Abstract
This study analyzed the behavior of an antiadhesive membrane molecule, CD43, in neutrophil polarization and locomotion. CD43 cross-linking by antibodies induced neutrophil locomotion, with CD43 molecules clustered at the uropod of polarized neutrophils. In contrast, CD11b/CD18 cross-linking by antibodies did not affect either cell polarization or locomotion. Stimulation of suspended or adherent neutrophils with chemotactic peptide results in cell polarization and locomotion and a concomitant redistribution of CD43 to the uropod. This process is entirely reversible. The study also investigated which actin-binding protein could be involved in CD43 lateral redistribution. -Actinin and moesin are preferentially adsorbed on Sepharose beads bearing a recombinant CD43 intracellular domain. Analysis by immunofluorescence confocal microscopy shows a codistribution of moesin during CD43 lateral redistribution. By contrast, -actinin is located at the leading edge, an area devoid of CD43. These results shed new light on the role of CD43 membrane redistribution, which appears to be directly related to neutrophil polarity and locomotion.
Neutrophils circulate in the blood as spherical resting cells. In response to inflammatory stimuli, neutrophils leave the blood vessel by diapedesis and locomote across the extracellular matrix to the inflamed area.1,2 Migrating cells acquire a polarized morphology in which the leading edge, where lamellipodia are generated, becomes differentiated from the rear, or uropod.3 Cell locomotion is a complex, still poorly understood, process.4-6 During locomotion, the leading edge extends in the direction of migration; this membrane extension is associated with F-actin polymerization.7-9 Contraction of the uropod, probably by a myosin II-dependent process, may also pull the cell forward toward the leading edge.10 11
Locomotion requires successive attachment and detachment of the cell from the substratum and is controlled by cell adhesiveness. Both adhesion at the front and release of the rear part of the cell regulate cell morphology and locomotion.12-15 The behavior of adhesion molecules, which mediate cellular attachment to the substratum, during cell locomotion, has been studied by various laboratories. Integrins on the adherent side of the cell connect the cytoskeleton with the extracellular matrix to produce the forces required for locomotion.16-18 Integrins remain attached to the substratum while the cell moves forward and end up at the rear of the cell.13,19 This rear attachment must then be released and new adhesion sites be created at the leading edge to ensure the continuation of cell movement. It has been proposed that integrins recycling and shedding17,19,20 and the proteolytic degradation of extracellular matrix21 are involved in these processes.
The possible function of uropod-localized membrane proteins—in particular antiadhesive molecules—in rear release has not been investigated. The main antiadhesive membrane molecule of leukocytes is CD43 (leukosialin, sialophorin). CD43 is a heavily sialylated O-glycosylated surface protein, which is a major component of the negatively charged repulsive barrier formed by the cell glycocalyx.22-25
The antiadhesive function of CD43 has been established by in vitro experiments in which CD43 transfection into CD43−cell lines diminishes cell adhesion,26 whereas, conversely, targeted disruption of the CD43 gene in CD43+ cells increases cell adhesion.27 In vivo rolling and adhesion of leukocytes to endothelial cells is enhanced in CD43-deficient mice compared to wild-type mice.28 Leukocyte emigration into tissues is, however, impaired in these mice, suggesting a positive role for CD43 in leukocyte adhesion and motility processes. Indeed, CD43 is also potentially an adhesion molecule; it bears the sialyl-Lewis x epitope and belongs to the mucin family of selectin counterreceptors. Various molecules, such as ICAM-1, E-selectin, or MHC-1, have been proposed as putative ligands. This dual antiadhesive versus adhesive function of CD43 has been recently reviewed.29
As far as neutrophil polarization and locomotion are concerned, we have previously shown that CD43 cross-linking by antibodies induces CD43 redistribution in a cap structure by a cytoskeleton-driven process. CD43 cross-linking induces the polarization of neutrophils in suspension, with an F-actin–rich lamellipodium and a myosin-rich uropod, similar to what is observed on adherent locomoting cells.30 CD43 has also been shown to be redistributed to the uropod of lymphocytes crawling on a monolayer of endothelial cells or on a protein-coated surface.31 While this work was in progress, the CD43 intracellular domain was shown to interact with the actin-binding protein members of the ezrin/radixin/moesin family (ERM).32 33
This work offers evidence for a role of CD43 clustering in neutrophil motility. Specifically, we show that CD43 cross-linking by antibodies induces neutrophil polarity and locomotion, concomitant with CD43 relocation to the uropod. We also describe CD43 redistribution to the uropod of either suspended or adherent neutrophils, following stimulation by chemotactic peptides. Kinetic analysis shows that CD43 redistribution parallels morphologic changes (cell polarization) and locomotion by a reversible process.
Because CD43 redistribution induced by cross-linking antibodies is driven by an actomyosin-dependent contractile process,30 we have investigated which actin-binding protein would link CD43 to the F-actin network. We have first analyzed in vitro the possible interactions of actin-binding proteins with CD43 by performing affinity chromatography of neutrophil cytosol on Sepharose beads bearing a recombinant CD43 intracellular domain. Among the actin-binding proteins tested, we found a preferential adsorption of α-actinin and moesin. The physiologic relevance of these interactions has been assessed by immunofluorescent analysis of adherent motile neutrophils. Moesin, but not α-actinin, is colocalized with CD43 at the rear of neutrophils, confirming the possible function of moesin as the cytoskeletal linker of CD43 to the F-actin network.
Materials and methods
Buffers and reagents
Hanks' balanced salt solution (HBSS) with or without Ca++/Mg++ (GIBCO, Paisley, Scotland), For-NLe-Leu-Phe-Nle-Tyr-Lys-OH 2.25 H2O (fNLPNTL) and N-Formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) were from Bachem (Bubendorf, Switzerland) and Sigma (St. Louis, MO), respectively. The 10−2 mol/L (fNLPNTL) or 10−3 mol/L (fMLP) stock solutions in DMSO were stored at −20°C and diluted to 10−9 mol/L (fNLPNTL) or 10−8 mol/L (fMLP) for cell stimulation. Corresponding dilutions of DMSO alone had no effect on cellular morphology and surface molecule distribution. Fluorescein isothiocyanate (FITC)-conjugated anti-CD11b mAb (clone Bear 1) and FITC-control mouse IgG1 were from Immunotech (Marseille, France). Anti-CD43 mAbs: clone MEM59 was from Biogenesis (Poole, UK) and clone L60 from Becton Dickinson (Bedford, MA). F(ab′)2 and Fab fragments of clone MEM59 anti-CD43 were obtained by pepsin or ficin digestion using Pierce (Rockford, IL) preparation kits according to the manufacturer's instructions. Fc fragments were removed by absorption on protein A-sepharose and purity of F(ab′)2 and Fab fragments assessed by polyacrylamide gel electrophoresis (PAGE) and silver staining. A rabbit polyclonal antiserum was raised by injecting the recombinant CD43 intracellular domain (see below) and antibodies purified by affinity on the recombinant molecule. Antimoesin mAb (clone 38) was from Transduction Laboratories (Lexington, KY), rabbit polyclonal antimoesin was a gift from Antony Bretscher (Cornell University, Ithaca, NY). Rabbit anti–α-actinin was from Sigma Immunochemicals and antivinculin mAb (clone V284) from Cymbus Bioscience (Southampton, UK). Polyclonal antifimbrin and antiezrin antisera were generous gifts from Monique Arpin (Curie Institute, Paris, France).
