The objective of this study was to investigate whether leukocytes could be recruited by rolling leukocytes in a human whole blood model system. In all experiments, either neutrophils, whole blood, or diluted blood was perfused over immobilized E-selectin. With isolated neutrophils (2 × 105/mL), the free-flowing neutrophils were captured by attached neutrophils to form secondary interactions that resulted in lines of rolling leukocytes. These secondary tethers accounted for 50% to 60% of all interactions and were eliminated by an L-selectin antibody, which also eliminated the lines of rolling leukocytes. Perfusion of whole blood or diluted blood revealed no lines of rolling leukocytes. The addition of red blood cells to isolated neutrophils either in a 1000:1 or a 10:1 ratio also inhibited lines of rolling leukocytes. Leukocytes were fluorescently labeled with rhodamine-6G so that leukocyte–leukocyte interactions could be studied in whole blood. A small number of secondary tethers (less than 20%) occurred and could be reduced by more than 80% with an L-selectin antibody. However, the overall impact on leukocyte recruitment was negligible. Similar experiments were performed using murine whole blood or isolated murine leukocytes. In the absence of red blood cells, murine leukocytes also formed lines of rolling leukocytes on E-selectin, and secondary tethers accounted for 50% of total interactions. However, when murine blood (diluted 1:5 with buffer) was perfused over E-selectin, secondary tethers accounted for only 13% of total interactions. These interactions were completely absent when blood was used from L-selectin–deficient mice. These data demonstrate for the first time that the importance of L-selectin–dependent leukocyte–leukocyte interactions is greatly reduced in whole blood and does not enhance overall recruitment of leukocytes in this physiologic milieu.
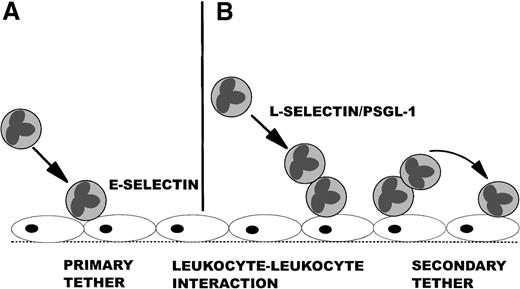
The recruitment of leukocytes from the mainstream of blood is a critical event in response to injury or foreign pathogen invasion. This process of leukocyte recruitment is a multistep cascade that involves leukocyte tethering followed by rolling, firm adhesion, and, finally, emigration from the vasculature. The selectins (P, E, and L) have been implicated as key mediators of the initial tethering and rolling events. P- and E-selectin are expressed by the endothelium and are thought to directly tether leukocytes.1-3 This interaction is known as primary tethering (Figure1A). By contrast, L-selectin and 1 of its ligands, P-selectin glycoprotein ligand-1 (PSGL-1), are found exclusively on leukocytes and can mediate leukocyte–leukocyte interactions.4-7 This event has been postulated to allow leukocytes rolling on endothelial monolayers, purified P-selectin, or purified E-selectin to capture or tether free-flowing leukocytes to the substratum. This event is termed secondary tethering (Figure 1B) and results in the formation of lines of leukocytes accumulating in the direction of flow.7,8 The importance of this phenomenon is that secondary tethers have been reported to account for 60% to 70% of recruitment of isolated neutrophil preparations in vitro.7 8
An overview of primary and secondary tethers.
The left side shows a primary tether of a leukocyte as it moves from the mainstream of blood and binds to E-selectin expressed on the endothelium (A), whereas the right side shows a leukocyte tethering to a rolling leukocyte through L-selectin and PSGL-1 (B), which captures the free-flowing leukocyte and causes it to interact with E-selectin on the endothelium (secondary tether).
An overview of primary and secondary tethers.
The left side shows a primary tether of a leukocyte as it moves from the mainstream of blood and binds to E-selectin expressed on the endothelium (A), whereas the right side shows a leukocyte tethering to a rolling leukocyte through L-selectin and PSGL-1 (B), which captures the free-flowing leukocyte and causes it to interact with E-selectin on the endothelium (secondary tether).
