The activation of blood cells, including T cells, triggers intracellular signals that control the expression of critical molecules, including cytokines and cytokine receptors. We show that T-cell receptor (TCR) ligation increases the cellular level of the protein linker for activation of T cells (LAT), a molecule critical for T-cell development and function. T-cell activation increased LAT messenger RNA, as determined by reverse transcription–polymerase chain reaction and by Northern blotting. The TCR-induced increase in LAT expression involved the activation of the serine/threonine kinases PKC and MEK, because inhibitors of these kinases blocked the increase in LAT. Accordingly, the PKC activator phorbol myristate acetate up-regulated LAT expression. Strikingly, the calcineurin inhibitors cyclosporin A (CsA) and FK506 strongly potentiated TCR-induced LAT expression, suggesting that the activation of calcineurin following TCR ligation negatively regulates LAT expression. Accordingly, Ca++ ionophores, which can activate calcineurin by increasing intracellular Ca++, blocked the TCR-induced increase in cellular LAT. CsA and FK506 blocked the Ca++ionophores' inhibitory effect on LAT expression. Notably, CsA and FK506 preferentially up-regulated TCR-induced LAT expression; under the same conditions, these compounds did not increase the expression of 14 other molecules that previously had been implicated in T-cell activation. These data show that TCR-induced LAT expression involves the activation of the PKC-Erk pathway and is negatively regulated by the activation of calcineurin. Furthermore, the potentiation of TCR-induced LAT expression by CsA and FK506 suggests that the action of these agents involves up-regulating the cellular level of critical signaling molecules. These findings may have important therapeutic implications.
Activated T cells are central to the immune response to infection, tumors, and allografts. T-cell activation in vivo is triggered by the interaction between the T-cell receptor (TCR) and its respective antigen presented in the context of the major histocompatibility complex. TCR engagement initiates an array of intracellular signals that lead to cell adhesion, proliferation, differentiation, and to the increase in the transcriptional activity of cytokine genes. TCR-triggered signals include the stimulation of protein tyrosine kinases (PTKs), the generation of inositol triphosphate and diacylglycerol, the activation of protein kinase C (PKC), the increase in intracellular Ca++, the activation of the Ras and Rho family of GTPases, and the activation of several mitogen-activated protein kinases (MAPKs).1,2 Stimulated MAPKs translocate from the cytoplasm to the nucleus, where they phosphorylate and activate several transcription factors.3,4 The increase in intracellular Ca++following TCR ligation stimulates key enzymes in the T-cell signal transduction cascade, such as the Ca++/calmodulin–dependent serine/threonine phosphatase calcineurin.5-10 Stimulated calcineurin regulates the activity of transcription factors and, in turn, the transcription of genes that encode cytokines, cytokine receptors, and protooncogenes.
Proteins that become phosphorylated on tyrosine residues following receptor ligation play important roles in the signal transduction pathways of various receptors, including TCR, and are critical for cell growth and differentiation.1,2 11-13 Such proteins can be divided into 2 groups: those with intrinsic enzymatic activity, such as PTKs, and those with no known enzymatic activity (collectively known as adapter or linker proteins). The earliest detectable biochemical event following TCR engagement is the activation of several PTKs, resulting in the transient tyrosine phosphorylation of numerous intracellular substrates. Among the PTKs that become rapidly phosphorylated on tyrosine following TCR ligation are those that belong to the Src family, the Syk/Zap-70 family, the Tec family, the Csk family, and the FAK family. Adapter molecules that become tyrosine-phosphorylated following TCR ligation include SLP-76, Vav, Cbl, and LAT. Despite their lack of enzymatic activity, adapter molecules are critical for TCR signaling because they propagate receptor signaling by bridging the various signaling molecules.
LAT (linker for activation of T cells) is a recently identified 36- to 38-kd transmembrane protein that becomes tyrosine-phosphorylated after the stimulation of various receptors, including TCR and CD28.14-25 LAT is a substrate for Src and Syk/Zap-70 PTKs and, upon tyrosine phosphorylation, binds the SH2 domains of several signaling molecules, including Grb2, Cbl, phospholipase C (PLC)-gamma, and Vav.14,18,21,22 Interestingly, LAT has also been shown to associate with the coreceptors CD4 and CD8, a process that appears to be important for LAT tyrosine phosphorylation by ZAP-70 upon TCR ligation.20 LAT association with CD8 required cytosolic cysteines C227 and C229 on CD8, because replacing these residues with alanines markedly diminished the association.20Transfection studies and studies in knockout mice strongly suggest that LAT is critical for T-cell development and function.14,18,.26
We examined the effect of T-cell stimulation on the expression of LAT in T cells and found that T-cell activation by TCR or CD28 ligation or by phorbol myristate acetate (PMA) treatment increased the expression of LAT. We also found that the PKC-Erk cascade is involved in up-regulating LAT expression in T cells. Ca++ ionophores did not increase the expression of LAT; however, they did block TCR- and PMA-induced up-regulation of LAT expression. Remarkably, the calcineurin inhibitors CsA and FK506, at concentrations that inhibited interleukin (IL)-2 production, did not block LAT expression; rather, both drugs potentiated a TCR-induced increase in LAT expression and blocked Ca++ ionophore-induced inhibition of LAT expression. Thus, LAT expression in T cells involves the activation of the PKC-Erk cascade and is negatively regulated by the increase in intracellular Ca++ and the activation of calcineurin. These results also show that the actions of CsA and FK506 on T cells involve the up-regulation of the expression of critical signaling molecules.
Materials and methods
Reagents
PD98059 and Ro-31-8220 were obtained from Calbiochem (San Diego, CA). Aprotinin, phenylmethylsulfonyl fluoride, H-7, sodium orthovanadate, PMA, Ca++ ionophore A23187, Ca++ionophore ionomycin, protease-free bovine serum albumin, and protein A-agarose beads were from Sigma (St Louis, MO). Cyclosporin A was obtained from Sandoz (East Hanover, NJ). FK506 was purchased from Fujisawa USA (Deerfield, IL). The LumiGLO chemiluminescent substrate kit and tetramethylbenzidine peroxidase substrate were obtained from KPL Laboratories (Gaithersburg, MD). The Oligotex messenger RNA (mRNA) mini kit was obtained from Qiagen (Santa Clarita, CA). Recombinant human IL-2 was obtained from Collaborative Biomedical Products (Bedford, MA). BrightStar-Plus positively charged nylon membranes, Northern blotting (Max-Gly) kit, and BrightStar BioDetect kit were obtained from Ambion (Austin, TX).
Antibodies
Antihuman CD28 monoclonal antibody (mAb) (Leu-28, clone L293) was purchased from Becton Dickinson (San Jose, CA). Antihuman CD3 mAb was from Ancell (Bayport, MN). Antiphosphotyrosine mAb PY20 was obtained from Transduction Laboratories (Lexington, KY). Anti-Csk and anti-LAT rabbit polyclonal Ab were from Santa Cruz (Santa Cruz, CA). Antihuman IL-2 polyclonal Ab and biotin-labeled antihuman IL-2 mAb were obtained from Endogen (Woburn, MA). Rabbit-antimouse IgG Ab, horseradish peroxidase (HRP)-conjugated donkey-antimouse Ig Ab, and HRP-conjugated donkey-antirabbit Ig Ab were from Jackson Immunoresearch Laboratories (Bar Harbor, ME). Goat-antirabbit IgG conjugated to Alexa 488 was purchased from Molecular Probes (Eugene, OR).
