Abstract
CD4 molecules serve as coreceptors for the T-cell receptor (TCR)/CD3 complex that are engaged coordinately with TCR and facilitate antigen-specific T-cell activation leading to interleukin 2 (IL-2) production and proliferation. However, cross-ligation of CD4 molecules prior to TCR stimulation has been shown to prime CD4 T cells to undergo apoptosis. Although in vivo and in vitro experiments have implicated the involvement of Fas/FasL interaction in this CD4 cross-linking (CD4XL)-induced apoptosis, detailed mechanisms to account for cell death induction have not been elucidated. In the present study, we demonstrate that CD4XL in purified T cells not only led to Fas up-regulation but also primed CD4 T cells to express FasL upon CD3 stimulation and rendered the T cells susceptible to Fas-mediated apoptosis. Notably, in addition to CD4+ T cells, CD4XL-induced sensitization for apoptosis was observed in CD8+ T cells as well and was associated with Bcl-x down-modulation. Both CD4 and CD8 T-cell subsets underwent apoptosis following cell–cell contact with FasL+ CD4 T cells. CD28 costimulation abrogated CD4XL/CD3-induced apoptosis with restoration of IL-2 production and prevented Bcl-x down-modulation. As CD4 molecules are the primary receptors for human immunodeficiency virus 1 (HIV-1), we conclude that HIV-1 envelope mediated CD4XL can lead to the generation of FasL-expressing CD4+ T cells that can lead to apoptosis of CD4 as well as CD8 T cells. These findings implicate a novel mechanism for CD8 T-cell depletion in HIV disease.
The current consensus of human immunodeficiency virus (HIV)-related cytopathicity in vivo is that CD4 T cells are depleted by two ways, one way is by direct cytopathicity and the other is by indirect killing of uninfected cells via mechanisms involving immune reactivity. The target cell population for the latter mechanism is not confined to the CD4+ T cells, as CD8+ T cells are also affected. Although the total number of circulating CD8 T cells are not significantly decreased, changes from CD8+CD28+ to CD8+CD28− phenotype,1accelerated apoptosis,2,3 and increased turnover with shorted telomere length4 have been reported for CD8 T cells during the course of HIV infection because of persistent viral burden. Repeated antigenic stimulation resulting in activation-induced cell death via apoptosis may account for the changes detected in the CD8 T-cell population. The expansion of particular viral antigen-specific cytotoxic T-lymphocyte (CTL) clones followed by depletion has been demonstrated in CD8 T cells from HIV-infected patients.5 Moreover, it has recently been shown that the adoptive transfer of ex vivo expanded HIV-specific CD8 CTLs resulted in rapid cell death in situ despite successful homing to the site of infection.6 It is thus likely that as yet unidentified mechanisms that lead to CD8 T-cell death may be operative at the site of HIV infection, especially in the peripheral lymphoid organs.
Contrary to the direct cytopathicity of HIV that was shown to result in cell death in HIV-infected Fas-defective T cells,7accumulating lines of evidence support the view that potential receptor/ligand interactions involved in the delivery of the death signal to uninfected cells could include Fas/FasL or others, such as tumor necrosis factor receptor (TNFR)/tumor necrosis factor-α (TNFα), and DR4, DR5/TRAIL.8-11 We as well as others12-19 have postulated that CD4 signaling as a result of HIV gp120 interaction with CD4 contributes to CD4 T-cell apoptosis via induction of these death-mediating molecules. It has been demonstrated that CD4 cross-linking (CD4XL) in murine12 or human13 CD4 T cells followed by T-cell receptor (TCR) stimulation results in apoptotic cell death. We have previously shown that CD4XL, if performed in the presence of monocytes/macrophages, is sufficient to induce CD4+ T-cell apoptosis without TCR stimulation.14 Subsequent studies have revealed the involvement of Fas/FasL interaction in this CD4XL-induced T-cell apoptosis. In the former system, both in vivo and in vitro studies have revealed that CD4XL-mediated T-cell apoptosis does not occur in Fas-defective T cells,15,16 and in the latter system, CD4XL in peripheral blood mononuclear cells (PBMCs) resulted in Fas expression in T cells17 and FasL expression in monocytes, resulting in monocyte-mediated Fas/FasL interaction-dependent CD4 T-cell apoptosis.18 In support of this observation, HIV-infected monocytes have been shown to express FasL and to engage in Fas-mediated CD4 T-cell killing.20 However, the fate of CD8 T cells in purified T cells that are activated by TCR/CD3 stimulation after CD4XL has not been examined. In the present study, we have addressed this point and report here that CD4XL primes both subsets of T cells for apoptosis following CD3 stimulation by generating FasL-expressing CD4+ T lymphocytes that, in turn, induce accelerated apoptosis of CD4 and CD8 T cells upon cell–cell contact.