In vivo, the importance of secondary tethers is more questionable. For example, Kunkel et al9 proposed that leukocyte–leukocyte interactions account for only 1.2% of all recruited leukocytes in mouse cremaster stimulated with tumor necrosis factor (TNF)-α. In that study, a secondary tether was defined as leukocytes that experience a major velocity change within 1 cell diameter of an already adherent leukocyte. In contrast to the line formation observed in vitro, Kunkel et al9 found that the leukocytes adhered in clusters. This cluster formation was also observed in L-selectin–deficient animals, suggesting that L-selectin may not be involved in leukocyte recruitment in this model.9 These data raised serious questions about the importance of leukocyte–leukocyte interactions enhancing leukocyte recruitment in vivo. However, there are numerous critical differences that potentially may explain the disparity between the aforementioned in vitro and in vivo studies. Because there are 1000 red blood cells surrounding each leukocyte, the likelihood of 1 leukocyte interacting with another leukocyte may be less likely in vivo than with isolated cells. A second critical difference is the use of human versus mouse models; there may be crucial species differences for L-selectin function in these 2 species. Indeed, Zollner et al10 demonstrated that human, not mouse, L-selectin can bind E-selectin. Whether similar differences exist for L-selectin/PSGL-1 interactions remains unknown.
Using a standard in vitro laminar flow chamber system, we systematically examined whether L-selectin–dependent leukocyte–leukocyte interactions occurred in human whole or diluted blood, whether this was an important mechanism of leukocyte recruitment in blood, whether the role of red blood cells had anything to do with respect to leukocyte–leukocyte interactions, and whether mouse leukocytes behaved similarly to human leukocytes.
Materials and methods
Reagents and antibodies
E-selectin was bought from R&D Systems (Minneapolis, MN). Bovine serum albumin and rhodamine-6G were obtained from Sigma Chemical (St. Louis, MO). Heparin (Hepalean) from porcine intestinal mucosa was from Organon Teknika (Toronto, ON, Canada). Hanks' balanced salt solution (HBSS) containing Ca++ and Mg2+ was obtained from Gibco BRL (Grand Island, NY). Blocking L-selectin antibody (Dreg-56) was a kind gift of Dr M. Jutila (Montana State University, Bozeman, MT).
Preparation of coverslips
Glass coverslips (Fisher Scientific, Ottawa, ON, Canada) were coated with 5 μg/mL soluble human E-selectin (R&D Systems) and incubated overnight at 4°C. To inhibit nonspecific interactions with glass, the coverslips were then incubated with 1% bovine serum albumin for 2 hours at 37°C. All leukocyte interactions could be inhibited with an E-selectin antibody in this system.11
Human blood cells for perfusion
Human neutrophils for perfusion over the protein-coated coverslips were isolated from acetate–citrate–dextrose anticoagulated blood by dextran sedimentation and density centrifugation on Ficoll–Hypaque.12 The neutrophils were resuspended in HBSS at a concentration of 2 × 105/mL. Whole blood for perfusion over protein-coated coverslips was collected from healthy donors and heparinized (30 U/mL) to prevent clotting. In whole blood, the total number of neutrophils ranged from 1.5 to 6.0 × 106/mL. To ensure that the larger neutrophil concentration present in whole blood was not a factor, we diluted whole blood 5-fold and 10-fold with HBSS. The dilution of whole blood reduced viscosity without altering the red blood cell-to-leukocyte ratio.