FITC- or tetrarhodamine isothiocyanate (TRITC)-conjugated F(ab′)2 fragments of goat antimouse F(ab′)2, FITC- or TRITC-conjugated Fab fragments of goat antimouse IgG (H+L) or TRITC-conjugated goat antirabbit IgG (minimal cross-reactivity with human, mouse, and rat serum proteins) were from Jackson Laboratories (West Grove, PA). Alexa 488-conjugated goat antimouse IgGs were from Molecular Probes (Eugene, OR). All antibodies were centrifuged before use, for 20 minutes at 11,600g, to remove immunoglobulin aggregates.
Normal goat serum (NGS) was from Sigma, and fetal calf serum (FCS) was from GIBCO. Protease inhibitors phenylmethanesulfonylfluoride (PMSF), diisopropyl-fluorophosphate (DFP), leupeptin, and chymostatin were from Sigma.
Neutrophil isolation and labeling
Neutrophils were prepared at room temperature from EDTA-anticoagulated blood from healthy adult volunteers. Neutrophils were isolated by a 1-step density gradient centrifugation, on Polymorphprep (Nycomed, Oslo, Norway), according to the manufacturer's instructions. Residual erythrocytes were lysed in 0.2% NaCl for 1 minute and the osmolarity of the medium then equilibrated by the addition of an equal volume of 1.6% NaCl. Cells were washed and resuspended in the culture medium.
When mentioned, cells were labeled sequentially with primary and secondary antibodies, for 30 minutes on ice, then washed twice with cold medium. Primary antibodies: anti-CD43 mAbs (clone MEM59, 50μg/mL), anti-CD11b (clone Bear1, 10 μg/mL). Secondary antibodies: TRITC-conjugated F(ab′)2 or Fab fragments of goat antimouse IgG.
Video analysis of cell locomotion and CD43 redistribution after antibody cross-linking
Prelabeled neutrophils (106/mL) were incubated at 37°C for 15 minutes in HBSS, then pelleted by centrifugation for 5 minutes. Cell suspension (5 μL), containing 106 cells, was placed between a slide and a round coverslip (25 mm diameter), both coated with FCS (for 2 hours at 37°C). The slide–coverslip preparation was sealed with paraffin and placed on the heated stage of a Wild-Leitz Diavert microscope with a 40 × objective. Cells were recorded on videotape for 10 minutes with a Sony CCD-IRIS, black and white camera. The outline of the cell at the initial and final position and the path traveled during 10 minutes was drawn on a transparency. Cells remaining totally or partially within the outline of the initial position are defined as stationary cells, and cells found outside after 10 minutes are defined as locomoting cells. The proportion of locomoting cells and their speed were determined by morphometry.
Analysis of CD43 distribution on fNLPNTL stimulation of suspended neutrophils
Prelabeled neutrophils were suspended at 2 × 106cells/mL and incubated at 37°C for 5 minutes in HBSS with Ca++/Mg++, then fNLPNTL (10−9mol/L) was added or not, for various times at 37°C. The incubation was stopped by addition of 3.7% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 minutes at room temperature. After 2 washes in PBS, cells were cytocentrifuged on a slide, mounted in fluoprep (BioMerieux, Marcy/Etoile, France) and analyzed by differential interference contrast (DIC) and fluorescence microscopy with a Wild-Leitz Diavert microscope.
Analysis of CD43 distribution on fMLP stimulation of adherent neutrophils
As previously described,34 neutrophils, prelabeled or not, were allowed to settle on coverslip bottom dishes precoated with 100 μg/mL human fibronectin (Collaborative Biomedical Products, Becton Dickinson), at 37°C for 5 minutes in the incubation medium (150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 20 mM HEPES, pH 7.4). Cells were then stimulated with a bath application of 10 nM fMLP at 37°C. After various times of incubation, cells were fixed in PBS containing 6.6% PFA and lysophosphatidyl-choline palmitoyl (LPCP) 0.1 mg/mL.
Where indicated, cells were labeled after the fixation step for CD43 (postlabeling). Fixed cells were incubated at room temperature for 2 hours in a blocking solution (PBS, 10% FCS). CD43 labeling was performed at room temperature with an antibody solution at 20 μg/mL (in PBS, 10% FCS). After washing in PBS, cells were incubated with TRITC-F(ab′)2 fragments of goat antimouse IgG (1/300, in PBS, 10% FCS) for 30 minutes, then washed in PBS.
DIC and fluorescence microscopy were performed on a Leica DMIRB microscope (Leica Microscopie and System, GmbH), equipped with a cooled CCD camera (Frame Transfer Pentamax camera with a 512 × 512 back-thinned EEV chip, No. 512 EFTB (Princeton Instruments) driven by Image-1/MetaMorph Imaging System software (Universal Imaging, West Chester, PA). Images were acquired using a 63 × oil immersion objective (1.4 NA).
Immunofluorescence analysis of CD43 extracellular and intracellular domain localization.
Nonlabeled neutrophils were activated by fMLP to locomote on fibronectin for 5 minutes at 37°C. Cells were fixed and permeabilized (PFA 6.6%, LPCP 0.1 mg/mL in PBS), incubated in the blocking solution, then labeled by successive 30-minute incubations, with the antibodies: anti-CD43 extracellular domain (clone L60), Alexa 488-conjugated goat antimouse, rabbit anti-CD43 intracellular domain, rhodamine-conjugated goat antirabbit antibodies. Fluorescence microscopy was performed as described above. There was no cross-reactivity between the different antibodies. The absence of cross-talk between fluorophores was assessed by the following method. We defined the acquisition parameters for imaging CD43 “extra” (Alexa 488 labeling) and CD43 “intra” (rhodamine labeling) using standard fluorescein and rhodamine filters. We acquired an image of Alexa 488 single-labeled cells using the rhodamine filter and reciprocally we acquired an image of rhodamine single-labeled cells using the fluorescein filter. In both cases there was no observable fluorescence cross-talk.