Cells
Resting T cells were isolated from the blood of healthy humans on Ficoll-Histopaque and washed 3 times in RPMI containing 10% fetal calf serum (FCS). The cells were then incubated with goat-antihuman IgG-coated Immulan beads according to the manufacturer's recommendations. Unbound cells (T cells) were collected, and adherent cells were removed by incubating the cells in a tissue culture flask for 30 minutes at 37°C. T-cell purity was at least 98% as determined by flow cytometry. Acute human T-cell leukemia (Jurkat) cells, clone E6-1, were obtained from American Type Culture Collection (Bethesda, MD) and maintained in suspension as described previously.19
Cell activation, preparation of cell lysates, and immunoblotting
T-cell activation was done as reported previously with some modification.19 27-30 Purified human normal resting T cells or Jurkat T cells were washed with RPMI containing 5% FCS (RPMI-5% FCS) and suspended in the same medium (4 × 106cells/mL). Meanwhile 96-well tissue culture plates were coated with the indicated concentrations of the antibodies for least 2 hours at 37°C. After washing the plates, 50 μl of the cell suspension (2 × 105 cells) were added to each well, and the plates were incubated for the indicated times at 37°C in a water-jacketed incubator. After incubation, the cells were immediately lysed with boiling 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Proteins in whole cell lysates (WCL) were separated by SDS-PAGE and electrotransferred onto PVDF membranes. Membranes were immunoblotted with HRP-conjugated antiphosphotyrosine mAb PY20 or with the specific antibodies followed by HRP-coupled donkey-antimouse Ig Ab or HRP-coupled donkey-antirabbit Ig Ab (1:40 000 dilution). The signals were visualized using the LumiGLO kit.
Interleukin-2 assay
The IL-2 level in the supernatants of overnight cultures was determined with a sandwich enzyme-linked immunosorbent assay technique using combinations of unlabeled and biotin-labeled antibodies as described previously.38
Reverse transcription-polymerase chain reaction
A total of 2 × 106 Jurkat T cells in RPMI-5% FCS were stimulated with 1 ng/mL of PMA or with 3 μg/mL of anti-CD3 mAb for 8 hours at 37°C. After incubation, the cells were washed and total RNA was extracted using the RNeasy mini kit according to the manufacturer's recommendations (Qiagen). Total RNA was reverse transcribed to generate first-strand complementary DNA (cDNA), and the resultant cDNA was amplified by polymerase chain reaction (PCR). Samples in agarose gels were visualized by ethidium bromide staining. The primer sequences for LAT were 5′-GTATCCAAGGGGCATCCAGTT-3′ (sense) and 5′-CCTCTTCCTCCACTTCCTCTG-3′ (antisense) and for glyceraldehyde phosphate dehydrogenase (GAPDH) were 5′-CCATGGAGAAGGCTGGGG-3′ (sense) and 5′-CAAAGTTGTCATGGATGACC-3′ (antisense).
Northern blotting
A total of 2 × 107 Jurkat T cells were incubated for 8 hours at 37°C in RPMI-5% FCS or in RPMI-5% FCS containing the indicated concentrations of PMA. After incubation, the cells were washed in phosphate-buffered saline (PBS), and poly A+ RNA was extracted using the Oligotex mRNA mini kit. The resultant mRNA was subjected to 1% agarose electrophoresis and then transferred to Ambion's BrightStar-Plus positively charged nylon membranes. The RNA was cross-linked to the membranes by baking the membranes at 80°C for 30 minutes. The membranes were then prehybridized for 4 hours at 50°C using the reagents provided in the Northern blotting (Max-Gly) kit. After prehybridization, the membranes were incubated for 16 hours at 50°C with a mixture of the following 5′-biotinylated LAT antisense oligonucleotides: 5′-GAACGTTCACGTAATCATCAATGGACTCCA-3′, 5′-GGCCTTTATTCTATTACACAGAGTAGGGCTGG-3′, and 5′-GGCCGTTTGAACTGGATGCCCCTTGGATAC-3′. After incubation, the membranes were extensively washed, and membrane-bound biotinylated probe was visualized using the BrightStar Biodetect kit. To ensure similar loading, the membranes were stripped of LAT oligonucleotides by boiling for 30 minutes in 0.1% SDS and then were reprobed with the following 5′ biotinylated β-actin antisense oligonucleotide: 5′-GGAAGGTGGACAGCGAGGCCAGGATGGAGC-3′.
Immunofluorescence confocal microscopy and flow cytometry
For stimulation with anti-CD3 mAb, 2 × 107Jurkat T cells in RPMI-5% FCS were incubated for 16 hours at 37°C in uncoated or anti-CD3 mAb-coated tissue culture dishes (100 × 20 mm Falcon polystyrene dishes; Becton Dickinson Labware, Franklin Lakes, NJ). Following incubation, nonadherent cells were aspirated and CD3-adherent cells were removed from the dishes by gentle pipetting, washed in PBS, and fixed for 60 minutes in 2% paraformaldehyde in PBS. The cells were then washed in PBS and were permeabilized for 5 minutes in 0.2% saponin in PBS. After permeabilization, the cells were washed in Tris-buffered saline containing 5% Tween (TBS/Tween). A total of 2 × 106 cells were incubated for 30 minutes at room temperature with 10 μg/mL anti-LAT Ab, washed in TBS/Tween, and then incubated for 30 minutes at room temperature with goat-antirabbit IgG conjugated to Alexa 488 diluted 1:300 in TBS/Tween. After incubation, the cells were washed in TBS/Tween and then analyzed on a Bio-Rad MRC 1024 confocal scanning laser microscope with inverted Nikon Eclipse TE300 microscope using Laser Sharp Version 3.2 software. Aliquots of the labeled cells used above were also examined for LAT expression by flow cytometry using the Becton Dickinson FACScan and the Cell Quest software.
Results
T-cell activation by CD3 ligation up-regulates the expression of LAT
To examine the effect of T-cell stimulation on the expression of LAT, we incubated purified human normal resting T cells or Jurkat T cells with the indicated concentrations of plate-immobilized anti-CD3 mAb for 16 hours at 37°C. Such incubation did not affect the number of the cells; nor did it affect the viability of the cells, as determined by trypan blue exclusion assay (data not shown). After incubation, the cells were immediately lysed with boiling SDS-PAGE sample buffer. The proteins in WCL were subjected to SDS-PAGE, electrotransferred to membranes, and blotted with anti-LAT mAb or, as a control, with anti-Csk Ab. In initial studies, we found that the expression of Csk in Jurkat T cells was resistant to the various treatments outlined in the present article. Anti-CD3 mAb reproducibly increased the level of total cellular LAT in human normal resting T cells (Figure 1A) and in the Jurkat T cell line (Figure 1B) in a dose-dependent manner. Although in some experiments Csk was slightly increased after CD3 ligation in human normal resting T cells, its level did not change in Jurkat T cells. LAT expression was also increased when T cells were incubated with anti-CD3 mAb for 16 hours at 37°C in suspension, albeit to a lesser extent than that with the plate-immobilized anti-CD3 mAb (data not shown). The results indicate that CD3 ligation initiates intracellular signals that up-regulate LAT expression in T cells.
Ligation of TCR or CD28 or PMA treatment up-regulates the expression of LAT in T cells.
Purified human normal resting T cells (A) or Jurkat T cells (B) were incubated for 16 hours at 37°C in 96-well tissue culture plates in RPMI-5% FCS (lane 1) or in RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb (lanes 2-5). After incubation, the cells were immediately lysed with 2× boiling SDS-PAGE sample buffer. After boiling, proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb (upper panel) or anti-Csk Ab (lower panel). (C) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or in RPMI-5% FCS containing the indicated concentrations of anti-CD28 mAb (lanes 2-5). Proteins were processed as described above. (D) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1), RPMI-5% FCS containing the indicated concentrations of PMA (lanes 2-4), or RPMI-5% FCS containing 4α-PMA (lanes 5-7). Proteins were processed as described above. (E) Purified human normal resting T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing the indicated concentrations of PMA (lanes 2-4). Proteins were processed as described above. (F) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing the indicated concentrations of the Ca++ ionophore A23187 (lanes 2-5). Proteins were processed as described above. Each of the experiments in this figure was performed at least 3 times with similar results.