Experimental protocol using human neutrophils
To study leukocyte interactions with selectins under shear conditions, a flow chamber assay was established as previously described.13 E-selectin coverslips were mounted into a polycarbonate chamber with parallel-plate geometry. The flow chamber was placed onto the stage of an inverted microscope (Zeiss, Canada), and coverslips were visualized at 100 × magnification using phase-contrast imagery. This yielded fields of view that measures 0.45 mm2. The stage area was enclosed in a warm air cabinet and maintained at 37°C. Blood or neutrophil suspensions were warmed to 37°C using a water bath. A syringe pump (Harvard Apparatus, Canada) was used to draw the cells through the flow chamber at an identical velocity for every experiment (2 mL/min). The relative viscosity of whole and diluted blood can be derived from the hematocrit,14 which allowed for calculations of relative shear forces within the flow chamber according to the formula R = (6μQ)/(B2W), in which μ is the relative viscosity of the blood, Q is the flow rate through the chamber, B is the chamber depth, and W is the chamber width. This has been used as the standard approach to calculate shear forces in these flow chambers by us11 and others.1 13
Isolated human neutrophils (2 × 105/mL), whole blood or blood diluted one fifth or one tenth with HBSS was perfused over the E-selectin–coated coverslip. The perfused blood was chased by HBSS to allow for visualization of leukocyte interactions as described.11 Five fields of view were recorded for each experiment and were played back for off-line analysis. The values for the 5 fields were averaged and treated as 1 value (n = 1). Each experiment was repeated a minimum of 4 times. In some experiments, the blood or neutrophil suspension was incubated at 37°C for 5 minutes before perfusion over the coverslip with the L-selectin antibody DREG-56 (3 μg/mL). Because heparin is known to bind to L-selectin15 and could possibly inhibit leukocyte–leukocyte interactions in the whole blood system, heparin (30, 3, or 0.3 U/mL) was added back to isolated neutrophils to determine whether this could have an impact on secondary tethers in the isolated neutrophil system. Finally, it is possible that plasma-derived proteins such as soluble L-selectin could inhibit L-selectin/PSGL-1 interactions. Therefore, in some experiments, neutrophils were resuspended in plasma and perfused over E-selectin.
Mouse blood for perfusion
Mice were anesthetized by intraperitoneal injection of a mixture of 10 mg/kg xylazine (MTC Pharmaceuticals, Cambridge, Ontario, Canada) and 200 mg/kg ketamine hydrochloride (Rogar/STB, London, Ontario, Canada). Blood was drawn from L-selectin–deficient mice (kind gift from Dr T. F. Tedder, Duke University Medical Center, Durham, NC) or from wild-type mice (C57Bl/6) by cardiac puncture into heparinized (30 U/mL) syringes. Because only a very small volume was obtained (less than 1 mL), we diluted the blood 5-fold with HBSS.
Experimental protocol using murine blood and isolated leukocytes
Diluted mouse blood (one fifth) labeled with rhodamine-6G (as described for human blood) was drawn over human E-selectin–coated coverslips for 2 minutes. To test for the effect of L-selectin, mice deficient in L-selectin were used. In a final series of experiments, red blood cells were removed from mouse leukocytes by lysis with distilled water resuspended in HBSS, and the white cells were perfused over E-selectin.
Experiments were recorded on a video cassette recorder (Panasonic, Secaucus, NJ) by a charge-coupled device (CCD) camera (Hatachi, Denshi, Japan) for off-line analysis. In some experiments, the leukocytes in human whole blood or diluted mouse blood were labeled with rhodamine-6G (0.1 mg/mL) perfused over E-selectin and visualized at 200 × magnification for 2 minutes using a low-light– sensitive CCD camera (Hamamatsu, Japan) and on an inverted microscope (Zeiss, Dan Mills, ON) with an AttoArc light source for epifluorescence. Total number of interacting cells was measured for each field of view and then averaged for each coverslip. Primary tethers were defined as flowing leukocytes that tethered directly to the selectin substrate, whereas secondary tethers were defined as leukocytes that tethered first to an attached leukocyte before interacting with the substrate. Line formation was defined as 4 or more leukocytes aligning on the substrate in the direction of flow and not more than 2 leukocyte diameters apart.
