Abstract
The stromal cell-derived factor-1 (SDF-1) is an alpha chemokine that binds to the CXCR4 receptor. Knock-out studies in mice demonstrate that this ligand-receptor pair is essential in hematopoiesis. One function of SDF-1 appears to be the regulation of migration of hematopoietic progenitor cells. We previously characterized signal transduction pathways induced by SDF-1 in human hematopoietic progenitors and found tyrosine phosphorylation of focal adhesion components, including the related adhesion focal tyrosine kinase (RAFTK), the adaptor molecule p130 Cas, and the cytoskeletal protein paxillin. To better understand the functional role of signaling molecules connecting the CXCR4 receptor to the process of hematopoietic migration, we studied SDF-1–mediated pathways in a model hematopoietic progenitor cell line (CTS), as well as in primary human bone marrow CD34+cells. We observed that several other focal adhesion components, including focal adhesion kinase (FAK) and the adaptor molecules Crk and Crk-L, are phosphorylated on SDF-1 stimulation. Using a series of specific small molecule inhibitors, both protein kinase C (PKC) and phosphoinositide-3 kinase (PI-3K) appeared to be required for SDF-1–mediated phosphorylation of focal adhesion proteins and the migration of both CTS and primary marrow CD34+ cells, whereas the mitogen-activated protein kinases ERK-1 and -2 were not. These studies further delineate the molecular pathways mediating hematopoietic progenitor migration and response to an essential chemokine, SDF-1.
Stromal cell-derived factor-1 (SDF-1) was initially characterized as a pre-B-cell stimulatory factor and cloned from mouse bone marrow stromal cells.1-4 SDF-1 is a CXC chemokine, secreted constitutively from several cell types.5 There are 2 isoforms of SDF-1, α and β, which are generated by differential splicing from a single gene.2 SDF-1 has been characterized as a highly efficient chemotactic factor for T cells, monocytes,6,7 pre-B cells,8 dendritic cells9 and hematopoietic progenitor cells,10-12and binds to the CXCR4 receptor. Like other α chemokine receptors, CXCR4 is a 7-transmembrane surface structure linked to G proteins.7,13,14 The chemotactic effect of SDF-1 on hematopoietic progenitor cells has been shown to be mediated by the CXCR4 receptor. Targeted disruption of either the SDF-1 or CXCR4 gene is lethal in mice and is accompanied by many severe defects, including the absence of both lymphoid and myeloid hematopoiesis in the fetal bone marrow.15,16 More recently, it was found that SDF-1 and the CXCR4 receptor play a critical role in the engraftment of hematopoietic stem cells to bone marrow.17 These results indicate that SDF-1/CXCR4 can regulate hematopoiesis by modulation of migration and homing of hematopoietic stem and progenitor cells.
Despite the increasingly prominent role of SDF-1/CXCR4 in the regulation of hematopoietic progenitor cells, there is scant information on the signal transduction pathways induced by SDF-1/CXCR4. SDF-1, binding to CXCR4, mobilizes calcium and reorganizes the actin structure. The chemotactic activity of SDF-1 is blocked by pertussis toxin.6,10,18 19 These results indicate that the CXCR4 receptor transmits signals through the Gαi-protein.
Various cytokines that induce migration modulate the formation and function of focal adhesions.20 These adhesions are cytoskeletal structures that form adherent contacts with the extracellular matrix. Formation of such focal adhesions and reorganization of the actin cytoskeleton have been shown to be associated with the phosphorylation of focal adhesion components.21,22 For example, the tyrosine phosphorylation of focal adhesion kinase (FAK),23,24 and of the adaptor proteins p130 Cas,25,26 Crk,25,27 and paxillin28 29 are directly associated with cell migration induced by cytokines or chemokines.
To characterize the signal transduction pathways associated with SDF-1–induced migration in hematopoietic progenitor cells, we first studied primitive bone marrow CD34+ cells and then a human hematopoietic progenitor cell line, CTS, which has been found to express a robust level of the CXCR4 receptor.18 CTS has been characterized as a multipotential progenitor cell line that can differentiate into either myeloid or lymphoid lineages.30We previously showed that SDF-1α stimulates migration and induces calcium flux and the tyrosine phosphorylation of related adhesion focal tyrosine kinase (RAFTK), paxillin, and p130 Cas in CTS cells.18 Here, we extend our previous study and report that SDF-1α induces the tyrosine phosphorylation of multiple adhesion proteins. In addition to RAFTK, paxillin, and p130 Cas, we observed that FAK, Crk, and Crk-L are also phosphorylated on SDF-1α stimulation. With respect to the functional roles of signaling molecules, both phosphoinositide-3 kinase (PI-3) and protein kinase C (PKC), but not p44/42 ERK, appeared to be required for the SDF-1α–induced tyrosine phosphorylation of focal adhesion proteins, and for the migration of CTS cells and of human bone marrow CD34+ progenitor cells.
Materials and methods
Reagents and antibodies
Recombinant SDF-1α was purchased from R&D Systems (Minneapolis, MN). Rabbit anti-RAFTK antibody (R-4250) was generated as described previously.31 32 Anti-FAK polyclonal antibody was generated from New Zealand white rabbits immunized with a bacterially expressed GST-fusion protein containing the C-terminal (750-end aa residues) of FAK cDNA, and was tested to react specifically with FAK. Rabbit anti-PLC-γ, and anti-Crk-L polyclonal antibodies were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The mouse antiphosphotyrosine monoclonal antibody (mAb) 4G10 was a generous gift from Dr Brian Druker (University of Oregon, Portland, OR), and rabbit anti-PI-3 kinase polyclonal antibodies were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Anti-Crk, anti-p130 Cas, anti-paxillin, and anti-phosphotyrosine mAb (PY20) were purchased from Transduction Laboratories (Lexington, KY). Normal rabbit serum and purified normal rabbit IgG or mouse IgG were purchased from Organon Teknika Corp (Westchester, PA). Wortmannin, a PI-3 kinase inhibitor; GF109203X, a PKC inhibitor; and PD98059, a MEK kinase inhibitor, were obtained from Calbiochem (La Jolla, CA). Electrophoresis reagents and nitrocellulose membrane were obtained from Bio-Rad Laboratories (Hercules, CA). Protein A-Sepharose CL-4B and Glutathione Sepharose 4B were obtained from Amersham Pharmacia (Piscataway, NJ). The protease inhibitors leupeptin, aprotinin, and alpha 1 antitrypsin and all other reagents were obtained from Sigma Chemical Co (St Louis, MO).
Cells and cell culture
The CTS cell line was a gift from Dr Takeyuki Sato (Chiba University, Chiba, Japan). The CTS hematopoietic cell line was established from a patient with acute myeloblastic leukemia (AML) and was grown at 37°C in 5% CO2 in RPMI-1640 medium containing 10% FCS, 50 μg/mL penicillin, and 50 μg/mL streptomycin, as described previously.30
Ligand stimulation of cells
CTS cells were starved in serum-free RPMI-1640 medium for 5 hours. During the last hour of starvation, 0.1 nmol/L of sodium vanadate was added. After starvation, cells were washed twice with serum-free RPMI-1640 medium and then resuspended at 15 × 106/mL. Cells were then stimulated in vitro with 20 nmol/L SDF-1α for different time periods at 37°C. After stimulation, cell lysates were prepared by lysis in lysis buffer (50 mmol/L HEPES, pH 7.0, 150 mmol/L NaCl, 10% glycerol, 1% Triton-X 100, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10 mmol/L sodium pyrophosphate, 100 mmol/L NaF, 10 mmol/L dithiothreitol, 1 mmol/L PMSF, 10 μg/mL of aprotinin, leupeptin and pepstatin, 10 mmol/L sodium orthovanadate). Total cell lysates (TCL) were clarified by centrifugation at 10 000g for 10 minutes. Protein concentrations were determined by protein assay (Bio-Rad Laboratories). To assess the effects of the PI-3 kinase inhibitor wortmannin, the PKC inhibitor GF109203X, or the MEK inhibitor PD98059 for ERK1/2, cells were preincubated with each of these compounds for 45 minutes before SDF-1α stimulation as described above.
