Previous cytogenetic studies of primary cutaneous T-cell lymphoma (CTCL) were based on limited numbers of patients and seldom showed consistent nonrandom chromosomal abnormalities. In this study, 54 tumor DNA samples from patients with CTCL were analyzed for loss of heterozygosity on 10q. Allelic loss was identified in 10 samples, all of which were from the 44 patients with mycosis fungoides (10/44 patients; 23%). Of the patients with allelic loss, 3 were among the 29 patients with early-stage myosis fungoides (T1 or T2) (3/29 patients; 10%), whereas the other 7 were among the 15 patients with advanced cutaneous disease (T3 or T4) (7/15 patients; 47%). The overlapping region of deletion was between 10q23 and 10q24. In addition, microsatellite instability (MSI) was present in 13 of the 54 samples (24%), 12 from patients with mycosis fungoides and 1 from a patient with Sezary syndrome. There was also an association between MSI and disease progression in patients with mycosis fungoides, with 6 of 15 (40%) patients with MSI having advanced cutaneous disease and only 6 of 29 (21%) having early-stage disease. Samples with allelic loss on 10q were analyzed for abnormalities of the tumor suppressor genePTEN (10q23.3). No tumor-specific mutations were detected, but homozygous deletion was found in 2 patients. Thus, we found loss of heterozygosity on 10q and MSI in advanced cutaneous stages of mycosis fungoides. These findings indicate that a tumor suppressor gene or genes in this region may be associated with disease progression. Furthermore, abnormalities of PTEN may be important in the pathogenesis of mycosis fungoides, but our data imply that this gene is rarely inactivated by small deletions or point mutations.
Primary cutaneous T-cell lymphoma (CTCL) represents a heterogeneous group of extranodal non-Hodgkin lymphomas of which mycosis fungoides is the most common. Patients with mycosis fungoides typically present with patches and plaques. Early cutaneous disease may be divided into stage T1, in which less than 10% of the body surface is affected, and T2, in which more than 10% is affected. Early-stage cutaneous mycosis fungoides may progress to tumor-stage disease (T3) or, rarely, to erythrodermic disease (T4). Hematologic involvement may also occur. In contrast, Sezary syndrome is characterized by erythroderma, the presence of more than 10% atypical circulating mononuclear cells at presentation, and a peripheral blood T-cell clone demonstrated by T-cell–receptor gene-analysis studies. Sezary syndrome has a poorer prognosis than mycosis fungoides, with a median survival of less than 3 years.
Few data are available on chromosomal abnormalities in CTCL. Several small cytogenetic studies identified structural chromosomal abnormalities involving 1p, 2p, 6q, and 10q,1-4 and multiple chromosomal abnormalities were found to be associated with a poor prognosis.1 Many factors have contributed to this paucity of genetic data. First, most cytogenetic studies have been based on analyses of peripheral blood lymphocytes, even though morphologic abnormalities are rare in mycosis fungoides. Second, growth of tumor cells from solid tissue is often poor because of a low mitotic index. Third, a large population of reactive lymphocytes is often present in cutaneous lesions, diluting the neoplastic population. Finally, studies of CTCL have concentrated on the detection of characteristic cytogenetic abnormalities found in nodal lymphomas. Specific translocations producing overexpression of oncogenes have been identified in several nodal lymphomas and leukemias but are rarely found in primary cutaneous lymphomas.5,6 However, abnormalities of tumor suppressor genes have been detected in CTCL. Overexpression of p53 is found in high-grade but rarely in low-grade cutaneous lymphoma,7,8 and some studies, including ours, identified p53 gene mutations in advanced CTCL.9 10
