSGL-1 were also observed in neutrophils that migrated toward a gradient of C5a (Figure2A). Similarly, PSGL-1 and ICAM-3 redistributed in neutrophils that transmigrated across a monolayer of endothelial cells activated by TNF-α (Figure 2B and 2C).
Polarization of PSGL-1 in neutrophils migrating toward a chemoattractant gradient or on endothelial cells.
(A) Neutrophils were allowed to adhere to FN80-coated coverslips for 10 minutes. C5a was then deposited at one edge of the bottom of the well (lower right-hand corner of photograph) and cells were allowed to migrate for 5 minutes. Cells were fixed and stained with anti-PSGL-1 KPL-1 mAb (green fluorescence). Epifluorescence and bright-field conditions were photographed on the same frame by means of double exposure. (B) Neutrophils were allowed to adhere to an endothelial cell monolayer activated by TNF-α for 5 minutes at 37°C. Cells were then fixed and double stained with anti-ICAM-3 TP1/25 mAb (green fluorescence) and anti-ICAM-1 MEM111 (red fluorescence) mAb. (C) Neutrophils were allowed to adhere to an endothelial cell monolayer activated by TNF-α for 5 minutes at 37°C. Cells were then fixed and stained with anti-PSGL-1 KPL-1 mAb. Epifluorescence and bright-field conditions were photographed on the same frame by means of double exposure.
Polarization of PSGL-1 in neutrophils migrating toward a chemoattractant gradient or on endothelial cells.
(A) Neutrophils were allowed to adhere to FN80-coated coverslips for 10 minutes. C5a was then deposited at one edge of the bottom of the well (lower right-hand corner of photograph) and cells were allowed to migrate for 5 minutes. Cells were fixed and stained with anti-PSGL-1 KPL-1 mAb (green fluorescence). Epifluorescence and bright-field conditions were photographed on the same frame by means of double exposure. (B) Neutrophils were allowed to adhere to an endothelial cell monolayer activated by TNF-α for 5 minutes at 37°C. Cells were then fixed and double stained with anti-ICAM-3 TP1/25 mAb (green fluorescence) and anti-ICAM-1 MEM111 (red fluorescence) mAb. (C) Neutrophils were allowed to adhere to an endothelial cell monolayer activated by TNF-α for 5 minutes at 37°C. Cells were then fixed and stained with anti-PSGL-1 KPL-1 mAb. Epifluorescence and bright-field conditions were photographed on the same frame by means of double exposure.
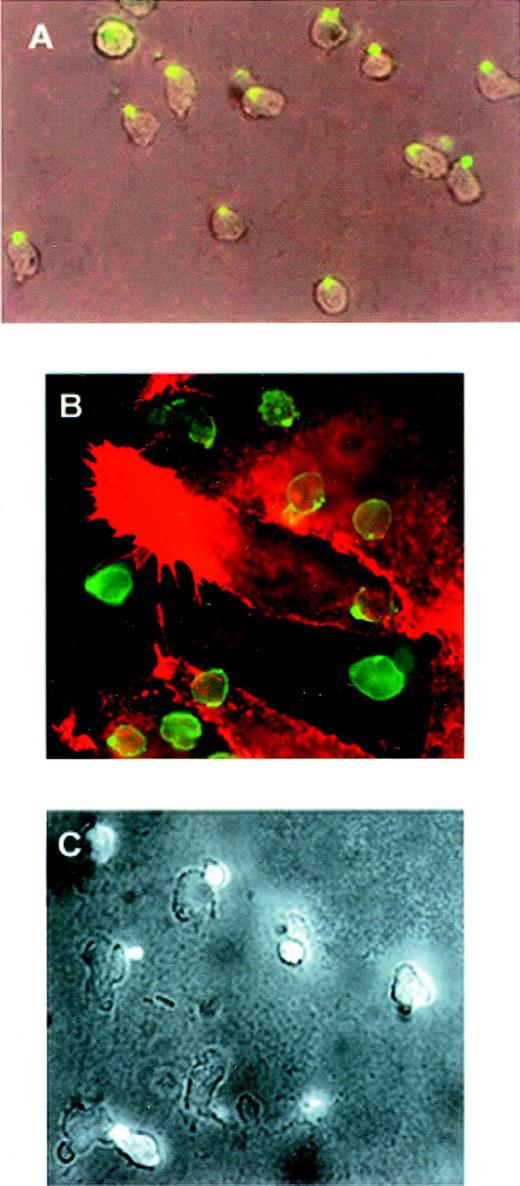
In studying possible mechanisms for the redistribution of these receptors, we found that moesin colocalized with PSGL-1 and ICAM-3 and was apparently also concentrated in uropods of neutrophils activated with IL-8 (Figure 3).