Time series acquisition.
Neutrophils were prelabeled with Fab fragments of anti CD43 antibodies (clone MEM59) and with TRITC-conjugated Fab fragments of goat antimouse antibodies. Cells were allowed to settle on fibronectin at 25°C for 5 minutes. Cells were then stimulated with a bath application of 10 nM fMLP. Time series acquisition was performed on the microscope stage at 25°C. Every 10 seconds over a period of 400 seconds, fluorescent images were recorded as previously described using a 40 × objective.
Adsorption of actin-binding proteins with rCD43intra-Sepharose beads
Sepharose beads bearing the recombinant intracellular part of CD43 (CD43intra).
A plasmid encoded for the whole intracytoplasmic CD43 sequence (PCD43intra, sequence shown in Figure 5) and expressed inEscherichia coli. The region coding for the intracellular domain of CD43 (from codon 283 to the end) was amplified from the full length CD43 complementary DNA (cDNA).35 Primers contained the appropriate restriction sites for in-frame insertion into the bacterial expression vector QE-12 (Qiagen, Courtaboeuf, France), which adds an N-terminal 6 His tag to the proteins coded by the insert. The 6 His-tagged CD43intra was expressed in E colistrain M15 (Qiagen) and purified as described on a nickel-charged chelating-agarose column (ProBond from Invitrogen, Leek, the Netherlands).36 The purified protein appeared on sodium dodecyl sulfate (SDS)-PAGE as a single 18 kd band, as expected from the sequence of the insert. This CD43intra preparation (1.5 mg), or 1.5 mg bovine serum albumin (BSA, Sigma) as a control, was coupled to 1 mL CNBr-Sepharose (Pharmacia, Upsala, Sweden) according to the manufacturer's instructions.
Cytosol adsorption.
Cytosol was obtained by sequential centrifugation after neutrophil lysis by nitrogen cavitation, as described.37 The cytosol was concentrated on amicon PM10 and frozen at −80°C. One milliliter of the concentrated cytosol (corresponding to 2.5 × 108 neutrophils) was first precleared for 1 hour with uncoupled Sepharose, then incubated with 150 μL of CD43intra- or BSA-Sepharose. After 1 hour at 4°C on roller and 5 washes in relaxation buffer (5 × 1 mL), specific elution was performed with 200 μL of recombinant CD43 (0.8 mg) followed by 2 times 200 μL of relaxation buffer. Elution was completed with 0.5 mol/L NaCl. All eluted fractions were boiled in unreduced sample buffer and analyzed by SDS-PAGE and Western blotting, using various antibodies directed to actin-binding proteins.
Immunofluorescence analysis of -actinin and moesin localization in relation to CD43 distribution
Neutrophils were allowed to locomote on fibronectin as described above. After different times of stimulation by fMLP at 37°C, cells were fixed and permeabilized by a 5-minute incubation (in 6.6% PFA and 0.1 mg/mL LPCP in PBS). Cells were then washed in 0.1 mol/L glycine in PBS and incubated for 2 hours in a blocking solution (PBS, 10% FCS). The following antibodies were then added sequentially (in PBS, 10% FCS): anti-CD43 (clone L60, 20 μg/mL), Alexa 488-conjugated goat antimouse (1/600), polyclonal rabbit antimoesin (1/300), TRITC-conjugated goat antirabbit IgG (1/300). Each labeling step was separated by 3 washes of 5 minutes in PBS. α-Actinin labeling was performed after cellular fixation for 5 minutes in 6.6% PFA, glutaraldehyde 0.7%, and saponin 250 μg/mL in PBS. Cells were then washed and incubated in the blocking solution. Cells were labeled with anti-α-actinin antibodies (1:50 in PBS, 10% FCS) and with TRITC-conjugated goat antirabbit IgG (1:300). Cellular fixation by glutaraldehyde preserves the structure of α-actinin but destroys CD43 epitopes, making double labeling impossible.
DIC and fluorescence microscopy were performed as described above. No cross-reactivity between the different antibodies and no cross-talk between the fluorophores were noted. Double-labeled cells were analyzed by confocal microscopy using an Axiovert 100 mol/L inverted microscope equipped with an LSM 510 laser scanning unit and a 63 × 1.4NA plan Apochromat objective (Carl Zeiss). CD43 fluorescence (Alexa 488) was excited with a 25 mW argon laser emitting at 488 nm, and the emission was collected by 650 long pass filter. Moesin fluorescence (rhodamine) was excited with a 1.0 mW helium/neon laser emitting at 543 nm and was collected by a 530 to 560 long pass filter. The 2 channels were scanned alternatively, having only 1 laser and 1 detector channel on at each time. No cross-talk between the fluorophores has been detected, using the method described above for CD43 “extra” and “intra” double labeling.
Results
CD43 cross-linking by antibodies induces neutrophil locomotion
We have previously shown that antibody cross-linking of CD43 at 37°C on suspended neutrophils induces the redistribution of CD43 into a cap structure and a front-to-tail polarization in 15% to 25% of the cells.30 To assess if CD43 cross-linking results in neutrophil locomotion, video microscopic analysis was performed on neutrophils treated with anti-CD43 antibodies, in the absence of stimulating agent. Cells were either unlabeled or treated with a mouse monoclonal anti-CD43 antibody (clone MEM59) followed by a bivalent F(ab′)2 secondary antibody (to cross-link CD43), or a monovalent Fab secondary antibody (to avoid cross-linking). Both F(ab′)2 and Fab secondary antibodies were TRITC conjugated. After 15 minutes of incubation at 37°C in HBSS Ca++/Mg++, to allow cross-linking and redistribution of membrane molecules, cells were mounted between FCS-coated coverslip and slide and video-analyzed, as described in “Materials and methods”.
The results of 3 independent experiments are shown in Table1. CD43 cross-linking resulted in locomotion of 73% neutrophils, as compared to the 22% of spontaneously motile cells in the absence of antibody. Induction of locomotion required CD43 cross-linking because it was not induced if cross-linking was avoided by using monovalent Fab secondary antibodies (22% of locomotion). Similar results were obtained with F(ab′)2 fragments of the anti-CD43 antibody, showing that this induction of locomotion is independent of Fc receptors.
By contrast, cross-linking of CD11b/CD18 integrins by anti-CD11b antibodies (clone Bear 1) and F(ab′)2 secondary antibodies resulted neither in neutrophil polarization nor in locomotion (data not shown). Cross-linked CD11b molecules became clustered in patches on round cells (Figure1). The anti-CD11b were nonblocking antibodies and their cross-linking did not prevent cells from adhering and locomoting on fibronectin when stimulated by fMLP (our unpublished data).