Several cytogenetic studies of CTCL identified abnormalities on 10q. A study of peripheral mononuclear cells in 5 patients with Sezary syndrome found clonal aberrations in all of them,3 and 4 patients had abnormalities of chromosome 10—structural rearrangements affecting bands 10q22-24 in 3 patients and monosomy of chromosome 10 in 1 patient. In a study of 11 patients with CTCL that used comparative genomic hybridization, 4 of the 11 had loss of 10q, with a minimal overlapping area at 10q25-26.4 Abnormalities on 10q have been found in hematologic malignancies: t(10;14)(q24;q11) was observed in 5% to 10% of patients with acute T-cell leukemias.11 A cytogenetic study of 159 patients with non-Hodgkin lymphoma detected abnormalities on 10q23-25 in 17 patients (10.7%),12 and a karyotype analysis of 201 lymphomas identified deletions on 10q in 6 of 57 patients (11%) with a B-cell lymphoma and t(14;18).13
Several putative tumor suppressor genes have been mapped to 10q.PTEN, previously known as MMAC1 (mutated in multiple advanced cancers), is a tumor suppressor gene on 10q23.3. Germline mutations of PTEN are found in Cowden disease,14which is an autosomal dominant cancer-predisposition syndrome associated with an elevated risk of tumors of the breast, thyroid, and skin. Somatic mutations in PTEN have been identified in a variety of sporadic malignancies, including glioblastomas, breast cancer, prostate cancer,15 and, more rarely, in non-Hodgkin lymphoma.16-18 Interestingly, PTEN knockout mice are susceptible to T-cell lymphomas and leukemias.19 Other candidate tumor suppressor genes include MXI1 on 10q25-26, which codes for a family of proteins that antagonize c-myc–induced transcription,20 and DMBT1 (10q25.3-q26), which is deleted in malignant brain tumors and encodes a protein that shares extensive homology with the scavenger-receptor cysteine-rich superfamily.21
Screening for loss of heterozygosity (LOH) is used to identify regions of chromosomal loss that may harbor tumor suppressor genes, and this method has been used extensively to map regions of chromosomal deletions in a number of solid malignancies. Microsatellite instability (MSI) may be identified by the same method. MSI is due to the expansion or retraction of small repeat sequences as a consequence of slippage of 1 DNA strand in relation to the other during DNA replication. Because such errors are normally corrected by enzymes encoded by DNA-mismatch repair genes, the hallmark of defects in the DNA-mismatch repair system is MSI. Germline mutations in mismatch repair genes occur in patients with hereditary nonpolyposis colorectal cancer syndrome (HNPCC), a familial cancer syndrome in which patients have a strong predisposition to colon cancer, gastric cancer, and endometrial carcinoma.22 MSI has been identified in various sporadic malignancies.23 It was detected in mucosa-associated lymphoid tissue lymphoma24 and in a subset of patients with chronic lymphocytic leukemia,25 but it is rarely found in other B-cell lymphomas.26 MSI was also identified in patients with T-cell lymphoma/leukemia, in whom it was associated with a poor prognosis.27
In view of the results of previous cytogenetic studies, we analyzed a large number of samples from patients with primary CTCL for LOH on 10q and MSI to fine map deletions in this chromosomal region and establish the prevalence of MSI.
Materials and methods
Samples
Fifty-four archival specimens of tumor and normal DNA from individual patients were selected. All tumor samples were chosen because either Southern blot analysis or T-cell–receptor gene analysis based on single-strand conformational polymorphism–polymerase chain reaction (SSCP-PCR) had shown a dominant tumor population.28 29 The samples were obtained from 44 patients with different cutaneous stages of mycosis fungoides (stage T1, n = 10; stage T2, n = 19; stage T3, n = 13; and stage T4, n = 2), 6 patients with Sezary syndrome, and 4 patients with other variants of CTCL, including 1 case of primary cutaneous CD30-positive large-cell anaplastic lymphoma, 1 of lymphomatoid papulosis, and 2 of large-cell pleomorphic CTCL (1 CD30 positive and 1 CD30 negative).
LOH and MSI analysis
Samples were analyzed for LOH on 10q by using oligonucleotide primers for 8 highly polymorphic dinucleotide repeat microsatellite markers from the Human Genethon Linkage Map30 in the region 10q22-26 limited by D10S210 (centromeric to 10q22) and D10S212 (telomeric to 10q26). The other markers and the approximate chromosomal bands corresponding to their position on 10q were, from centromere to telomere, D10S215 (10q23), D10S541 (10q23), D10S185 (10q23-24), D10S187 (10q24), D10S209 (10q24), and D10S587 (10q24-25).