Redistribution of PSGL-1, ICAM-3, and moesin to the uropod of polarized neutrophils.
Neutrophils adhering to FN80 were stimulated with IL-8 for 5 minutes. Cells were then fixed and stained. (A) ICAM-3. (B) PSGL-1. Inset shows colocalization of PSGL-1 (a) and ICAM-3 (b). (C) Moesin. Inset shows colocalization of moesin (c) and ICAM-3 (d).
Redistribution of PSGL-1, ICAM-3, and moesin to the uropod of polarized neutrophils.
Neutrophils adhering to FN80 were stimulated with IL-8 for 5 minutes. Cells were then fixed and stained. (A) ICAM-3. (B) PSGL-1. Inset shows colocalization of PSGL-1 (a) and ICAM-3 (b). (C) Moesin. Inset shows colocalization of moesin (c) and ICAM-3 (d).
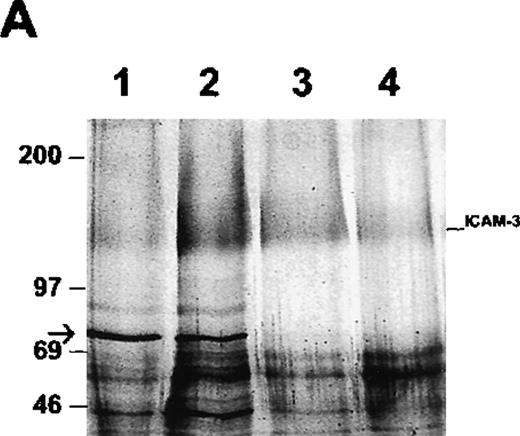
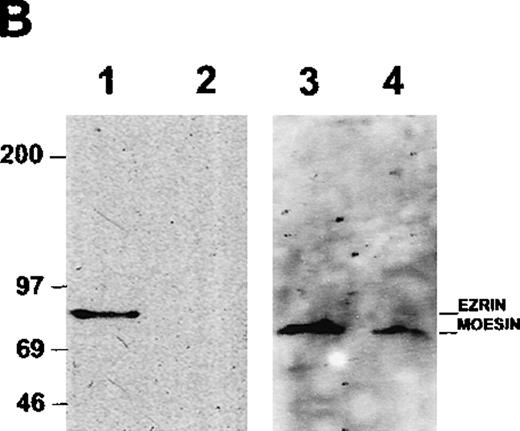
Coelution of ICAM-3 and moesin from human neutrophils
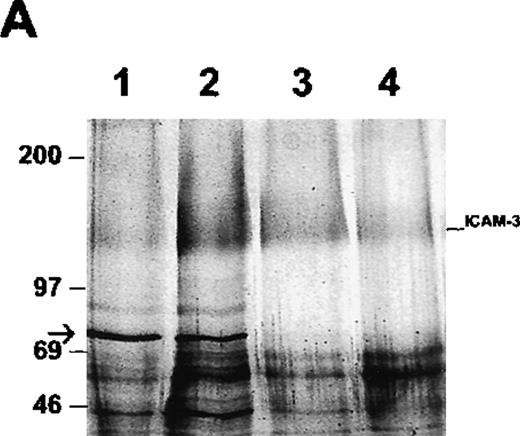
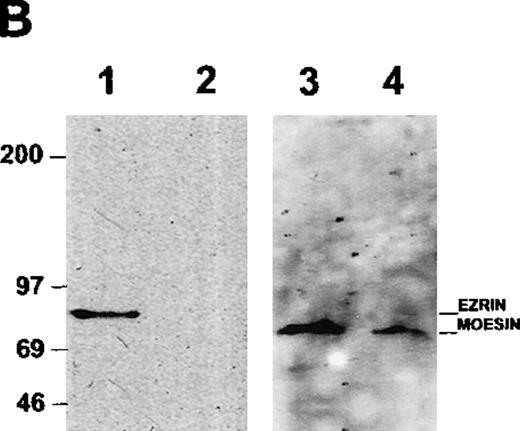
To determine whether ICAM-3 is associated with moesin in neutrophils, ICAM-3 was isolated from lysates by antibody-affinity chromatography. A protein of 78 kd eluted at approximately pH 11 and was partly separated from ICAM-3, which eluted at pH 11.5 as a broad band of nearly 125 kd (Figure 4A). When the pooled fraction containing ICAM-3 was analyzed by immunoblotting, moesin was detected (Figure 4B). This immunoreactive polypeptide corresponded to the 78-kd band on the gel (Figure 4A). The original lysate of neutrophils reacted with antibodies to both moesin and ezrin,34 although higher amounts of moesin than of ezrin were detected with specific antibodies (data not shown). Ezrin was not detected in the ICAM-3 fraction (Figure 4B). To