Ligation of TCR or CD28 or PMA treatment up-regulates the expression of LAT in T cells.
Purified human normal resting T cells (A) or Jurkat T cells (B) were incubated for 16 hours at 37°C in 96-well tissue culture plates in RPMI-5% FCS (lane 1) or in RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb (lanes 2-5). After incubation, the cells were immediately lysed with 2× boiling SDS-PAGE sample buffer. After boiling, proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb (upper panel) or anti-Csk Ab (lower panel). (C) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or in RPMI-5% FCS containing the indicated concentrations of anti-CD28 mAb (lanes 2-5). Proteins were processed as described above. (D) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1), RPMI-5% FCS containing the indicated concentrations of PMA (lanes 2-4), or RPMI-5% FCS containing 4α-PMA (lanes 5-7). Proteins were processed as described above. (E) Purified human normal resting T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing the indicated concentrations of PMA (lanes 2-4). Proteins were processed as described above. (F) Jurkat T cells were incubated as described above for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing the indicated concentrations of the Ca++ ionophore A23187 (lanes 2-5). Proteins were processed as described above. Each of the experiments in this figure was performed at least 3 times with similar results.
T cells can also be activated for gene expression through the ligation of costimulatory molecules such as CD28 and by pharmacologic agents such as PMA and Ca++ ionophore. Therefore, we examined the effect of these stimuli on LAT expression in T cells. Ligating CD28 only weakly increased the level of LAT in T cells (Figure 1C). PMA, a membrane-permeable phorbol ester that binds to and directly activates PKC, strongly increased the expression of LAT in Jurkat T cells (Figure1D) and in human normal resting T cells (Figure 1E). The control inactive phorbol 4α-PMA, however, did not increase LAT expression (Figure 1D, lanes 5-7). Interestingly, the Ca++ ionophore A23187, which increases intracellular Ca++ by mechanisms that bypass molecules activated early after receptor ligation, failed to increase the level of LAT (Figure 1F). Similar results were obtained with the Ca++ ionophore ionomycin (data not shown). Together, the data show that T-cell activation increases the cellular level of LAT. Furthermore, the data suggest that the activation of PKC, but not the increase in intracellular Ca++, is sufficient to initiate intracellular signals that lead to increased LAT expression. Because the CD28-induced increase in LAT expression was relatively low compared with that induced by CD3 and by PMA, we chose to focus on CD3- and PMA-induced up-regulation of LAT expression.
T-cell activation up-regulates the mRNA for LAT
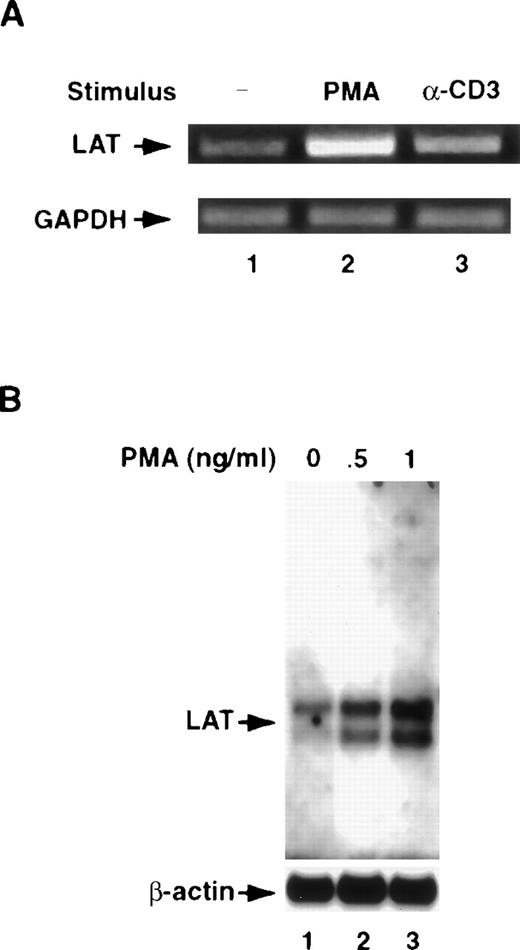
To determine whether T-cell activation up-regulates LAT mRNA, we stimulated the cells for 8 hours at 37°C with anti-CD3 mAb or PMA and then examined the cells for the level of the mRNA for LAT by the reverse transcription (RT)-PCR technique and by Northern blotting. For RT-PCR, total RNA was extracted from unstimulated and stimulated cells, reverse transcribed to generate first-strand cDNA, and the resultant cDNA was amplified by PCR. Samples in agarose gels were visualized by ethidium bromide staining. As shown in Figure2A, both anti-CD3 mAb and PMA increased the mRNA for LAT. As a control, we measured the level of the mRNA for GAPDH. No increase in the level of mRNA for this gene was seen in cells treated with either anti-CD3 mAb or PMA (Figure 2A). To confirm these data, poly A+ RNA was isolated from unstimulated and stimulated Jurkat T cells and then used in Northern blotting as described in “Materials and methods.” As shown in Figure 2B, Jurkat T-cell activation increased the level of LAT mRNA. Whether the increase in the level of LAT mRNA after T-cell activation is the result of the increase in gene transcription or due to enhanced mRNA stability is subject to further investigation.
T-cell activation up-regulates the level of the mRNA for LAT.
(A) Jurkat T cells were incubated as described in the legend for Figure1 for 8 hours at 37°C with RPMI-5% FCS (lane 1), RPMI-5% FCS containing 1 ng/mL of PMA (lane 2), or RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (α-CD3; lane 3). After washing, total RNA was extracted, reverse transcribed to generate first-strand cDNA, and the resultant cDNA amplified by PCR using LAT- or GAPDH-specific primers, as described in “Materials and methods.” GAPDH gene expression was analyzed to control for mRNA integrity and loading. PCR products were visualized by ethidium bromide staining of the gel. This experiment was repeated 3 times with similar results. (B) A total of 2 × 107 Jurkat T cells were incubated for 8 hours at 37°C in RPMI-5% FCS or in RPMI-5% FCS containing the indicated concentrations of PMA. After incubation, the cells were washed in PBS, and poly A+ RNA was extracted using the Oligotex mRNA mini kit according to the manufacturer's recommendations. The resultant mRNA was subjected to 1% agarose electrophoresis and then transferred to positively charged nylon membranes. The membranes were then prehybridized for 4 hours at 50°C using the reagents provided in the Northern blotting (Max-Gly) kit. After prehybridization, the membranes were incubated for 16 hours at 50°C with a mixture of 5′-biotinylated LAT antisense oligonucleotides (upper panel). After incubation, the membranes were extensively washed, and the membrane-bound biotinylated probe was visualized as described in “Materials and methods.” To ensure equal loading, the membranes were stripped of LAT oligonucleotides by boiling for 30 minutes in 0.1% SDS and were then reprobed with a 5′-biotinylated β-actin antisense oligonucleotide. This experiment was repeated twice with similar results.
T-cell activation up-regulates the level of the mRNA for LAT.
