Abstract
During cell migration, integrin attachments to the substratum provide the means to generate the traction and force necessary to achieve locomotion. Once the cell has moved over these attachments, however, it is equally important that integrins detach from the substratum. The fate of integrins after detachment may include release from the cell, lateral diffusion across the cell surface, or endocytosis and redelivery to the cell surface. Polymorphonuclear neutrophils (PMNs) become stuck on the extracellular matrix proteins fibronectin and vitronectin when their intracellular free calcium concentration ([Ca++]i) is buffered. Taking advantage of this feature of PMN migration, we investigated the fate of integrins to differentiate among various models of migration. We demonstrate that 5β1, one of the fibronectin-binding integrins, is responsible for immobilization of [Ca++]i-buffered PMNs on fibronectin. We find that 5 and β1 are in endocytic vesicles in PMNs and that 5 colocalizes with a marker for an endocytic recycling compartment. When [Ca++]i is buffered, 5 and β1 become concentrated in clusters in the rear of the adherent cells, suggesting that [Ca++]i transients are required for 5β1 detachment from the substratum. Inhibition of 5β1 detachment by buffering [Ca++]i results in the depletion of 5 from both endocytic vesicles and the recycling compartment, providing compelling evidence that integrins are normally recycled by way of endocytosis and intracellular trafficking during cell migration. This model is further refined by our demonstration that the endocytic recycling compartment reorients to retain its localization just behind the leading lamella as PMNs migrate, indicating that membrane recycling during neutrophil migration has directionality.
Neutrophils migrate from the blood stream, through the vascular endothelium and connective tissue to sites of inflammation or infection. This process occurs after stimulation by chemoattractants generated by bacteria (eg, N-formylated peptides) or the immune system (eg, complement component C5a). After chemoattractant stimulation, neutrophils activate several intracellular signaling pathways,1 including rapid and repeated changes in the intracellular free calcium concentration ([Ca++]i).2,3 Previous studies have shown that these transients are required for motility on fibronectin and vitronectin,4,5 substrates encountered in the connective tissue stroma. On vitronectin, this loss of motility has been shown to be due to the clustering of αvβ3 integrin in the rear of [Ca++]i-buffered cells.6 Both the loss of motility and the clustering of αvβ3 found in [Ca++]i-buffered neutrophils on vitronectin can be mimicked by the addition of inhibitors of the serine/threonine phosphatase, calcineurin.7 Although it has been suggested that serine/threonine phosphatase inhibitors may play a role in the attachment events of some fibronectin-binding proteins,8the motility of neutrophils on fibronectin is not affected by calcineurin inhibitory peptides.7
Neutrophils contain a number of integrins, of which the best characterized belong to the β2 (CD18) family.9,10Integrins in this family are involved in the attachment of neutrophils to the endothelium,11 as well as attachment to a number of proteins in the connective tissue stroma.12-14 Neutrophils have also been shown to contain α5β1 integrin15,16 42that is known to bind to fibronectin with a high affinity.
During neutrophil migration on a flat surface, the cell sends out numerous pseudopods, some of which adhere to the substrate. The body of the cell then proceeds forward in the direction of the newly formed attachment. To continue moving, the cell must make attachments to the substrate that can be released as it moves forward.5,17There are several mechanisms used by cells to affect this release without actively regulating integrin/substrate interactions:1 by using interactions that are reversible over the time required for a cell to move forward over an attachment site,18-20 by leaving behind pieces of adherent membrane as the cell moves forward,21,22 or by digesting the extracellular matrix proteins to which it is attached.23While migrating neutrophils use these unregulated mechanisms for some adhesive interactions, they actively regulate their interactions with vitronectin and fibronectin, using transient increases in [Ca++]i to disrupt tight integrin/substrate interactions so that the cells can continue moving.5 24
Various fates are plausible for integrins that undergo regulated release from the substrate. It has been proposed in the case of fibroblasts that newly released integrins disperse on the cell surface to be used again in adhesions toward the front of the cell.21,25 Another possibility is that newly released integrins become endocytosed, then transported to the cell surface where they can diffuse to sites of attachment. A related possibility is that integrins are endocytosed at the cell rear and are transported in a directed manner toward the cell front.26 27 This oriented recycling of integrins, whereby endocytosis occurs near the uropod and exocytosis occurs near the leading lamellae, provides a possible mechanism by which a cell could maintain a gradient of adhesiveness along its axis.
In a previous study, we used a shearing procedure,28 which removes the upper surface of the cell and leaves behind only the lower adherent membrane. In this way, proteins directly involved in cell-substrate interactions were studied. For polymorphonuclear neutrophils (PMNs) crawling on vitronectin, we found that αvβ3 integrins were present primarily near the leading edge on the adherent membrane of polarized cells.6 Confocal microscopy on whole cells showed that αvβ3 integrins were also in endocytic vesicles. When these cells were loaded with the cytoplasmic calcium buffer quin2, the αvβ3 integrins were found in clusters on the adherent membrane at the rear of the cell. These clusters were also found in cells in which calcineurin was inhibited, suggesting that calcium was acting through calcineurin to break up integrin clusters.
In this paper, we examined which integrin on PMNs is responsible for [Ca++]i-sensitive adhesion on fibronectin. We show that antibodies to α5β1 integrins, but not against αvβ3 integrins, restore motility to [Ca++]i-buffered cells on fibronectin. We also show that α5 integrins become clustered at the rear of [Ca++]i-buffered cells on fibronectin. We directly demonstrate that α5 integrins are internalized in motile PMNs and are found colocalized with a marker of an endocytic recycling compartment (ERC), strongly suggesting that integrins are indeed recycled via endocytosis during PMN migration. Finally, using a fluorescent label of the ERC, we establish that the ERC is located toward the front of polarized PMNs and reorients during migration to maintain this localization.
Materials and methods
Materials
Fibronectin and vitronectin were purchased from GIBCO BRL (Gaithersburg, MD). Monoclonal antibodies CLB-705(α5), JB1a(β1), JB55(α5β1), CLB-701(α6), and MAB1962 (β2) were purchased from Chemicon (Temecula, CA). LM609 (αvβ3), IB4 (β2), and MAB44 (β2) were gifts from D. Cheresh (Scripps Research Institute, La Jolla, CA), S. D. Wright (Merck Research Laboratories, Rahway, NJ), and A. Huttenlocher (University of Illinois at Urbana-Champaign, Urbana, IL), respectively. Fluorescent-labeled secondary antibodies were purchased from Pierce Scientific (Rockford, IL). Quin2/AM was purchased from Molecular Probes (Eugene, OR). The calcineurin inhibitory peptide, CN412, was a gift from C. Klee (NIH, Bethesda, MD). N-formyl-methyl-leucyl-phenylalanine (fMLP) was purchased from Sigma (St Louis, MO).
Neutrophil isolation
Polymorphonuclear leukocytes (neutrophils) were isolated from whole blood donated by healthy volunteers by a single-step separation over a ficoll-hypaque solution (GIBCO BRL). Contaminating erythrocytes were lysed by a 30-second hypotonic shock. Cells were then rinsed with phosphate-buffered saline (PBS) and resuspended in incubation buffer (150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L glucose, 20 mmol/L HEPES pH 7.4).
Intracellular calcium buffering
For calcium-buffering experiments, neutrophils were incubated in the presence of 50 μmol/L quin2/AM solution as described previously.5 Briefly, the solution was prepared by adding 3 μL of a 50 μmol/L quin2/AM stock solution in anhydrous DMSO to 4 μL of a 25% w/v solution of pluronic-F127 in water. This mixture was then added to 60 μL of heat-inactivated fetal calf serum, followed by 2.9 mL of incubation medium with mixing. The solution was then added to 3 mL of incubation medium containing 5 × 106cells/mL. The cells were incubated in this mixture with gentle mixing for 40 minutes at room temperature. After incubation, the cells were washed twice in PBS and resuspended in incubation medium. After this treatment, Ca++ transients stop and the basal [Ca++]i levels drop to approximately 100 nmol/L.2 We can mimic the effects of quin2/AM buffering by other Ca++-chelating agents (eg, BAPTA/AM), as well as by keeping the PMNs in Ca++-free medium in the presence of EGTA.2,24 The effects of quin2/AM on motility can be attributed to the Ca++-buffering properties of quin2 as opposed to the acetoxymethylester because loading cells with high concentrations of quene-1/AM, a pH-sensitive relative of quin2/AM, has no effect on neutrophil migration.2
Calcineurin inhibition
A peptide inhibitor of calcineurin, CN412, was delivered to the cytoplasm by an endocytosis/hypo-osmotic shock procedure.7Cells were incubated with 100 μmol/L CN412 for 30 minutes at 37°C to allow endocytosis of the calcineurin inhibitor peptide. Cells were then subjected to osmotic shock in water without added salts for 30 seconds to disrupt endocytic vesicles, thereby introducing the peptide into the cytoplasm. Control cells were subjected to osmotic shock in the absence of the peptide. The cells were then rinsed thoroughly with PBS and resuspended in incubation buffer. After the osmotic shock, an estimated 1% to 5% of the external concentration of the peptide is present in the cytoplasm.7 Successful CN412 loading was verified by confirming that migration on vitronectin was inhibited in the same batch of cells (data not shown).
Motility assays
Neutrophils were maintained in incubation buffer at 15°C to prevent clumping and loss of the cytoplasmic CN412 or Quin2. Neutrophils (103-104 cells) were then incubated ± antibodies for 15 minutes at 37°C before starting the experiment. The neutrophils were plated onto the glass coverslip area of the experimental chamber,24 which had been previously coated with fibronectin (0.1 mg/mL) for 1 hour. The neutrophils were maintained at 37°C and allowed to attach for 5 minutes. The medium was removed and replaced with incubation buffer with or without 5 μg/mL antibody. After 5 minutes, the chemoattractant fMLP (10 nmol/L) was added. The experimental chamber was then placed on a microscope stage maintained at 37°C. Five minutes after the application of fMLP, cell motility was monitored in the continued presence of antibody or peptide using a Leitz Diavert microscope (Wetzlar, Germany) equipped with Nomarski differential interference contrast (DIC) optics. A video camera (CCD-72; Dage-MTI Inc, Michigan City, IN) and an optical memory disk recorder (Panasonic; Matsushita Electronics Corp, Osaka, Japan) were used to record single frames every 10 seconds for a period of 200 seconds. Migrating cells were defined as those in which both the leading edge and tail of the cell were observed to move at least 7 μm from their initial position in 200 seconds.24 Separate dishes were used for each treatment, and 3 sequential fields were recorded from each dish. In most experiments, 2 or more dishes were used for each treatment condition. The percentage motile cells (number of motile cells divided by the number of cells observed) was determined for each treatment group in each experiment. Experiments were repeated with fresh preparations of neutrophils on several days.