exclude a possible nonspecific effect, the pooled fractions were also analyzed in additional control experiments by immunoblotting with antibodies to GRP78, an immunoglobulin-binding and possible contaminating protein with a molecular mass similar to that of moesin. No reactivity was observed (data not shown).
Association of ICAM-3 and moesin in neutrophils.
ICAM-3 was isolated from human neutrophil lysates by antibody-affinity chromatography. (A) A polypeptide of 78 kd (arrow) eluted in fractions at pH 11.0 (lanes 1-2) while ICAM-3 coeluted at pH 11.0 (lane 2) and separated from the 78-kd polypeptide at pH 11.5 (lanes 3-4). (B) Western blot analysis of purified recombinant ezrin (lane 1) and the pooled ICAM-3 fraction (lane 2) with ezrin antibodies and the same pooled ICAM-3 fraction (lane 3) and recombinant moesin (lane 4) with moesin antibodies. A strong signal for moesin showed that the 78-kd polypeptide coeluting with ICAM-3 (Figure 4A) corresponded to moesin.
Association of ICAM-3 and moesin in neutrophils.
ICAM-3 was isolated from human neutrophil lysates by antibody-affinity chromatography. (A) A polypeptide of 78 kd (arrow) eluted in fractions at pH 11.0 (lanes 1-2) while ICAM-3 coeluted at pH 11.0 (lane 2) and separated from the 78-kd polypeptide at pH 11.5 (lanes 3-4). (B) Western blot analysis of purified recombinant ezrin (lane 1) and the pooled ICAM-3 fraction (lane 2) with ezrin antibodies and the same pooled ICAM-3 fraction (lane 3) and recombinant moesin (lane 4) with moesin antibodies. A strong signal for moesin showed that the 78-kd polypeptide coeluting with ICAM-3 (Figure 4A) corresponded to moesin.