(A) Jurkat T cells were incubated as described in the legend for Figure1 for 8 hours at 37°C with RPMI-5% FCS (lane 1), RPMI-5% FCS containing 1 ng/mL of PMA (lane 2), or RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (α-CD3; lane 3). After washing, total RNA was extracted, reverse transcribed to generate first-strand cDNA, and the resultant cDNA amplified by PCR using LAT- or GAPDH-specific primers, as described in “Materials and methods.” GAPDH gene expression was analyzed to control for mRNA integrity and loading. PCR products were visualized by ethidium bromide staining of the gel. This experiment was repeated 3 times with similar results. (B) A total of 2 × 107 Jurkat T cells were incubated for 8 hours at 37°C in RPMI-5% FCS or in RPMI-5% FCS containing the indicated concentrations of PMA. After incubation, the cells were washed in PBS, and poly A+ RNA was extracted using the Oligotex mRNA mini kit according to the manufacturer's recommendations. The resultant mRNA was subjected to 1% agarose electrophoresis and then transferred to positively charged nylon membranes. The membranes were then prehybridized for 4 hours at 50°C using the reagents provided in the Northern blotting (Max-Gly) kit. After prehybridization, the membranes were incubated for 16 hours at 50°C with a mixture of 5′-biotinylated LAT antisense oligonucleotides (upper panel). After incubation, the membranes were extensively washed, and the membrane-bound biotinylated probe was visualized as described in “Materials and methods.” To ensure equal loading, the membranes were stripped of LAT oligonucleotides by boiling for 30 minutes in 0.1% SDS and were then reprobed with a 5′-biotinylated β-actin antisense oligonucleotide. This experiment was repeated twice with similar results.
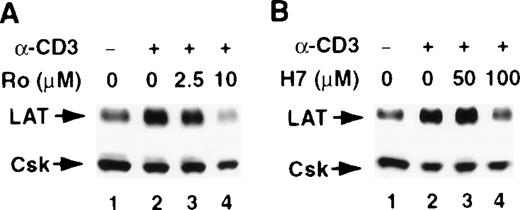
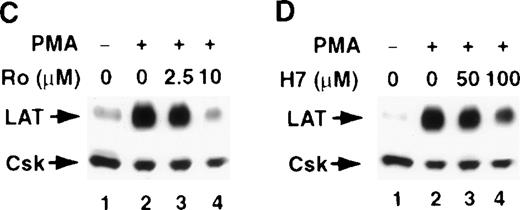
The involvement of PKC in the stimulus-induced up-regulation of LAT expression
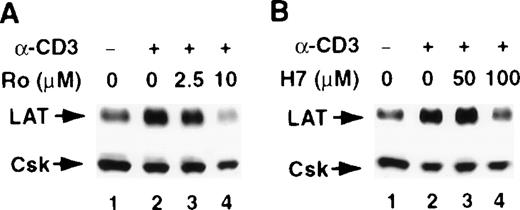
T-cell activation through CD3 has been shown to activate the serine/threonine kinase PKC.31,32 Furthermore, the increase in LAT expression after PMA treatment suggests a role for PKC in regulating LAT expression, because PKC is the intracellular receptor for PMA.33 Therefore, we examined the involvement of PKC in regulating LAT expression by investigating the effect of the PKC inhibitors H-7 and Ro-31-8220 on a stimulus-induced increase in LAT expression. Both PKC inhibitors blocked the CD3- and PMA-induced increase in LAT expression (Figure 3, upper panels). These blockers had no effect on the viability of the cells, as determined by trypan blue exclusion assay (data not shown). Blotting the membranes with anti-Csk Ab confirmed that a similar amount of WCL was loaded in each lane, because the level of Csk was only slightly decreased in the presence of the PKC inhibitors (Figure 3, lower panels). These results implicate PKC in the signaling pathways that up-regulate LAT expression.
The involvement of PKC in the stimulus-induced up-regulation of LAT expression.
Jurkat T cells were pretreated with the indicated concentrations of the PKC inhibitors Ro-31-8220 (A and C, lanes 3 and 4) or H-7 RPMI-5% FCS (B and D, lanes 3 and 4) for 2 hours at 37°C and then stimulated in the continuous presence of inhibitors with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (A and B) or RPMI-5% FCS containing 1 ng/mL of PMA (C and D) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results.
The involvement of PKC in the stimulus-induced up-regulation of LAT expression.
Jurkat T cells were pretreated with the indicated concentrations of the PKC inhibitors Ro-31-8220 (A and C, lanes 3 and 4) or H-7 RPMI-5% FCS (B and D, lanes 3 and 4) for 2 hours at 37°C and then stimulated in the continuous presence of inhibitors with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (A and B) or RPMI-5% FCS containing 1 ng/mL of PMA (C and D) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results.
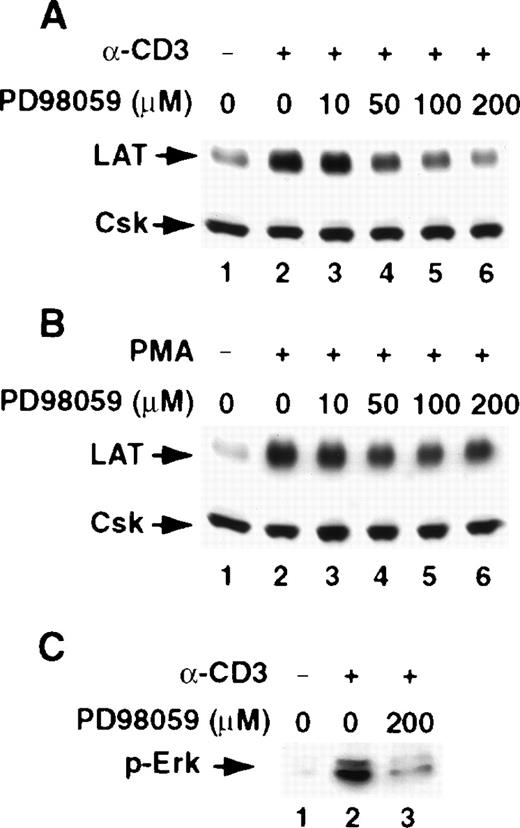
The MAPK MEK is involved in regulating LAT expression in T cells
TCR-initiated signals in T cells have been shown to activate the MAPK mitogen-activated protein kinase/extracellular signal-regulated protein kinase kinase (MEK–Erk) cascade, leading to the phosphorylation and activation of transcription factors. Similarly, PMA-activated PKC has been shown to activate the Ras-Raf pathway, which in turn links PKC to the MEK–Erk pathway. To further define the pathways that regulate LAT expression in T cells, we examined the effect of the selective MEK blocker PD98059 on a stimulus-induced increase in LAT level. PD98059 blocked the CD3- and PMA-induced increase in LAT expression in a dose-dependent manner, suggesting that the MEK–Erk cascade is involved in regulating LAT expression (Figure4A and 4B). The inhibition of LAT expression by PD98059 correlated with the inhibition of MEK activation by PD98059, as determined by the decrease in the tyrosine phosphorylation of Erk (Figure 4C). We do not know why PD98059 was more effective in inhibiting CD3-induced LAT expression than inhibiting PMA-induced LAT expression.
The MEK inhibitor PD98059 blocks activation-induced up-regulation of LAT expression.
(A) Jurkat T cells were pretreated with the indicated concentrations of the MEK inhibitor PD98059 in RPMI-5% FCS (A and B, lanes 3-6) for 2 hours at 37°C and then stimulated in the continuous presence of the inhibitor with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (A) or RPMI-5% FCS containing 1 ng/mL of PMA (B) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 4 times with similar results. (C) Inhibition of Erk phosphorylation by PD98059. Jurkat T cells were incubated in RPMI-5% FCS (lanes 1 and 2) or RPMI-5% FCS containing 200 μM of PD98059 (lane 3) for 16 hours at 37°C. After incubation, the cells were washed with RPMI-0.001% bovine serum albumin and then suspended in the same media. The cells were then left untreated (lane 1) or were stimulated with 1 μg/mL of anti-CD3 mAb for 15 minutes (lanes 2 and 3). After incubation, the cells were immediately lysed by adding an equal volume of 2× boiling SDS-PAGE sample buffer, and the proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-phospho-Erk mAb. This experiment was repeated 3 times with similar results.
The MEK inhibitor PD98059 blocks activation-induced up-regulation of LAT expression.