Immunoprecipitation and Western blot analysis
For the immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein A-Sepharose for 1 hour at 4°C. After the removal of protein A-Sepharose by brief centrifugation, the solution was incubated with different primary antibodies as detailed below for each experiment for 4 hours or overnight at 4°C. Immunoprecipitations of the antibody-antigen complexes were performed by incubation for 2 hours at 4°C with 75 μL of protein A-Sepharose (10% suspension). Nonspecific bound proteins were removed by washing the Sepharose beads 2 times with HNTG buffer (50 mmol/L HEPES, pH 7.0, 150 mmol/L NaCl, 10% glycerol, 0.1% Triton-X 100, 1 mmol/L PMSF, 10 μg/mL aprotinin, leupeptin, and pepstatin, 10 mmol/L sodium orthovanadate) and 1 time with phosphate-buffered saline (PBS). Bound proteins were solubilized in 40 μL of 2 × Laemmli sample buffer and further analyzed by immunoblotting. Samples were separated on 8% to 12% SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 2 hours at room temperature (RT) or 4°C overnight. Immunoreactive bands were visualized using horseradish peroxidase (HRP)-conjugated secondary antibody and the enhanced chemiluminescent (ECL) system (Amersham Pharmacia). Immunoreactive bands were quantitated by scanning the blot under a Model GS-700 Imaging Densitometer (Bio-Rad). Control lanes were assigned a value of 1, and the quantitations of the immunoreactive bands were expressed as multiples of the control, based on the densitometry values. Each experiment was repeated at least 3 times and the presented blots are representative of these experiments.
Assays of phosphoinositide-3 kinase activity
SDF-1α–stimulated or –unstimulated cells were lysed in ice-cold lysis buffer containing 137 mmol/L NaCl, 20 mmol/L Tris-HCl (pH 7.4), 1 mmol/L MgCl2, 1 mmol/L sodium orthovanadate, 10% glycerol, 1% NP-40, and 1 mmol/L PMSF. Immunoprecipitation was performed using antiphosphotyrosine mAb (PY20) (for phosphotyrosine-associated PI-3–kinase activity). Immunoprecipitates were washed 3 times with lysis buffer, 3 times with buffer containing 0.1 mol/L Tris-HCl (pH 7.4), 5 mmol/L LiCl, and 0.1 mmol/L sodium orthovanadate, 2 times with TNE buffer containing 10 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 5 mmol/L EDTA, and 0.1 mmol/L sodium orthovanadate. Samples were resuspended in 20 μL TNE buffer, 20 μL phosphoinositol (10 μg; Avanti Polar Lipids, Alabaster, AL), and 10 μL ATP mix (1 mmol/L HEPES, 10 μmol/L ATP, 1 μmol/L MgCl2, 10 μmol/L γ32P-ATP), then incubated at 37°C for 10 minutes. The reaction was stopped by adding 40 μL of 3 mol/L HCl and 160 μL chloroform:methanol (1:1 vol/vol). Lipids were separated on oxalate-impregnated silica thin-layer chromatography (TLC) plates with a solvent system of chloroform:methanol:water:ammonium hydroxide (28%) (35:35:3.5:7). TLC plates were dried and subjected to autoradiography at −80°C.
Preparation of human bone marrow cells
Light-density bone marrow mononuclear cells were obtained from normal consenting donors and depleted of adherent cells as previously described.33
Cell separation or isolation by immunomagnetic beads
Nonadherent light-density bone marrow cells were resuspended in ice-cold PBS with 1% FCS and 0.5% BSA (isolation medium). CD34+ cells were selected by the Dynal CD34 Progenitor Cell Selection System (Dynalbeads™ M-450 CD34 and DETACHaBEAD™ CD34, Dynal Inc, Lake Success, NY) according to the manufacturer's instructions. The beads binding on the cells were detached and the cells were washed with and resuspended in a medium for the migration assays (see below). The purity of the CD34+ cells selected by this method was found to be more than 95%.
Chemotaxis assays
Chemotaxis assays were performed in triplicate using 5-μm pore filters (Transwell, 24-well cell clusters; Costar, Boston, MA) as described previously.11 Briefly, the filters were rinsed with migration medium (RPMI-1640 with 0.5% BSA for the CTS cells; complete α-medium with 0.5% BSA for the CD34+ bone marrow cells) and the supernatant was aspirated immediately before loading the cells. Either 2 × 105 CTS cells or 1.5 × 105 CD34+ cells suspended in 100 μL migration medium were loaded into each Transwell filter. Filters were then carefully transferred to another well containing 650 μL migration medium with 20 nmol/L SDF-1α (R&D Systems). The plates were incubated at 37°C in 5% CO2 for 3.5 hours. Next, the upper chambers were carefully removed and the cells in the bottom chambers were collected. The cells were washed and resuspended in proper volume and quantitated for viable cells using the trypan blue exclusion method. To assess the effects of wortmannin, GF109203X, or PD98059, the cells were pre-incubated with various concentrations of these inhibitors for 45 minutes, and then the chemotaxic assays were performed, as described above.
Statistical analysis
The results are expressed as the mean ± SD of data obtained from 3 or more experiments performed in duplicate or triplicate. Statistical significance was determined using the Student t test.
Results
Stromal cell-derived factor-1 stimulation induces activation of phosphoinositide-3 kinase and the tyrosine phosphorylation of PLC-γ in CTS cells
Cytokine or chemokine-induced cell migration is a complex process, mediated by multiple signaling mechanisms. PI-3 kinase, PKC, or MAPK pathways have been reported to be involved in cytokine or chemokine-induced migration in various cell types.34-39 We assessed whether these signaling pathways were involved in SDF-1α–induced migration in hematopoietic progenitor cells.
Using CXCR4 receptor-transfected L1.2 mouse pre-B cells, we previously showed that SDF-1α stimulation via the CXCR4 receptor selectively activated p44/42 ERK kinase, but not p38 or JNK kinase.36In CTS cells, we previously observed a similar activation of p44/42 ERK by SDF-1α.18 Here, we examined the effects of SDF-1α on PI-3 kinase activity and on the tyrosine phosphorylation of PLC-γ in CTS cells.
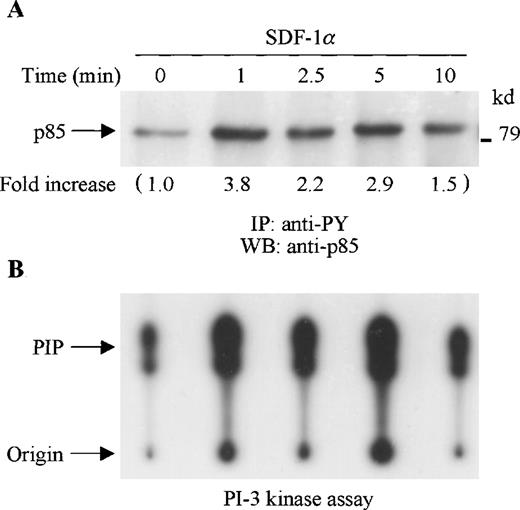
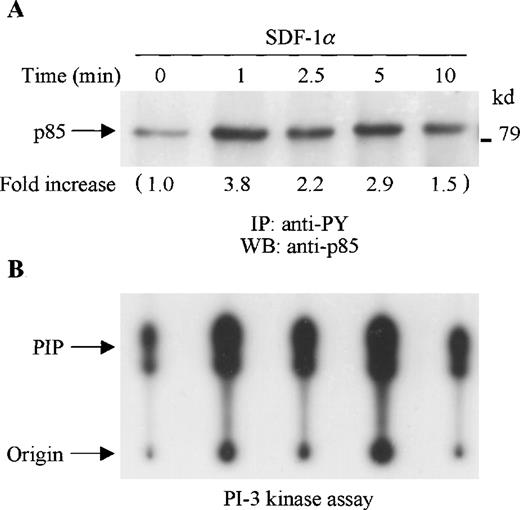
SDF-1α stimulation of CTS cells induced tyrosine phosphorylation of the p85 PI-3 kinase subunit as detected by immunoblotting of anti-phosphotyrosine (PY) immunoprecipitates with anti-p85 anti-body (Figure 1A). To determine whether SDF-1α stimulated PI-3 kinase activity, cell lysates from SDF-1α stimulated or unstimulated CTS cells were immunoprecipitated with anti-PY antibody. The tyrosine phosphorylation-associated PI-3 kinase activity was measured by an in vitro PI-3 kinase assay. As shown in Figure 1B, PI-3 kinase activity was enhanced by SDF-1α stimulation in a time course that paralleled p85 tyrosine phosphorylation.