Statistics
All data are presented as mean ± SEM. Student t test was used to compare between groups. Statistical significance was set atP < .05.
Results
Line formation with isolated neutrophils is L-selectin dependent
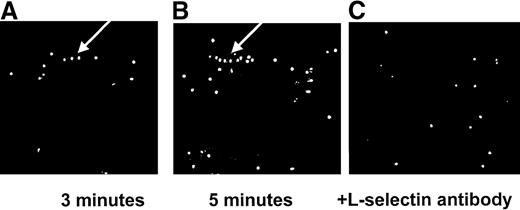
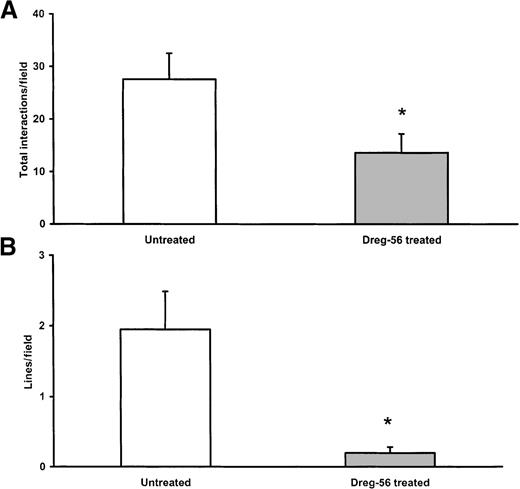
Figures 2A and 2B show the development of lines of neutrophils, a hallmark feature of secondary interactions, over the first 5 minutes on immobilized E-selectin. Neutrophils were seen to interact briefly with an already attached neutrophil (primary tether) and to form a secondary tether within 2 leukocyte diameters of the initial neutrophil. This process occurred repeatedly such that a line of rolling leukocytes formed. At 3 minutes, 3 neutrophils in a row can be clearly seen, and at 5 minutes, the number has more than doubled (arrow). It is now generally accepted that these secondary interactions are mediated by L-selectin. Indeed, Figure 2C illustrates 2 important points—the addition of the L-selectin antibody completely abolished the line of rolling neutrophils, and this impacted on the total number of neutrophils recruited. The cumulative data are summarized in Figure3. Approximately 30 neutrophils were seen per field of view, and L-selectin antibody reduced the total number of cells by approximately 50% to 60% (Figure 3A). On average, 2 very notable lines of rolling neutrophils were observed per field of view from isolated neutrophil experiments, and an L-selectin antibody essentially eliminated this pattern of neutrophil recruitment (Figure3B). These data illustrate that the majority of cells accumulated as a result of L-selectin and that these interactions are responsible for the neutrophil line formation. Not shown is the fact that the L-selectin antibody abolished secondary tethers in the isolated neutrophil system.
Interactions of isolated neutrophils with immobilized E-selectin.
Line formation is seen at 3 minutes (A). This line is increased in length by 5 minutes (B). The L-selectin antibody (C) eliminates this line formation. The arrow indicates line formation.
Interactions of isolated neutrophils with immobilized E-selectin.
Line formation is seen at 3 minutes (A). This line is increased in length by 5 minutes (B). The L-selectin antibody (C) eliminates this line formation. The arrow indicates line formation.
Role of L-selectin in isolated neutrophil recruitment.
Total interactions (A) and line formation (B) with isolated neutrophils (n = 4). The L-selectin antibody significantly reduced interactions and line formation. *P < .05 compared to untreated.
Role of L-selectin in isolated neutrophil recruitment.
Total interactions (A) and line formation (B) with isolated neutrophils (n = 4). The L-selectin antibody significantly reduced interactions and line formation. *P < .05 compared to untreated.