(A) Jurkat T cells were pretreated with the indicated concentrations of the MEK inhibitor PD98059 in RPMI-5% FCS (A and B, lanes 3-6) for 2 hours at 37°C and then stimulated in the continuous presence of the inhibitor with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb (A) or RPMI-5% FCS containing 1 ng/mL of PMA (B) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 4 times with similar results. (C) Inhibition of Erk phosphorylation by PD98059. Jurkat T cells were incubated in RPMI-5% FCS (lanes 1 and 2) or RPMI-5% FCS containing 200 μM of PD98059 (lane 3) for 16 hours at 37°C. After incubation, the cells were washed with RPMI-0.001% bovine serum albumin and then suspended in the same media. The cells were then left untreated (lane 1) or were stimulated with 1 μg/mL of anti-CD3 mAb for 15 minutes (lanes 2 and 3). After incubation, the cells were immediately lysed by adding an equal volume of 2× boiling SDS-PAGE sample buffer, and the proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-phospho-Erk mAb. This experiment was repeated 3 times with similar results.
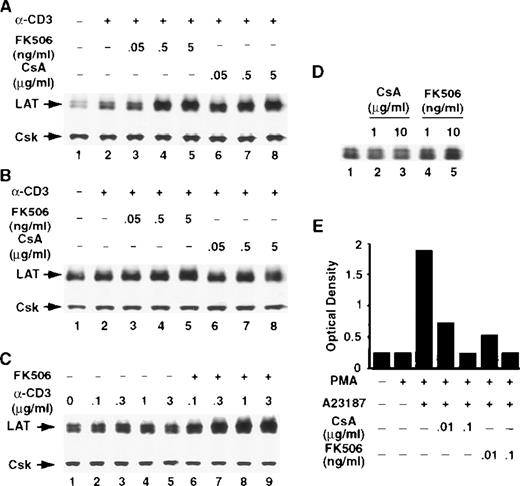
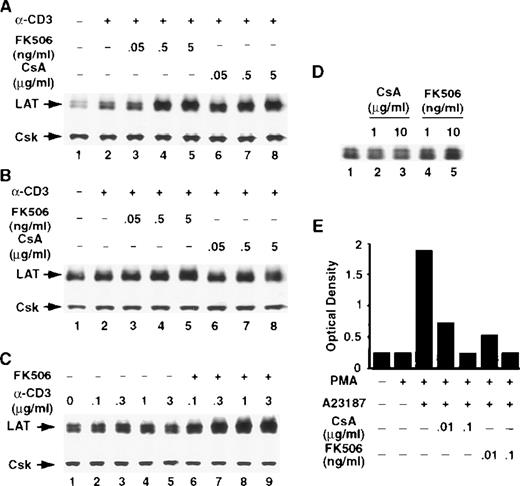
The calcineurin inhibitors CsA and FK506 potentiate the TCR-induced increase in LAT expression in T cells
The serine/threonine phosphatase calcineurin, also known as phosphatase 2B, is a key enzyme in TCR signal transduction pathways and has been implicated in regulating receptor-induced gene expression in T cells. The calcineurin inhibitors CsA and FK506 are widely used to block calcineurin activation. Both agents form complexes with intracellular proteins, which bind calcineurin, leading to the inhibition of its phosphatase activity.34 Thus, to examine the involvement of calcineurin in regulating receptor-induced LAT expression, T cells were stimulated with anti-CD3 mAb in the continuous presence of the indicated concentrations of CsA or FK506. Remarkably, both compounds potentiated CD3-induced LAT expression in Jurkat T cells (Figure 5A) and in human normal resting T cells (Figure 5B) in a dose-dependent manner. The effect of FK506 was dependent on the concentrations of the anti-CD3 mAb used to stimulate T cells (Figure 5C). CsA or FK506 alone increased the basal level of LAT only slightly, if at all, suggesting that inhibiting the activation of calcineurin is not by itself sufficient to modulate LAT expression, but that calcineurin inhibition appears to modulate receptor-induced LAT expression (Figure 5D). Interestingly, the effect of CsA and FK506 on LAT expression was evident at concentrations of CsA and FK506 that effectively blocked stimulus-induced IL-2 production (Figure 5E), indicating that the concentrations of FK506 and CsA used in the present studies effectively blocked calcineurin activation. These results strongly suggest that calcineurin activation negatively regulates LAT expression in T cells.
The calcineurin inhibitors CsA and FK506 potentiate TCR-induced increase in LAT expression.
Jurkat T cells (A) or purified human normal resting T cells (B) were stimulated for 16 hours at 37°C with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb in the absence (lane 2) or presence of the indicated concentrations of FK506 (lanes 3-5) or CsA (lanes 6-8). After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results. (C) Jurkat T cells were stimulated for 16 hours at 37°C with RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb in the absence (lanes 2-5) or presence (lanes 6-9) of FK506. Proteins were processed as described above. (D) Jurkat T cells were incubated for 16 hours at 37°C in RPMI-5% FCS containing the indicated concentrations of CsA (lanes 2 and 3) or FK506 (lanes 4 and 5). (E) IL-2 level in the supernatants of overnight cultures of Jurkat T cells stimulated for 16 hours at 37°C with 1 ng/mL of PMA or with the combination of PMA and 1 μM Ca++ ionophore in the absence or presence of the indicated concentrations of CsA or FK506. IL-2 level was determined with a sandwich enzyme-linked immunosorbent assay technique using combinations of unlabeled and biotin-labeled antibodies, as described in “Materials and methods.” This experiment was repeated twice with similar results.
The calcineurin inhibitors CsA and FK506 potentiate TCR-induced increase in LAT expression.
Jurkat T cells (A) or purified human normal resting T cells (B) were stimulated for 16 hours at 37°C with RPMI-5% FCS containing 1 μg/mL of anti-CD3 mAb in the absence (lane 2) or presence of the indicated concentrations of FK506 (lanes 3-5) or CsA (lanes 6-8). After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results. (C) Jurkat T cells were stimulated for 16 hours at 37°C with RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb in the absence (lanes 2-5) or presence (lanes 6-9) of FK506. Proteins were processed as described above. (D) Jurkat T cells were incubated for 16 hours at 37°C in RPMI-5% FCS containing the indicated concentrations of CsA (lanes 2 and 3) or FK506 (lanes 4 and 5). (E) IL-2 level in the supernatants of overnight cultures of Jurkat T cells stimulated for 16 hours at 37°C with 1 ng/mL of PMA or with the combination of PMA and 1 μM Ca++ ionophore in the absence or presence of the indicated concentrations of CsA or FK506. IL-2 level was determined with a sandwich enzyme-linked immunosorbent assay technique using combinations of unlabeled and biotin-labeled antibodies, as described in “Materials and methods.” This experiment was repeated twice with similar results.