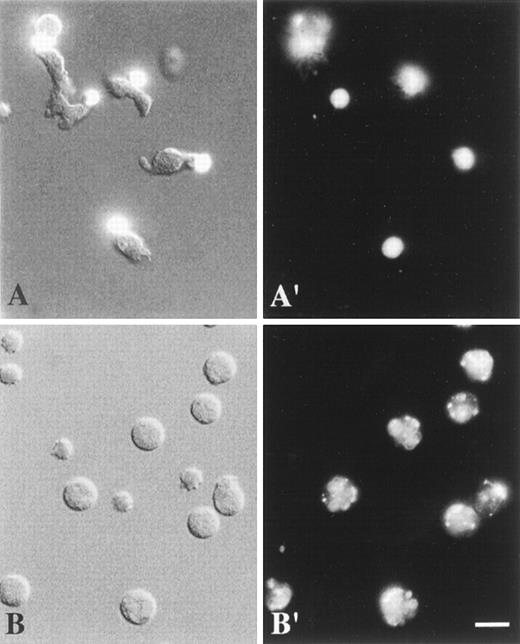
Combined DIC and fluorescence image (A), DIC image (B), and fluorescence images (A′, B′) of living neutrophils.
Cells were labeled at 4°C with anti-CD43 mAbs (A, A′) or anti-CD11b mAbs (B, B′) and TRITC-conjugated F(ab′)2 fragments of secondary antibodies. They were analyzed at 37°C as described in “Materials and methods”. (A, A′) Polarized, locomoting neutrophils with CD43 caps located at the uropods. (B, B′) Round, stationary neutrophils with uniformly patched CD11b. Scale bar = 10 μm. (Panel A, the top left corner cell is round, nonpolarized, and nonmotile.)
Combined DIC and fluorescence image (A), DIC image (B), and fluorescence images (A′, B′) of living neutrophils.
Cells were labeled at 4°C with anti-CD43 mAbs (A, A′) or anti-CD11b mAbs (B, B′) and TRITC-conjugated F(ab′)2 fragments of secondary antibodies. They were analyzed at 37°C as described in “Materials and methods”. (A, A′) Polarized, locomoting neutrophils with CD43 caps located at the uropods. (B, B′) Round, stationary neutrophils with uniformly patched CD11b. Scale bar = 10 μm. (Panel A, the top left corner cell is round, nonpolarized, and nonmotile.)
CD43 redistribution is related to neutrophil polarization and locomotion
Cell locomotion and cell morphology are functionally related because polarization is a prerequisite for locomotion. The relationship between CD43 redistribution and the cell morphology changes and locomotion was explored. From movies obtained during the locomotion assay (from the previous paragraph), cells were examined for the different patterns of CD43 distribution (cap, patch, uniform), for the corresponding cell morphologies (distinct polarization or not), and for their locomotor activity.
As shown in Table 2, during CD43 cross-linking experiments, CD43 molecules were capped on 94% (± 4) of the total cell population; 78% (± 13) of these capped cells were polarized and motile, with CD43 caps always located at the uropod. Only 6% (± 4) of the total cell population had redistributed CD43 in a patch structure; 17% (± 29) of this patched cell population was motile (which represents only 1% of the total cell population), regardless of whether cells were polarized or not. In summary, cell polarity and locomotion appear to be related to CD43 relocation at the uropod. The same correlation was observed for spontaneously migrating cells (in the case of CD43 labeling with monovalent antibodies).
The proportion of polarized cells in this system was higher than the previously reported 15% to 25% of front-tail polarized cells, observed during CD43 cross-linking on cells in suspension.30 Indeed, we observed that, after CD43 cross-linking, the contact of neutrophils with serum- or gelatin-coated (data not shown) slide and coverslip increased the number of polarized cells.
Chemotactic stimulation of neutrophils induces CD43 redistribution to the cellular uropod, concomitant with cell polarization and locomotion
Chemotactic stimulation of suspended neutrophils.
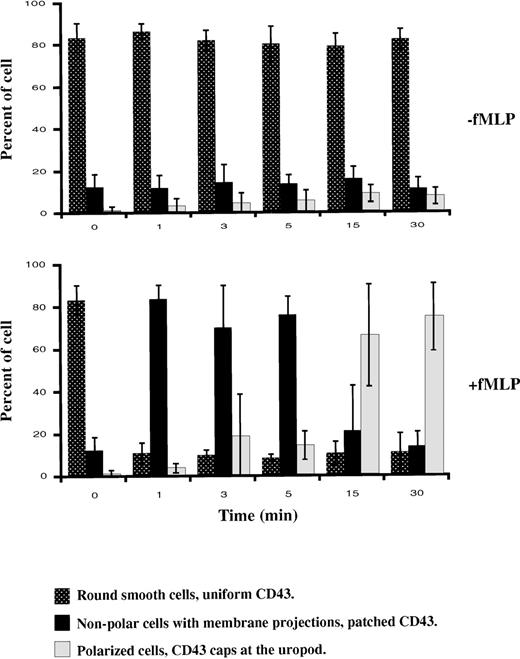
To assess if chemotactic peptide-induced cell polarization results in surface redistribution of CD43, we analyzed the effect of fNLPNTL on suspended cells, which had been fluorescently labeled with anti-CD43 mAb (clone MEM59) and TRITC-Fab fragments of secondary antibodies. After various times of incubation with or without chemotactic peptide (fNLPNTL 10−9 mol/L) at 37°C, cells were PFA fixed and analyzed by microscopy. Three distinct patterns of CD43 distribution (uniform, patched, or capped) were observed that each corresponded to a distinct cellular morphology (round smooth, nonpolar with membrane projection, or polarized, respectively). Figure2 summarizes 3 independent experiments. Initially (time 0), more than 80% of cells were round without protrusions and showed a uniform CD43 distribution. After 1 minute of chemotactic stimulation, 84% of the cells became ruffled and showed a patched CD43 distribution. Patching of CD43 clearly preceded cell polarization, which was observed in a majority of cells after 15 minutes of stimulation. After 30 minutes of incubation at 37°C with the chemotactic peptide, 75% of the cells were polarized with CD43 caps located at the uropod, whereas this phenotype was observed in only 8% of unstimulated cells. A similar redistribution of CD43 to the uropod was observed when the secondary antibody was omitted, or when cells labeled with Fab or F(ab′)2 fragments of the primary anti-CD43 monoclonal antibody were stimulated by fNLPNTL at 37°C (data not shown). By contrast, in the absence of fNLPNTL, CD43 labeling with anti-CD43 mAb and monovalent secondary antibody did not induce either CD43 redistribution or morphologic changes when neutrophils were incubated at 37°C for 0 to 30 minutes.