PCR was performed in a reaction mixture of 20μL, including 20 pmol of each oligonucleotide primer, 0.2 mmol/L deoxyadenosine triphosphate, 0.2 mmol/L deoxyguanosine triphosphate, 0.2 mmol/L deoxythymidine triphosphate (Amersham-Pharmacia Biotech, Little Chalfont, UK), 0.03 mmol/L deoxycytidine triphosphate (dCTP), 3.7 × 103 Bq α-phosphorus 33–labeled dCTP (1.11 × 1014 Bq/mmol), 50 ng of sample DNA, 1 × PCR buffer containing 1.5 mmol/L magnesium chloride, 1.25 U Taq polymerase (Amersham-Pharmacia Biotech), and 1.25 U Taq antibody (Clontech, Basingstoke, UK). Twenty-five cycles of PCR were performed in a DNA thermal cycler (model 9700; Perkin-Elmer, Warrington, UK). Each cycle consisted of denaturing at 94°C for 20 seconds, annealing at 55°C to 65°C (according to the temperature midpoint for each oligonucleotide primer pair) for 20 seconds, and extension at 72°C for 50 seconds.
A negative control reaction containing no DNA was examined for each PCR assay. PCR products were diluted 2-fold with stop solution (95% formamide, 20 mmol/L EDTA, 0.05% xylene cyanol FF, and 0.05% bromophenol blue) and denatured for 10 minutes at 95°C. They were then loaded onto denaturing 6% polyacrylamide gels (Gibco BRL, Paisley, UK) containing 7 mol/L urea (United States Biochemicals, Cleveland, OH) and electrophoresed by using a S2 sequencing gel apparatus (Gibco BRL). The gel was dried on 3-mm Whatman paper and exposed to x-ray film (Genetic Research Instrumentation, Braintree, UK) with an intensifying screen (Appligene Oncor, Walford, UK) at room temperature for 48 to 72 hours.
Determination of LOH and MSI
All samples in which 2 distinct alleles of similar intensity were present in the normal DNA were considered to be informative. Signal intensities were examined visually by 2 independent observers who had no knowledge of the histologic type of the tumor or the patient's clinical details.
LOH was scored as positive when a clear reduction in signal intensity was detected in 1 of the alleles of the tumor DNA compared with the paired normal DNA. To quantify signal intensities, samples were analyzed by using Eagle-eye II with Q-gel software (Stratagene, La Jolla, CA). A reduction of more than 50% in 1 of the alleles in the tumor DNA compared with the paired normal DNA was defined as LOH. All samples showing LOH were subjected to repeat amplification and analysis for confirmation.
MSI was defined as the presence of novel alleles in tumor DNA compared with the corresponding normal DNA. MSI at more than 40% of the markers analyzed or at 3 or more in our study using 8 markers was considered to represent a highly unstable phenotype (MSI-H). MSI at less than 40% of markers was scored as a low level of instability (MSI-L).31
When a marker showed both allelic loss (apparent LOH) and the presence of novel fragments (MSI), it was scored positive for MSI and noninformative for LOH. Markers were scored as either LOH or MSI but not both, consistent with accepted criteria.31
SSCP analysis of the PTEN gene
Samples showing LOH on 10q were analyzed for PTEN mutations by using SSCP-PCR. Ten intronic primer pairs flanking exons 1 to 9 of the PTEN gene were used as previously described.18The PCR reaction was performed as described above. Six percent dimethyl sulfoxide was added for the primer pairs of exon 1 and 5. For this reaction, 33 cycles were performed. PCR products were diluted as described previously. Samples were then denatured for 10 minutes at 95°C and then cooled rapidly in a dry-ice ethanol bath. Samples were loaded in turn on nondenaturing polyacrylamide gels containing 0%, 5%, and 10% glycerol and electrophoresed at 4 W overnight. Polyacrylamide gels were processed as described earlier, and autoradiographs were examined for conformational change indicated by a band shift compared with the wild type.
Homozygous deletion of PTEN
Samples with LOH on 10q were also examined for homozygous deletions of PTEN. For this analysis, we looked for apparent retention of heterozygosity at the intragenic PTENmicrosatellite marker D10S2491 in tumors with LOH in the markers flanking PTEN (D10S215 and D10S541).32The apparent retention of heterozygosity is due to amplification of DNA from normal cells contaminating the tumor specimen. This technique was shown to detect homozygous deletion of genes accurately when compared with Southern blot analysis and fluorescent in situ hybridization (FISH).33 PCR reactions were performed as described previously for LOH analysis. Twenty-five cycles were performed, with annealing at 55°C.