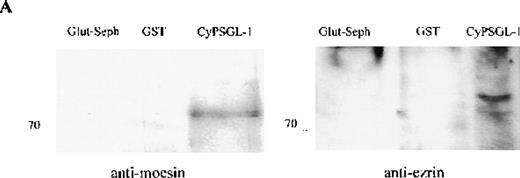
Interaction of ERM proteins with the cytoplasmic region of PSGL-1
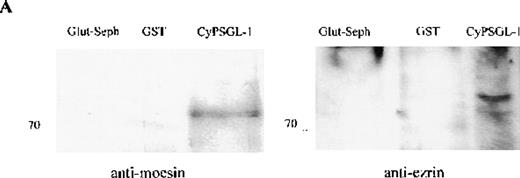
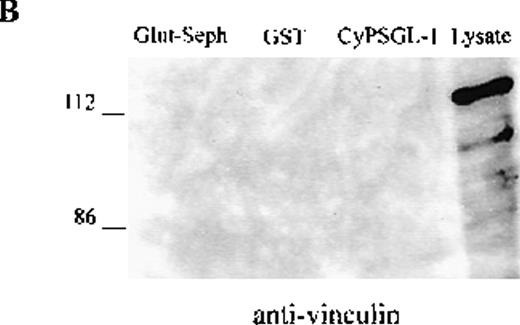
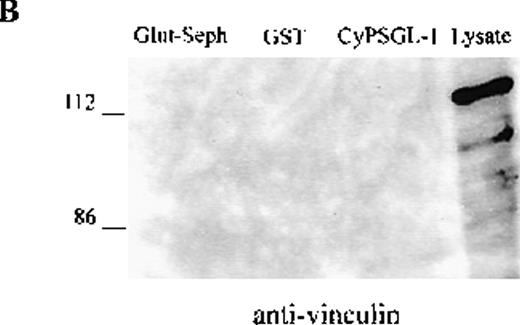
To investigate further the association between adhesion proteins and moesin, GST-fusion proteins of the cytoplasmic domains of PSGL-1 and ICAM-3 were prepared. Instead of neutrophils, HL-60 cells were metabolically labeled with 35S met-cys to search for labeled proteins in the lysate that would bind to the cytoplasmic domains of these 2 membrane proteins. Several radiolabeled polypeptides were recovered from the GST-PSGL-1– and ICAM-3–glutathione-Sepharose but not from control beads containing either GST or no ligand that included polypeptides of 75-78 kd (data not shown). In parallel experiments, lysates of unlabeled HL-60 cells were incubated with the GST-PSGL-1 cytoplasmic-domain fusion protein, and bound proteins were analyzed by Western blot assessment using moesin and ezrin antibodies. As shown in Figure5, the cytoplasmic region of PSGL-1 bound 78- and 81-kd polypeptides that were identified as moesin and ezrin, respectively (Figure 5A). In contrast, none of the proteins bound to PSGL-1 corresponded to vinculin (Figure 5B).
Western blot analysis of proteins interacting with the cytoplasmic tail of PSGL-1 in HL-60 cells.
HL-60 cell lysates were incubated with equivalent amounts of glutathione-Sepharose, glutathione S-transferase (GST), and GST-CyPSGL-1 fusion protein bound to glutathione-Sepharose 4B beads. Each immunoprecipitate was immunoblotted after 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with antimoesin 95/2 polyclonal antibody (pAb), antiezrin 90/3 pAb (A), or antivinculin mAb (B). Cell lysate blotted with antivinculin was included as the positive control.
Western blot analysis of proteins interacting with the cytoplasmic tail of PSGL-1 in HL-60 cells.
HL-60 cell lysates were incubated with equivalent amounts of glutathione-Sepharose, glutathione S-transferase (GST), and GST-CyPSGL-1 fusion protein bound to glutathione-Sepharose 4B beads. Each immunoprecipitate was immunoblotted after 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with antimoesin 95/2 polyclonal antibody (pAb), antiezrin 90/3 pAb (A), or antivinculin mAb (B). Cell lysate blotted with antivinculin was included as the positive control.
Interaction of PSGL-1 and ICAM-3 with the amino-terminal domain of moesin
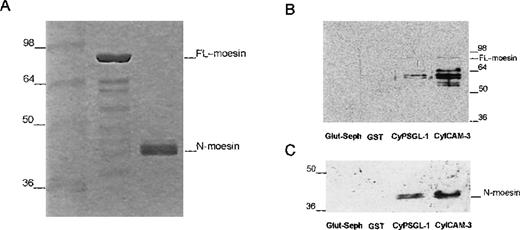
Several studies have shown that the binding of moesin and related proteins to membrane proteins requires additional factors that may include a conformational change to expose potential binding sites.28,40 41 When purified soluble recombinant full-length moesin or the isolated amino-terminal domain proteins were added to beads containing GST-fusion proteins of the cytoplasmic domains of PSGL-1 and ICAM-3, we found only trace amounts of full-length moesin (Figure 6); however, there were much stronger signals with smaller immunoreactive polypeptides (∼60 kd). In contrast to the weak binding observed for full-length moesin, the N-terminal domain of moesin bound strongly to the cytoplasmic domain of both PSGL-1 and ICAM-3 (Figure 6C). This result suggests that the bound immunoreactive polypeptides shown in Figure 6B represented enriched degradation products that contaminated the purified full-length moesin preparation.