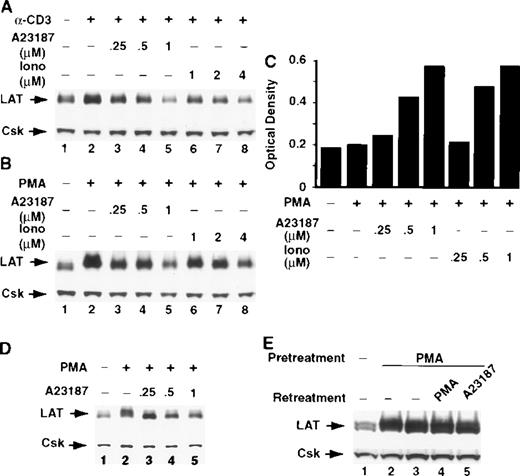
Ca++ ionophores block stimulus-induced up-regulation of LAT expression
The increase in intracellular Ca++ after T-cell activation regulates the activity of various enzymes, including the protein phosphatase calcineurin. Ca++ ionophores mobilize Ca++ from intracellular stores and induce Ca++influx by mechanisms that bypass signals activated early in TCR signaling, leading to a prolonged and sustained increase in intracellular Ca++. As described, the potentiation of TCR-induced LAT expression by CsA and FK506 strongly suggests that calcineurin negatively regulates LAT expression. To further examine this possibility, we determined whether Ca++ ionophores would down-regulate a stimulus-induced increase in LAT expression. The Ca++ ionophores A23187 and ionomycin blocked TCR- and PMA-induced expression of LAT in Jurkat T cells in a dose-dependent manner (Figure 6A and 6B). The vehicle dimethyl sulfoxide at a concentration of 1% (20 times more than that found in the 1-μM ionophore A23187 solution) did not block stimulus-induced up-regulation of LAT expression (data not shown). Notably, the inhibition of LAT expression was evident at concentrations of A23187 and ionomycin (Figure 6C) that synergized with PMA in inducing IL-2 expression, suggesting that the increase in intracellular Ca++ and the subsequent activation of calcineurin have opposing roles in IL-2 and LAT expression. The Ca++ionophores' effect was not limited to the Jurkat T cells, because Ca++ ionophore A23187 also blocked stimulus-induced up-regulation of LAT expression in human normal resting T cells in a dose-dependent manner (Figure 6D). Notably, the LAT level in cells pretreated for 16 hours with PMA did not decrease when the cells were subsequently incubated for up to 8 hours with Ca++ionophore A23187, suggesting that Ca++ ionophores do not down-regulate the newly expressed LAT by activating enzymes such as proteases (Figure 6E, compare lanes 2 and 5); however, this issue is subject to further investigation. These results show that Ca++ ionophores trigger signals that down-regulate receptor-induced LAT expression in T cells.
Ca++ ionophores completely block activation-induced up-regulation of LAT expression.
Jurkat T cells were incubated with RPMI-5% FCS containing 1μg/mL of anti-CD3 mAb (A) or RPMI-5% FCS containing 1 ng/mL of PMA (B) in the absence (A and B, lane 2) or presence of the indicated concentrations of the Ca++ ionophores A23187 (A and B, lanes 3-5) or ionomycin (Iono; A and B, lanes 6-8) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb. This experiment was repeated 3 times with similar results. (C) Jurkat T cells were incubated with RPMI-5% FCS containing 1 ng/mL of PMA in the presence of the indicated concentrations of Ca++ ionophore A23187 or ionomycin (Iono) for 16 hours at 37°C. After incubation, the tubes were centrifuged and the supernatants were collected and analyzed for IL-2. (D) Purified human normal resting T cells were incubated with RPMI-5% FCS (lane 1) or RPMI-5% FCS containing 1 ng/mL of PMA in the absence (lane 2) or presence of the indicated concentrations of the Ca++ ionophore A23187 (lanes 3-5). (E) Jurkat T cells were incubated for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing 1 ng/mL of PMA (lane 2-5). After incubation, PMA-pretreated cells were washed and then incubated for 8 hours at 37°C with RPMI-5% FCS (lane 3), RPMI-5% FCS containing 1 ng/mL of PMA (lane 4), or RPMI-5% FCS containing 1 μM of Ca++ ionophore A23187 (lane 5). This experiment was repeated twice with similar results.
Ca++ ionophores completely block activation-induced up-regulation of LAT expression.
Jurkat T cells were incubated with RPMI-5% FCS containing 1μg/mL of anti-CD3 mAb (A) or RPMI-5% FCS containing 1 ng/mL of PMA (B) in the absence (A and B, lane 2) or presence of the indicated concentrations of the Ca++ ionophores A23187 (A and B, lanes 3-5) or ionomycin (Iono; A and B, lanes 6-8) for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb. This experiment was repeated 3 times with similar results. (C) Jurkat T cells were incubated with RPMI-5% FCS containing 1 ng/mL of PMA in the presence of the indicated concentrations of Ca++ ionophore A23187 or ionomycin (Iono) for 16 hours at 37°C. After incubation, the tubes were centrifuged and the supernatants were collected and analyzed for IL-2. (D) Purified human normal resting T cells were incubated with RPMI-5% FCS (lane 1) or RPMI-5% FCS containing 1 ng/mL of PMA in the absence (lane 2) or presence of the indicated concentrations of the Ca++ ionophore A23187 (lanes 3-5). (E) Jurkat T cells were incubated for 16 hours at 37°C in RPMI-5% FCS (lane 1) or RPMI-5% FCS containing 1 ng/mL of PMA (lane 2-5). After incubation, PMA-pretreated cells were washed and then incubated for 8 hours at 37°C with RPMI-5% FCS (lane 3), RPMI-5% FCS containing 1 ng/mL of PMA (lane 4), or RPMI-5% FCS containing 1 μM of Ca++ ionophore A23187 (lane 5). This experiment was repeated twice with similar results.
CsA and FK506 block Ca++ ionophore–mediated inhibition of activation-induced up-regulation of LAT expression
The data strongly implicate calcineurin in down-regulating CD3-induced LAT expression. To further examine the involvement of calcineurin in the inhibition of LAT expression by the Ca++ionophore A23187, T cells were treated with PMA and Ca++ionophore A23187 in the absence or presence of CsA or FK506. Both CsA and FK506 blocked Ca++ ionophore A23187–induced inhibition of LAT expression in a dose-dependent manner (Figure7). These results further implicate calcineurin in negatively regulating LAT expression in T cells.
CsA and FK506 block Ca++ ionophore–mediated inhibition of stimulus-induced up-regulation of LAT.
Jurkat T cells were pretreated with the indicated concentrations of FK506 (lanes 4-6) or CsA (lanes 7-9) for 1 hour at 37°C and then stimulated in the continuous presence of the inhibitors with the combinations of 1 ng/mL of PMA and 1 μM of Ca++ ionophore A23187 for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results.
CsA and FK506 block Ca++ ionophore–mediated inhibition of stimulus-induced up-regulation of LAT.
Jurkat T cells were pretreated with the indicated concentrations of FK506 (lanes 4-6) or CsA (lanes 7-9) for 1 hour at 37°C and then stimulated in the continuous presence of the inhibitors with the combinations of 1 ng/mL of PMA and 1 μM of Ca++ ionophore A23187 for 16 hours at 37°C. After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with anti-LAT mAb or anti-Csk Ab. This experiment was repeated 3 times with similar results.
Inhibition of calcineurin preferentially potentiates TCR-induced expression of LAT
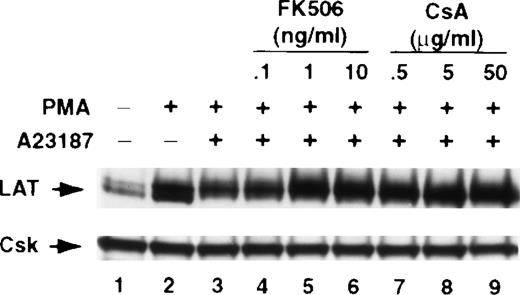
To examine whether inhibiting calcineurin activation enhances TCR-mediated expression of signaling molecules other than LAT, we stimulated Jurkat T cells in the absence or presence of FK506 using the same conditions described above for LAT. The cellular level of 14 signaling proteins that previously have been implicated in T-cell activation was determined by immunoblotting as described previously. These signaling proteins were arbitrarily selected, because the antibodies for these molecules were available in our laboratory at the time of the study. T-cell activation did not have a detectable effect on the cellular levels of Csk, Erk, FAK, Grb2, PLC-γ, or PKCα (Figure 8). T-cell activation, however, increased the expression of some of the molecules shown in Figure8.35-38 Thus, TCR ligation increased the expression of Cbl, Fyn, Vav, Lck, Pyk2, SLP-76, paxillin, and ZAP-70 (Figure 8). FK506 by itself did not induce a detectable increase in the levels of any of the molecules (Figure 8, compare lanes 1 and 8). Strikingly, under the conditions described in the present study, FK506 strongly and reproducibly increased the level of LAT but not of any of the other molecules shown in Figure 8. In some experiments, however, a slight increase was seen in the level of SLP-76 and Pyk2. Interestingly, FK506 blocked TCR-induced increase in Fyn, Lck, paxillin, and ZAP-70, suggesting that the activation of calcineurin may be important for the TCR-induced expression of these molecules. Similar results were obtained with CsA (data not shown).