Results
LOH at 10q
All samples were informative for at least 6 of the 8 microsatellite markers. LOH was observed in 10 of 54 samples (19%). The degree of reduction in allelic signal intensity was frequently greater than 50% and approached 100% in some instances. Examples of allelic loss are shown in Figure 1.
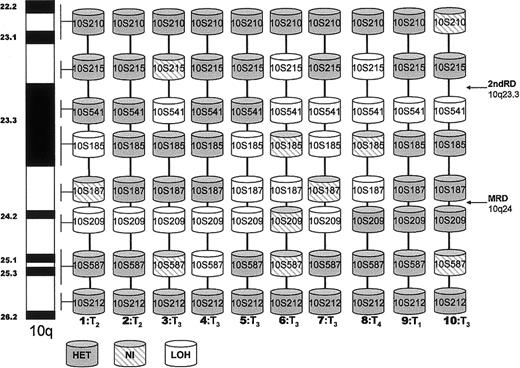
Loss of heterozygosity on 10q in CTCL.
Diagrammatic representation of LOH detected by using 8 microsatellite markers on 10q 22-26 in 10 patients with mycosis fungoides. NI, noninformative; HET, retention of heterozygosity; T1-4, cutaneous stage MF; MRD, minimal region of deletion; 2ndRD, second region of deletion.
Loss of heterozygosity on 10q in CTCL.
Diagrammatic representation of LOH detected by using 8 microsatellite markers on 10q 22-26 in 10 patients with mycosis fungoides. NI, noninformative; HET, retention of heterozygosity; T1-4, cutaneous stage MF; MRD, minimal region of deletion; 2ndRD, second region of deletion.
All tumor samples showing LOH were from patients with mycosis fungoides (10/44 patients; 23%). Three of the 10 samples with LOH were from among the 29 patients with early-stage disease (T1 or T2) (3/29 patients; 10%), whereas 7 were from among the 15 patients with advanced cutaneous disease (T3 or T4) (7/15 patients; 47%). LOH was not found in samples from patients with Sezary syndrome or other variants of CTCL.
The deletion map shown in Figure 2 provides a diagrammatic representation of LOH in the 10 samples from patients. The map delineates a region of deletion between 10q23 and 10q24. Eight samples had deletions at 10q24 flanked by D10S209 and D10S187 (the minimal region of deletion). A second region of deletion at 10q23.3 bound by D10S215 and D10S541 was present in 6 patient samples. ThePTEN gene has been mapped to this region and is closely flanked by these microsatellite markers.
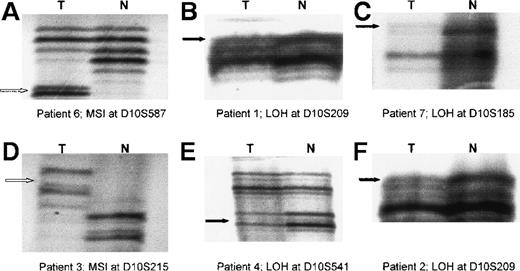
Radiographs showing loss of heterozygosity (LOH) and microsatellite instability (MSI) in primary cutaneous T-cell lymphoma (CTCL).
Examples are shown of MSI and LOH on 10q detected by using microsatellite markers (D10S587, D10S209, D10S185, D10S215, and D10S541) in tumor samples from 6 patients with CTCL. The black arrow indicates allelic loss (LOH) in tumor (T) DNA with corresponding normal (N) alleles. The white arrow indicates novel alleles (MSI) in T DNA with corresponding N alleles.
Radiographs showing loss of heterozygosity (LOH) and microsatellite instability (MSI) in primary cutaneous T-cell lymphoma (CTCL).
Examples are shown of MSI and LOH on 10q detected by using microsatellite markers (D10S587, D10S209, D10S185, D10S215, and D10S541) in tumor samples from 6 patients with CTCL. The black arrow indicates allelic loss (LOH) in tumor (T) DNA with corresponding normal (N) alleles. The white arrow indicates novel alleles (MSI) in T DNA with corresponding N alleles.