Flow cytometry
To verify α5β1 integrin expression on PMNs, cells were incubated in incubation buffer containing saturating amounts of primary monoclonal antibody in the presence of 10% normal goat serum ± 10 nmol/L fMLP for 1 hour on ice. Binding experiments were used to determine the concentration of each antibody required for saturation of all binding sites on the cells (not shown). The cells were rinsed with PBS and incubated in 5 μg/mL FITC-goat antimouse IgG for 1 hour on ice. The cells were then rinsed well with PBS. For all flow cytometry measurements, PMNs were resuspended at 1 × 106cells/mL, and fluorescence was measured using a FACScan (Becton Dickinson, San Jose, CA) flow cytometer. Cell analysis was gated on forward and side scatter. The fluorescence intensity of PMNs incubated with the control antibody (MOPC21, dotted lines) was set to an arbitrary number and all other samples were measured relative to this value. In this way, contributions from nonspecific binding of the antibodies and cellular autofluorescence are accounted for. For each condition 104 cells were measured.
Results similar to those presented later in Figure 2 were obtained when monoclonal antibody VC5, which is the same isotype as the irrelevant control antibody (MOPC21), or a different secondary antibody, Alexa488-goat antimouse (Molecular Probes), was used (data not shown).
Immunofluorescence
Where indicated, cells were loaded with either quin2/AM or the calcineurin inhibitory peptide CN412. The cells were plated for 5 minutes at 37°C on coverslip dishes that had been previously coated for 1 hour with a solution of 100 μg/mL fibronectin (GIBCO BRL). Cells were then stimulated for 5 minutes with 10 nmol/L fMLP, fixed, and permeabilized simultaneously by incubation with 6.6% paraformaldehyde/0.05% gluteraldehyde/0.25 mg/mL saponin in PBS for 2 minutes at 37°C. Nonspecific binding sites were blocked for 10 minutes with PBS containing 10% calf serum (blocking buffer). To visualize F-actin, samples were stained with 1 U/5 μL FITC-conjugated phalloidin (Molecular Probes). For indirect immunofluorescence, cells were incubated with primary antibody for at least 1 hour at room temperature, washed extensively, then incubated with the appropriate secondary for an hour. Images of fluorescent-labeled cells were obtained using a Leica DMIRB (Leica Mikroscopie und Systeme GmbH, Germany) equipped with a 63 × 1.32 numerical aperture objective. Images were acquired with a Princeton Instruments (Princeton, NJ) cooled CCD camera driven by Image-1/MetaMorph Imaging System software (Universal Imaging Corporation, PA). Alternatively, cells were visualized on a Bio-Rad MRC600 laser scanning confocal microscope (Bio-Rad Microscience, Cambridge, MA) and a z-stack was obtained. Maximum projection images were produced using Image-1/MetaMorph Imaging System software.
Labeling polymorphonuclear neutrophils with Cy3-VC5 and C6-NBD-gal
A nonfunction blocking monoclonal anti-α5 antibody (VC5, Pharmingen) was directly conjugated to the fluorophore Cy3 (Amersham) according to the manufacturer's instructions. To block nonspecific and Fc receptor binding sites, PMNs were first incubated in the presence of 25 μg/mL of an irrelevant isotype-matched antibody (MOPC21, Sigma) for 10 minutes on ice. Surface-expressed α5 were then labeled by incubation with 10 μg/mL Cy3-VC5 in the continuing presence of MOPC21 for an additional 30 minutes on ice. For some experiments, during the last 30 seconds of incubation with the Cy3-VC5 and MOPC21 antibodies, the plasma membrane of PMNs was labeled with C6-NBD-gal as described below.
N-([6-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino] hexanoyl) sphingosyl phosphocholine (C6-NBD-gal) was prepared as described previously.29 C6-NBD-gal/lipid vesicles (100 μmol/L total lipid) were prepared by injecting an ethanolic solution of a 1:1 mixture of C6-NBD-gal and dioleylphosphatidylcholine (DOPC; 2.5 mmol/L total lipid; Avanti Polar Lipids, Inc, Albaster, AL) into 150 mmol/L NaCl, 20 mmol/L HEPES, pH 7.4. The plasma membrane of PMNs was labeled by resuspending the cells into a 1:30 dilution of the stock C6-NBD-gal/lipid vesicle solution in incubation buffer. PMNs were then incubated for 30 seconds at room temperature, washed once with incubation buffer, then placed on ice until ready for use. For some experiments, C6-NBD-gal was removed from the plasma membrane by incubating the cells in the presence of serum-containing medium (back exchange medium).30
When simultaneous DIC and fluorescence images were required, time-lapse microscopy of C6-NBD-gal-labeled cells was accomplished with a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss Inc, Jena, Germany) with the pinhole opened to maximize the depth of field.
Results
Effect of function-blocking anti-integrin antibodies on neutrophil motility
As previously shown,4,5 neutrophil motility on fibronectin is inhibited when [Ca++]itransients are suppressed by the intracellular calcium buffer quin2. In a previous study, time-lapse video microscopy revealed that cells loaded with quin2 are able to send out pseudopods, but they are unable to detach their uropods from fibronectin-coated surfaces.2To determine whether α5β1 integrin is responsible for [Ca++]i-sensitive motility of PMNs on fibronectin, we measured the ability of function-blocking antibodies to restore motility to [Ca++]i-buffered cells.
When [Ca++]i-buffered cells were incubated in a blocking polyclonal antibody to α5β1, motility was restored to near control levels (Figure1A). Incubation with a polyclonal antibody to αvβ3 had no effect on this motility. To further characterize the integrin responsible for [Ca++]i-sensitive motility on fibronectin, we measured the ability of function-blocking monoclonal antibodies to restore motility to [Ca++]i-buffered cells (Figure 1B). Monoclonal antibodies to α5 and β1 or the α5β1 complex were able to restore motility in [Ca++]i-buffered cells. Both control and [Ca++]i-buffered PMNs that are treated with these antibodies exhibit the typical amoeboid-like motility displayed by untreated control cells. β2 integrins are the most abundant integrins on neutrophils, but antibodies to β2 integrins do not restore motility to [Ca++]i-buffered neutrophils on fibronectin, indicating that they are not the [Ca++]i-sensitive fibronectin-binding integrin (data not shown). In adhesion assays, monoclonal antibodies against either α5β1 or β2 integrins alone only partially inhibit adhesion of PMNs to fibronectin; addition of both anti-α5β1 and anti-β2 antibodies is necessary to completely abrogate adhesion (not shown). Thus, β2 integrins are presumably responsible for adhesion and motility when α5β1 integrins are blocked, but the β2 attachments do not exhibit [Ca++]i-sensitivity.
Motility restoration of calcium-buffered neutrophils on fibronectin with polyclonal antibodies.
(A) Where indicated, cells were [Ca++]i-buffered by a 40 minutes' incubation in 50 μmol/L Quin2/AM. The cells were then incubated with a 1:2000 dilution of the indicated antisera, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antisera. (B) Motility restoration of calcium-buffered neutrophils on fibronectin with monoclonal antibodies. Where indicated, neutrophils were [Ca++]i-buffered with Quin2/AM as in (A), incubated with a 5 μg/mL solution of the indicated monoclonal IgG, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antibody. Cells able to move more than 7 μm in 200 seconds were considered motile. In each case, more than 150 cells were assayed. The data shown are mean values ± SEM.
Motility restoration of calcium-buffered neutrophils on fibronectin with polyclonal antibodies.
(A) Where indicated, cells were [Ca++]i-buffered by a 40 minutes' incubation in 50 μmol/L Quin2/AM. The cells were then incubated with a 1:2000 dilution of the indicated antisera, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antisera. (B) Motility restoration of calcium-buffered neutrophils on fibronectin with monoclonal antibodies. Where indicated, neutrophils were [Ca++]i-buffered with Quin2/AM as in (A), incubated with a 5 μg/mL solution of the indicated monoclonal IgG, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antibody. Cells able to move more than 7 μm in 200 seconds were considered motile. In each case, more than 150 cells were assayed. The data shown are mean values ± SEM.
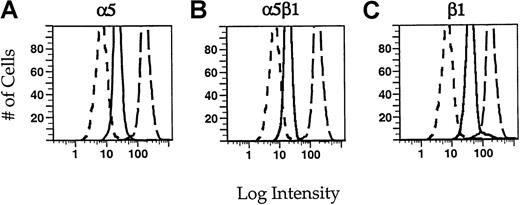
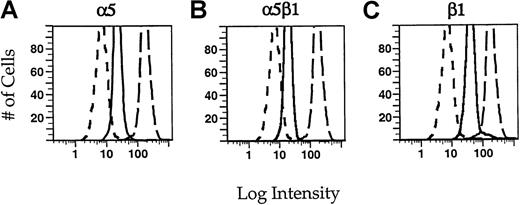
Identification of integrins by flow cytometry.
Neutrophils were incubated in 5 μg/mL of the monoclonal antibodies JB55 (α5) (A), CLB-705 (α5β1) (B), or JB1a (β1) (C) in the presence of fMLP (solid lines). For comparison, cells were incubated with an irrelevant control antibody MOPC21 (short dashes) or with the monoclonal antibody against β2 integrin, IB4 (long dashes). The cells were rinsed with PBS and then incubated in a fluorescein-conjugated secondary antibody. The cells were fixed with paraformaldehyde before flow cytometry. Intensity histograms for each of the monoclonal antibodies are shown. For each condition, 1 × 104cells were measured.
Identification of integrins by flow cytometry.