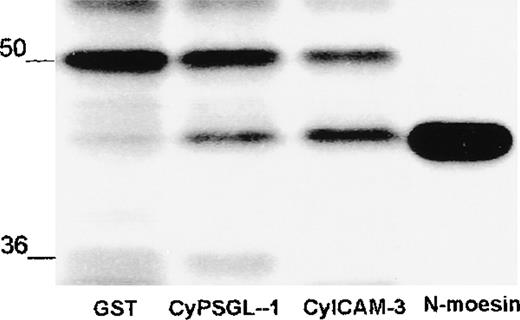
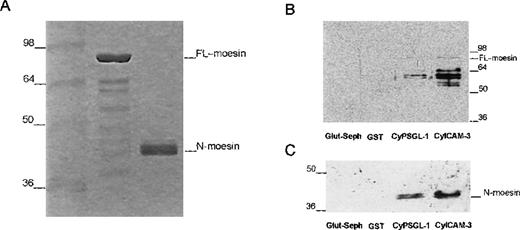
Interaction of recombinant moesin with the cytoplasmic region of PSGL-1 and ICAM-3.
(A) Coomassie-blue–stained 12% SDS-PAGE results with recombinant full-length moesin (moesin) and the amino-terminal domain of moesin (N-moesin) purified from bacteria. Small amounts of contaminating peptides are present. (B) Glutathione-Sepharose (Glut-Seph), GST, or GST-fusion proteins containing the cytoplasmic domain of PSGL-1 (CyPSGL-1) or ICAM-3 (CyICAM-3) linked to glutathione-Sepharose beads were incubated with purified recombinant full-length moesin. GST, GST-fusion proteins, and other bound proteins were eluted together from the beads with Tris buffer containing 20 mmol/L of glutathione. Eluted proteins were resolved by 10% SDS-PAGE followed by immunoblotting with 95/2 pAb specific for moesin. (C) Binding assay of the amino-terminal region of moesin (N-moesin) to the cytoplasmic tail of PSGL-1 and ICAM-3 was performed as described above (Figure 6B); proteins were separated by 10% SDS-PAGE and immunoblotted with the 95/2 pAb. To detect N-terminal moesin, a chemiluminescence system and a longer exposure than that used to obtain the results shown in Figure 6B were required because of the lower reactivity of the 95/2 pAb for the amino-terminal fragment compared with the full-length form of moesin. Molecular masses in kilodaltons are indicated on the left in Figure 6A and 6C and on the right in Figure 6B.
Interaction of recombinant moesin with the cytoplasmic region of PSGL-1 and ICAM-3.
(A) Coomassie-blue–stained 12% SDS-PAGE results with recombinant full-length moesin (moesin) and the amino-terminal domain of moesin (N-moesin) purified from bacteria. Small amounts of contaminating peptides are present. (B) Glutathione-Sepharose (Glut-Seph), GST, or GST-fusion proteins containing the cytoplasmic domain of PSGL-1 (CyPSGL-1) or ICAM-3 (CyICAM-3) linked to glutathione-Sepharose beads were incubated with purified recombinant full-length moesin. GST, GST-fusion proteins, and other bound proteins were eluted together from the beads with Tris buffer containing 20 mmol/L of glutathione. Eluted proteins were resolved by 10% SDS-PAGE followed by immunoblotting with 95/2 pAb specific for moesin. (C) Binding assay of the amino-terminal region of moesin (N-moesin) to the cytoplasmic tail of PSGL-1 and ICAM-3 was performed as described above (Figure 6B); proteins were separated by 10% SDS-PAGE and immunoblotted with the 95/2 pAb. To detect N-terminal moesin, a chemiluminescence system and a longer exposure than that used to obtain the results shown in Figure 6B were required because of the lower reactivity of the 95/2 pAb for the amino-terminal fragment compared with the full-length form of moesin. Molecular masses in kilodaltons are indicated on the left in Figure 6A and 6C and on the right in Figure 6B.