Materials and methods
Cells and culture conditions
PBMCs from HIV-seronegative healthy persons were isolated by Ficoll-Hypaque density gradient centrifugation (Accurate Chemicals, Westbury, NY). T cells were separated from PBMCs by Human T Cell Enrichment Columns (R&D Systems, Minneapolis, MN), using high affinity negative selection. Resulting cells were > 95% CD3+ and contained < 1% of CD19+ or CD14+ cells by immunofluorescence. In some experiments, CD8+ and CD8− T cells were purified by incubation of peripheral blood T cells (PBTs) with magnetic beads (Dynal, Oslo, Norway). The CD8− T cells were designated as CD4 T cells in these studies. RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies), 2 mmol/L l-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin was used for all cultures.
Reagents and monoclonal antibodies
Reagents and sources were as follows: monoclonal antibody (mAb) to CD3 (clone HIT3a, Pharmingen, San Diego, CA), to CD4 (clone RPA-T4, Pharmingen), to CD28 (clone CD28.2, Pharmingen); goat antimouse immunoglobulin (GAM; Jackson ImmunoResearch Laboratory, West Grove, PA); and mouse immunoglobulin from nonimmune animals (mIg; Jackson ImmunoResearch Laboratory). The soluble Fas-immunoglobulin G (IgG) chimeric protein (sFas) was prepared as described,21utilizing plasmids containing a fusion gene of human Fas extracellular domain (nucleotides 165-713) with human IgG1 fragment (hFas-hIgG-pcDNA I, a gift from Dr J. D. Mountz, University of Alabama at Birmingham, Birmingham, AL). The mAb to human FasL, 4H9 IgG1, was kindly provided by MBL Ltd, Nagoya, Japan; mAbs to human interferon-γ (IFNγ) and TNFα were a gift from Dr Y. Ohmoto (Otsuka Pharmaceutical, Tokushima, Japan).
Priming and induction of apoptosis
T cells were isolated and CD4XL was performed as described previously.14 In brief, cells were treated with anti-CD4 mAb at concentrations of 10 μg/1 × 106 cells for 40 minutes at 4°C. Cells were then cultured in round-bottom 12 × 75 mm polystyrene tubes (Falcon 2054, Becton Dickinson Lab Ware, Bedford, MA) coated with GAM immunoglobulin for 40 minutes, after which time cells were transferred to noncoated tubes. Nonimmune mIg-treated cells were used as controls for anti-CD4 mAb treatment. Anti-CD3 mAb (0.1 μg/mL) with or without anti-CD28 mAb (1.0 μg/mL) was added at 40 minutes or at 24 hours after CD4XL, and cells were incubated for an additional 18 hours. In some experiments, the CD8 T cells were separated from the CD4XL-treated CD4 T cells by a polycarbonate transwell culture membrane insert of 6.5-mm diameter and 3.0-μm pore size (Costar, Cambridge, MA); CD4 T cells were placed in the bottom chamber and CD8 T cells were placed in the upper chamber. We confirmed that these inserts did not affect the viability of either subset.