Neutrophils were incubated in 5 μg/mL of the monoclonal antibodies JB55 (α5) (A), CLB-705 (α5β1) (B), or JB1a (β1) (C) in the presence of fMLP (solid lines). For comparison, cells were incubated with an irrelevant control antibody MOPC21 (short dashes) or with the monoclonal antibody against β2 integrin, IB4 (long dashes). The cells were rinsed with PBS and then incubated in a fluorescein-conjugated secondary antibody. The cells were fixed with paraformaldehyde before flow cytometry. Intensity histograms for each of the monoclonal antibodies are shown. For each condition, 1 × 104cells were measured.
Because the β1 integrin subunit can also form heterodimers with the α6 integrin chain, we measured the ability of blocking antibodies to α6 integrin to restore neutrophil motility on fibronectin. [Ca++]i-buffered cells that were incubated in antibody to α6 integrin showed no increase in motility (data not shown). Similarly, the monoclonal antibody to αvβ3 (LM609), which has been shown to restore [Ca++]i-sensitive motility on vitronectin,31 was not able to restore motility to these cells on fibronectin. These results indicate that the fibronectin-binding integrin α5β1 is responsible for [Ca++]i-sensitive motility of PMNs on fibronectin substrates.
5β1 expression on polymorphonuclear neutrophils
We used flow cytometry to confirm that α5β1 integrin is indeed expressed in neutrophils. We performed binding assays on each of the antibodies used in these studies to determine the concentration of antibodies necessary to saturate all binding sites (data not shown), thereby allowing us to make estimates of the relative concentrations of α5β1 integrin compared with other integrins. Integrins on PMNs were labeled with saturating concentrations of primary antibodies, followed by fluorescently conjugated secondary antibody (Figure 2). PMNs express significant amounts of β1 integrin subunits (Figure 2C). The fluorescence intensity seen using antibodies to α5 (A) or α5β1 (B) integrin is slightly less than 50% of that associated with the β1 integrin subunit alone. This is consistent with the association of other α chains with β1. Because it has been shown that α6β1 integrin is also present in neutrophils,15,32 we used antibodies to the α6 integrin subunit (CLB-701) to determine what concentration of β1 was due to this complex. The fluorescence intensity of cells labeled with anti-α6 integrin antibody accounts for approximately 60% of the β1 concentration (data not shown). Because the α5β1 integrin heterodimer accounts for 40% to 45% of the β1 integrin subunit and α6β1 accounts for approximately 60%, α5β1 and α6β1 appear to comprise the major β1 containing integrins in neutrophils. The fluorescence intensity associated with α5β1 integrins is approximately 10% of that associated with β2 integrins (compare solid with long-dashed lines in Figure 2B). Neutrophils express approximately 4 to 5 × 105 β2 integrins,33 so on the basis of the relative fluorescence intensities, we can estimate that neutrophils express approximately 4 to 5 × 104 α5β1 integrins. It should be noted that this calculated expression level of α5β1 integrin is only a rough estimate because it was obtained using indirect immunofluorescence. Nevertheless, these results show that PMNs have detectable amounts of α5β1 and that α5β1 is at much lower expression levels than the β2 integrins.
Immunofluorescence of 5 and β1 in polarized polymorphonuclear neutrophils
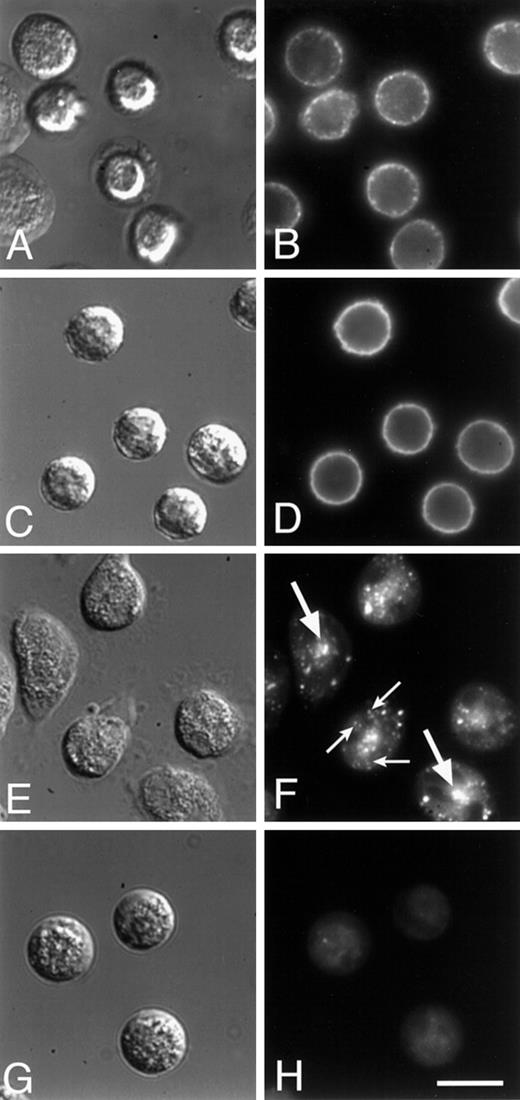
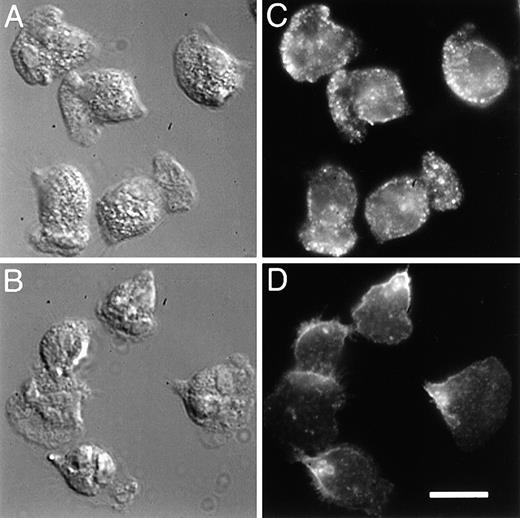
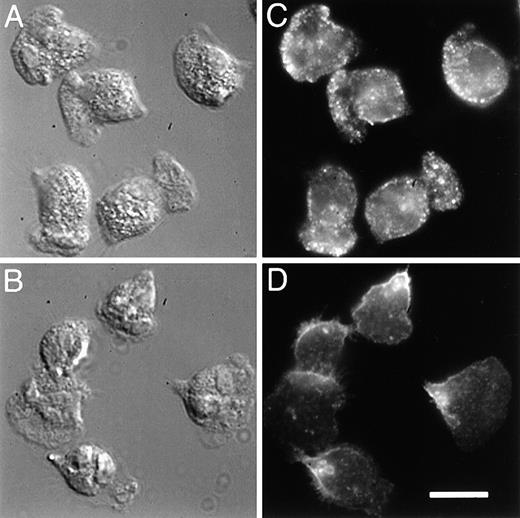
To determine the localization of integrins in fMLP-stimulated neutrophils, we compared the distribution of α5 integrins with that of the predominant integrins expressed on PMNs, the β2 integrins. Localization of the α5 integrin was attained by labeling fixed and permeabilized cells with a monoclonal antibody to the extracellular domain of α5, followed by visualization with a fluorophore-conjugated secondary antibody. With this procedure, we found that this integrin is localized toward the leading edge of the cells (Figure3C). Much of the leading edge staining is lost when immunofluorescence is performed on nonpermeabilized cells, possibly because tight attachment at the front of the cells excludes the antibody. The punctate fluorescence seen throughout the cell is also lost when immunofluorescence is performed on nonpermeabilized cells, and this also may be because antibody is excluded from the lower adherent surface or because the integrin is located in intracellular vesicles (see below). In contrast to the α5 integrin, the β2 integrins are generally found in the back third of both permeabilized (Figure 3D) and nonpermeabilized cells (not shown), and this integrin is never seen in a band at the leading edge. The image shown in Figure3D was obtained with MAB1962 (Chemicon) and differs somewhat from that obtained with other antibodies against β2 (eg, IB4 and MAB44). With these latter antibodies, much of the fluorescence in permeabilized cells is derived from β2 integrins in intracellular vesicles, making it difficult to discern the surface distribution of this integrin. All the antibodies tested consistently gave a distribution for β2 that was toward the back third of the cells.
5 integrin, compared with β2, is enhanced toward the front of polarized PMNs.
PMNs were plated on fibronectin, stimulated with 10 nmol/L fMLP, and then simultaneously fixed and permeabilized with 6.6% paraformaldehyde/0.05% gluteraldehyde in PBS containing 0.25 mg/mL saponin. Samples were incubated with 5 μg/mL of either a monoclonal antibody to the extracellular domain of α5 integrin (VC5, panel C), or a monoclonal antibody to the extracellular domain of β2 integrin (MAB1962, panel D). The samples were rinsed with PBS and then incubated with a TRITC-conjugated secondary antibody. The leading edge of the cells can be determined morphologically from differential interference contrast (DIC) images (A, B). Bar = 10 μm.
5 integrin, compared with β2, is enhanced toward the front of polarized PMNs.
PMNs were plated on fibronectin, stimulated with 10 nmol/L fMLP, and then simultaneously fixed and permeabilized with 6.6% paraformaldehyde/0.05% gluteraldehyde in PBS containing 0.25 mg/mL saponin. Samples were incubated with 5 μg/mL of either a monoclonal antibody to the extracellular domain of α5 integrin (VC5, panel C), or a monoclonal antibody to the extracellular domain of β2 integrin (MAB1962, panel D). The samples were rinsed with PBS and then incubated with a TRITC-conjugated secondary antibody. The leading edge of the cells can be determined morphologically from differential interference contrast (DIC) images (A, B). Bar = 10 μm.