The observed frequencies of LOH at each marker ranged from 2% at 10S587 to 11% at 10S209. In most samples, the allelic losses involved a contiguous region and the degree of signal reduction of the lost allele was consistent for each marker analyzed. However, in 1 sample, the pattern observed was of hemizygosity, with LOH at markers D10S541 (10q23) and D10S209 (10q24) and retention of heterozygosity between them (Figure 2, patient 3).
MSI at 10q
MSI was observed in 13 tumor samples (24%); examples are shown in Figure 1. Twelve of these samples were from patients with mycosis fungoides (27%), and the other was from a patient with Sezary syndrome (17%). MSI was also associated with disease progression in mycosis fungoides: it was present in 6 of 15 patients (40%) with advanced skin disease but in only 6 of 29 patients (21%) with early-stage disease.
Nine of 13 samples showed MSI at only 1 marker. However, 4 (7%) had MSI at more than 2 markers, indicating a highly unstable phenotype (MSI-H). Three of these samples were from patients with mycosis fungoides, 1 of whom had early-stage disease and 2 of whom had advanced cutaneous disease. The other sample with MSI-H was from the patient with Sezary syndrome. Five samples with MSI had LOH on 10q at separate markers. The remaining 8 samples with MSI did not show LOH on 10q, even though our studies used 8 dinucleotide markers.
SSCP analysis of the PTEN gene
Nine primer sets designed to amplify the entire coding region ofPTEN were used to analyze all tumor samples with LOH on 10q23 for point mutations or small deletions or insertions in PTEN. No conformational band shifts were detected, even though we analyzed PCR products with polyacrylamide gels containing 0%, 5%, and 10% glycerol.
Homozygous deletions of PTEN
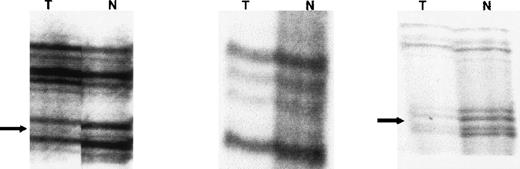
The samples from patients 6 and 8 showed LOH with microsatellite markers flanking the PTEN gene (D10S215 and D10S541). Using these flanking markers and the intragenic microsatellite marker D10S2491, we demonstrated homozygous deletion of PTEN in these samples. Figure 3 shows homozygous deletion of PTEN in the sample from patient 6. The radiographs demonstrate LOH at D10S215 and D10S541, with a 50% to 70% reduction in signal intensity in the smaller allele compared with the normal control in both these markers flanking PTEN. The reduction in signal intensity was not 100% because of the presence of normal DNA contaminating the tumor sample. There was retention of heterozygosity at the PTEN intragenic marker D10S2491. This presumably occurred because of loss of both PTEN alleles in the tumor DNA (homozygous deletion) so that only the contaminating normal DNA was amplified to produce 2 alleles of low signal intensity. This method of detecting homozygous deletion has been shown to be as reliable as Southern blot analysis and FISH.33
Radiographs from patient 6 showing homozygous deletion ofPTEN gene.
LOH was found at D10S215 and D10S541, with a 50% to 70% reduction in signal intensity in 1 allele compared with the normal control. The reduction in signal intensity was not 100% because of the presence of normal DNA contaminating the tumor sample. There was retention of heterozygosity at the PTEN intragenic marker D10S2491. This presumably occurred because of loss of both PTEN alleles in the tumor DNA (homozygous deletion) so that only the contaminating normal DNA was amplified to produce 2 alleles with low signal intensity.
Radiographs from patient 6 showing homozygous deletion ofPTEN gene.
LOH was found at D10S215 and D10S541, with a 50% to 70% reduction in signal intensity in 1 allele compared with the normal control. The reduction in signal intensity was not 100% because of the presence of normal DNA contaminating the tumor sample. There was retention of heterozygosity at the PTEN intragenic marker D10S2491. This presumably occurred because of loss of both PTEN alleles in the tumor DNA (homozygous deletion) so that only the contaminating normal DNA was amplified to produce 2 alleles with low signal intensity.