SDF-1 stimulation results in increased tyrosine phosphorylation and activation of PI-3 kinase in CTS cells. (A) Total cell lysates, prepared from SDF-1α–stimulated CTS cells for the indicated times, were immunoprecipitated with anti-PY antibody (PY20). The immune complexes were subjected to immunoblot analysis with anti-p85 antibody. The changes in the immunoblotted bands of p85 are indicated (by fold increase) based on the densitometry values. (B) Total cell lysates were immunoprecipitated with anti-PY antibody (PY20). PI-3 kinase activity was measured by a PI-3 kinase assay as described in “Materials and methods.”
SDF-1 stimulation results in increased tyrosine phosphorylation and activation of PI-3 kinase in CTS cells. (A) Total cell lysates, prepared from SDF-1α–stimulated CTS cells for the indicated times, were immunoprecipitated with anti-PY antibody (PY20). The immune complexes were subjected to immunoblot analysis with anti-p85 antibody. The changes in the immunoblotted bands of p85 are indicated (by fold increase) based on the densitometry values. (B) Total cell lysates were immunoprecipitated with anti-PY antibody (PY20). PI-3 kinase activity was measured by a PI-3 kinase assay as described in “Materials and methods.”
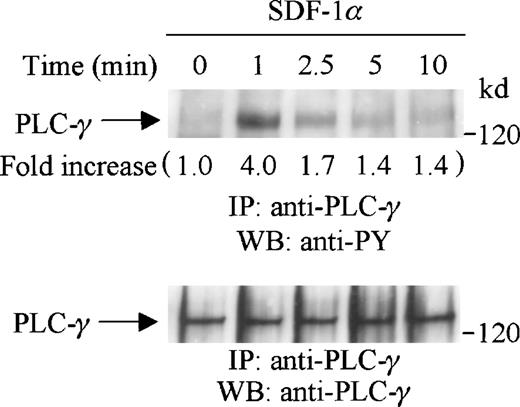
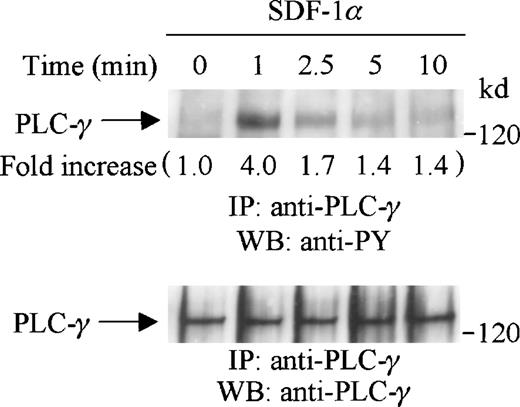
PLC-γ has been shown to play an important role in G-protein coupled receptor signaling and is an upstream mediator for PKC activation.40 41 We thus investigated if PLC-γ was tyrosine-phosphorylated after SDF-1α stimulation of CTS cells. Cell lysates from SDF-1α stimulated or unstimulated CTS cells were immunoprecipitated with anti-PLC-γ antibody and subjected to serial immunoblotting with anti-PY antibody and anti-PLC-γ antibody. We observed that PLC-γ was significantly tyrosine-phosphorylated on SDF-1α stimulation (Figure 2, upper panel). The tyrosine phosphorylation of PLC-γ was rapid and transient, reached a maximum at 1 minute, and returned to a basal level at 5 to 10 minutes after stimulation. Equal amounts of PLC-γ were present in each lane (Figure 2, lower panel). The tyrosine phosphorylation of PLC-γ in response to SDF-1α stimulation suggested that the PKC pathway may be involved in CXCR4 receptor signaling.
SDF-1 stimulation induces tyrosine phosphorylation of PLC-γ in CTS cells. CTS cells were serum-starved and stimulated with SDF-1α for the indicated times. Total cell lysates were immunoprecipitated with anti-PLC-γ antibody, and subjected to serial immunoblotting with anti-PY antibody (4G10) (upper panel) and anti-PLC-γ antibody (lower panel). The increase in tyrosine phosphorylation of PLC-γ is indicated (by fold increase) based on the densitometry values.
SDF-1 stimulation induces tyrosine phosphorylation of PLC-γ in CTS cells. CTS cells were serum-starved and stimulated with SDF-1α for the indicated times. Total cell lysates were immunoprecipitated with anti-PLC-γ antibody, and subjected to serial immunoblotting with anti-PY antibody (4G10) (upper panel) and anti-PLC-γ antibody (lower panel). The increase in tyrosine phosphorylation of PLC-γ is indicated (by fold increase) based on the densitometry values.
Stromal cell-derived factor-1 stimulates tyrosine phosphorylation of focal adhesion kinase and its association with p130 Cas and paxillin
To determine whether FAK is tyrosine-phosphorylated after SDF-1α stimulation, CTS cells were serum-starved and stimulated with SDF-1α for the indicated times (Figure 3). Cell lysates were immunoprecipitated with a specific anti-FAK antibody, and the immunoprecipitates were then analyzed by Western blotting with anti-PY antibody. As shown in Figure 3, SDF-1α stimulation induced a rapid and transient tyrosine phosphorylation of FAK. The maximum tyrosine phosphorylation of FAK was detected as early as 1 minute after the addition of 20 nmol/L of SDF-1. Thereafter, tyrosine phosphorylation of FAK declined gradually to almost baseline levels after 5 minutes (Figure 3, upper panel). We verified that similar amounts of FAK were recovered from the lysates of cells untreated or treated with SDF-1α for various times (Figure 3, lower panel).
SDF-1 treatment induced the tyrosine phosphorylation of FAK in CTS cells. Total cell lysates from CTS cells unstimulated or stimulated with SDF-1α for the indicated times were immunoprecipitated with anti-FAK antibody. The immune complexes were subjected to immunoblotting analysis with anti-PY antibody (4G10) (upper panel) and anti-FAK antibody (lower panel). The increase in tyrosine phosphorylation of FAK is indicated (by fold increase) based on the densitometry values.
SDF-1 treatment induced the tyrosine phosphorylation of FAK in CTS cells. Total cell lysates from CTS cells unstimulated or stimulated with SDF-1α for the indicated times were immunoprecipitated with anti-FAK antibody. The immune complexes were subjected to immunoblotting analysis with anti-PY antibody (4G10) (upper panel) and anti-FAK antibody (lower panel). The increase in tyrosine phosphorylation of FAK is indicated (by fold increase) based on the densitometry values.
