The concept of tolerogenic dendritic cells (DCs) came from experiments in mice showing that thymic DCs mediated clonal deletion of emerging autoreactive T cells within thymus.1The finding that lymphoid precursors give rise to both T cells and CD8+CD11b− DCs within the thymus suggests the existence of a lymphoid pathway, in addition to a well-established myeloid DC pathway giving rise to CD8−CD11b+DCs.2 In mouse spleen, both CD8−CD11b+ myeloid DCs and CD8+CD11b− lymphoid DCs were identified. The finding that lymphoid DCs express higher levels of self-peptide–MHC class II complex3 and FAS-binding protein4suggests that lymphoid DCs may be tolerogenic for T cells, in contrast to immunogenic myeloid DCs. This hypothesis was not, however, supported by studies showing that CD8+CD11b− lymphoid DCs produce a high level of IL-12 and induce potent TH1 response to foreign antigens.5-8 The existence of tolerogenic or TH2-inducing DCs in peripheral tissues was, however, further suggested by studies of DCs from Peyer's patches, liver, and lung. These DCs were shown to preferentially induce TH2 responses, in contrast to splenic DCs and bone-marrow–derived DCs that preferentially induce TH1 responses.9-11 The TH2-inducing function of Peyer's patch DCs and liver DCs may contribute to the mechanism underlying tolerance to food antigens or to allo-liver transplants, respectively.
TH2-inducing dendritic cell precursors (pre-DC2) are derived from hematopoietic stem cells (HSC) in bone marrow.
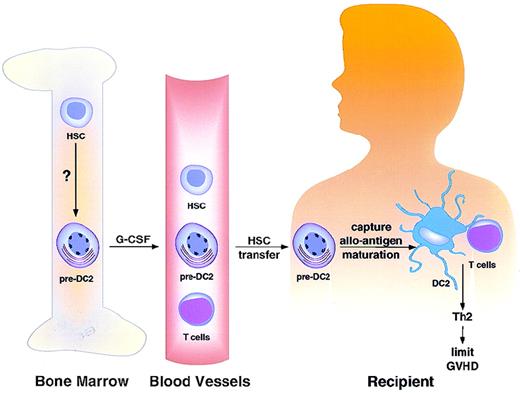
Signals regulating HSC differentiation into pre-DC2 are currently unknown. G-CSF appears to mobilize bone marrow pre-DC2 into peripheral blood. Recipients of blood stem cell transplantation from G-CSF–treated donors received 5- to 6-fold more pre-DC2 than did recipients of bone marrow stem cell products. Pre-DC2 may capture alloantigen and undergo maturation after transfer into the host. These DC2 may present alloantigen to donor T cells and induce them to undergo TH2 differentiation and to limit GVHD.
TH2-inducing dendritic cell precursors (pre-DC2) are derived from hematopoietic stem cells (HSC) in bone marrow.
Signals regulating HSC differentiation into pre-DC2 are currently unknown. G-CSF appears to mobilize bone marrow pre-DC2 into peripheral blood. Recipients of blood stem cell transplantation from G-CSF–treated donors received 5- to 6-fold more pre-DC2 than did recipients of bone marrow stem cell products. Pre-DC2 may capture alloantigen and undergo maturation after transfer into the host. These DC2 may present alloantigen to donor T cells and induce them to undergo TH2 differentiation and to limit GVHD.
To find tolerogenic or TH2-inducing DCs in humans and to develop a way to grow and manipulate these DCs for use in immunotherapy for autoimmune diseases and GVHD have been an immunologist's fantasy. Recently, human lymphoid DCs (DC2) have been generated from human blood CD4+IL-3Rα++CD11c− precursors (pre-DC2) in culture with IL-3 and CD40-ligand.13-17 In contrast to CD40-ligand–activated monocyte-derived DC1 that produce a large amount of IL-12 and induce TH1 differentiation, CD40-ligand–activated DC2 produce a lower amount of IL-12 and induce TH2 differentiation.13 Although TH1-inducing myeloid DC1 and CD11c+ blood DCs have been used in immunotherapy for certain human cancers,18-21 potential application of TH2-inducing DC2 in immunotherapy for autoimmune diseases and GVHD has been limited. This is because the frequency of pre-DC2 in human blood is low (0.2% to 0.8%), and the identity and function of mature DC2 in vivo is unclear.
In this issue of Blood, Dr Arpinati and colleagues report a major advancement in human DC biology, which may pave the way toward DC2-based immunotherapy for autoimmune disease and GVHD.22They show that G-CSF treatment, which is widely used to mobilize hematopoietic stem cells into blood for stem cell transplantation, induced a more than 5-fold increase in blood pre-DC2 numbers. Interestingly, the number of blood myeloid CD11c+DC were found unchanged in the same donor. The authors further showed that while DC1 derived from CD11c+ blood immature DCs cultured with GM-CSF, IL-3, and TNF-a preferentially induced TH1 differentiation, pre-DC2–derived DC2 cultured with the same cytokines preferentially induced TH2 differentiation. Together with a recent study by Rissoan et al,13 Arpinati's study suggests that T helper cell differentiation depends not only on the maturation stage of DCs, but also on the type of DCs.
The question is what the functional consequence is of transferring more DC2 or pre-DC2 into patients. This study showed that recipients of blood stem cell preparations from G-CSF–treated donors received 5- to 6-fold more pre-DC2 than did recipients of bone marrow stem cell products. Interestingly, human G-CSF–mobilized blood stem cells do not cause a higher incidence of GVHD than marrow grafts,23,24despite containing 10-fold more T cells.25 In mice, pretreatment of donor mice with G-CSF enhances TH2-cytokine production and reduces severity of experimental GVHD.26 27 These studies suggest that pre-DC2 in G-CSF–treated blood may capture alloantigen and undergo maturation after transfer into the host. These DC2 may present alloantigen to donor T cells and induce them to undergo TH2 differentiation and to limit GVHD (Figure).
The combination of G-CSF treatment and blood leukapheresis may offer, for the first time, the possibility of generating sufficient DC2s for immunotherapy of certain autoimmune diseases and GVHD. Understanding the developmental pathway and regulation of pre-DC2 from hematopoietic stem cells will be the next critical step in generating a large number of DC2 for immunotherapy. Because the identity of mature DC2 in vivo and the fate of the transferred pre-DC2 in recipient patients are unclear, a direct correlation between pre-DC2 transfer with an increased TH2 differentiation and a decreased GVHD remains to be established.
Acknowledgments
We thank Dr M. Andonian for graphic works and M. Gilliet for critical reading of the manuscript. DNAX Research Institute is fully supported by Schering-Plough.
Reprints:Yong-Jun Liu, Department of Immunobiology, DNAX Research Institute of Molecular and Cellular Biology, 901 California Avenue, Palo Alto, CA 94306.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.