Lack of lines of rolling leukocytes with whole or diluted blood
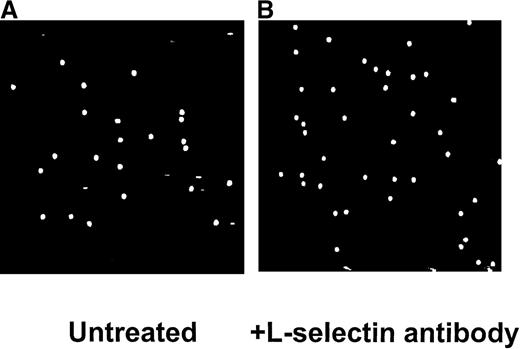
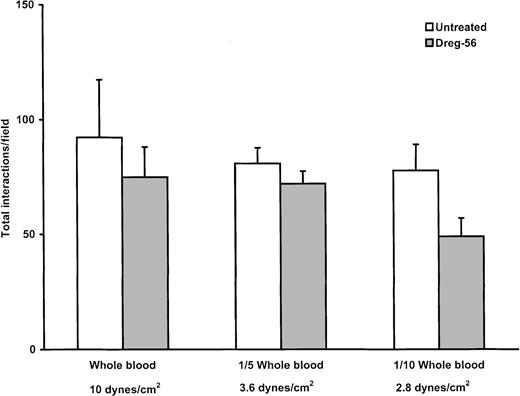
In contrast to isolated neutrophils, no lines of rolling leukocytes were noted in whole blood or in blood diluted one fifth or one tenth with buffer. Figure 4A illustrates the pattern of leukocyte recruitment after perfusion of untreated one-fifth diluted blood. Figure 4B demonstrates that in this system, the addition of L-selectin antibody did not affect the amount or pattern of leukocyte accumulation. The cumulative data are summarized in Figure5. After perfusion of whole blood or diluted blood (one fifth and one tenth), approximately 80 to 100 rolling leukocytes could be seen interacting with E-selectin per field of view. The addition of L-selectin antibody did not significantly reduce leukocyte recruitment in either whole blood or diluted blood. Because the same perfusion rate was used with whole blood and the 2 diluted blood samples, the relative shear force was reduced from approximately 10 dynes/cm2 to less than 3 dynes/cm2, suggesting that the importance of L-selectin did not change over this range of shear forces.
Interactions of one fifth diluted blood with immobilized E-selectin.
No lines were observed with untreated blood (A). Inhibition of L-selectin had no effect on the pattern of leukocyte interaction (B).
Interactions of one fifth diluted blood with immobilized E-selectin.
No lines were observed with untreated blood (A). Inhibition of L-selectin had no effect on the pattern of leukocyte interaction (B).
Total interactions with blood on immobilized E-selectin (n = 4 for each group).
The number of interactions was maintained with whole blood and blood diluted one fifth and one tenth, regardless of the presence or absence of L-selectin antibody.
Total interactions with blood on immobilized E-selectin (n = 4 for each group).
The number of interactions was maintained with whole blood and blood diluted one fifth and one tenth, regardless of the presence or absence of L-selectin antibody.
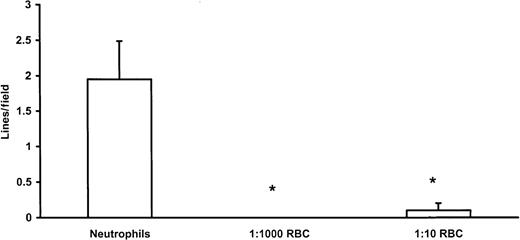
To determine whether the absence of lines of rolling leukocytes was predicated on the presence of red blood cells, we added back to isolated neutrophils various amounts of red blood cells from the same donor to achieve final concentrations of 1 neutrophil/1000 red blood cells (predicted physiologic concentration) or 1 neutrophil/10 red blood cells. Figure 6 reveals that the lines of rolling cells noted with isolated neutrophils were not apparent at the 1:1000 neutrophil-to-red blood cell ratio. Interestingly, even a ratio of 1:10 neutrophil-to-red blood cell was sufficient to reduce significantly the lines of rolling leukocytes.