As seen in Figure 4A and B, [Ca++]i-buffered PMNs have a morphology that is quite distinct from the control cells shown in Figures 3A and B. After calcium buffering with quin2/AM, time-lapse video microscopy showed that PMNs continue to extend pseudopodia, but they are unable to detach at the rear, and consequently they become elongated (data not shown and Marks and Maxfield2). In these cells, there is a marked decrease in punctate α5 staining throughout the cell (compare Figure 4C with Figure 3C). In contrast to untreated cells (Figure 3C), most of the α5 is found accumulated in clusters at the rear of [Ca++]i-buffered cells (Figure 4C); the localization of the β2 integrins toward the rear of the cells is unaffected by [Ca++]i-buffering (compare Figure 3D with 4D). From 2 different days experiments, an average of 81% of [Ca++]i-buffered cells (n = 86) became elongated and 86% of these elongated cells showed an accumulation of α5 in the uropod.
[Ca++]i-buffering affects the localization 5 but not β2 integrin in PMNs.
PMNs were loaded with quin2/AM as described in “Materials and methods,” then plated onto fibronectin, stimulated with fMLP, and fixed and permeabilized as for Figure 3. α5 (C) and β2 (D) integrins were visualized by indirect immunofluorescence with monoclonal antibodies and a TRITC-conjugated secondary. DIC images are shown in panels A and B. Bar = 10 μm.
[Ca++]i-buffering affects the localization 5 but not β2 integrin in PMNs.
PMNs were loaded with quin2/AM as described in “Materials and methods,” then plated onto fibronectin, stimulated with fMLP, and fixed and permeabilized as for Figure 3. α5 (C) and β2 (D) integrins were visualized by indirect immunofluorescence with monoclonal antibodies and a TRITC-conjugated secondary. DIC images are shown in panels A and B. Bar = 10 μm.
When a monoclonal antibody to β1 is used, we found that the protein is also found toward the leading edge, but there is additional staining throughout the cell (not shown) presumably because of β1 integrins associated with α6 subunits. As with the α5 integrin, calcium-buffering of neutrophils caused a significant amount of the β1 integrin to be found in clusters toward the rear of the cell (not shown). To avoid the contribution of α6β1 integrin, all subsequent experiments were performed using an anti-α5 antibody.
It has been shown previously that calcineurin inhibition causes cells to become stuck on vitronectin but not on fibronectin.7 We have shown that calcium transients act through the calcium-dependent phosphatase calcineurin to break up αvβ3 integrin clusters in cells on vitronectin.6 We introduced the calcineurin inhibitory peptide CN412 into neutrophils using an osmotic shock technique7 and tested whether calcineurin inhibition has any effect on α5β1 localization in cells on fibronectin. To demonstrate that we had successfully introduced the peptide into the cells, we verified that migration on vitronectin was inhibited (data not shown). When cells are plated onto fibronectin, the localization of α5 integrin is unaffected by inhibition of calcineurin (not shown). Thus, in contrast to the effects on αvβ3 distribution for cells migrating on vitronectin, there is no effect of calcineurin inhibition on the distribution of α5β1 for cells migrating on fibronectin.
Immunolocalization of actin-associated proteins
Talin and α-actinin are proteins that link integrins to the actin cytoskeleton.34-36 We have shown previously that both talin and α-actinin colocalize with integrin clusters at the rear of cells that have been [Ca++]i-buffered on vitronectin.6 Here we used monoclonal antibodies to both talin and α-actinin to determine whether calcium buffering causes a redistribution of talin and α-actinin to the rear of cells attempting to migrate on fibronectin (Figure 5). Both talin (Figure 5C) and α-actinin (data not shown) are found colocalized with F-actin (Figure 5B) near the leading edge of motile cells on fibronectin, but in cells that are [Ca++]i-buffered, F-actin and talin are found in the rear of the cells, as well as in the leading lamella when one exists (Figure 5D and F). Inhibition of calcineurin in cells crawling on fibronectin had no effect on the localization of either F-actin or talin (data not shown). These findings suggest the presence of transient adhesion complexes in cells on fibronectin that require elevations in free calcium to be disassembled. Unlike adhesion complexes in cells on vitronectin, disassembly of adhesion complexes on fibronectin is apparently independent of calcineurin.
[Ca++]i-buffering causes a redistribution of F-actin and talin in PMNs.
PMNs were either loaded with quin2/AM (D-F) or not (A-C), then prepared for immunofluorescence as described. Samples were incubated in 5 μg/mL of a monoclonal antibody to talin, rinsed, and then stained with a TRITC-conjugated secondary antibody and FITC-conjugated phalloidin to visualize F-actin. In control cells (A-C), talin (C) is found colocalized with F-actin (B) predominately at the leading edge of motile cells. In contrast, when cells are [Ca++]i-buffered both talin (F) and F-actin (E) are found at the rear of cells as well as in the leading lamella if one exists. DIC images are shown (A, D). Bar = 10 μm.
[Ca++]i-buffering causes a redistribution of F-actin and talin in PMNs.
PMNs were either loaded with quin2/AM (D-F) or not (A-C), then prepared for immunofluorescence as described. Samples were incubated in 5 μg/mL of a monoclonal antibody to talin, rinsed, and then stained with a TRITC-conjugated secondary antibody and FITC-conjugated phalloidin to visualize F-actin. In control cells (A-C), talin (C) is found colocalized with F-actin (B) predominately at the leading edge of motile cells. In contrast, when cells are [Ca++]i-buffered both talin (F) and F-actin (E) are found at the rear of cells as well as in the leading lamella if one exists. DIC images are shown (A, D). Bar = 10 μm.
Confocal microscopy of 5 integrin
To determine whether the punctate α5β1 integrin staining that we observed via wide-field fluorescence microscopy (Figure 3) was due to integrin localized in intracellular vesicles or in focal contacts on the lower adherent surface of neutrophils, we used confocal microscopy to distinguish between intracellular integrins and those on the surface. In motile cells (Figure 6A-D), a large portion of the anti-α5 staining is seen throughout the cytoplasm of the cell and at the leading edge. The cytoplasmic α5 is seen in a large number of intracellular vesicles and in a perinuclear compartment. To show that the vesicles and this compartment are intracellular, Figure 6C shows a single horizontal plane through the cells shown in Figure 6A; this plane corresponds to the plane marked by the arrows in Figure 6B and is clearly above the adherent surface of the cell. Figure 6D shows a single x-z slice through one of the cells along the axis indicated by arrows in Figure 6A.
Confocal imaging of 5 integrin in control and [Ca++]i-buffered PMNs plated on fibronectin.
PMNs were either loaded with quin2/AM (E-H) or not (A-D), then prepared for immunofluorescence as in Figure 3. Samples were incubated with 10 μg/mL of a monoclonal antibody to the extracellular domain of α5 integrin, rinsed with PBS, and then incubated with a TRITC-conjugated secondary antibody. The samples were viewed using a Bio-Rad MRC 600 laser scanning confocal microscope and vertical sections were obtained. x-y (A, E) and x-z (B, F) projections of both control (A-D) and quin2-buffered (E-H) cells are shown. Panels C and G show the localization of α5 at a single x-y slice at the depth indicated by the arrows in panels B and F, respectively. Panels D and H show a single x-z slice along the axis of the cells indicated by the arrows in panels A and E, respectively. Bar = 10 μm.
Confocal imaging of 5 integrin in control and [Ca++]i-buffered PMNs plated on fibronectin.
PMNs were either loaded with quin2/AM (E-H) or not (A-D), then prepared for immunofluorescence as in Figure 3. Samples were incubated with 10 μg/mL of a monoclonal antibody to the extracellular domain of α5 integrin, rinsed with PBS, and then incubated with a TRITC-conjugated secondary antibody. The samples were viewed using a Bio-Rad MRC 600 laser scanning confocal microscope and vertical sections were obtained. x-y (A, E) and x-z (B, F) projections of both control (A-D) and quin2-buffered (E-H) cells are shown. Panels C and G show the localization of α5 at a single x-y slice at the depth indicated by the arrows in panels B and F, respectively. Panels D and H show a single x-z slice along the axis of the cells indicated by the arrows in panels A and E, respectively. Bar = 10 μm.
Cells that have been calcium buffered with quin2 show significantly less α5 in intracellular vesicles inside the cell (Figure 6E-H). There is a large amount of α5 on the lower surface of the cell, accumulated in the pronounced uropod. There is still some α5 found in intracellular vesicles and in the perinuclear compartment, but it is markedly less than that seen in control cells. This depletion of α5 integrin from intracellular vesicles in calcium-buffered cells could be due to an inhibition of either normal endocytic trafficking or α5 integrin release from integrin clusters. The latter interpretation is supported by the finding that Ca++ buffering has no effect on the rates of endocytosis and recycling of bulk membrane in neutrophils (L.M.P. and F.R.M., unpublished results).
