To the editor:
HFE polymorphism and accurate diagnosis of C282Y hereditary hemochromatosis carriers
Mutations in the HFE gene are responsible for more than 90% of the cases of hereditary hemochromatosis in the Caucasian population.1-3 Three mutations have been reported to contribute to disease, including C282Y, H63D, and S65C.1 2
We read with interest and concern 2 articles published recently with respect to the accuracy of hereditary hemochromatosis DNA testing.4,5 Each of these articles describes the presence of a G→A polymorphism at nucleotide 6117 within the primer sequence for the amplification of exon 4, which contains the C282Y mutation.1 (Genbank #Z92910 has been updated since the publication of Jeffrey et al [1999] and Somerville et al [1999]. These nucleotide numbers represent the current nucleotide numbers in the HFE gene. All numbers are from genomic sequence.) Data from each of these articles indicate that reduced or nonpriming of the allele can occur in the presence of this polymorphism. If nonpriming does occur, then heterozygous samples (C282Y/+) could appear to be homozygous (C282Y/C282Y), and a disproportionate number of homozygotes would be diagnosed.
The current standard method for detecting mutation status in our laboratory uses the original Feder et al1 primers (forward, 5′-TGGCAAGGGTAAACAGATCC-3′; reverse, 5′-CTCAGGCACTCCTCTCAACC-3′), followed by RsaI restriction digestion, and agarose gel electrophoresis (Figure). The authors in Jeffrey et al4 suggested utilizing an alternative reverse primer for amplification of exon 4 (reverse, 5′-TACCTCCTCAGGCACTCCTC-3′) in combination with the original forward primer described by Feder et al1 to avoid the polymorphic site.
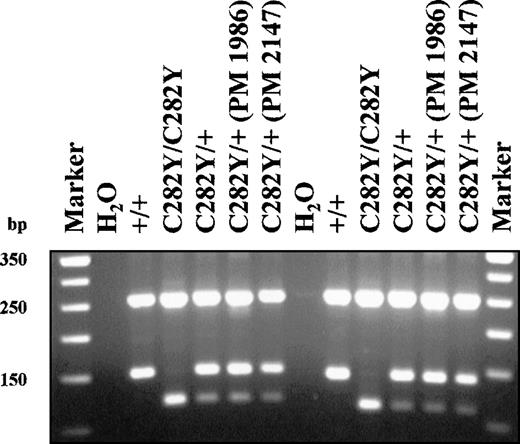
HFE C282Y mutation detection assay.
Lanes 1 and 14 contain 50 bp marker as indicated at the left of the figure. Lanes 2-7 contain samples as indicated above the gel. All reactions were performed with the original Feder et al (1996) primers. Lanes 8-13 contain the samples indicated above the gel. All reactions were performed with the original Feder et al2 forward primer and the Jeffrey et al4 reverse primer. Known polymorphic samples are indicated (PM 1986, PM 2147) and were prepared in the Molecular Diagnostic Lab of the University of Alberta as described in Somerville et al.5 All other DNA samples were obtained from either blood or buccal cells and were prepared in the MSU DNA Diagnostic Laboratory by a standard alkaline lysis procedure. Each PCR reaction contains 12.5 pmoles each primer, 1× Rediload (Research Genetics), 10 mmol/L dNTPs, 1× GeneAmp PCR buffer (Perkin-Elmer: 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.01% w/v gelatin), and 0.25 U Taq DNA polymerase in a total reaction volume of 25 μL. Conditions for amplification: 95°C, 5′; 30 cycles of 94°C, 30"; 63°C, 30"; 72°C 30"; hold at 4°C. All PCR reactions were performed in a Perkin-Elmer 9600 thermocycler. PCR products (390 bp) are digested with RsaI to give 250 bp + 140 bp for a normal result, or 250 bp + 140 bp + 111 bp + 29 bp for a C282Y carrier, or 250 bp + 111 bp + 29 bp for a mutant C282Y homozygote. Restriction digestion products are run on a 3% Nu-Sieve 3:1 (FMC BioProducts) agarose gel in 0.5× TBE.
HFE C282Y mutation detection assay.