Patterns of cell morphology.
Cells were labeled with anti-CD43 mAb and TRITC-Fab fragments of secondary antibodies, then incubated in suspension at 37°C with or without fNLPNTL (10−9 mol/L). After 1, 3, 5, 15, and 30 minutes, cells were PFA fixed and the different patterns of cell morphology associated with CD43 distribution were recorded by DIC and fluorescence microscopy. The results are expressed in percent (mean ± SD) of the different patterns observed in 3 independent experiments.
Patterns of cell morphology.
Cells were labeled with anti-CD43 mAb and TRITC-Fab fragments of secondary antibodies, then incubated in suspension at 37°C with or without fNLPNTL (10−9 mol/L). After 1, 3, 5, 15, and 30 minutes, cells were PFA fixed and the different patterns of cell morphology associated with CD43 distribution were recorded by DIC and fluorescence microscopy. The results are expressed in percent (mean ± SD) of the different patterns observed in 3 independent experiments.
Chemotactic stimulation of adherent neutrophils.
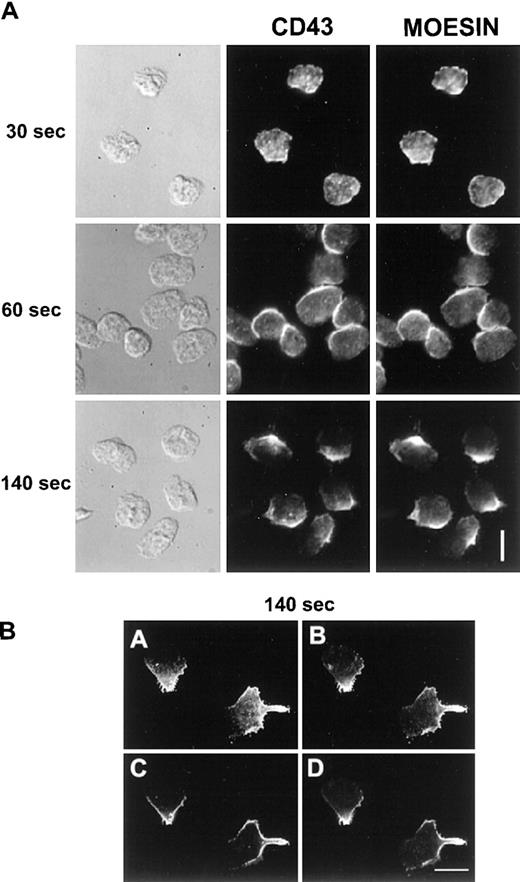
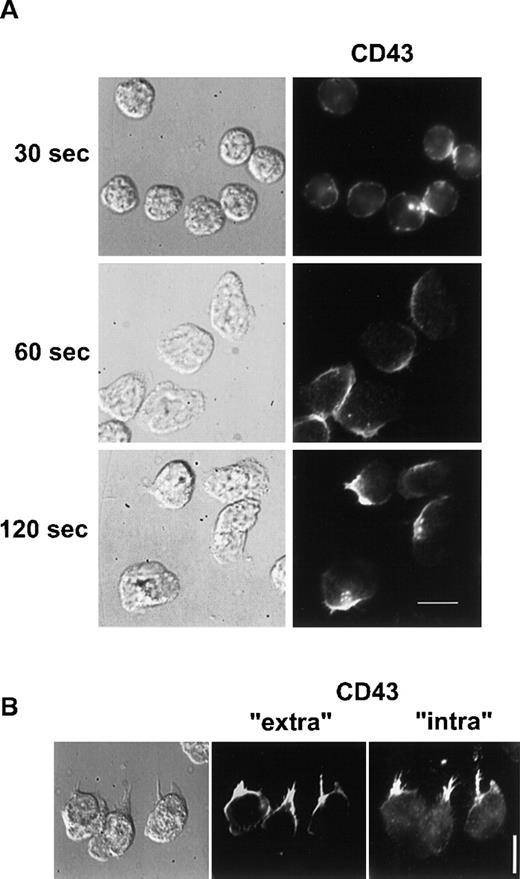
We then investigated if CD43 was also redistributed to the uropod when neutrophils were crawling on an adherent surface. Unlabeled neutrophils, adherent to human fibronectin, were stimulated with 10 nM fMLP for various times. Cells were then fixed and postlabeled with anti-CD43 as described in “Materials and methods”. As shown in Figure 3A, 30 seconds after the chemotactic peptide stimulation, neutrophils presented a ruffled morphology and CD43 was partially redistributed. After 60 seconds, neutrophils started to spread on the substratum and CD43 redistribution progressed. After 2 minutes of stimulation, locomoting neutrophils were polarized and had redistributed most of their CD43 to their uropods, leaving a faint CD43 labeling of the cell body.
CD43 distribution in neutrophils developing locomotory activity on fibronectin.
(A) Neutrophils were allowed to settle on fibronectin for 5 minutes at 37°C and were stimulated by fMLP for 30, 60, and 120 seconds. Cells were then fixed and labeled for CD43. (B) After 5 minutes of locomotion on fibronectin, at 37°C, cells were fixed and double labeled for CD43 extracellular domain (Alexa 488) and for CD43 intracellular domain (rhodamine). Scale bars = 10 μm.
CD43 distribution in neutrophils developing locomotory activity on fibronectin.
(A) Neutrophils were allowed to settle on fibronectin for 5 minutes at 37°C and were stimulated by fMLP for 30, 60, and 120 seconds. Cells were then fixed and labeled for CD43. (B) After 5 minutes of locomotion on fibronectin, at 37°C, cells were fixed and double labeled for CD43 extracellular domain (Alexa 488) and for CD43 intracellular domain (rhodamine). Scale bars = 10 μm.
To demonstrate that CD43 uropod localization was not the result of a specific shedding of CD43 at the leading edge, we analyzed the respective distribution of CD43 intracellular and extracellular domains on neutrophils crawling on fibronectin. We performed double labeling of CD43 extracellular domain (clone L60, which does not label the residual cell-associated CD43 after CD43 shedding23) and of CD43 intracellular domain (with polyclonal antibodies recognizing shedding-independent intracellular epitopes). As shown in Figure3B, both fluorescent markers were very similarly distributed at the uropod. This result confirms the hypothesis of the redistribution of CD43 toward the uropod.