Effect of red blood cell addition to line formation with neutrophils on immobilized E-selectin (n = 4).
Lines of neutrophils were noted with isolated neutrophils but were significantly reduced by the addition of red blood cells in the ratios of 1000 or 10 red blood cells for every neutrophil. *P < .05, compared to neutrophils alone.
Effect of red blood cell addition to line formation with neutrophils on immobilized E-selectin (n = 4).
Lines of neutrophils were noted with isolated neutrophils but were significantly reduced by the addition of red blood cells in the ratios of 1000 or 10 red blood cells for every neutrophil. *P < .05, compared to neutrophils alone.
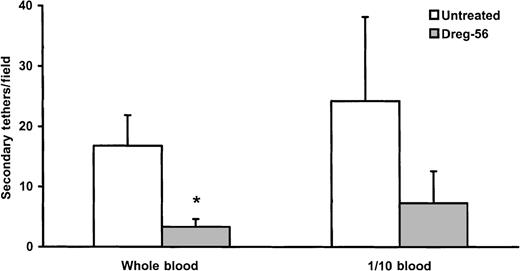
Rhodamine 6G treatment of whole blood permitted direct visualization of leukocyte–leukocyte interactions. Fewer than 20 secondary interactions (less than 25% of total interactions; primary plus secondary) could be seen during the perfusion period (Figure7). The addition of L-selectin antibody reduced the secondary interactions by approximately 75% to 80%. As already stated (Figure 5), this reduction in the leukocyte-assisted capture of flowing leukocytes with L-selectin antibody was of minor consequence because the total number of accumulated cells was not reduced by this treatment (Figure 5). Secondary tethers occurred with similar frequency in diluted blood (one tenth) as in whole blood, and the majority of these interactions were again inhibitable with L-selectin antibody (Figure 7).
Secondary interactions with whole blood and one-tenth blood (n = 4-17).
There were similar numbers of secondary tethers between the whole and one-tenth diluted blood. L-selectin antibody significantly reduced the number of secondary tethers in whole blood. *P < .05, compared to untreated.
Secondary interactions with whole blood and one-tenth blood (n = 4-17).
There were similar numbers of secondary tethers between the whole and one-tenth diluted blood. L-selectin antibody significantly reduced the number of secondary tethers in whole blood. *P < .05, compared to untreated.
Heparin and plasma do not affect line formation with neutrophils
In a final series of human leukocyte experiments, we ensured that heparin did not affect line formation because the whole blood had to be heparinized and this anticoagulant has been purported to inhibit L-selectin function.15 We added heparin in varying concentrations directly to isolated human neutrophils and noted that line formation still occurred, excluding heparin as the variable responsible for the lack of line formation in whole blood (Table1).
To ensure that endogenous proteins in plasma (eg, soluble L-selectin or soluble PSGL-1) were not altering the neutrophil-assisted capture of free-flowing neutrophils, isolated neutrophils were resuspended in either HBSS or plasma. The results revealed that the same number of secondary interactions were noted per field of view, leading to similar numbers of lines of rolling leukocytes (Table2).
Isolated murine leukocytes form secondary tethers
Lines of rolling leukocytes were clearly visible on E-selectin using isolated murine leukocytes (data not shown). Table3 summarizes that approximately 50% of total tethers were secondary, similar to what we reported with isolated human neutrophils. By contrast, primary tethers were the dominant interaction when mouse blood was used. Only 13% of the total interactions were secondary (Table 3). An absence of L-selectin (L-selectin–deficient mice) resulted in no secondary tethers (Table3). No lines were observed when diluted mouse blood from wild-type or L-selectin–deficient mice was perfused over E-selectin (data not shown).