Lanes 1 and 14 contain 50 bp marker as indicated at the left of the figure. Lanes 2-7 contain samples as indicated above the gel. All reactions were performed with the original Feder et al (1996) primers. Lanes 8-13 contain the samples indicated above the gel. All reactions were performed with the original Feder et al2 forward primer and the Jeffrey et al4 reverse primer. Known polymorphic samples are indicated (PM 1986, PM 2147) and were prepared in the Molecular Diagnostic Lab of the University of Alberta as described in Somerville et al.5 All other DNA samples were obtained from either blood or buccal cells and were prepared in the MSU DNA Diagnostic Laboratory by a standard alkaline lysis procedure. Each PCR reaction contains 12.5 pmoles each primer, 1× Rediload (Research Genetics), 10 mmol/L dNTPs, 1× GeneAmp PCR buffer (Perkin-Elmer: 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.01% w/v gelatin), and 0.25 U Taq DNA polymerase in a total reaction volume of 25 μL. Conditions for amplification: 95°C, 5′; 30 cycles of 94°C, 30"; 63°C, 30"; 72°C 30"; hold at 4°C. All PCR reactions were performed in a Perkin-Elmer 9600 thermocycler. PCR products (390 bp) are digested with RsaI to give 250 bp + 140 bp for a normal result, or 250 bp + 140 bp + 111 bp + 29 bp for a C282Y carrier, or 250 bp + 111 bp + 29 bp for a mutant C282Y homozygote. Restriction digestion products are run on a 3% Nu-Sieve 3:1 (FMC BioProducts) agarose gel in 0.5× TBE.
We retrospectively investigated 310 samples from patients originally tested in our laboratory that we diagnosed as C282Y homozygotes. We utilized the primer combination suggested above, so that the polymorphism (G→A at nucleotide 6117) described would not be within the primer sequence, in order to avoid potentially nonpriming of the allele containing the polymorphism. Of the 310 samples we tested with this new primer combination, no discrepancies were found in our analyses (Figure). Based on these results, all patients originally diagnosed in our laboratory as C282Y homozygotes are truly homozygous for this mutation.
We then requested polymorphic samples from each of the 2 reporting laboratories. One laboratory would not provide us any samples, citing patent issues; however, the Molecular Diagnostic Lab of the University of Alberta kindly provided 2 samples containing the G→A polymorphism at 6117 for our analysis.5 The figure illustrates results obtained from these 2 samples, as well as control samples from our laboratory utilizing both the original Feder et al1 primers and the primer set described in Jeffrey et al.4 As you can see, no differences or discrepancies were found among these samples. The same results were obtained using either set of primers. Thus, the primers and conditions we are utilizing amplify all alleles correctly and do not lead to misdiagnosis/overdiagnosis of this condition.
Since our standard annealing temperature is 63°C, we thought perhaps lowering this temperature and therefore decreasing the stringency of the polymerase chain reaction (PCR) might produce different results, allowing the polymorphic allele to amplify more readily; however, we analyzed 20 samples, including the known polymorphic samples at the much lower annealing temperature of 56°C with both sets of primers and also obtained the same expected results (data not shown). These data further indicate that the annealing temperature we currently utilize (63°C) is producing the correct results and that the PCR is not too stringent. Temperatures above 63°C significantly reduce the amount of HFE product produced, and it is advised not to utilize such a stringent condition (data not shown).
The incidence of the polymorphism in the general Caucasian population is not known. However, in the hemochromatosis referral population, the polymorphism carrier frequency is estimated to be between 4-21%.4 5 Additional studies are required to fully understand the incidence and any potential ramifications of this polymorphism.
The prevalence of hereditary hemochromatosis in the Caucasian population is estimated to be 1:250-1:400.1 The Jeffrey et al (1999) group indicates that the original incidence of disease in their population study was 1:168, which is much higher than has typically been reported. With the change in their protocol, the incidence of HH in this same population is now 1:327, which is within the expected range for the C282Y allele frequency.
It is not completely clear how these discrepancies in diagnosis could occur; however, several possibilities exist. PCR conditions, although based upon the Feder et al1 study, can vary from laboratory to laboratory (magnesium concentration, annealing temperatures, thermocyclers, source of DNA, method of DNA preparation, etc). While our studies here suggest that the source of DNA and method of DNA preparation may not play a significant role (DNAs were prepared from different source and in different laboratories), employing annealing temperatures above 63°C may decrease the amount ofHFE product produced. As well, the populations studied may be somewhat different, and the incidence of the polymorphism may vary among different ethnic groups. Further study of this polymorphism is warranted, and careful, stringent analysis ofHFE alleles must be performed to accurately diagnose individuals with mutations in the HFE gene.
Acknowledgments
The authors are thankful to Karen Friderici for critical reading of the manuscript. We also thank the Molecular Diagnostic Laboratory of the University of Alberta for kindly providing known polymorphic samples.