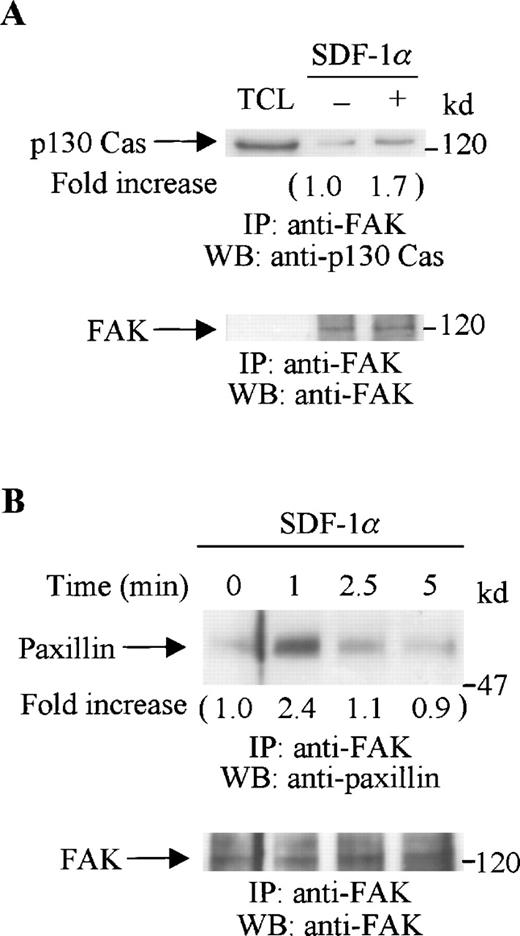
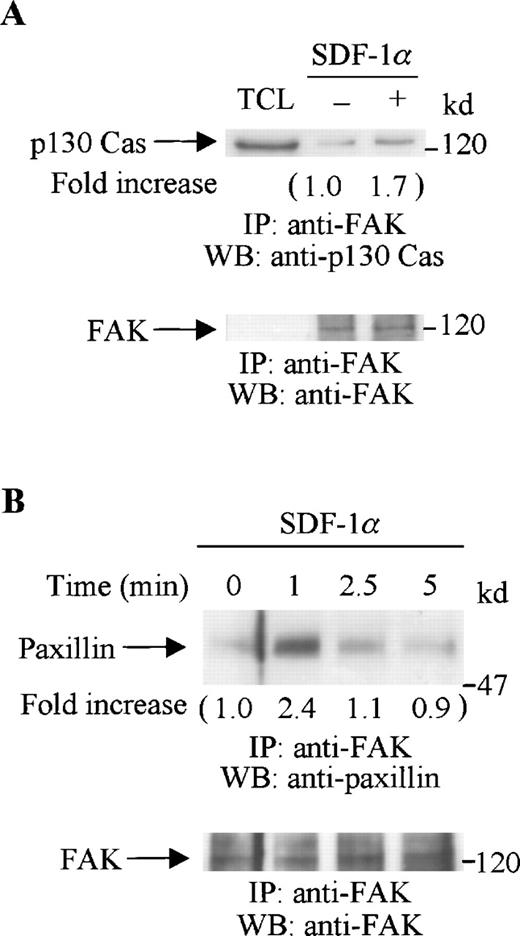
Tyrosine-phosphorylated FAK can form a complex with other focal adhesion proteins. The adaptor protein, p130 Cas, has been shown to associate with FAK and mediate cell migration.26 The cytoskeletal protein, paxillin, is also a substrate of FAK.42 Our previous study showed that, in CTS cells, both p130 Cas and paxillin were tyrosine phosphorylated on SDF-1α stimulation.18 Therefore, we now investigated if FAK associated with either p130 Cas or paxillin. Serum-starved CTS cells were stimulated with SDF-1α for various times. Cell lysates from unstimulated or SDF-1α stimulated CTS cells were immunoprecipitated with anti-FAK polyclonal antibody. The immunoprecipitates were resolved on SDS-PAGE gels, followed by immunoblotting with anti-p130 Cas (Figure4A, upper panel) or anti-paxillin antibody (Figure 4B, upper panel). SDF-1α stimulation enhanced the association of FAK both with p130 Cas (Figure 4A, upper panel) and with paxillin (Figure 4B, upper panel). Similar amounts of FAK were recovered from the lysates of cells untreated or treated with SDF-1α (Figure 4A and B, lower panels).
SDF-1 stimulation enhanced the association of FAK with p130 Cas or paxillin. CTS cells unstimulated or stimulated with SDF-1 for 1 minute were lysed in a lysis buffer and immunoprecipitated with anti-FAK antibody. (A) The immune complexes were resolved on 8% SDS-PAGE and immunoblotted with anti-p130 Cas (upper panel), anti-FAK (lower panel). (B) The immunocomplexes were resolved on 8% SDS-PAGE and immunoblotted with anti-paxillin antibody (upper panel) or anti-FAK antibody (lower panel). TCL represents total cell lysates. The changes in the association of FAK with p130 Cas (A) or with paxillin (B) are indicated (by fold increase) based on the densitometry values.
SDF-1 stimulation enhanced the association of FAK with p130 Cas or paxillin. CTS cells unstimulated or stimulated with SDF-1 for 1 minute were lysed in a lysis buffer and immunoprecipitated with anti-FAK antibody. (A) The immune complexes were resolved on 8% SDS-PAGE and immunoblotted with anti-p130 Cas (upper panel), anti-FAK (lower panel). (B) The immunocomplexes were resolved on 8% SDS-PAGE and immunoblotted with anti-paxillin antibody (upper panel) or anti-FAK antibody (lower panel). TCL represents total cell lysates. The changes in the association of FAK with p130 Cas (A) or with paxillin (B) are indicated (by fold increase) based on the densitometry values.
These results indicate that SDF-1α stimulation induces the tyrosine phosphorylation of FAK and its association with p130 Cas and paxillin in hematopoietic progenitor cells.
Stromal cell-derived factor-1 induces the tyrosine phosphorylation of Crk and Crk-L and enhances their association with p130 Cas
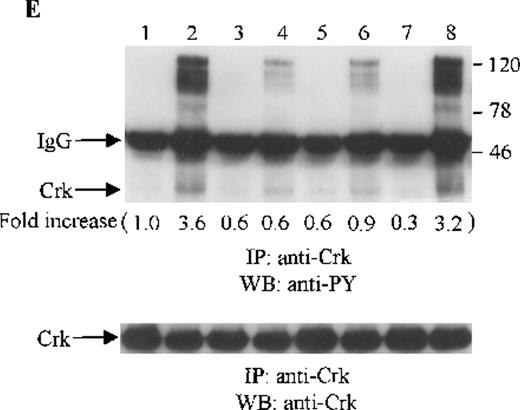
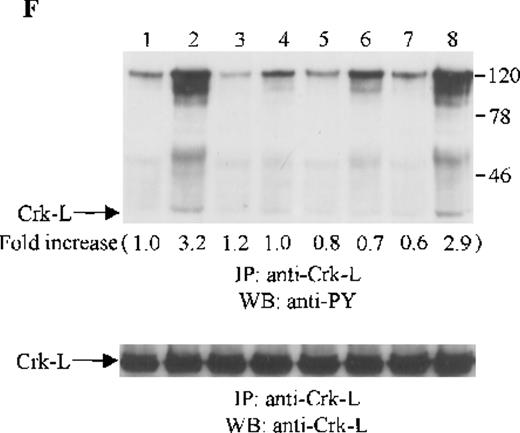
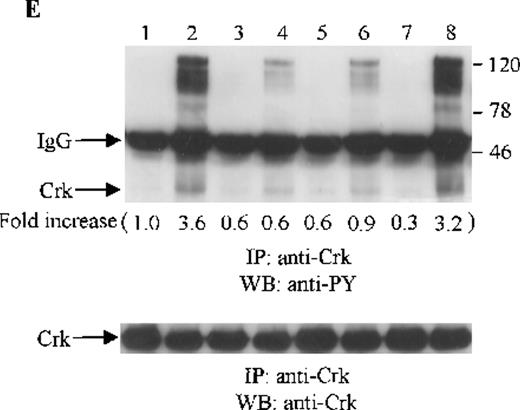
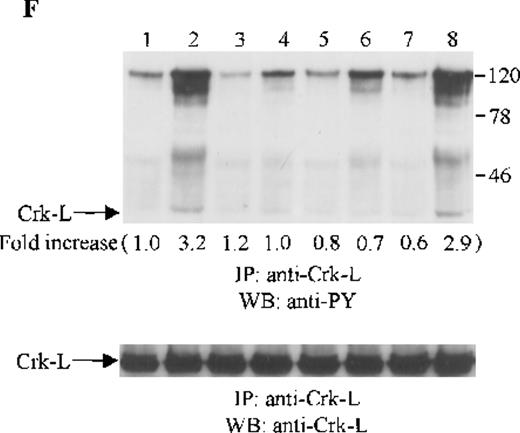
Crk and Crk-L are structurally similar, but encoded by separate genes.43 Both Crk and Crk-L are composed of 1 SH2 and 2 SH3 domains. Crk and Crk-L share overall 60% amino acid similarity and their SH2 and SH3 domains are highly conserved.43 Crk and Crk-L have been shown to function as adaptor proteins, linking different proteins in signaling.44 Thus, we sought to determine whether the adaptor protein Crk or Crk-L is tyrosine phosphorylated in CTS cells on SDF-1α stimulation. Cell lysates from unstimulated or SDF-1α stimulated CTS cells were immunoprecipitated with anti-Crk or anti-Crk-L, followed by immunoblotting with anti-PY antibody. As shown in Figure 5E and F, SDF-1α stimulation resulted in the tyrosine phosphorylation of both Crk (Figure 5E) and Crk-L (Figure 5F). Moreover, we observed that several proteins which coimmunoprecipitated with Crk or Crk-L were also tyrosine phosphorylated. We verified that similar amounts of Crk (Figure 5E, lower panel) or Crk-L (Figure 5F, lower panel) were recovered from the lysates of cells untreated or treated with SDF-1α.