Intracellular localization of the 5 integrin subunit
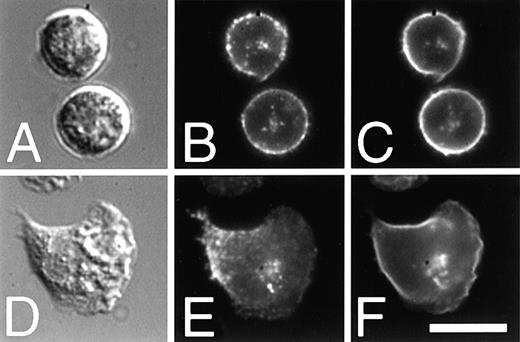
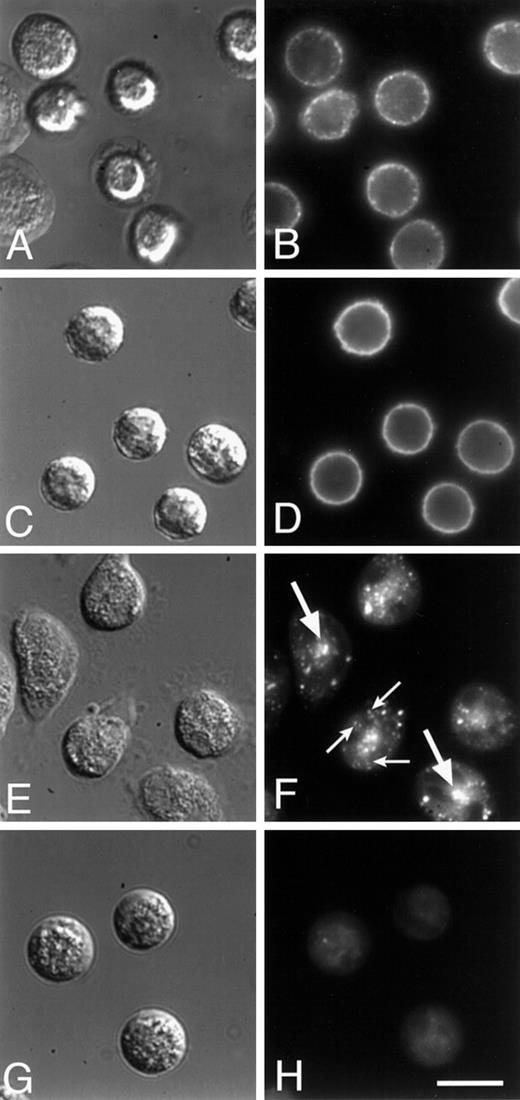
The localization of α5β1 in intracellular vesicles suggests that this integrin may be in endocytic compartments and thus may be recycled during cell migration. Many cell surface proteins, such as the LDL and transferrin receptors, as well as bulk lipid membrane, recycle along a well-characterized pathway.37 In CHO cells that have been transfected with the human transferrin receptor,38fluorescently labeled transferrin has been used to characterize the endocytic recycling pathway of the transferrin receptor.29,39 After endocytosis but before exiting the cell, the transferrin receptor accumulates in a pericentriolar ERC that is separate and distinguishable from sorting endosomes.40Together, sorting endosomes and the ERC comprise the early endosome system. It has been shown previously that the fluorescent lipid analog, C6-NBD-gal, as well as several other lipid analogues, follow an endocytic recycling pathway that is indistinguishable from that followed by the transferrin receptor in CHO and other cells.29 Because PMNs do not express significant amounts of the transferrin receptor, we used C6-NBD-gal as a marker of the ERC in PMNs and looked for colocalization with the α5 subunit.
When PMNs are labeled with C6-NBD-gal and maintained on ice, the C6-NBD-gal labeling is restricted to the plasma membrane (Figure 7D). After warming the cells to 37°C, some of the C6-NBD-gal is endocytosed from the plasma membrane and is seen accumulated in an intracellular perinuclear compartment (Figure 7F and Figure8C). In Figure 7, panels F and H, C6-NBD-gal was removed from the plasma membrane by incubation in back exchange medium. Immediately after the plasma membrane-associated C6-NBD-gal was removed, arrays of vesicles (small arrows, Figure 7F) are seen that appear to either be emanating from or converging into the central compartment. When PMNs are incubated for longer times (30-60 minutes) in back exchange medium, C6-NBD-gal can be chased out of the perinuclear compartment and out of the cell (Figure 7H), confirming that C6-NBD-gal indeed labels a recycling compartment in these cells. The kinetics with which C6-NBD-gal leaves PMNs is similar to that of C6-NBD-gal exocytosis from CHO cells (data not shown), suggesting that the C6-NBD-gal–labeled compartments in each of these cells are analogous.
C6-NBD-gal on the surface of PMNs is endocytosed and trafficked through an endocytic recycling compartment (ERC) before being transported back out to the cell surface.
PMNs were labeled with the fluorescent lipid analogue C6-NBD-gal (C-F) or with a Cy3-conjugated, nonfunction-blocking monoclonal antibody to α5 (Cy3-VC5; A, B), then maintained at 4°C (A-D) or warmed to 37°C for 30 minutes (E-H). After this warm-up, cells were incubated for an additional 2 minutes (E, F) or 30 minutes (G, H) in back exchange medium. Cy3-VC5 and C6-NBD-gal are initially found on the plasma membrane (A-D). After a 30-minute incubation at 37°C, both Cy3-VC5 and C6-NBD-gal accumulate in a central compartment (see Figure8). Two minutes after back exchange of C6-NBD-gal from the plasma membrane, a central compartment is clearly visible in the cells (large arrows, panel F) and in some cases C6-NBD-gal–labeled vesicles (small arrows, panel F) appear to be emanating from the central compartment. By 30 minutes after warm-up, almost all of the C6-NBD-gal has been returned to the plasma membrane and been back exchanged into the medium (H). DIC images are shown in (A, C, E, and G). Bar = 10 μm.
C6-NBD-gal on the surface of PMNs is endocytosed and trafficked through an endocytic recycling compartment (ERC) before being transported back out to the cell surface.
PMNs were labeled with the fluorescent lipid analogue C6-NBD-gal (C-F) or with a Cy3-conjugated, nonfunction-blocking monoclonal antibody to α5 (Cy3-VC5; A, B), then maintained at 4°C (A-D) or warmed to 37°C for 30 minutes (E-H). After this warm-up, cells were incubated for an additional 2 minutes (E, F) or 30 minutes (G, H) in back exchange medium. Cy3-VC5 and C6-NBD-gal are initially found on the plasma membrane (A-D). After a 30-minute incubation at 37°C, both Cy3-VC5 and C6-NBD-gal accumulate in a central compartment (see Figure8). Two minutes after back exchange of C6-NBD-gal from the plasma membrane, a central compartment is clearly visible in the cells (large arrows, panel F) and in some cases C6-NBD-gal–labeled vesicles (small arrows, panel F) appear to be emanating from the central compartment. By 30 minutes after warm-up, almost all of the C6-NBD-gal has been returned to the plasma membrane and been back exchanged into the medium (H). DIC images are shown in (A, C, E, and G). Bar = 10 μm.
5 integrin colocalizes with a lipid marker of the ERC.
PMNs were incubated with Cy3-VC5 and MOPC21 for 30 minutes on ice, then labeled with C6-NBD-gal. Cells were washed well, incubated at 37°C for 30 minutes, then plated onto fibronectin-coated coverslip dishes. PMNs were incubated in the presence (D-F) or absence (A-C) of fMLP before fixation with 2% paraformaldehyde. Cy3-VC5 (B, E) is seen on the plasma membrane, as well as accumulated in a centrally located cluster inside the cells. The intracellular clusters of α5 colocalize with a compartment labeled with C6-NBD-gal (C, F) in both unstimulated (A-C) and stimulated (D-F) cells. DIC images are shown in (A, D). Bar = 10 μm.
5 integrin colocalizes with a lipid marker of the ERC.
PMNs were incubated with Cy3-VC5 and MOPC21 for 30 minutes on ice, then labeled with C6-NBD-gal. Cells were washed well, incubated at 37°C for 30 minutes, then plated onto fibronectin-coated coverslip dishes. PMNs were incubated in the presence (D-F) or absence (A-C) of fMLP before fixation with 2% paraformaldehyde. Cy3-VC5 (B, E) is seen on the plasma membrane, as well as accumulated in a centrally located cluster inside the cells. The intracellular clusters of α5 colocalize with a compartment labeled with C6-NBD-gal (C, F) in both unstimulated (A-C) and stimulated (D-F) cells. DIC images are shown in (A, D). Bar = 10 μm.
As with the C6-NBD-gal labeling, α5 labeled with a directly conjugated nonblocking antibody (Cy3-VC5) is restricted to the plasma membrane for PMNs maintained on ice (Figure 7B). After a warm-up to 37°C, α5 is found in a perinuclear compartment that colocalizes with that labeled by C6-NBD-gal in both resting (Figure 8A-C) and stimulated (Figure 8D-F) cells. The colocalization of the α5 integrin subunit with a bulk membrane marker in PMNs demonstrates that integrins are found in an ERC. In apparent contrast to Figure 3, α5 integrin is absent from the leading edge of the polarized cell shown in Figure 8E. For this experiment, only those α5β1 initially expressed on the surface of PMNs were labeled. Excess antibody was removed by washing the cells before stimulating the cells to migrate. Thus, integrins that are newly delivered to the cell surface after stimulation would not be labeled by the directly conjugated antibody. That the front of the cell is not labeled is consistent with our model because that is where we would expect new integrins to be delivered (see Figure 10).