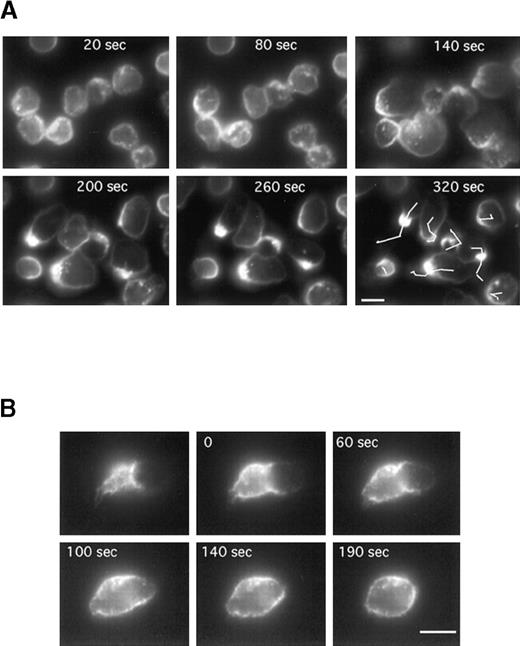
To follow CD43 redistribution by video microscopy in real time, neutrophils were prelabeled for CD43 (with Fab fragments of primary and secondary antibodies) before the locomotion assay. Neutrophils were induced to locomote on the microscope stage at 25°C to slow down the process, and a fluorescent image was recorded every 10 seconds for 400 seconds. Figure 4A represents the fluorescent images (at 60-second intervals) of a representative time series acquisition of migrating neutrophils. Here again CD43 clearly redistributed to the uropod. Until 120 seconds after fMLP stimulation, neutrophils were stationary but displayed a ruffled morphology with CD43 partially redistributed. At 140 seconds after fMLP stimulation, neutrophils started to spread, then to locomote. This induction of locomotion paralleled a clear redistribution of CD43 to the uropod. We obtained the same result when the coverslips were coated with human vitronectin (data not shown).
Time series of neutrophils developing or losing polarity and locomotor activity on a fibronectin-coated surface.
Cells were labeled for CD43 (with Fab fragments of primary and secondary antibodies) at 4°C, then allowed to settle on the fibronectin-coated surface for 5 minutes at 25°C. fMLP (10 nM) was applied and fluorescence images were acquired every 10 seconds. (A) Images have been selected at 60-second intervals. The lines in the last panel represent the tracks of the cell centroids during the 320 seconds of stimulation. (B) The first image represents the uropod of a neutrophil after 5 minutes of locomotion. The cells were then washed in a medium without fMLP. In B, images have been selected at the indicated times (0, 60, 140, and 190 seconds). Scale bar = 10 μm.
Time series of neutrophils developing or losing polarity and locomotor activity on a fibronectin-coated surface.
Cells were labeled for CD43 (with Fab fragments of primary and secondary antibodies) at 4°C, then allowed to settle on the fibronectin-coated surface for 5 minutes at 25°C. fMLP (10 nM) was applied and fluorescence images were acquired every 10 seconds. (A) Images have been selected at 60-second intervals. The lines in the last panel represent the tracks of the cell centroids during the 320 seconds of stimulation. (B) The first image represents the uropod of a neutrophil after 5 minutes of locomotion. The cells were then washed in a medium without fMLP. In B, images have been selected at the indicated times (0, 60, 140, and 190 seconds). Scale bar = 10 μm.
Figure 4B shows that CD43 redistribution is reversible. Cells were labeled for CD43 (with Fab fragments of primary and secondary antibodies), then allowed to locomote on fibronectin as described above. After 5 minutes of locomotion, cells were washed and incubated in the medium without fMLP to allow the cells to revert to a spherical shape. The first panel of Figure 4B represents the fluorescent uropod of a cell before the removal of fMLP. After the washes, pictures were taken at the indicated times (0, 60, 100, 140, and 190 seconds) over a period of 190 seconds. As shown in Figure 4B, CD43 molecules originally clustered at the uropod progressively returned to a uniform distribution all around the cell when fMLP was washed off.
Actin-binding proteins interact with the intracytoplasmic tail of CD43
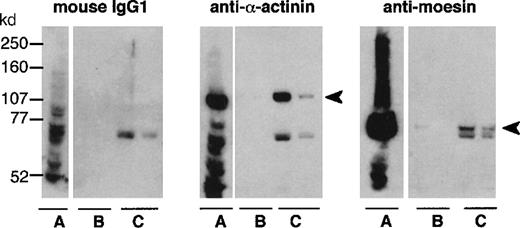
Neutrophil cytosol was applied to a Sepharose column bearing either the recombinant intracellular portion of CD43 (Figure5) or BSA, which was chosen as a control because of its similar charge (BSA isoelectric point of 5.4-5.8, as compared to 5.81 for rCD43intra). Among proteins specifically eluted with purified rCD43intra from CD43intra-Sepharose (Figure6, lane C), but not the BSA-Sepharose column (Figure 6, lane B), we identified α-actinin and moesin by Western blotting. An unidentified band, slightly below the moesin band, reacted nonspecifically with mouse IgGs. Faint bands were also observed in the CD43intra eluate but not the BSA eluate with antiezrin and antiradixin antibodies (data not shown). Because of the high level of homology (70-80%) between ERM proteins, we cannot exclude a cross-reaction of these antibodies with moesin. Western blot analysis of neutrophil cytosol showed that it mainly contains moesin, a very low amount of ezrin, and no detectable radixin (data not shown). Antivinculin revealed a similar eluted band in samples eluted either from CD43intra or the BSA eluate, showing nonspecific binding of vinculin to Sepharose columns. Fimbrin was not detected in either eluate, although clearly detected in the whole cytosol with the antifimbrin polyclonal antibody (data not shown).
Sequence of recombinant CD43intra (137aa).
The sequence of CD43 intracytoplasmic tail begins at threonine n22. It was translated from DNA sequence CD43 (base 943-1293).
Sequence of recombinant CD43intra (137aa).
The sequence of CD43 intracytoplasmic tail begins at threonine n22. It was translated from DNA sequence CD43 (base 943-1293).
Analysis of actin-binding proteins binding to rCD43intra-Sepharose.
Whole cytosol (A) and the 2 first fractions eluted with purified rCD43intra from BSA-Sepharose (B) or from rCD43intra-Sepharose (C) were submitted to 7.5% acrylamide-PAGE electrophoresis and analyzed by Western blotting with control mouse IgG1, anti–α-actinin or antimoesin.
Analysis of actin-binding proteins binding to rCD43intra-Sepharose.
Whole cytosol (A) and the 2 first fractions eluted with purified rCD43intra from BSA-Sepharose (B) or from rCD43intra-Sepharose (C) were submitted to 7.5% acrylamide-PAGE electrophoresis and analyzed by Western blotting with control mouse IgG1, anti–α-actinin or antimoesin.