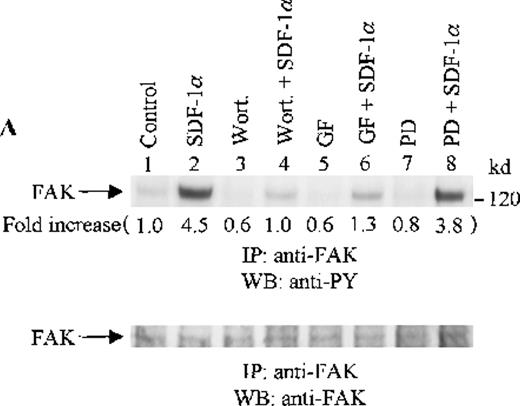
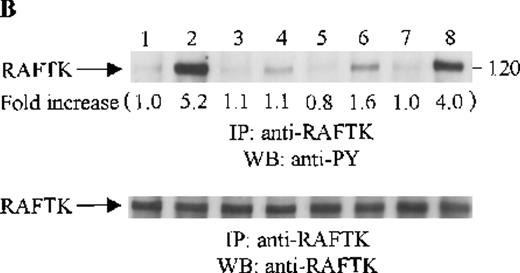
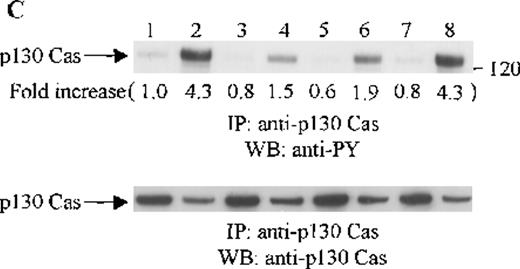
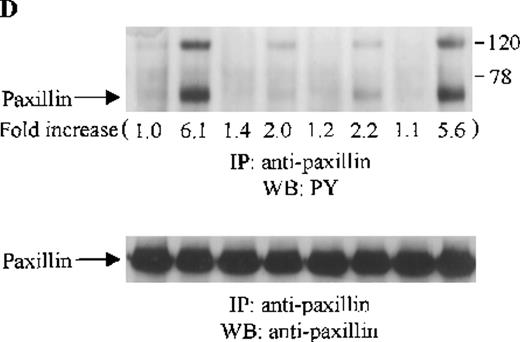
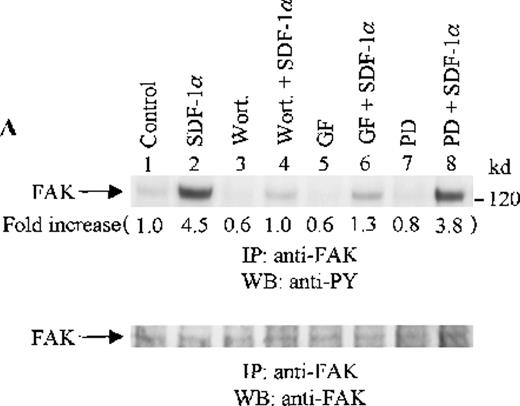
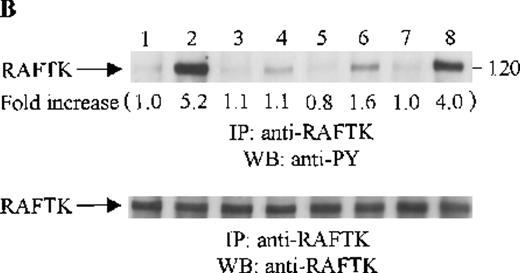
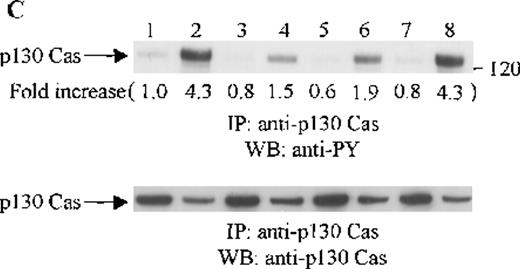
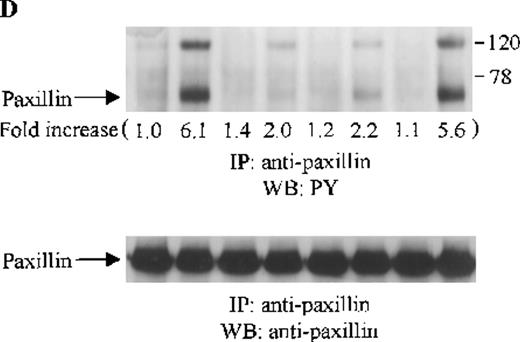
Effects of wortmannin, GF109203X, or PD98059 on the SDF-1–induced tyrosine phosphorylation of various adhesion proteins in CTS cells. Serum-starved CTS cells were incubated without or with 100 nmol/L wortmannin, 3 μmol/L GF109203X, or 20 μmol/L PD98059 (PD) for 45 minutes, then stimulated with SDF-1α (20 nmol/L) or left untreated for 1 minute at 37°C. Total cell lysates were immunoprecipitated with antibodies for FAK (A), RAFTK (B), p130 Cas (C), paxillin (D), Crk (E), or Crk-L (F), respectively. The immunoprecipitates were subjected to immunoblot analysis with anti-PY antibody (4G10) (upper panels), followed by reprobing with the same antibody used for each of the specific immunoprecipitations (lower panels). Lanes 1 to 8 represent the control, untreated cells with SDF-1α stimulation (SDF-1α), wortmannin-treated cells without (Wort.) or with SDF-1α (Wort.+SDF-1α) stimulation, GF109203X-treated cells without (GF) or with SDF-1α (GF+SDF-1α) stimulation, PD98059-treated cells without (PD) or with SDF-1α (PD+SDF-1α) stimulation, respectively. The increase in tyrosine phosphorylation of each adhesion protein is indicated (by fold increase) based on the densitometry values.
Effects of wortmannin, GF109203X, or PD98059 on the SDF-1–induced tyrosine phosphorylation of various adhesion proteins in CTS cells. Serum-starved CTS cells were incubated without or with 100 nmol/L wortmannin, 3 μmol/L GF109203X, or 20 μmol/L PD98059 (PD) for 45 minutes, then stimulated with SDF-1α (20 nmol/L) or left untreated for 1 minute at 37°C. Total cell lysates were immunoprecipitated with antibodies for FAK (A), RAFTK (B), p130 Cas (C), paxillin (D), Crk (E), or Crk-L (F), respectively. The immunoprecipitates were subjected to immunoblot analysis with anti-PY antibody (4G10) (upper panels), followed by reprobing with the same antibody used for each of the specific immunoprecipitations (lower panels). Lanes 1 to 8 represent the control, untreated cells with SDF-1α stimulation (SDF-1α), wortmannin-treated cells without (Wort.) or with SDF-1α (Wort.+SDF-1α) stimulation, GF109203X-treated cells without (GF) or with SDF-1α (GF+SDF-1α) stimulation, PD98059-treated cells without (PD) or with SDF-1α (PD+SDF-1α) stimulation, respectively. The increase in tyrosine phosphorylation of each adhesion protein is indicated (by fold increase) based on the densitometry values.