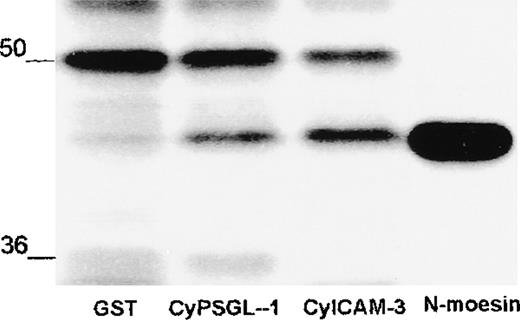
The amino-terminal domain of moesin made in bacteria contains several additional amino acid residues that remain attached after cleavage by thrombin. To exclude a contribution of this additional structure to binding, we tested the same domain of moesin made by in vitro translation. The amino acid sequence of this fragment corresponded precisely to the sequence of moesin from amino acid residues 1 to 310. As shown in Figure 7, the radiolabeled probe associated with PSGL-1 and ICAM-3 but did not bind to the control GST.
Association of in vitro translated amino-terminal moesin with PSGL-1 and ICAM-3.
In vitro translated amino-terminal moesin (N-moesin) was incubated with GST or GST-fusion proteins containing the cytoplasmic domain of PSGL-1 or ICAM-3 linked to glutathione-Sepharose beads. After incubation, Sepharose beads were centrifuged and washed and bound proteins were boiled in sample buffer and analyzed by means of 10% SDS-PAGE and autoradiography. N-moesin labeled with sulfur 35 was loaded as a control in the last lane. Autoradiography showed nonspecific binding of a polypeptide of about 50 kd and specific binding of a labeled polypeptide of the size of N-moesin to PSGL-1 and ICAM-3. Molecular masses in kilodaltons are indicated on the left.
Association of in vitro translated amino-terminal moesin with PSGL-1 and ICAM-3.
In vitro translated amino-terminal moesin (N-moesin) was incubated with GST or GST-fusion proteins containing the cytoplasmic domain of PSGL-1 or ICAM-3 linked to glutathione-Sepharose beads. After incubation, Sepharose beads were centrifuged and washed and bound proteins were boiled in sample buffer and analyzed by means of 10% SDS-PAGE and autoradiography. N-moesin labeled with sulfur 35 was loaded as a control in the last lane. Autoradiography showed nonspecific binding of a polypeptide of about 50 kd and specific binding of a labeled polypeptide of the size of N-moesin to PSGL-1 and ICAM-3. Molecular masses in kilodaltons are indicated on the left.
Discussion
During acute inflammatory responses, selectins mediate initial rolling of neutrophils along the endothelial surface.42,43This is thought to facilitate juxtacrine signaling by positioning leukocytes near locally generated chemoattractants. These mediators, which are either synthesized or presented by activated endothelial cells, activate leukocyte integrins. The signals generated through selectins, combined with those triggered by chemoattractants, result in the integrin-mediated tight adhesion of leukocytes, followed by their transendothelial migration.42,43 Free-flowing leukocytes can also attach to and roll on monolayers of adherent leukocytes under hydrodynamic shear stress, and PSGL-1 can serve as the ligand for L-selectin in neutrophil-neutrophil interactions.44,45 The redistribution of PSGL-1, ICAM-3, and other adhesion molecules to the uropod may therefore enable activated cells to make contact with additional leukocytes and to increase neutrophil recruitment. The concentration of PSGL-1 in the uropod may also facilitate interaction with and recruitment of platelets.22
L-selectin and other adhesion receptors involved in initiation of the leukocyte-endothelial cell adhesion cascade, such as PSGL-1, are concentrated in microvillous tips.21,46 This preferential localization in microvilli is thought to facilitate contact with the endothelium.46 Studies have shown that the uropod of leukocytes is highly enriched in microvilli and microspikes.47 This suggests that accumulation of adhesion molecules, such as PSGL-1, in the uropod of neutrophils stimulated by chemoattractants serves to capture other leukocytes.