Localization and orientation of the endocytic recycling compartment during polymorphonuclear neutrophils migration
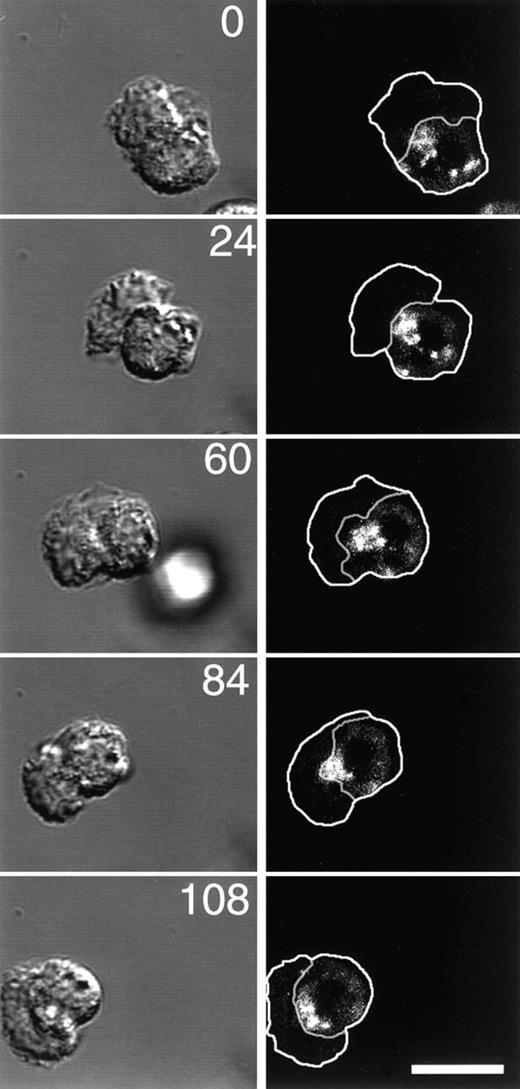
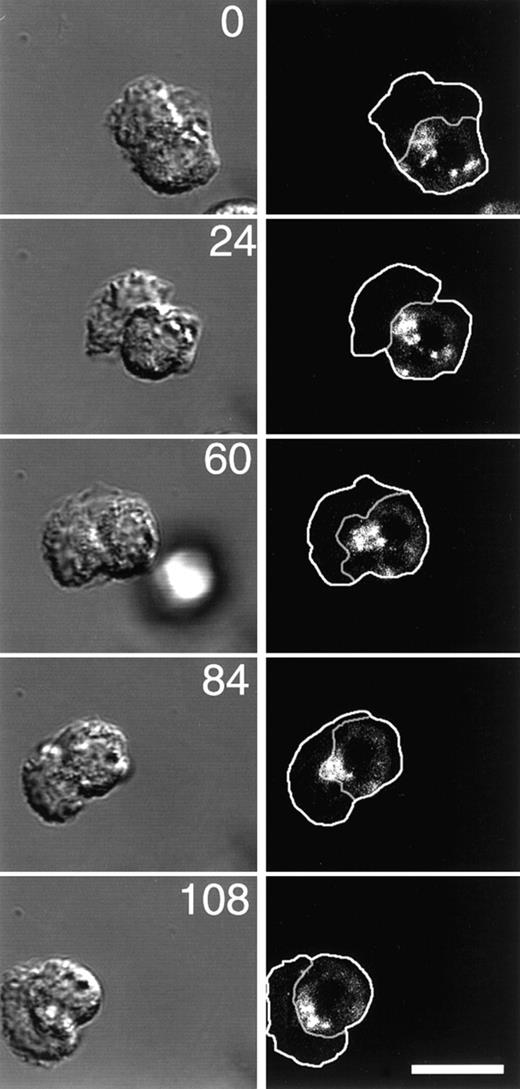
Because it has been reported that the nucleus and MTOC of migrating PMNs reorient as the cell moves and because in many cell types the ERC is closely associated with the MTOC, we used time-lapse microscopy to determine the localization of the ERC during PMN migration. The plasma membrane and ERC of PMNs were labeled with C6-NBD-gal as above, then C6-NBD-gal was back exchanged from the plasma membrane just before plating and stimulating the cells. Immediately after stimulation, DIC and fluorescence time-lapse images were acquired. The outline of the cell, as well as the border between the cell body and the leading lamella, shown in the right panels in Figure9, were determined from the DIC images on the left. The large active leading lamella of the PMN appears slightly out-of-focus and irregular by DIC. The appearance of leading lamellae of PMNs is unlike the flat and smooth appearance of leading lamellae of other cell types, yet they are still clearly distinguishable from the sharply focused granule-containing cell bodies. Time-lapse fluorescence microscopy of motile cells revealed that the ERC is almost always (43/45 cells) located just behind the leading lamellae in migrating PMNs. In all but 1 cell (12/13) that made a dramatic change in direction during the experiment, the ERC reoriented as the cell migrated so that it retained its position just behind the lamella (Figure 9). This asymmetric localization of the ERC is in contrast to the central localization found in resting cells (see Figure 7F), and it in effect imparts a polarity to the cell's recycling mechanism that may play an important role in cell migration (see “Discussion”).
The ERC is localized just behind the leading lamella of motile PMNs and reorients to retain this position as the cells move.
The plasma membrane of PMNs was labeled with C6-NBD-gal as described in “Materials and methods.” The ERC was labeled by incubating C6-NBD-gal–labeled PMNs for 10 minutes at 37°C. To remove C6-NBD-gal from the plasma membrane, yet retain C6-NBD-gal labeling of the recycling compartment, cells were incubated for an additional 10 minutes on ice in back exchange medium. PMNs were plated onto fibronectin-coated coverslip dishes, stimulated with fMLP, then imaged with a Zeiss LSM510 confocal microscope. DIC and fluorescence images were acquired simultaneously at the indicated time points (numbers represent time in seconds). Outlines of the cells and the boundary between the cell body and the lamella were obtained from the DIC images, then transferred to the fluorescence images. Bar = 10 μm.
The ERC is localized just behind the leading lamella of motile PMNs and reorients to retain this position as the cells move.
The plasma membrane of PMNs was labeled with C6-NBD-gal as described in “Materials and methods.” The ERC was labeled by incubating C6-NBD-gal–labeled PMNs for 10 minutes at 37°C. To remove C6-NBD-gal from the plasma membrane, yet retain C6-NBD-gal labeling of the recycling compartment, cells were incubated for an additional 10 minutes on ice in back exchange medium. PMNs were plated onto fibronectin-coated coverslip dishes, stimulated with fMLP, then imaged with a Zeiss LSM510 confocal microscope. DIC and fluorescence images were acquired simultaneously at the indicated time points (numbers represent time in seconds). Outlines of the cells and the boundary between the cell body and the lamella were obtained from the DIC images, then transferred to the fluorescence images. Bar = 10 μm.
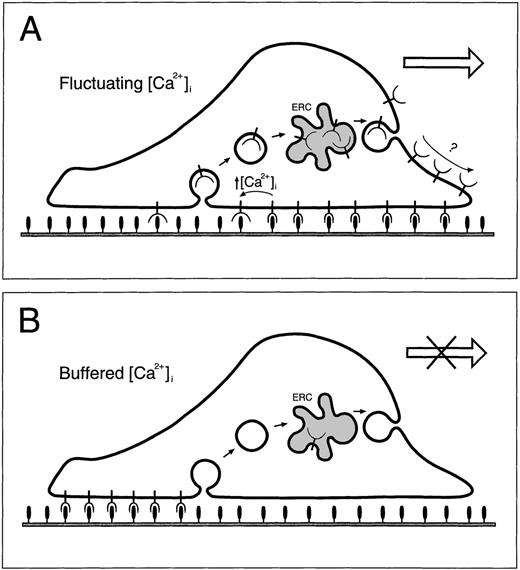
Diagram of regulation of PMN motility on fibronectin.
In panel A, α5β1 integrins (cup shapes on stems) at the leading edge form tight attachments to the fibronectin substrate (filled ovals on stems). During migration, PMNs move forward over the attachment sites while forming new attachments toward the front and releasing old ones toward the rear. The released integrins near the rear are internalized into endocytic vesicles (open circles inside cell) then delivered via the ERC (gray freeform inside cell) toward the front of the cell. Eventual delivery of integrins to attachment sites occurs by an unknown mechanism. When intracellular Ca++ is buffered (panel B), old attachments cannot be released causing α5β1 integrins to accumulate at the back of the cell and become depleted from endocytic compartments.
Diagram of regulation of PMN motility on fibronectin.
In panel A, α5β1 integrins (cup shapes on stems) at the leading edge form tight attachments to the fibronectin substrate (filled ovals on stems). During migration, PMNs move forward over the attachment sites while forming new attachments toward the front and releasing old ones toward the rear. The released integrins near the rear are internalized into endocytic vesicles (open circles inside cell) then delivered via the ERC (gray freeform inside cell) toward the front of the cell. Eventual delivery of integrins to attachment sites occurs by an unknown mechanism. When intracellular Ca++ is buffered (panel B), old attachments cannot be released causing α5β1 integrins to accumulate at the back of the cell and become depleted from endocytic compartments.
Discussion
Previous studies have shown that transient increases in intracellular calcium are required for neutrophil motility on both vitronectin and fibronectin.5 The [Ca++]i-sensitive adhesion molecule for vitronectin has been shown to be an αvβ3 integrin,31and de-adhesion events on this substrate are regulated in part by the calcium-activation of protein phosphatase 2B (calcineurin).7 To determine whether α5β1 integrin was involved in the loss of neutrophil motility on fibronectin, we tested a number of antibodies for their ability to restore motility to [Ca++]i-buffered cells. Function-blocking antibodies to α5 and β1 integrin subunits, as well as antibodies to the α5β1 heterodimer, restored motility to these cells, whereas antibodies to αvβ3 or to α6 integrin did not. The presence of antibodies to α5β1 integrins does not completely inhibit cell adhesion on fibronectin (data not shown), indicating that there are other molecules present on the cell that are capable of mediating attachment to fibronectin. However, these additional fibronectin-binding molecules are apparently not involved in the immobilization of [Ca++]i-buffered cells. β2 integrins, the most abundant integrins found in neutrophils, are able to attach to fibronectin,9 and these are presumably responsible for adhesion when α5β1 is blocked. Time-lapse video microscopy showed that PMNs continue to extend lamellae forward, and these lamellae are able to attach to the substrate even after [Ca++]i is buffered (data not shown and ref.2), further suggesting that adhesion to fibronectin can be mediated by β2 integrins. The addition of function-blocking β2 antibodies, such as IB4,41 was unable to restore motility to [Ca++]i-buffered cells on fibronectin (not shown). Even without [Ca++]i-buffering, cells that had been treated with IB4 did not spread or polarize and were unable to move on fibronectin (data not shown). Anti-β2 integrin antibodies similarly inhibit spreading and motility on a glass surface10 and fibrin.42
PMN migration on fibronectin-coated surfaces in the presence of function-blocking anti-α5β1 antibodies could indicate that α5β1 is not normally required for migration on this substrate. However, it should be noted that both the time-lapse microscopy assay used here and modified Boyden chamber assays are essentially static in that these assays do not require PMNs to move against any fluid pressure. Thus, the physiologic role of the higher affinity attachments mediated by α5β1 may not be properly assessed. Although α5β1 may not berequired for PMN migration, it has been reported that α5β1 is involved in the regulation of human neutrophil migration through fibronectin,16 fibrin and plasma clots,42,43and human synovial fibroblasts in vitro.44 Similarly, β1 integrins have been reported to regulate extravascular migration of rat PMNs in vivo.45 A role for α5β1 in PMN migration is further supported by reports that α5β1 is involved in crosstalk with other integrins,46-48 including β2 integrins.42 It has been shown that fMLP-stimulated PMNs are unable to migrate through fibrin, whereas LTB4-stimulated PMNs are able to migrate.42 Loike et al42 show that this difference is due to the differential regulation of α5β1 and its subsequent regulation of β2 integrins. Clearly, PMN migration is controlled by a complex multireceptor regulatory mechanism that may be necessary for directing the accumulation of these cells at specific anatomic sites.