Localization of actin-binding proteins during CD43 redistribution
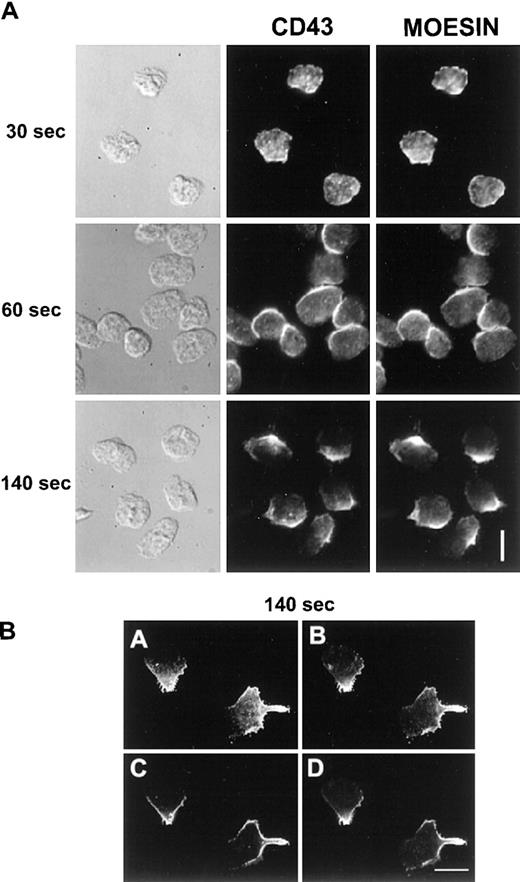
Neutrophils were allowed to locomote for various time at 37°C on fibronectin (30, 60, and 140 seconds). Cells were then fixed and permeabilized and blocked with FCS, then double immunofluorescence of CD43 and moesin was carried out. Figure 7A represents the matching DIC, CD43 fluorescence (Alexa 488), and moesin fluorescence (TRITC) images. We observed a colocalization between CD43 and moesin, both molecules following the same kinetics of redistribution. After 30 seconds of stimulation they were both partially redistributed and colocalized, before any morphologic polarization. When cells were polarized and motile, moesin and CD43 colocalized at the uropod, whereas a small proportion of these molecules remained in other cell areas. To more clearly delineate CD43 and moesin distribution, we performed confocal microscopic analysis of double-labeled cells as shown in Figure 7B. Nineteen images were acquired every 0.5 μm. Panels A and B represent a summation of the 19 planes of the stack, for CD43 and for moesin imaging, respectively. Panels C and D represent a single plane from the middle of the stack for CD43 and for moesin imaging, respectively. Moesin labeling was located very close to the plasma membrane and again perfectly colocalized with CD43.
CD43 (Alexa 488) and moesin (TRITC) double labeling of migrating neutrophils.
Cells, stimulated by fMLP, were allowed to locomote on fibronectin as described in “Materials and methods”. Cells were then fixed and permeabilized after 30, 60, or 140 seconds of locomotion. After washes and saturation steps, cells were double labeled for CD43 and moesin. A represents DIC and fluorescent images as indicated. B represents confocal images of CD43 (A, C) and of moesin (B, D). A and B represent a summation of the 19 planes of the stack; C and D represent a single plane from the middle of the stack. Scale bars = 10 μm.
CD43 (Alexa 488) and moesin (TRITC) double labeling of migrating neutrophils.
Cells, stimulated by fMLP, were allowed to locomote on fibronectin as described in “Materials and methods”. Cells were then fixed and permeabilized after 30, 60, or 140 seconds of locomotion. After washes and saturation steps, cells were double labeled for CD43 and moesin. A represents DIC and fluorescent images as indicated. B represents confocal images of CD43 (A, C) and of moesin (B, D). A and B represent a summation of the 19 planes of the stack; C and D represent a single plane from the middle of the stack. Scale bars = 10 μm.
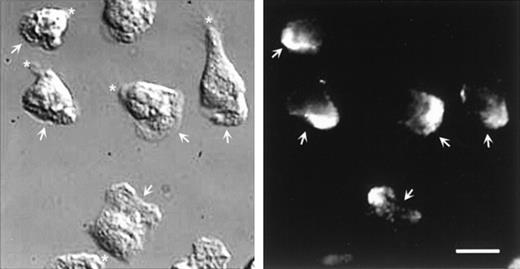
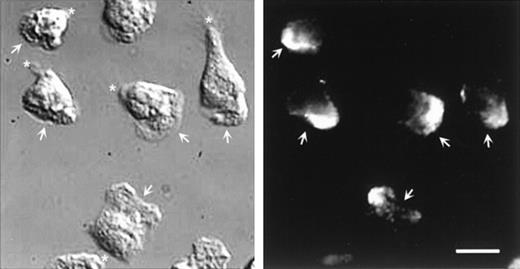
Double labeling of CD43 and α-actinin could not be performed because the respective staining conditions were not compatible. However, parallel staining of these molecules allowed us to exclude their colocalization. Figure 8 shows that α-actinin is located at the leading edge of polarized neutrophils and is not detectable in the uropod, where CD43 molecules are concentrated.
DIC and fluorescent images of neutrophils labeled for -actinin.
Cells, stimulated by fMLP, were allowed to locomote on fibronectin for 2 minutes. Cells were then fixed and permeabilized and labeled for α-actinin. Arrows indicate the position of the lamellipodium; asterisk indicates the uropod position. Scale bar = 10μm.
DIC and fluorescent images of neutrophils labeled for -actinin.
Cells, stimulated by fMLP, were allowed to locomote on fibronectin for 2 minutes. Cells were then fixed and permeabilized and labeled for α-actinin. Arrows indicate the position of the lamellipodium; asterisk indicates the uropod position. Scale bar = 10μm.
Discussion
Antibody cross-linking of CD43 induces CD43 capping and triggers neutrophil polarization and locomotion. This induction of migration is associated with a polar redistribution of CD43 to the uropod. These phenomenon are Fc independent because they were also observed when F(ab′)2 fragments of anti-CD43 antibodies were used instead of whole IgGs. It is the first description of an induction of neutrophil locomotion by antibody cross-linking of a defined membrane molecule, namely CD43, in the absence of other stimulating agents. Under the same conditions, antibody cross-linking of other receptors such as the integrin CD11b/CD18 and HLA molecules (data not shown) results, in our hands, in a patched distribution of the receptors and does not induce either morphologic changes or cell motility.