Discussion
There is a large body of evidence demonstrating that the initial or primary tether of a leukocyte to endothelium and subsequent rolling is mediated by the selectin family of adhesion molecules, including P-selectin1,2,16 and E-selectin3 on endothelium and L-selectin17,18 on leukocytes. Laminar flow chambers have been instrumental in this regard; perfusion of isolated leukocytes over endothelium-expressing selectins or over immobilized selectins has clearly demonstrated the importance of selectins in this initial attachment.1,3 A consistent observation in these types of studies (regardless of substratum) is the formation of lines of rolling leukocytes. Close examination of this phenomenon revealed that free-flowing leukocytes frequently collided with a rolling leukocyte, were captured by the rolling cell, and were secondarily tethered to the endothelium or the immobilized selectin.7,8 This resulted in 2 rolling leukocytes (Figure 1B) that would in turn recruit additional free-flowing leukocytes to roll by the same process until a line of rolling leukocytes had formed. It was demonstrated that L-selectin and P-selectin glycoprotein ligand-1 (PSGL-1) were the 2 molecules responsible for this phenomenon.7,8 Secondary tethers forming lines of rolling leukocytes were potentially very important because they accounted for 60% to 70% of total neutrophil,8 eosinophil,19monocyte,20 or SKW3 T-cell8 recruitment in the laminar flow chambers.
In this study, we report that perfusion of human whole blood or diluted blood through the flow chamber revealed few secondary tethers and a lack of lines of rolling leukocytes. The reintroduction of red blood cells (but not of plasma or heparin) to isolated neutrophils was sufficient to disrupt and prevent secondary tethers from occurring and thereby prevented the characteristic lines of rolling leukocytes. A number of key differences may explain the striking discrepancy in behavior between isolated neutrophils versus whole blood. The viscosity of whole blood is clearly much higher than the viscosity of isolated neutrophils. This elevated viscosity would increase the shear stress imposed on the leukocytes and could potentially disrupt or prevent secondary tethers from successfully forming. Indeed, increasing the viscosity of the perfusate with the addition of red blood cells to isolated neutrophils eliminated secondary tethers. However, when whole blood was diluted with buffer one fifth or one tenth, thereby reducing the viscosity and the shear stress by more than 75% (Figure 4) without altering the leukocyte-to-red blood cell ratio, no increase in secondary tethers or lines of rolling leukocytes was observed. These data suggest that across a reasonably broad range of viscosity and shear forces, secondary tethers do not contribute to overall leukocyte recruitment in blood. Although we cannot exclude the possibility that the viscosity associated with isolated neutrophils in buffer is required to allow for secondary tethers, this does not diminish the observation that in the physiologic setting secondary tethers are less likely to occur.
The presence of red blood cells may be responsible for the less significant role of secondary tethers for leukocyte recruitment from whole blood. There are approximately 1000 red blood cells for each leukocyte in whole blood, and it is possible that with such large numbers of erythrocytes surrounding each leukocyte, red blood cells could physically interfere with leukocyte–leukocyte interactions. Indeed, when red blood cells were added back to isolated neutrophils in the physiologic ratio of 1000 erythrocytes to 1 leukocyte, line formation was abolished. The addition of 10 red blood cells for every leukocyte also interfered with line formation, albeit not to the same extent. As mentioned previously, the reintroduction of red blood cells would also increase the viscosity, which could have a direct impact on the ability of leukocytes to interact with other leukocytes. However, simply diluting the whole blood with buffer, but maintaining the red blood cell-to-leukocyte ratio at the present ratio in whole blood, did not increase the importance of secondary tethers supporting the view that the very large number of red blood cells for each leukocyte may decrease the likelihood of leukocyte–leukocyte interactions in whole blood. Another factor that may contribute to the reduction in secondary tethers in the physiologic setting may be soluble L-selectin and P-selectin that could bind PSGL-1 and prevent leukocyte–leukocyte interactions. This seems a less likely possibility because isolated neutrophils reconstituted in plasma had at least as many secondary tethers and lines of rolling neutrophils as neutrophils perfused in buffer.