The clustering of α5β1 integrins in the uropod of neutrophils adherent on fibronectin is similar to the clustering of αvβ3 integrin in cells on vitronectin.6 In both cases, the integrin clusters colocalize with the cytoskeletal proteins actin, talin and α-actinin. When cells are migrating on vitronectin, calcium acting through calcineurin is apparently necessary to break up these clusters, allowing for de-adhesion of the cell from the substrate and continued motility. In the case of α5β1 integrin adhesion to fibronectin, calcineurin inhibition has no effect. Therefore, although calcium is involved in the de-adhesion of PMNs from both of these substrates, calcium regulation of de-adhesion likely occurs through distinct pathways.
There are several possible mechanisms for calcium-activated breakup of α5β1 integrin clusters. Modulation of [Ca++]i could be involved in the regulation of integrin affinity through covalent modifications (eg, phosphorylation/dephosphorylation), as proposed for αvβ3 clusters. Another possibility is that rises in intracellular calcium activate a calcium-dependent protease, such as calpain. Activated calpain could either directly cleave the integrin or it could disrupt integrin/cytoskeleton linkages. Although calpain-dependent migration has been observed for CHO cell lines,22 calpain inhibitors do not seem to have an effect on PMN motility on either fibronectin (unpublished results, L.M.P. and F.R.M.) or fibrinogen (oral personal communication, A. Huttenlocher, August 1999). A third possibility is that mechanical forces are activated by calcium and that these mechanical forces are important in the dissociation of adhesion complexes. There is abundant evidence that mechanical forces affect cell adhesion.49-52 In fibroblasts, inhibition of cytoskeletal tension causes an inhibition of focal adhesion breakdown.50 Calcium is required for activation of myosin light chain kinase, which is involved in the contraction of nonmuscle cells via myosin II.53 In fibroblasts, myosin II and activated calmodulin are preferentially distributed toward the rear of migrating cells, where they can play a critical role in tail retraction.54-56 The cytoskeletal tension generated by uropod contraction may similarly play an important role in breaking the integrin/fibronectin interactions in neutrophils. Blunting of [Ca++]i transients could weaken the contractile forces and allow the integrin clusters to remain intact. Therefore, in neutrophils the mechanism of calcium-sensitive integrin cluster breakup may well involve a combination of covalent modifications and changes in mechanical forces.
As stated previously, there are 2 principle fates of integrins during cell migration. Integrins can either be left behind on the substratum or they can remain associated with the motile cell and be reused to form new contacts with the substratum. For integrins that remain associated with the cell, detachment from the substratum can be followed by either diffusion across the cell surface to new sites of attachment21,25 or endocytosis and recycling back to the cell surface.26 27 This recycling could occur in an oriented fashion such that integrins are taken in at the back of the cell, then delivered toward the leading edge. Exocytosis of integrin-containing vesicles near the leading edge would provide a constant supply of new integrins for use in tight attachments at the cell front. In this way, oriented recycling may help to maintain an adhesion gradient across the adherent surfaces of motile cells. Alternatively, endocytosis and exocytosis may occur uniformly across the cell. In this scenario, exocytosed integrins would need to diffuse to new sites of attachment.
In our previous work6 we presented data consistent with a model for αvβ3 integrin regulation during neutrophil motility in which (1) integrins bind to the substrate near the leading edge of the cell, (2) the cell moves forward over the site of the attachment, (3) during a [Ca++]i transient, the integrin/substrate attachment is broken and the integrin is free to be endocytosed, and (4) internalized integrins are recycled forward (see Figure 10). This was based on the observations of αvβ3 integrins in endocytic vesicles and at the front of the adherent membrane in intact migrating cells. In [Ca++]i-buffered cells, αvβ3 integrins were found only on the rear adherent membrane and they did not enter endosomes. The preferential insertion of recycling αvβ3 integrins toward the front of the cells was inferred from their distribution, but it was not shown directly.
In this paper, we have shown that α5β1 integrins are the [Ca++]i-regulatable integrin in PMNs migrating on fibronectin and we have provided evidence supporting the idea that PMNs recycle their integrins via endocytosis and reinsertion in the plasma membrane. Specifically, we have shown by immunofluorescence and confocal microscopy of fixed and permeabilized cells that α5 is distributed throughout PMNs in intracellular vesicles and in a perinuclear ERC. When PMNs are Ca++-buffered, integrins are found clustered in the uropod of cells because the cells are apparently unable to disassemble the adhesion complex (Figure 10B). Such an inability to detach integrin from the substrate would necessarily inhibit any putative endocytosis of the integrin. That α5 is depleted from the vesicles and from the central compartment of Ca++-buffered cells strongly suggests that the maintenance of the steady state distribution of α5 is dependent on the endocytosis and recycling of this integrin. This idea is further supported by a separate set of experiments utilizing the fluorescent lipid analogue C6-NBD-gal as a marker for the ERC. In these experiments, we show that PMNs, like CHO cells, contain a well-organized ERC. The morphology and location of the ERC in PMNs resemble those of the compartment that can be labeled by anti-α5 antibodies in fixed cells. Using directly conjugated antibodies to α5, we demonstrate for the first time that cell surface α5 becomes endocytosed and delivered to an ERC in migrating PMNs. Collectively, these data provide compelling evidence for the idea that during PMN migration integrins are recycled via endocytosis and redelivery to the cell surface. Because the ERC in PMNs is located just behind the leading lamella and because the leading lamella has been shown to be free of vesicles, reinsertion of integrins in the plasma membrane of these cells is likely to occur toward the front of the cell, but not within the lamella where attachments are being formed. Thus, the ultimate delivery of integrins to attachment sites must proceed by a mechanism other than endocytic trafficking, possibly via passive diffusion across the dorsal surface of the lamella or by an active cytoskeleton-driven process.57 This is in contrast to the mechanism proposed for recycling of integrins in motile fibroblasts, namely that, after detachment from the substratum, integrins are delivered to the front of the cell entirely by diffusion across the plasma membrane.21 25
It has been proposed that migrating cells may maintain an adhesive gradient by delivering adhesion molecules to the cell surface via oriented vesicle transport from the rear of the cell toward the leading edge26,27; however, there has been little direct evidence to support this hypothesis. Our observation that the recycling compartment is almost always located just behind the leading lamella necessitates at least some degree of polarized vesicle trafficking. That is, newly endocytosed vesicles from the rear of the cell that are destined for delivery to the recycling compartment must travel in a directed fashion toward the front of the cell simply because that is where the recycling compartment resides. The directional delivery of membrane proteins from the ERC of polarized epithelial cells is a well-documented phenomenon.58 Ongoing experiments in our laboratory are aimed at detecting the direction of delivery of vesicles in migrating PMNs. Even in the absence of directional delivery, emitted vesicles would be more likely to insert into the plasma membrane just behind the leading edge because of the location of the ERC.
In summary, we have identified α5β1 as the integrin responsible for [Ca++]i-regulatable PMN migration on fibronectin, and we have shown that this integrin is found in endocytic vesicles as well as an ERC. This is the first demonstration that integrins undergo endocytosis and trafficking through an ERC in motile PMNs. These findings and the distribution of α5β1 in control and [Ca++]i-buffered PMNs support a model of integrin recycling that relies on endocytosis and reinsertion in the plasma membrane. Finally, we have shown here that the ERC of migrating PMNs exhibits an asymmetry in its subcellular localization and that this feature is tightly correlated with the direction of cell motility. These observations are consistent with a model involving directed endocytic recycling during cell migration.
Acknowledgments
We thank Drs Stephanie Seveau, Sushmita Mukherjee, and Bob Vasquez for careful reading of the manuscript and insightful comments. We also thank Drs John Mandeville and Richik Ghosh for discussions, C. Klee (NIH) for calcineurin inhibitory peptide, and D. Cheresh (Scripps Research Institute), S. D. Wright (Merck Research Laboratories, Rahway, NJ), and A. Huttenlocher (University of Illinois at Urbana-Champaign, Urbana, Illinois) for antibodies.
Supported by National Institutes of Health grants GM34770 (F.R.M.) and AI40253 (B.H.) and training grants AG00189 (M.A.L.) and GM19078 (L.M.P.).