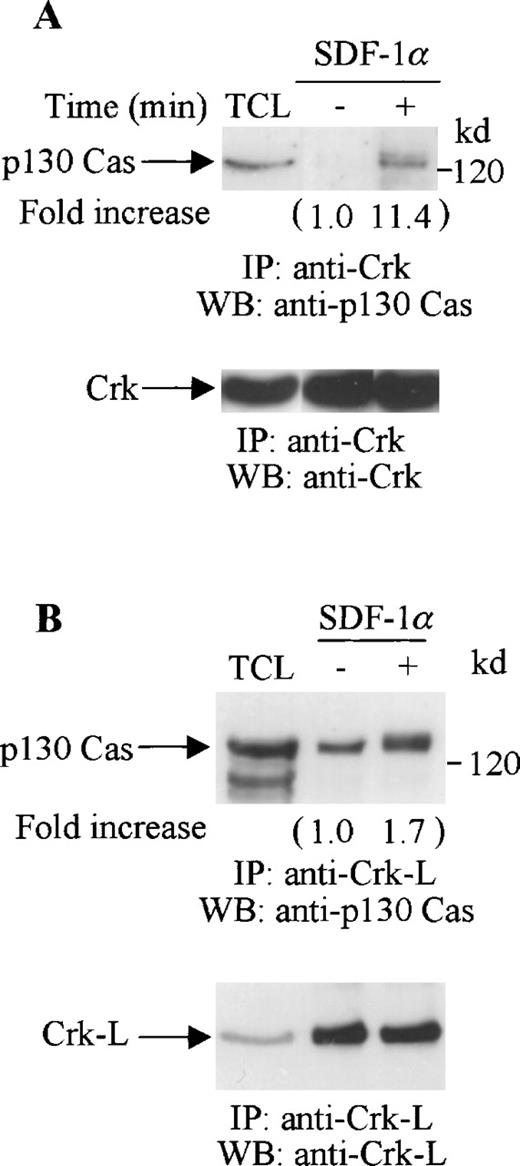
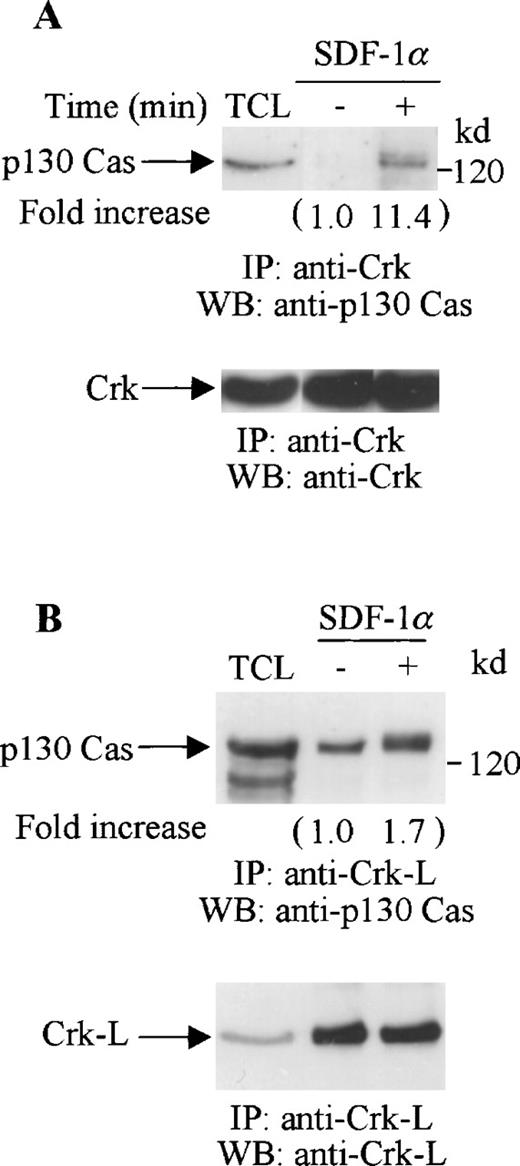
Both Crk and Crk-L have been reported to associate with p130 Cas.25,45 46 Thus, we investigated if the tyrosine-phosphorylated proteins that coimmunoprecipitated with Crk or Crk-L included p130 Cas. Cell lysates from unstimulated or SDF-1α–stimulated CTS cells were immunoprecipitated with anti-Crk (Figure 6A) or anti-Crk-L (Figure 6B), and then immunoblotted with p130 Cas. We observed a constitutive association between p130 Cas and Crk-L but not between p130 Cas and Crk in the unstimulated CTS cells. However, SDF-1α stimulation enhanced the association of both Crk and Crk-L with p130 Cas (Figure 6A and B, upper panels). We verified that similar amounts of Crk (Figure 6A, lower panel) or Crk-L (Figure 6B, lower panel) were recovered from the lysates of the untreated or SDF-1 treated cells.
Association of p130 Cas with Crk or Crk-L on stimulation with SDF-1 in CTS cells. Total cell lysates from unstimulated or SDF-1α–stimulated CTS cells were immunoprecipitated with anti-Crk antibody (A) or anti-Crk-L antibody (B). The immune complexes were resolved on 10% SDS-PAGE and immunoblotted with anti-p130 Cas antibody (A and B, upper panels). The immunoblots were stripped and reblotted with anti-Crk (A, lower panel) or anti-Crk-L antibody (B, lower panel). TCL represents total cell lysates. The changes in the association of Crk (A) or Crk-L (B) with p130 are indicated (by fold increase) based on the densitometry values.
Association of p130 Cas with Crk or Crk-L on stimulation with SDF-1 in CTS cells. Total cell lysates from unstimulated or SDF-1α–stimulated CTS cells were immunoprecipitated with anti-Crk antibody (A) or anti-Crk-L antibody (B). The immune complexes were resolved on 10% SDS-PAGE and immunoblotted with anti-p130 Cas antibody (A and B, upper panels). The immunoblots were stripped and reblotted with anti-Crk (A, lower panel) or anti-Crk-L antibody (B, lower panel). TCL represents total cell lysates. The changes in the association of Crk (A) or Crk-L (B) with p130 are indicated (by fold increase) based on the densitometry values.
These results indicate that both Crk and Crk-L are involved in CXCR4 receptor signaling in hematopoietic progenitor cells. SDF-1α stimulated the tyrosine phosphorylation of both Crk and Crk-L and induced their association with p130 Cas.
Effects of signaling inhibitors on stromal cell-derived factor-1–induced tyrosine phosphorylation of various adhesion proteins in CTS cells
To assess the functional roles of PI-3 kinase, PKC, and p44/42 ERK, before stimulation with SDF-1α, CTS cells were pretreated with 100 nmol/L wortmannin, a PI-3 kinase inhibitor; 3 μmol/L GF109203X, a PKC inhibitor; or 20 μmol/L PD98059, a MEK kinase inhibitor, respectively. Changes in the tyrosine phosphorylation of focal adhesion proteins on inhibitor treatment were investigated by immunoprecipitation with specific antibodies for these proteins and by immunoblotting with anti-PY antibody. As shown in Figure 5, the SDF-1α–induced tyrosine phosphorylation of FAK (A, upper panel), RAFTK (B, upper panel), p130 Cas (C, upper panel), paxillin (D, upper panel), Crk (E, upper panel), or Crk-L (F, upper panel) was significantly reduced by pretreatment with wortmannin or GF109203X but not PD98059. These results indicate that the tyrosine phosphorylation of these focal adhesion molecules was dependent on the activation of PI-3 kinase or PKC but not on that of MEK/p44/42 ERK. When the protein loading controls in each immunoblot were examined by reprobing with the same antibody used for the immunoprecipitation, we observed that the SDF-1α–treated sample showed an apparent decrease in p130 Cas protein level, along with an increase in the tyrosine phosphorylation of this protein (Figure 5C, lower panel). However, similar amounts of protein were recovered in each lane of the other immunoblots (Figure5A, B, D, E, and F, lower panels).
Inhibition of phosphoinositide-3 kinase or protein kinase C, but not MEK, inhibits stromal cell-derived factor-1α–induced migration of CTS cells or primary bone marrow CD34+ progenitor cells.