This induction of cell locomotion by cross-linking of a surface molecule is reminiscent of the motile behavior of B lymphocytes triggered by the capping of membrane immunoglobulins.38Also, antibody cross-linking of αLβ2 and α4β1 integrins has been reported to stimulate T-lymphocyte locomotion, but only on surfaces coated with extracellular matrix proteins, suggesting that a costimulation by adhesive substrates is required in that process.39 Cross-linking by antibodies may mimic cross-linking by substratum-bound molecules. More recently, simultaneous cross-linking of CD3 and CD2 by antibodies was shown to result in a clustering of both molecules at the T-lymphocyte uropod and to stimulate cell migration within 3-dimensional collagen matrices, presumably via specific T-cell activation.40 We did not address here the question of a possible effect of adhesion on CD43 redistribution induced by antibody cross-linking. We found that the proportion of polarized cells following anti-CD43 cross-linking increased from 15% to 25% in suspended neutrophils30 to 73% when cells were placed between 2 coated slides for locomotion assays. This suggests that the contact with a surface facilitates polarization and locomotion. A similar observation has been reported during the capping of surface Igs, where B-lymphocyte polarization was decreased if cells were not allowed to settle onto a surface.41
A second important point is that neutrophil stimulation by chemotactic peptide, in the presence or in the absence of an anti-CD43 mAb, results in CD43 membrane redistribution to the uropod and cell polarization, CD43 molecules being always concentrated at the uropods. This redistribution is observed for both suspended and adherent cells. A proportion of CD43 molecules are known to be shed from neutrophil surface during neutrophil activation and adhesion.23 The observed CD43 asymmetric distribution could thus result from a localized shedding of CD43 molecules at the leading edge. Our results using a polyclonal antibody directed against the intracellular domain of CD43 exclude this hypothesis and show that all CD43 molecules are concentrated at the uropod (whether they had released their extracellular portion or not). CD43 redistribution to uropods has also been described on T lymphocytes polarized by chemokines or spontaneously polarized when in contact with endothelial cells.31 A similar clustering of another membrane sialomucin, PSGL-1, has recently been observed at the uropod of neutrophils activated by nanomolar concentrations of fMLP.42 We conclude that the leukocyte polarization process may include lateral redistribution of specific membrane glycoproteins to the uropod, although the function of this phenomenon is still unknown.
It is tempting to establish a connection between the 2 following observations: (1) CD43 redistribution induced by cross-linking antibodies results in cell locomotion, with CD43 caps located at the uropod, with CD43 redistribution preceding and appearing to trigger cell locomotion; and (2) spontaneously locomoting cells or cells activated by chemotactic peptides redistribute CD43 to the uropods. In both cases, the induction of locomotion results in CD43 redistribution to the uropod. Altogether, these sets of observations strongly suggest that CD43 redistribution and motility are closely related processes.
CD43 clustering on the cell membrane is mediated by an actomyosin-dependent contractile process during CD43 cross-linking by antibodies.30 We investigated the interactions of CD43 with cytoskeletal proteins likely to be involved in CD43 redistribution during neutrophil motility. Among the various cytoskeleton molecules present in neutrophil cytosol, we found that moesin and α-actinin, but not vinculin or fimbrin, were preferentially adsorbed on the recombinant intracellular portion of CD43 bound to Sepharose. Immunofluorescence analysis, however, precluded that an interaction of CD43 with α-actinin would mediate CD43 lateral redistribution at the cell surface of neutrophils. Indeed α-actinin was localized at the leading edge of the polarized neutrophil and was not detected in the CD43-containing uropod. On the other hand, a nearly perfect colocalization of moesin with CD43 was observed. We conclude that moesin bridges CD43 to the cytoskeleton and mediates CD43 redistribution to the uropod, or that CD43 and moesin both belong to a membrane and submembrane structure that is organized during neutrophil motility. This conclusion is in accordance with several reported data showing that ERM family members appear as F-actin linkers to the plasma membrane of neutrophils,43 ERM colocalize with CD43 and CD44 at the cleavage furrow of dividing lymphocytes,44 and ERM molecules interact specifically with CD44 and CD43 intracellular domains.32 33
Our observation of a relationship between CD43 redistribution to the tail and neutrophil motility is relevant to the general function of uropods in cell locomotion. Two hypothesis based on CD43 and motility properties can be put forward:
1. In the antiadhesive hypothesis, a repulsive uropod would facilitate the release of adhesion at the rear of the cell. The concentration of a negatively charged repulsive molecule such as CD43 in the uropod could fulfill this function. Furthermore, CD43 redistribution may clear negatively charged antiadhesive molecules from the leading edge of polarized neutrophils.24 45
2. In the adhesive hypothesis, an adhesive uropod would allow the cell to lean on the substratum when exerting forward traction forces. Indeed, transient interactions of the uropod with collagen fibers have been described on T cells migrating through 3-dimensional collagen lattices.46 In this model, CD43 would interact positively with the substratum. This would allow, as previously proposed,46 “the uropod to stabilize the cell's position for the formation of new contacts at the leading edge, pushing of the cell body forward.” Putative ligands of CD43 could be the positively charged heparin-binding domains present on most proteins of the extracellular matrix, such as fibronectin, laminin, or vitronectin.
Each of these hypotheses may be coupled to the idea of an important role of rear contractions in cell movement. CD43 has been shown to concentrate in the cleavage furrow of dividing cells,44where strong myosin II-dependent contractions occur. CD43 localization in the myosin II-rich uropod during cell locomotion suggests that the anchoring of actomyosin fibers to transmembrane molecules such as CD43 could promote propulsive contractions involved in locomotion. Indeed, whether CD43 was cross-linked by antibodies or not, cell migration was associated with a striking localization of CD43 molecules at the rear of the cell, a localization that never changed during locomotion.
Acknowledgments
We thank Dr Verena Niggli (Institute of Pathology, Bern, Switzerland), Dr Monique Arpin (Curie Institute, Paris, France), and Dr Anthony Bretscher (Cornell University, Ithaca, NY) for helpful discussion; Drs Lynda Pierini and Robert Eddy for their critical reading of the manuscript; Dr Minoru Fukuda for providing the CD43 cDNA; and Virginie Cuelhe and Nicole Bureaud for expert technical assistance.
Supported by fellowships ASTF 8598 and ASTF 8713 to S.S. from the European Molecular Biology Organization (EMBO), Heidelberg, Germany; and N° MLD/CM/ML-CN3/97 from the Association pour la Recherche contre le Cancer (ARC), Paris, France. This work was also supported in part by National Institutes of Health grant GM34770 (F.R.M.).
Reprints:Stephanie Seveau, Department of Biochemistry, Weill Medical College of Cornell University, 1300 York Avenue, New York, NY 10021; e-mail: sseveau@mail.med.cornell.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.