FK506 preferentially potentiates TCR-induced expression of LAT.
Jurkat T cells were incubated for 16 hours at 37°C with RPMI-5% FCS (lane 1), RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb in the absence (lanes 2-4) or presence (lanes 5-7) of 5 ng/mL of FK506, or with RPMI-5% FCS containing 5 ng/mL of FK506 (lane 8). After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with the indicated antibody. This experiment was repeated 4 times with similar results.
FK506 preferentially potentiates TCR-induced expression of LAT.
Jurkat T cells were incubated for 16 hours at 37°C with RPMI-5% FCS (lane 1), RPMI-5% FCS containing the indicated concentrations of anti-CD3 mAb in the absence (lanes 2-4) or presence (lanes 5-7) of 5 ng/mL of FK506, or with RPMI-5% FCS containing 5 ng/mL of FK506 (lane 8). After incubation, the cells were immediately lysed with boiling 2× SDS-PAGE sample buffer. Proteins in WCL were separated by SDS-PAGE and then transferred to membranes and immunoblotted with the indicated antibody. This experiment was repeated 4 times with similar results.
LAT expressed upon CD3 ligation and FK506 treatment distributes normally in the cell
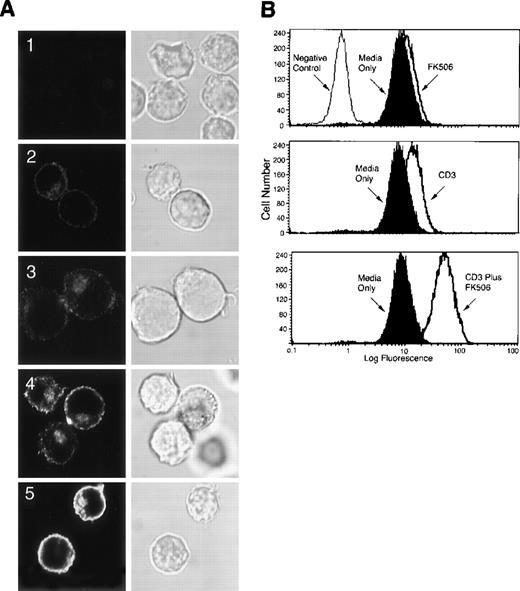
To examine whether the overexpressed LAT after CD3 ligation and FK506 treatment distributes normally in the cell, Jurkat T cells were incubated with the anti-CD3 mAb in the presence or absence of FK506 for 16 hours at 37°C. After washing, the cells were fixed, permeabilized, labeled with anti-LAT Ab, and then were stained with goat-antirabbit IgG conjugated to Alexa 488. The cells were then examined for LAT distribution by confocal scanning laser microscopy. As reported previously,14 LAT localized in unstimulated cells to the cell membrane and to a juxtanuclear intracellular compartment (Figure 9A2). FK506 alone induced only a slight increase in LAT expression (Figure 9A3). As reported above, anti-CD3 mAb increased LAT expression (Figure 9A4), which was markedly increased in the presence of FK506 (Figure 9A5). Notably, although LAT staining was stronger in cells stimulated with anti-CD3 mAb in the presence or absence of FK506 than in resting cells, the pattern of LAT staining in the stimulated cells was similar to that in the unstimulated cells. Thus, the overexpressed LAT localizes normally in the cells. The normal distribution of the overexpressed LAT suggests that the newly expressed LAT localizes to areas important for its involvement in TCR signaling pathways. Flow cytometry performed on aliquots of the labeled cells that were used in Figure 9A confirmed the increase in LAT expression (Figure 9B).
LAT expressed upon CD3 ligation and FK506 treatment distributes normally in the cell.
(A) A total of 2 × 107 Jurkat T cells in RPMI-5% FCS were incubated for 16 hours at 37°C in uncoated tissue culture dishes in the absence (A2) or presence (A3) of 5 ng/mL of FK506. For CD3 stimulation, Jurkat T cells in RPMI-5% FCS were incubated for 16 hours at 37°C in anti-CD3 mAb-coated tissue culture dishes in the absence (A4) or presence (A1 and A5) of 5 ng/mL of FK506. Following incubation, cells were removed from the dishes by gentle pipetting, fixed in suspension with 2% paraformaldehyde, and permeabilized with saponin. After permeabilization, the cells were incubated for 30 minutes at room temperature with 10 μg/mL of normal rabbit Ig (A1) or anti-LAT Ab (A2-A5), washed, and then incubated for 30 minutes at room temperature with goat-antirabbit IgG conjugated to Alexa 488. After incubation, the cells were washed in TBS/Tween and then analyzed by confocal scanning laser microscopy. Both immnuofluorescence (left panel) and corresponding differential interference contrast images (right panel) are shown. Original magnification, ×600. (B) Aliquots of the labeled cells in (A) were examined for LAT expression by flow cytometry. These experiments were repeated twice with similar results.
LAT expressed upon CD3 ligation and FK506 treatment distributes normally in the cell.
(A) A total of 2 × 107 Jurkat T cells in RPMI-5% FCS were incubated for 16 hours at 37°C in uncoated tissue culture dishes in the absence (A2) or presence (A3) of 5 ng/mL of FK506. For CD3 stimulation, Jurkat T cells in RPMI-5% FCS were incubated for 16 hours at 37°C in anti-CD3 mAb-coated tissue culture dishes in the absence (A4) or presence (A1 and A5) of 5 ng/mL of FK506. Following incubation, cells were removed from the dishes by gentle pipetting, fixed in suspension with 2% paraformaldehyde, and permeabilized with saponin. After permeabilization, the cells were incubated for 30 minutes at room temperature with 10 μg/mL of normal rabbit Ig (A1) or anti-LAT Ab (A2-A5), washed, and then incubated for 30 minutes at room temperature with goat-antirabbit IgG conjugated to Alexa 488. After incubation, the cells were washed in TBS/Tween and then analyzed by confocal scanning laser microscopy. Both immnuofluorescence (left panel) and corresponding differential interference contrast images (right panel) are shown. Original magnification, ×600. (B) Aliquots of the labeled cells in (A) were examined for LAT expression by flow cytometry. These experiments were repeated twice with similar results.
Discussion
Proteins that become tyrosine-phosphorylated following TCR engagement are critical for the function of T cells. To better understand the involvement of the phosphoprotein LAT in T cell processes, we studied the effect of T-cell activation on the expression of LAT and examined the molecular mechanisms that regulate receptor-induced LAT expression. We showed in this report that TCR ligation initiated signals that led to the up-regulation of LAT expression in human normal resting T cells and in the Jurkat T-cell line. We also showed that the signals that regulate TCR-induced increase in LAT expression included the activation of the PKC-Erk pathway and the Ca++–calcineurin pathway. Our results strongly suggest that these pathways play opposing roles in regulating LAT expression in T cells.