Discussion
We identified LOH on 10q in 23% of patients with mycosis fungoides and showed an association with disease progression, since 47% of the patients with advanced cutaneous disease had chromosomal loss on 10q. In addition, MSI was present in 27% of samples from patients with mycosis fungoides. The region of deletion was identified at 10q23-24. Mutational studies of the candidate tumor suppressor gene PTEN(10q23.3) that used exon-specific primers in samples with LOH on 10q did not reveal any abnormal conformations, which suggests that inactivation of the PTEN gene by point mutations or small deletions is rare in mycosis fungoides. However, homozygous deletion ofPTEN was identified in 2 patients by using an intragenic microsatellite marker.
All samples with LOH were from patients with mycosis fungoides (10/44 samples; 23%). To our knowledge, there are no previously published data on LOH in mycosis fungoides. LOH was not found in samples from patients with Sezary syndrome or other variants of CTCL. These normal findings in Sezary syndrome contrast with previous reports of deletions on 10q in 3 of 5 patients3 and 4 of 7 patients,4 respectively, with Sezary syndrome. However, in our diagnostic criteria for Sezary syndrome, we included the presence of greater than 10% Sezary cells in the peripheral circulation and a peripheral blood T-cell clone demonstrated by T-cell–receptor gene-analysis studies at presentation.34 Patients with erythroderma who did not meet these criteria were classified as having erythrodermic mycosis fungoides (T4), with or without hematologic involvement (B0 or B1), in accordance with the tumor-node-metastasis-blood (TNMB) classification. These diagnostic criteria may differ from those in other studies, such as that by Karenko et al,4 who described 4 patients with Sezary syndrome evolving from mycosis fungoides, 3 of whom had allelic loss on 10q. By our criteria, these patients would have been classified as having erythrodermic mycosis fungoides with hematologic involvement (T4B1). Interestingly, we identified LOH in 1 of 2 patients with erythrodermic mycosis fungoides.
In particular, we found an association between LOH and advanced cutaneous stages of mycosis fungoides, with LOH present in 47% of patients with T3 or T4 stage disease but in only 10% of patients with T1 or T2 stage disease. Although this finding could reflect a smaller population of clonal T-cells in early-stage disease, our methods involved analysis of samples with a dominant tumor population to eliminate this potential bias. However, this analysis may have preselected patients with a large tumor burden and may therefore not have provided a true representation of early-stage disease. To eliminate this bias, tumor cells in early cutaneous lesions could be microdissected from surrounding tissues before DNA extraction.
One tumor sample showed hemizygosity. This finding might be explained by the presence of 2 subclones—1 with LOH at 10q23 and the other with LOH at 10q24—but this is unlikely because the reduction in signal intensities at both these regions exceeded 70%. Furthermore, T-cell–receptor gene-analysis studies showed a monoclonal population in this sample, thereby effectively excluding the presence of 2 separate lymphoid clones. To account for a monoclonal population with 2 regions of deletion on 10q, it may be postulated that 2 mitotic events (recombination or deletion accompanied by chromosome nondisjunction) affecting 10q23 and 10q24 occurred simultaneously.35
The rationale for analyzing tumors for LOH is the identification of sites of putative tumor suppressor genes. Previous studies have found higher rates of LOH than of inactivation of tumor suppressor genes. This may be due to methodologic problems in identifying tumor-specific gene mutations or because tumor suppressor genes may be inactivated by a variety of mechanisms, including small deletions, point mutations, homozygous deletion, and hypermethylation of promoter sites. The clinical importance of LOH is unknown, and its correlation with prognosis has, in some instances, produced conflicting results.36 37
There are no universal criteria for scoring LOH and MSI in noncolonic malignancies. We interpreted allelic loss in association with novel bands as positive for MSI and noninformative for LOH (Figure 1, radiograph A); thus, in individual patients, markers were scored positive for either LOH or MSI but not both, in keeping with the criteria for MSI of the National Cancer Institute.31 The detection of MSI in 27% of patients may have therefore resulted in underscoring of LOH. However, we tried to limit this by using 8 dinucleotide markers on 10q. The finding of LOH in 5 of 13 patients with MSI highlights the relevance of using multiple markers.