Reprints:Frederick R. Maxfield, Department of Biochemistry, Weill Medical College of Cornell University, 1300 York Ave, New York, NY 10021.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Motility restoration of calcium-buffered neutrophils on fibronectin with polyclonal antibodies. / (A) Where indicated, cells were [Ca++]i-buffered by a 40 minutes' incubation in 50 μmol/L Quin2/AM. The cells were then incubated with a 1:2000 dilution of the indicated antisera, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antisera. (B) Motility restoration of calcium-buffered neutrophils on fibronectin with monoclonal antibodies. Where indicated, neutrophils were [Ca++]i-buffered with Quin2/AM as in (A), incubated with a 5 μg/mL solution of the indicated monoclonal IgG, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antibody. Cells able to move more than 7 μm in 200 seconds were considered motile. In each case, more than 150 cells were assayed. The data shown are mean values ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726001x.jpeg?Expires=1765949337&Signature=agk1a1e0PGg5Xq-AWiwu4inWakZ3TqHhQUWdeflZNby0BVT~qJfz5EFkkbEM~zCJRIhJczBLqyvZVzc1vgMNxWSV8VVSSU47fL~u37Ln7caI9z5UNV0gHNCVT-jeZYX6ZvrcmUGJ3NCNiFCRujDYArcVgIX42DuKX8-j486EBk06XESmTQibFAHuiDQNYbKKaaDoVxwvP85U0OImt05pEtvjxwKDrMVBMnf9RwiqgwZEa4SqVidgpt74oHKh76Oz0SwZMcWajUWcftjs7edCFnc2NXOJSj1mEWC5qrSC-kv4OjmFQ4sqFv~bRK~qL5ANgUY8YfsnvQhV~OtnlsfNFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. [Ca++]i-buffering affects the localization 5 but not β2 integrin in PMNs. / PMNs were loaded with quin2/AM as described in “Materials and methods,” then plated onto fibronectin, stimulated with fMLP, and fixed and permeabilized as for Figure 3. α5 (C) and β2 (D) integrins were visualized by indirect immunofluorescence with monoclonal antibodies and a TRITC-conjugated secondary. DIC images are shown in panels A and B. Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726004y.jpeg?Expires=1765949337&Signature=z5XG5m5fjdna02ifftGCY6ZuSOB7jnhlXYwu~4leTv1sMNSbIC8q67SfRQZXDXMkJ5x3g2kQ9mdWUK~RRAbqRQDRTAiecgCG-uc7upjaCq8q7SaZ2J2km1~Zr6Vn6oCPMNa04T3b88LtXHjE236uYAeliWFcwLBfHIwGY~qfkgFxVMVzcjxsaaIvvcqinqNV08aL0edhA61-fjBxKGHtHtMmb17g2~eewdDB1E4vrW~XaU25c3VpmFmFg8ScscSJoWmsWK4BVae5REPtVbQFDrgbihS1IddoNzI7yQgTY2QyMAH6OaUfR11mTmnN5coI7l4asQfVOWyaOfzdjPXaVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. [Ca++]i-buffering causes a redistribution of F-actin and talin in PMNs. / PMNs were either loaded with quin2/AM (D-F) or not (A-C), then prepared for immunofluorescence as described. Samples were incubated in 5 μg/mL of a monoclonal antibody to talin, rinsed, and then stained with a TRITC-conjugated secondary antibody and FITC-conjugated phalloidin to visualize F-actin. In control cells (A-C), talin (C) is found colocalized with F-actin (B) predominately at the leading edge of motile cells. In contrast, when cells are [Ca++]i-buffered both talin (F) and F-actin (E) are found at the rear of cells as well as in the leading lamella if one exists. DIC images are shown (A, D). Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726005y.jpeg?Expires=1765949337&Signature=TzVUl84B8zgNO0EL-e~GTC2bqFCeC1tE2BpCpd39Ke6FUc4eyJQ1CR~pkqvu6ZJxZ4bWHJ1I-BceRxBSBz3RZtEwfYlHRQxbPD5BN64IPakZHyvOsZ5Dyh9jueHFzqIgVzOfSDNy0H~vvU9-3-gzzlZP2VPs-8lT01DeIwBTzoM4RtCu6qHivGZ81iZU166NCPN7-UKyBFEHvpqszFrj5Aag4kw-U4tc3DJSPDzo8~D-J0uccrRnfVdFPoSrziHF~itKkrtfRqrcOZaJO9mM4bQI5W3HuPmkNA9dGe1G4IdqFqGFCbkaJ6moGcf2bc3wFNVNUUWX2I1ddLLhn5VI~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Confocal imaging of 5 integrin in control and [Ca++]i-buffered PMNs plated on fibronectin. / PMNs were either loaded with quin2/AM (E-H) or not (A-D), then prepared for immunofluorescence as in Figure 3. Samples were incubated with 10 μg/mL of a monoclonal antibody to the extracellular domain of α5 integrin, rinsed with PBS, and then incubated with a TRITC-conjugated secondary antibody. The samples were viewed using a Bio-Rad MRC 600 laser scanning confocal microscope and vertical sections were obtained. x-y (A, E) and x-z (B, F) projections of both control (A-D) and quin2-buffered (E-H) cells are shown. Panels C and G show the localization of α5 at a single x-y slice at the depth indicated by the arrows in panels B and F, respectively. Panels D and H show a single x-z slice along the axis of the cells indicated by the arrows in panels A and E, respectively. Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726006y.jpeg?Expires=1765949337&Signature=AV275IWbAj8tOvrgwgDOP21IbsNcpWPD~LG1qQv1CbJ5JORrHN1AE-CTM-Ep6MGLrk074zK4AWbQoyjVV0PsqBoBdxsImyw5xZ2mBobyF~idGR4AFIW~VEuoCSekoeOVyyRAx7iKYvED9Nxaq4yGCV7mOkahoOzsIm8dyBFHAAr9eBNGrTCjV7Kon6GIYiwi9HlQSBUrgc7dOz0EQOvX6uZAyrGA3vVNGMCKFouKjFJ5P2Mn9UWSDbqx1CLJFYXEMIKkMB4NB04wfTqmeLi0uM~AQ6WC97Go3uowoJKCeMOe880241KRnbrvcyK0To3~m4oRwdGFj2SjxL9hZKdtXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 1. Motility restoration of calcium-buffered neutrophils on fibronectin with polyclonal antibodies. / (A) Where indicated, cells were [Ca++]i-buffered by a 40 minutes' incubation in 50 μmol/L Quin2/AM. The cells were then incubated with a 1:2000 dilution of the indicated antisera, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antisera. (B) Motility restoration of calcium-buffered neutrophils on fibronectin with monoclonal antibodies. Where indicated, neutrophils were [Ca++]i-buffered with Quin2/AM as in (A), incubated with a 5 μg/mL solution of the indicated monoclonal IgG, rinsed, plated on a fibronectin-coated coverslip dish, and stimulated with 10 nmol/L fMLP in the continued presence of antibody. Cells able to move more than 7 μm in 200 seconds were considered motile. In each case, more than 150 cells were assayed. The data shown are mean values ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726001x.jpeg?Expires=1766332717&Signature=kp4EPWdVfl1dgGbn-GFlGQPZInt5BZopDChsXc61Xpg2TcELPd7lQXqfl7vLVaoaoPrsw02PrsClxEGHNGhk9ucXL1vL-WkZIBYhSWEnSSC6I11Dc4NxuFy1gsHuuQbrx1jblEnInw9naskl9xRPuN~GWIvf~Ll78XozKyeUvXXcinaKNOU80IpxE-jYzSMW4hZUpBQIXyT1ghLRpA4MkU~WAaZkbX~8t-yBgEghNWTTIr~sn6B9iLvvwSkNjn1cOoO2MRWk7ZPzgFd8j60PwRw7fZzqeBG9dZoX~~Z8Ms2SGvMyKSklYzJHNSWWBddBMFc~uSN~urSxmebuEXl3GA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. [Ca++]i-buffering affects the localization 5 but not β2 integrin in PMNs. / PMNs were loaded with quin2/AM as described in “Materials and methods,” then plated onto fibronectin, stimulated with fMLP, and fixed and permeabilized as for Figure 3. α5 (C) and β2 (D) integrins were visualized by indirect immunofluorescence with monoclonal antibodies and a TRITC-conjugated secondary. DIC images are shown in panels A and B. Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726004y.jpeg?Expires=1766332717&Signature=1Q4NzBlD7MfI69Y0in~rg-FaccDhUQZWAIHlFz6iO1QaWBjCv2IxbydgHU4ns2LtgXe1pv02Yk~XaJomvvyambJGs~Jr2O3Bpb-054m-pvZNqJ3RuEXyRtLnDxvmsdI-9bkl-5zz3leMfyJw0RcH1meVmmXxpr1drpob1demeYmfLEQjYPRMYRujicEnGjZIC4jyE8H-NVF-9lhBC8mjbO31ckrMR3sfhSAZ6maj7QUOZyEnz7Ds7iGLHK8lM5FchXN6L2pFij5rF0eW013mKF5VGWiDliIzE2m5loob-tzJYrSSLRrjXCcxK1KSUou2xN~sYNJa3mjG3almnUHNMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. [Ca++]i-buffering causes a redistribution of F-actin and talin in PMNs. / PMNs were either loaded with quin2/AM (D-F) or not (A-C), then prepared for immunofluorescence as described. Samples were incubated in 5 μg/mL of a monoclonal antibody to talin, rinsed, and then stained with a TRITC-conjugated secondary antibody and FITC-conjugated phalloidin to visualize F-actin. In control cells (A-C), talin (C) is found colocalized with F-actin (B) predominately at the leading edge of motile cells. In contrast, when cells are [Ca++]i-buffered both talin (F) and F-actin (E) are found at the rear of cells as well as in the leading lamella if one exists. DIC images are shown (A, D). Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726005y.jpeg?Expires=1766332717&Signature=m7htOsc7LckGJKPlHdsycAprM92HaX07NzurvTxgqaVY63tvXN9ekDnmExnlRDbrkLgKJyylVyUcKbO0TpnrGEk2NheAai-i83xnex5Th4YiYhJ4iQ6VsjtcrCZz9Sfyskd60E6ORBmZLAvshVs2PhmWSRv-NHm7FpeTfjwMPrmEe1KK59MGmFnjFqXNSzaKLdg0Li2lFNIEoKAE7Tldl-8D-DdtERK~N7dcopVci5fqGJxbWRB7qqGYPqNWKKAn7d5kG-LstPXJTEekWUqzVBzqLZ9CkPeN3KNTeIoL5tH32tLSdiWvUiQ~zI5WgA2G~XCkqCoKvgIMnTJ1pGoMBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Confocal imaging of 5 integrin in control and [Ca++]i-buffered PMNs plated on fibronectin. / PMNs were either loaded with quin2/AM (E-H) or not (A-D), then prepared for immunofluorescence as in Figure 3. Samples were incubated with 10 μg/mL of a monoclonal antibody to the extracellular domain of α5 integrin, rinsed with PBS, and then incubated with a TRITC-conjugated secondary antibody. The samples were viewed using a Bio-Rad MRC 600 laser scanning confocal microscope and vertical sections were obtained. x-y (A, E) and x-z (B, F) projections of both control (A-D) and quin2-buffered (E-H) cells are shown. Panels C and G show the localization of α5 at a single x-y slice at the depth indicated by the arrows in panels B and F, respectively. Panels D and H show a single x-z slice along the axis of the cells indicated by the arrows in panels A and E, respectively. Bar = 10 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2471/4/m_bloo00726006y.jpeg?Expires=1766332717&Signature=kNpirxaEyu0U6wP3eLsc2luSd8Fi6VEeb-N6DnwRLS-oBoAKEjH1N21GovA1yiXvCg19r4HhFDPrxIbs-aPuk5dHBbI1LDm4ALJ135Ya0byScVEM8~jF6VJKXyA~sAHzKLcitGKv3gLM5NLwNPLiJtMbAqK3bs3UxE6VQzNtSTM7fAfghQzUttCabGblJlJkdYR-2kQ796VTnW1po3~febALlI4Gaipwc2irmId5QnPVhvOWm84v5xZjPG76qXgwIVMFdjqDJ0nbvEOcuTIdS4gFfmG23WkkQz-Thb1ZL4ChhkH1bnUtriO1cjjhhAhMue14Oz4~EYm-qXGdKdgZrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)