Chemoattractants induce the full range of neutrophil activation events, including chemotaxis, oxygen free-radical production, and degranulation.6,7 An early event is a change in morphologic conformation and, as we observed in this study, polarization and redistribution to the uropod of several adhesion molecules and members of the ERM protein family, which link membrane F-actin. Activated and adherent neutrophils move on the endothelial surface and extravasate by migrating through endothelial clefts. Our data show that both crawling neutrophils and those migrating through a monolayer of endothelial cells have polarized morphologic features, with ICAM-3 and PSGL-1 redistributed to the uropod. These results concur with those of previous studies, which found that neutrophils stimulated by fMLP transmigrated across the endothelium and engaged other neutrophils through uropod-mediated contacts.48
Protrusions of the plasma membrane in lymphocytes and neutrophils, described morphologically as microvilli or microspikes, have not been studied in detail. These structures, unlike microvilli on the apical surface of brush-border epithelia in the gut or kidney, do not contain villin or fimbrin. They are presumably more closely related to filopodia, microspikes, and retraction fibers of fibroblasts in culture.29 It is well established that these microextensions contain actin filaments and one or more members of a family of proteins (the ERM proteins) that include moesin, ezrin, and radixin. At least one of these proteins appears to be necessary for either the formation or maintenance of such microextensions.33 All 3 proteins bind F-actin at a site in the C-terminal domain that must be activated or unmasked, since no interaction has been observed with intact, undenatured full-length molecules.40,41 49
In cells, moesin, ezrin, and radixin form complexes with CD44, CD43, ICAM-1, ICAM-2, and ICAM-3,24,25,50-53 and direct low-affinity associations between recombinant full-length proteins and CD44 and ICAM-1 have been studied in vitro.52,53 The in vitro data in this study show that PSGL-1 and ICAM-3 bind poorly to full-length moesin but strongly to the isolated amino-terminal domain. This result is reminiscent of the strong interaction between actin filaments and the isolated C-terminal domain40 and may indicate that a conformational change is necessary to expose functionally important binding sites in the N-terminal domain. In the native configuration, N- and C-terminal domains of moesin associate in a manner that prevents F-actin binding.26-28,40 41 The same intramolecular association may prevent interactions with membrane proteins, as we observed in this study.
The binding of ICAM-3 and PSGL-1 to moesin from cell lysates but not from bacteria may be explained by the fact that, in neutrophils and HL-60 cells, a fraction of the moesin molecules can be structurally altered by phosphorylation or by formation of a complex with polyphosphoinositides. For example, phosphorylation by Rho-dependent kinase or protein kinase C disrupts the intramolecular association between N- and C-terminal domains, suggesting regulation by small GTPases of the Rho family and other signaling pathways,49,54,55 and cosedimentation of highly purified moesin from human platelets with F-actin was observed in vitro when the molecule was both phosphorylated at thr558 and when it formed a complex with phospholipids.40,41 A study has found that tyrosine phosphorylation is required for the interaction between the N-terminal domain of ezrin and phosphatidylinositol 3-kinase,56 suggesting yet other mechanisms for activation. Although our in vitro binding data are consistent with this simple mechanism and a direct interaction between receptor proteins and moesin or ezrin, another possibility has not been excluded. Links between transmembrane proteins and the actin cytoskeleton in cells also develop by insertion of additional proteins containing the PDZ domain that interact with membrane proteins on one hand and moesin or ezrin on the other.57 58 This provides additional possibilities for regulation. Further work is required to determine whether ICAM-3 or PSGL-1 are among the receptors that use this mechanism.
Acknowledgments
We thank R. González-Amaro, M. Montoya, and M. Vicente-Manzanares for critical reading of the manuscript and E. Fernández Villareal, M. J. Borque, and Lourdes Herreros for help in providing reagents and technical advice.
Supported by grants SAF 99/0034-CO1 and 2FD97-0680-C02-02 from the Ministerio de Educación y Cultura (to F.S.-M.) and 08/0011/1997 from the Comunidad Autónoma de Madrid. G.K. is an Established Investigator of the American Heart Association, funded by grant CB-204 from the American Cancer Society.
Reprints:Francisco Sánchez-Madrid, Hospital de la Princesa, Servicio de Inmunologı́a, C/ Diego de León 62, Madrid 28006, Spain; e-mail: smadrid/princesa@hup.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.