We identified samples with instability at 3 or more markers (> 40%) as the highly unstable phenotype MSI-H that is characteristic of HNPCC kindred. Samples with MSI at less than 40% of markers were defined as MSI-L. However, we used dinucleotide-repeat microsatellite markers, which are inherently more stable than trinucleotide and tetranucleotide repeats and therefore less sensitive for detecting MSI-L. Conversely, dinucleotide markers are less stable than mononucleotide repeats, which are more reliable for identifying the MSI-H phenotype.31Furthermore, the importance of MSI in sporadic malignancies has not been established and it is not clear whether the criteria used to identify MSI in HNPCC cancers are relevant in these disorders.
The detection of MSI in 27% of patients with mycosis fungoides and 1 with Sezary syndrome is novel because there are no previously reported studies of MSI in CTCL. The majority had MSI at only 1 microsatellite (9 of 13 patients). Similar low levels of MSI have been detected in a variety of sporadic malignancies, including lymphomas,23-25 but their importance has not yet been elucidated.31 We also identified high levels of instability characteristic of patients with HNPCC in 4 of our patient samples. Further studies of MSI, using a panel of markers including mononucleotide, dinucleotide, and tetranucleotide repeats, are required to assess the frequency and importance of MSI-L and MSI-H in CTCL.
Some tumor suppressor genes contain intragenic microsatellites, and it has been hypothesized that defects in mismatch repair genes may lead to MSI within these tumor suppressor genes, resulting in gene inactivation.31 Interestingly, PTEN contains such intragenic microsatellites. Five of the 10 patients with LOH on 10q also had MSI, and all 5 had deletions flanking the PTEN gene.
These findings led us to analyze samples with LOH on 10q for abnormalities of PTEN (10q23.3). We did not identify any small deletions or point mutations in PTEN in the 10 patients with LOH on 10q by using SSCP-PCR analysis. However, we did detect homozygous deletion of PTEN in 2 patients (20%) by using intragenic microsatellite markers. Abnormalities of PTEN have been observed in patients with non-Hodgkin lymphoma.16-18An analysis of 29 cases of non-Hodgkin lymphoma for PTENmutations found only 1 mutation, an 11-base-pair deletion.16 A study of 170 primary lymphoid malignancies detected point mutations in the PTEN gene in 2 of 39 patients with B-cell lymphomas.17 A study of 65 primary lymphomas found PTEN mutations in 3 patients (4.6%), all of whom had large-B-cell lymphoma. One of these patients had a base substitution in codon 16 of exon 1, and the other 2 had small deletions in intron 7.18 An alternative mechanism of inactivation of tumor suppressor genes is hypermethylation of the promotor region. Unfortunately, the DNA sequence of the promotor region for PTENhas not yet been published, but such aberrant methylation may represent an important mechanism of PTEN inactivation,38 and it is possible that we underestimated the prevalence of PTENinactivation in CTCL.
Eight of our samples with LOH had a region of chromosomal loss at 10q24, distal to the PTEN locus. One sample showed hemizygosity, with 1 deletion in the region of PTEN at 10q23.3 and the other at 10q24. These data suggest that other tumor suppressor genes on 10q may also be important in the pathogenesis of cutaneous lymphoma. As mentioned earlier, candidate genes close to this region include DMBT1 at 10q25.3-q26 and MXI1 at 10q25-q26. However, no candidate tumor suppressor genes have yet been identified on 10q24.
Our results indicate a relatively high rate of LOH on 10q23-24 (23%) and MSI (27%) in mycosis fungoides. In addition, LOH on 10q appears to be associated with disease progression in mycosis fungoides, although this must be confirmed by analyzing microdissected tumor samples from patients with early-stage disease. These data imply that a tumor suppressor gene or genes in this region may be inactivated in mycosis fungoides. We analyzed the candidate tumor suppressor gene PTEN(10q23.3) for inactivation and identified homozygous deletion in 20% of patients with LOH on 10q, but we found no evidence of small deletions or point mutations. Further studies of PTENinactivation are warranted to clarify its role in primary cutaneous lymphoma.
Funded by a Fellowship Grant awarded to J.S. by the Special Trustees of St Thomas' Hospital.
Reprints:Julia Scarisbrick, Skin Tumour Unit, John's Institute Dermatology, St Thomas' Hospital, Lambeth Palace Road, London, SE1 7EH, United Kingdom; e-mail:juliascarisbrick@doctors.org.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.