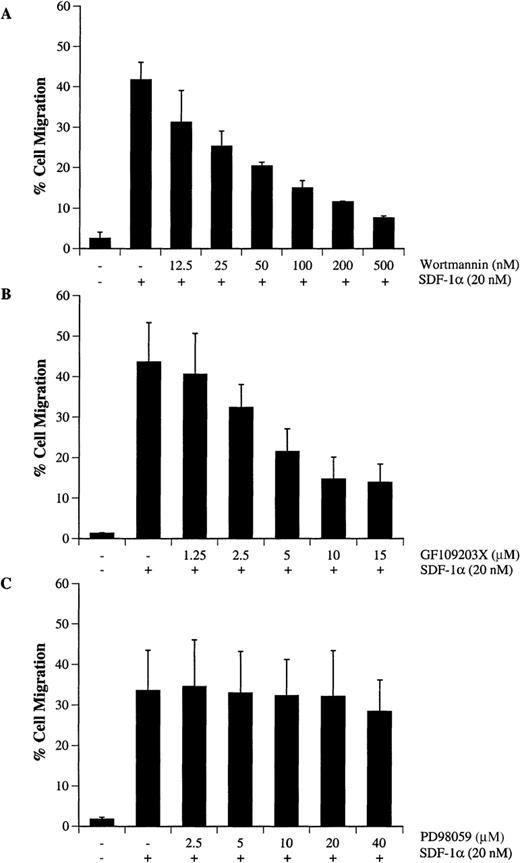
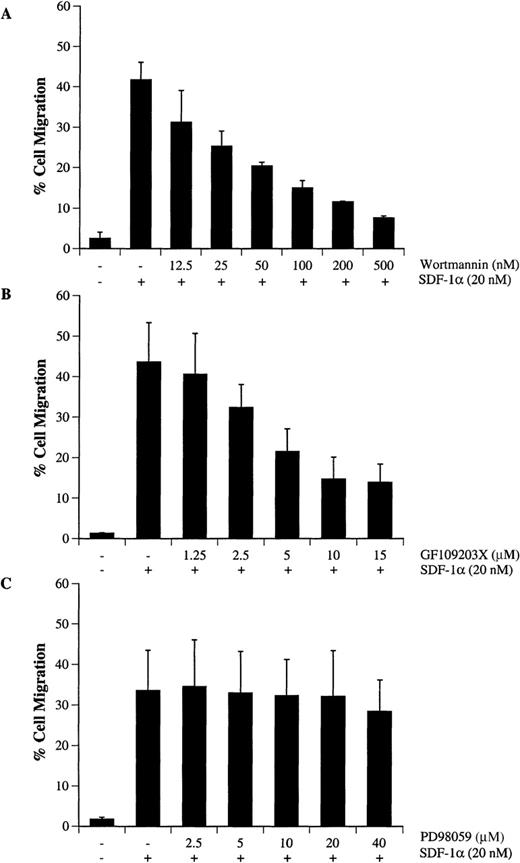
Activation of PI-3 kinase, PKC, and p44/42 ERK after SDF-1α treatment indicated that CXCR4 signaling involves multiple pathways. To determine the functional role of PI-3 kinase, PKC or ERK in SDF-1α–induced migration of hematopoietic progenitor cells, CTS cells were pretreated with different concentrations of wortmannin (Figure7A), GF109203X (Figure 7B), or PD98059 (Figure 7C). Cell migration in response to SDF-1α was examined by a Transwell migration assay as described in “Materials and methods.” We observed that treatment with wortmannin (Figure 7A) or GF109203X (Figure 7B) significantly inhibited SDF-1α–induced migration in a dose-dependent manner. However, treatment with PD98059 over a concentration range of 1 to 40 μmol/L did not alter cell migration (Figure 7C). Because the specificity of wortmannin, at low doses, was recently questioned,47 we also tested the ability of LY294002,48 a competitive inhibitor of PI-3 kinase, to confirm this observed effect. Similar to the results with wortmannin, LY294002 treatment inhibited cell migration in a dose-dependent manner (data not shown).
Wortmannin or GF109203X but not PD98059 inhibits SDF-1–-induced migration of CTS cells. CTS cells were treated with either carrier (DMSO) alone, various concentrations of wortmannin (A), GF109203X (B), or PD98059 (C) for 45 minutes. The cell migration in response to SDF-1α (20 nmol/L) was measured in a chemotactic assay as described in “Materials and methods.” Cell migration is shown as the percentage of cell input. The data shown represent the mean ± SD of 3 separate experiments.
Wortmannin or GF109203X but not PD98059 inhibits SDF-1–-induced migration of CTS cells. CTS cells were treated with either carrier (DMSO) alone, various concentrations of wortmannin (A), GF109203X (B), or PD98059 (C) for 45 minutes. The cell migration in response to SDF-1α (20 nmol/L) was measured in a chemotactic assay as described in “Materials and methods.” Cell migration is shown as the percentage of cell input. The data shown represent the mean ± SD of 3 separate experiments.
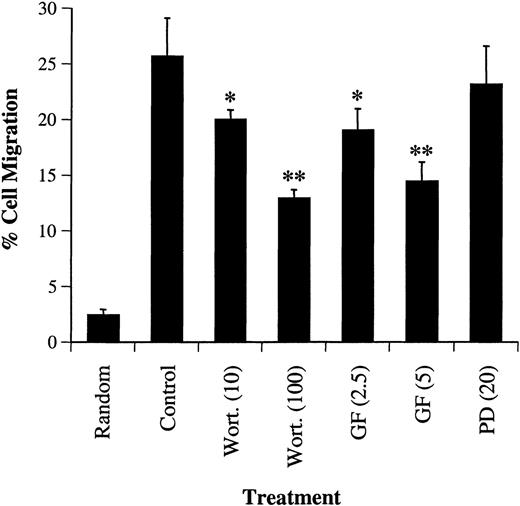
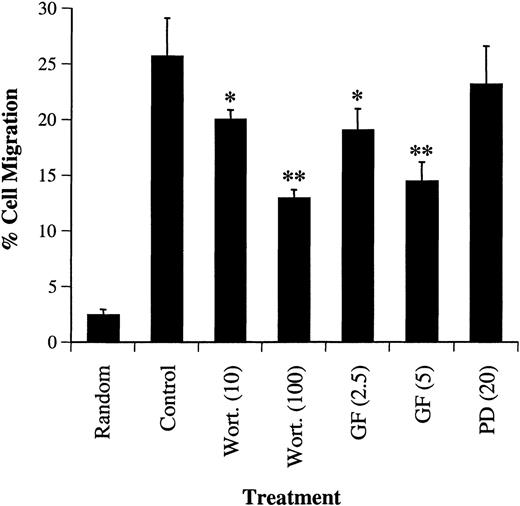
We next examined the effects of wortmannin, GF109203X, or PD98059 on the migration of primary bone marrow CD34+ cells in response to SDF-1α. CD34+ cells, isolated from normal human bone marrow, were pretreated with each inhibitor and tested in a Transwell migration assay. As shown in Figure8, similar to that observed in CTS cells, treatment with wortmannin or GF109203X, but not PD98059, inhibited the SDF-1α–induced migration of CD34+ progenitor cells in response to SDF-1α.
Effects of the PI-3 kinase inhibitor, wortmannin, PKC inhibitor, GF109203X or MEK kinase inhibitor, PD98059 on SDF-1–induced migration of CD34+ marrow cells. CD34+ cells were treated with either carrier alone or various concentrations (shown in nanomolars) of wortmannin (Wort.), GF109203X (GF, μM}, or PD98059 (PD, μM) for 45 minutes. The cell migration in response to SDF-1α (20 nmol/L) or medium alone (random) was measured in a chemotactic assay as described in “Materials and methods.” Cell migration was shown as the percentage of cell input. The data shown represent the mean ± SD of 3 separate experiments. * and ** show statistical significance compared with control (cells pretreated with carrier, DMSO) and are assessed asP < .05 and P < .01, respectively.
Effects of the PI-3 kinase inhibitor, wortmannin, PKC inhibitor, GF109203X or MEK kinase inhibitor, PD98059 on SDF-1–induced migration of CD34+ marrow cells. CD34+ cells were treated with either carrier alone or various concentrations (shown in nanomolars) of wortmannin (Wort.), GF109203X (GF, μM}, or PD98059 (PD, μM) for 45 minutes. The cell migration in response to SDF-1α (20 nmol/L) or medium alone (random) was measured in a chemotactic assay as described in “Materials and methods.” Cell migration was shown as the percentage of cell input. The data shown represent the mean ± SD of 3 separate experiments. * and ** show statistical significance compared with control (cells pretreated with carrier, DMSO) and are assessed asP < .05 and P < .01, respectively.
Discussion
The molecular mechanisms that regulate hematopoietic progenitor cell migration are not well characterized. On the basis of studies in mice null for either the CXCR4 receptor or its cognate ligand SDF-1, it appears that this chemokine is a major and essential physiological regulator of effective hematopoiesis, because such mice have an absence of both lymphoid and myeloid hematopoiesis.15,16 Moreover, in vitro studies with human progenitors have demonstrated a potent chemotactic effect of SDF-1.10-12 With this background, we sought to characterize the signaling pathways triggered after CXCR4 activation by SDF-1α, first in a model hematopoietic cell line, CTS, and then using primary bone marrow CD34+ cells.