In this study, we have amalgamated the beneficial aspects of an in vitro laminar flow chamber, which permits excellent visualization of leukocyte–leukocyte interactions using human leukocytes, with the in vivo attributes of leukocyte–leukocyte interactions in whole blood. Nevertheless, this still is not the in vivo situation wherein the postcapillary venules are narrower and have more variable geometry. Nevertheless, our data are in line with the only study to our knowledge to address the importance of secondary interactions in vivo.9 Kunkel et al9 examined secondary tethers in an autoperfused murine cremaster preparation stimulated with TNF-α, and they defined a secondary tether as a leukocyte that experienced a reduction in velocity within 1 cell diameter of an already adherent leukocyte. Although this might actually overestimate secondary tethers, the authors still concluded that leukocyte–leukocyte interactions could only account for 1.2% of all recruited leukocytes in this inflammatory situation. One could argue that with high concentrations of TNF-α administration, L-selectin was shed, thereby eliminating L-selectin–dependent secondary tethers. However, in our system in which inflammatory mediators were not introduced, our data agree with the work of Kunkel et al9in concluding that secondary tethers play a minor role in leukocyte recruitment in whole blood. Finally, the fact that L-selectin antibody or L-selectin deficiency is inhibitory under only some but not all in vivo conditions21 22 is consistent with the induction of a vascular L-selectin ligand under those specific conditions. If L-selectin antibody inhibited leukocyte–leukocyte interactions, one would predict that it would be an effective inhibitor under all conditions (as L-selectin/PSGL-1 are constitutively expressed).
Our own study suggested that human leukocyte–leukocyte secondary tethers are more frequent (approximately 20%) than the 1.2% murine leukocyte–leukocyte secondary tethers reported by Kunkel et al.9 Although there may be different affinities between PSGL-1 and L-selectin in human versus mouse systems, we were able to observe that secondary tethers were similar (50%) when either isolated murine or human leukocytes were perfused through the laminar flow chamber. However, when murine blood was perfused through the laminar flow chamber, secondary tethers were decreased to approximately 13% (in line with the 20% seen in human blood) and did not occur when blood from L-selectin– deficient mice was used. Therefore, it appears that the secondary interactions are similar in frequency within the human and mouse systems. However, this study does not completely exclude a role for L-selectin/PSGL-1–dependent leukocyte–leukocyte interactions potentially playing a role in leukocyte aggregation in places such as capillaries, which could have an impact on downstream rolling events. Indeed, it remains unclear why in a number of animal models L-selectin antibody reduces P-selectin–dependent rolling by approximately 60%, despite the fact that L-selectin is not thought to be a ligand for P-selectin.23-25
In conclusion, our results suggest that the secondary tethers observed in isolated leukocyte populations, which account for a significant amount of leukocyte recruitment in vitro, play a less dominant role in human whole blood. Our data also suggest that red blood cells may interfere with or impede the number of leukocyte–leukocyte interactions that occur. This may be the result of a simple physical barrier function of the red blood cell decreasing leukocyte–leukocyte interactions. Alternatively, other physical properties, such as the increased hydrodynamic dispersal forces imparted on a leukocyte by red blood cells as it briefly tethers to a rolling leukocyte, may prevent subsequent tethering to the endothelium. It is now well established that red blood cells facilitate selectin-dependent leukocyte rolling on endothelium.26 27 This event is thought to be caused by the erythrocytes increasing the lateral displacement of leukocytes toward the endothelial surface. The decrease in leukocyte secondary tethers in the presence of red blood cells further underscores the importance of erythrocytes in leukocyte–endothelium interactions.
Supported by a grant from the Medical Research Council. P.K. is a Medical Research Council Scientist and an Alberta Heritage Foundation for Medical Research Senior Scholar. D.M. is a fellow with the Alberta Heritage Foundation for Medical Research.
Reprints:Paul Kubes, Immunology Research Group, Department of Medical Physiology, Faculty of Medicine, University of Calgary, Calgary, Alberta, T2N 4N1, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.