TCR ligation induces the tyrosine phosphorylation and the activation of PLC, leading to the generation of diacylglycerol and inositol triphosphate. Diacylglycerol binds to PKC, leading to its activation, whereas inositol triphosphate binds receptors on intracellular Ca++ stores and leads to the rapid release of Ca++ from the stores and to Ca++ influx. A number of studies have shown that the activation of cellular PKC in T cells induces gene expression. For example, PKC activation has been shown to up-regulate the expression of several immediate response genes (transcription factors), including c-fos and Elk-1.39,40Our results, indeed, strongly implicate PKC in the signaling pathways that up-regulate LAT expression in T cells: (1) PMA, a potent activator of PKC, strongly up-regulated LAT expression, and (2) the PKC inhibitors H-7 and Ro-31-8220 blocked a CD3- and PMA-induced increase in LAT expression. Activated PKC has been shown to stimulate the small GTPase Ras,41 which in turn activates the serine/threonine kinase Raf.3,4,42 Activated Raf phosphorylates and activates the MAPK MEK, which in turn phosphorylates and activates the MAPK Erk.4 Phosphorylated Erk translocates to the nucleus, where it phosphorylates and activates transcription factors such as c-fos and Elk-1, leading to the initiation of gene transcription.3 4 The inhibition of the CD3-induced increase in LAT expression by the specific MEK inhibitor PD98059 (Figure 4) suggests that PKC is linking CD3-initiated signals to the MEK-Erk cascade to up-regulate LAT expression in T cells. The increase in intracellular Ca++ following TCR ligation leads to the activation of several enzymes, including calcineurin, a key molecule in T-cell activation. We showed experimental evidence that implicates calcineurin in negatively regulating TCR-induced LAT expression: (1) the calcineurin inhibitors CsA and FK506 enhanced TCR-induced up-regulation of LAT expression, (2) the Ca++ ionophores A23187 and ionomycin blocked TCR-induced increase in LAT expression, and (3) CsA and FK506 inhibited Ca++ ionophores' effect on TCR-induced LAT expression. The fact that 2 structurally different compounds (CsA and FK506) exerted similar effects on LAT expression suggests that the actions of these compounds on the expression of LAT are not nonspecific.
The calcineurin inhibitors CsA and FK506 are widely used to prevent allograft rejection in humans and are currently the keystone of most immunosuppressive regimens used in clinical organ transplantation.43 In certain models, both compounds have also been associated with the induction of tolerance to the allograft. Both CsA and FK506 bind with high affinity to intracellular receptors collectively known as immunophilins. CsA, a cyclic undecapeptide, binds to members of the cyclophilins family, whereas the structurally unrelated FK506, a macrolide lactone, binds to the family of FK506-binding proteins. The binding of the drug-immunophilin complex to the Ca++/calmodulin–activated calcineurin inhibits the catalytic activity of calcineurin and in turn blocks the expression of several regulatory cytokines and growth factors, including IL-2, IL-4, and tumor necrosis factor α. These lymphokines are critical for T-cell proliferation, stimulate antibody production, induce maturation of cytotoxic effector cells, and activate various accessory cells, such as phagocytes. Recent studies have also shown that FK506 and CsA also augment receptor-induced production of regulatory lymphokines, including granulocyte-macrophage colony-stimulating factor, interferon-γ, transforming growth factor-β, IL-5, and IL-13 in T cells,44-46 thereby suggesting that calcineurin negatively regulates the expression of certain genes. However, the molecular mechanisms by which calcineurin inhibition by CsA and FK506 led to the increase in lymphokine production are not clear. Importantly, CsA and FK506 have been associated with cancer progression and with deleterious adverse effects that limit the drugs' clinical utility, including neurotoxicity, hepatotoxicity, and nephrotoxicity.47-49 The mechanisms underlying CsA and FK506 toxicity and tumor promotion are not clear. Our data showing that CsA and FK506 up-regulate TCR-induced LAT expression indicate that although the inhibition of calcineurin by CsA and FK506 blocks certain aspects of TCR signaling important for cytokine gene expression, other aspects of TCR signaling, normally suppressed by calcineurin, are up-regulated. This, in turn, leads to the exaggerated expression of critical signaling molecules. The effect of enhanced LAT expression by CsA and FK506 on T cells awaits further investigation.
The precise mechanisms by which LAT functions in T cells are not yet fully clear. LAT has been shown to become tyrosine-phosphorylated following TCR and CD28 ligation.14-20 Such phosphorylation promotes the association of LAT with several signaling molecules, a process thought to be critical for transducing receptor signaling.14,15,18,20 Thus, mutants of LAT lacking 2 tyrosine residues abort LAT binding with signaling molecules and in turn inhibit receptor-induced activation of the transcriptional factors activating protein (AP)-1 and NF-AT.14,18 LAT has also been shown to associate with the coreceptors CD4 and CD8, a process that appears to be important for LAT tyrosine phosphorylation by ZAP-70 upon TCR ligation.20 Recent studies also suggest that LAT not only promotes positive signaling through PLC-gamma, Vav, and SLP-76, but also may inhibit negative signaling by down-regulating TCR-induced Cbl tyrosine phosphorylation.17 These results, together with the fact that LAT is involved in different cellular processes and that it becomes tyrosine-phosphorylated after the ligation of distinct receptors, suggest that this molecule may be involved in regulating multiple distinct signaling pathways. Thus, the TCR-induced up-regulation of LAT expression shown in this report could be important for TCR and for T-cell function.
Previous transfection studies suggest that LAT is involved in regulating IL-2 gene expression in T cells. For example, the transient transfection of LAT mutants into Jurkat T cells blocked TCR-induced increase in the transcription of AP-1– or NF-AT–promoter driven constructs.14 Similarly, the transient expression of LAT in the LAT-deficient Jurkat T cells, J.CaM2 and ANJ3, restored TCR-inducible NF-AT activation and IL-2 promoter-driven gene expression.15 18 Our data, however, suggest an inverse relationship between LAT and IL-2 expression in T cells. Thus, CsA and FK506, which down-regulate TCR-induced IL-2 production, potentiated LAT expression. Furthermore, Ca++ ionophores, which in combination with PMA increase IL-2 production, decreased LAT expression. We do not know the reasons for the apparent discrepancy between our data and those previously reported on the importance of LAT for IL-2 production. We can speculate, however, that the level of LAT that is present at the time of initial TCR ligation may be sufficient to promote the activation of downstream signaling pathways and IL-2 gene transcription, a process that when started may not require the persistent presence of LAT. This issue, however, needs further investigation.
We have recently reported that TCR ligation increases the expression of the PTK Pyk2 by mechanisms that also involve the activation of the PKC–Erk cascade.38 As with LAT expression, Pyk2 expression was also down-regulated by the Ca++ ionophores A23187 and ionomycin. However, in contrast to the strong and reproducible enhancement of LAT expression by CsA and FK506, these agents only slightly (if at all) increased Pyk2 expression. Furthermore, CsA and FK506 only partially blocked the inhibitory effect of the Ca++ ionophores A23187 and ionomycin on TCR-induced Pyk2 expression, whereas both compounds effectively blocked the Ca++ ionophores' effect on LAT expression. These results suggest that the mechanism by which Ca++ ionophores A23187 and ionomycin regulated Pyk2 expression are different, at least in part, from those that regulated LAT expression.
Finally, our data depict a model for the regulation of TCR-initiated expression of certain proteins in which the PKC–Erk cascade and the Ca++/calmodulin–calcineurin cascade positively and negatively regulate protein expression, respectively. Because both cascades are activated following TCR ligation, it is not clear how these 2 cascades coordinate their actions to regulate LAT expression, a process that may be coordinated sequentially or temporally.
Acknowledgments
We thank Drs Robert Conhaim and Donna Peters for assistance with the microscopy studies, John Fechner for assistance with flow cytometery studies, and Jackie Schultz for excellent technical assistance. We also thank Andrea Schmick for editorial assistance.
Reprints:Majed M. Hamawy, H4/749, Department of Surgery, 600 Highland Ave, Madison, WI 53792; e-mail:hamawy@surgery.wisc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.