Our studies focused on components of the focal adhesion complex. Focal adhesions are important structures that mediate cell adhesion and migration. They consist of a constellation of signaling molecules and cytoskeletal proteins and are believed to be essential in migration.20,49,50 Our previous studies demonstrated that SDF-1α induced calcium flux in CTS cells, followed by the tyrosine phosphorylation of RAFTK, paxillin, and p130 Cas.18 Calcium flux is the signature initial change in G-protein coupled receptors.5 RAFTK and the related FAK are well-recognized platform kinases that have multiple binding motifs for a number of different signaling molecules and act as putative bridges to transmit signals to the cytoskeleton.51,52 RAFTK and FAK are highly homologous, sharing an overall 45% amino acid sequence identity, with 60% identity in the catalytic domain. Several tyrosine residues appear to be conserved between RAFTK and FAK, including an Src family tyrosine kinase SH2-binding site. Both RAFTK and FAK contain proline-rich motifs capable of SH3 domain interaction.32,53 Given the high degree of structural and amino acid sequence similarity, RAFTK and FAK may have some similar or even exchangeable functions. Recently, it has been shown that RAFTK can phosphorylate FAK and functions as an agonist-dependent regulator of FAK.54 FAK appears to play a more important role than RAFTK in regulating cell migration.55 Our studies demonstrated that both FAK and RAFTK were phosphorylated on SDF-1α treatment of CTS cells, and this phosphorylation was followed by their association with 2 other important focal adhesion molecules, p130 Cas and paxillin (Dutt et al18 and our unpublished data). These results indicated that both RAFTK and FAK are involved in CXCR4 signaling induced by SDF-1α in hematopoietic progenitor cells.
With the use of CXCR4-transfected L1.2 cells, our previous study demonstrated that SDF-1α stimulation induced the tyrosine phosphorylation of Crk and its association with paxillin and RAFTK.36 In the current study, we observed that not only Crk but also Crk-L was tyrosine phosphorylated in CTS cells on SDF-1α stimulation. Crk-L has a high homology to Crk and contains a similar domain structure, SH2-SH3-SH3.43,44 Like Crk, Crk-L has been shown to be involved in focal adhesion formation by interacting with several focal adhesion proteins, including p130 Cas, paxillin, Abl, and Bcr-Abl.44 Crk-L is most abundantly expressed in hematopoietic cells,56 and regulates integrin-mediated cell adhesion.57,58 Although Crk-L has similar in vitro binding characteristics with Crk,43,44 there are a few differences to be noted.45,59 One example of these differences is the observation that Crk-L but not Crk constitutively associates with p130 Cas in Bcr-Abl transformed leukemia cells.45 Consistent with this observation, we demonstrated that there was a constitutive association of Crk-L, but not Crk, with p130 Cas in CTS cells. However, SDF-1α stimulation significantly enhanced the association of both Crk and Crk-L with p130 Cas. Our results indicated that both Crk and Crk-L, through their interaction with p130 Cas, were involved in CXCR4 signaling.
p130 Cas was initially identified as a prominent tyrosine-phosphorylated substrate of the oncoproteins v-src and v-crk.60 p130 Cas contains an SH3 domain and numerous potential SH2 domain docking sites, and also serves as a docking protein for the recruitment of proteins involved in protein-tyrosine kinase-mediated signaling pathways. p130 Cas localizes to focal adhesions and interacts with other focal adhesion components.61 It has been shown that the association of p130 Cas with FAK26 or Crk25 is critical for cell migration. This study, combined with our previous studies,18,36 demonstrate that SDF-1α stimulates the tyrosine phosphorylation of p130 Cas and enhances its association with focal adhesion proteins, including RAFTK, FAK, Crk, and Crk-L. These results indicate that p130 Cas may play an important role in CXCR4 signaling and cell migration. The apparent decrease in the protein levels of p130 Cas after SDF-1α stimulation (Figure 5C, lower panel) could be due to p130 Cas redistribution to actin-rich cytoskeletal complexes in the Triton-insoluble fractions on its phosphorylation, as reported previously.60,62 63 However, further study is needed to elucidate the functional significance of p130 Cas redistribution in CXCR4 signaling on SDF-1α stimulation.
Our data indicate that multiple adhesion proteins participated in SDF-1α induced signaling in hematopoietic progenitor cells. The changes in phosphorylation and interaction of these structural components may be important for the cell adhesion and migration mediated by SDF-1α in hematopoietic cells. Thus, we sought to define which upstream signaling components may be critical for these observed changes and the related cell migration induced by SDF-1α.
Accumulating data have implicated that multiple signaling mechanisms exist to regulate cell migration. MAP kinase (p44/42 ERK),38,39 PI-3 kinase,34-36 and PKC37 signaling pathways have been shown to regulate the cell migration induced by chemokines or cytokines. Consistent with our previous observation in CXCR4-transfected L1.2 cells,36 we found that SDF-1α activated PI-3 kinase in CTS cells (Figure 1). Additionally, we observed that SDF-1α significantly induced the tyrosine phosphorylation of PLC-γ (Figure 2). Phosphorylated PLC-γ hydrolyzes phosphatidylinositol diphosphate to the second-messenger molecules IP3 and DAG, which in turn activate PKC.40,41 Our previous study demonstrated that SDF-1α also stimulated p44/42 ERK activity in CTS cells.18 To investigate the functional roles of PI-3 kinase, PKC, and p44/42 ERK in the SDF-1α–induced phosphorylation of focal adhesion components and cell migration, we used a series of compounds that have been used as specific inhibitors for PI-3 kinase (wortmannin or LY294002), for PKC (GF109203X), or for ERK (PD98059, MEK inhibitor). Our results demonstrated that inhibition of PI-3 kinase or PKC significantly decreased the tyrosine phosphorylation of various focal adhesion proteins in response to SDF-1α stimulation, including FAK, RAFTK, p130 Cas, paxillin, Crk, and Crk-L. Inhibition of PI-3 kinase or PKC also decreased cell migration in response to SDF-1α, both in CTS and primary bone marrow CD34+ cells. These results suggest that PI-3 kinase and PKC are both required for the SDF-1α–induced cell migration.
Although activation of the p44/42 ERK signaling pathway has been shown to promote cell motility either by regulating gene expression38 or directly activating myosin light chain kinase,39 this was not the case in SDF-1α–induced cell migration. Our studies using the MEK inhibitor PD98059 demonstrated that inhibition of MEK kinase upstream of p44/42 ERK did not inhibit SDF-1α–induced migration in CTS cells or CD34+ bone marrow progenitors. Furthermore, treatment with PD98059 could not interfere with the SDF-1α–induced tyrosine phosphorylation of various focal adhesion proteins (Figure 5). These results imply that the ERK signaling pathway is not involved in the focal adhesion formation and migration induced by SDF-1α in hematopoietic progenitor cells.
In summary, our experiments, first done in the CTS cell line and then confirmed in primary bone marrow CD34+ cells, indicate that both PI-3 kinase and PKC are functionally important in inducing SDF-1α–mediated progenitor cell migration, whereas p44/42 ERK is not. The CXCR4 receptor has also been characterized as the primary coreceptor for certain strains of HIV, and binds the envelope glycoproteins gp120/160 with high affinity. Furthermore, such binding results in the activation of certain downstream signaling molecules, including RAFTK/Pyk2.64 65 With better characterization of these signal transduction pathways triggered by SDF-1 in hematopoietic progenitor cells, we now can study in the context of HIV infection whether such pathways are conserved or whether dysregulated hematopoiesis might occur because of aberrant signaling on gp120/160 binding to CXCR4.
Acknowledgments
We thank our colleague William C. Hatch for his technical assistance. We are grateful to Janet Delahanty for editing this manuscript and for preparation of the figures, as well as Daniel Kelley for his assistance with the figures. Finally, we appreciate Delroy Heath for facilitating our receipt of the needed reagents for the experiments.
Supported in part by NIH grants HL 53745-02, HL 55187-01, HL 51456-02, and HL 55445-01.
Reprints:Jerome E. Groopman, Division of Experimental Medicine, Harvard Institutes of Medicine, Beth Israel Deaconess Medical Center, 4 Blackfan Circle, Boston, MA 02115; e-mail:jgroopma@caregroup.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.