Measurement of apoptosis
To analyze cell surface phenotype in conjunction with apoptosis, we used the annexin V binding method, which detects phosphatidylserine that is preferentially expressed on the surface of apoptotic cells,22 utilizing a commercially available kit (Apoptosis Detection Kit, R&D Systems) according to the manufacturer's protocol. Surface labeling for the CD8 molecule was performed simultaneously with annexin V binding. In selected experiments, the presence of fragmented nuclei was also confirmed by a propidium iodide staining technique as described.23
Determination of lymphocyte surface and cytoplasmic antigen expression
Lymphocyte surface markers were identified by triple-color immunofluorescence with the use of various combinations of mAbs. The mAbs were conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or peridinin chlorophyll A (PerCP) as indicated: PE-CD4 (IgG1), PerCP- or PE-CD8 (IgG2a), FITC-CD14 (IgG2b), FITC- or PE-CD19 (IgG1), and PerCP-CD45 (IgG1); all mAbs and relevant isotype-matched control mAbs were purchased from Becton Dickinson (San Jose, CA). FITC-Fas (clone UB2, IgG1) was obtained from Immunotech (Westbrook, ME). Surface labeling was performed at room temperature for 15 minutes in cells suspended in staining buffer (HBSS containing 1% FCS and 0.1% sodium azide). For intracytoplasmic measurement of Bcl-2, Bcl-x, and cytokines IFNγ, TNFα, or interleukin 2 (IL-2)], cells were fixed and permeabilized for 40 minutes with ORTHO PermeaFIX (Johnson & Johnson, Raritan, NJ) following surface labeling. After permeabilization, cells were incubated for 20 minutes on ice with FITC-labeled antihuman Bcl-2 (IgG1, DAKO, Carpinteria, CA) diluted 1:50 in HBSS, which was predetermined to be the optimal concentration. Intracytoplasmic labeling of Bcl-x was performed with the use of polyclonal rabbit anti–Bcl-x S/L antibody (1:5 dilution in HBSS, 20 minutes on ice; Santa Cruz Biotechnology, Santa Cruz, CA). After washing twice with HBSS, cells were incubated with cyanine 5-conjugated antirabbit F(ab′)2 antibody (1:50 dilution in HBSS, 20 minutes on ice; Jackson ImmunoResearch). For intracytoplasmic cytokine detection, cells were incubated with 10 μg/mL Brefeldin A (Sigma Chemical, St. Louis, MO), and harvested cells were treated for 20 minutes at 4°C with PE-IFNγ (IgG1), PE-TNFα (IgG1), or PE-IL-2 (IgG2a); all mAbs and relevant isotype-matched control mAbs were purchased from Pharmingen. For selected experiments, we assessed surface binding of annexin V and anti-CD8 mAb simultaneously with intracytoplasmic cytokine detection. Cells were washed twice with HBSS, resuspended in 1% paraformaldehyde HBSS, and analyzed by flow cytometry with the use of the Epics Elite instrument (Coulter, Hialeah, FL).
CD95L messenger RNA determination by reverse transcriptase PCR
For the quantitation of CD95L messenger RNA (mRNA), PCR-assisted mRNA amplification (RT-PCR) was performed as described in detail elsewhere.24 In brief, total cellular RNA was extracted from 2 × 106 cells with Ultraspec RNAzol (Biotecx Laboratories, Houston, TX) according to the manufacturer's protocol. Reverse transcription of RNA to complementary DNA (cDNA) was performed, according to the protocol of the Gene-Amp RNA PCR kit (Perkin Elmer Cetus, Norwalk, CT). CD95L-specific primer pairs and β-actin were purchased from Research Genetics (Huntsville, AL). Primer sequences for CD95L 5′ sense and 3′ antisense were CTGGTGGCTCTGGTTGGAAT and GTTTAGGGGCTGGTTGTTGC, respectively [size of amplified fragments, 506 base pairs (bp)]. PCR was performed in a GeneAmp PCR system 9600 (Perkin Elmer Cetus) for 30 cycles of 1 minute of denaturation at 94°C, 1.5 minutes of annealing at 55°C, and 1.5 minutes of extension at 72°C. A final extension period of 10 minutes followed the 30 cycles. β-Actin was used as an internal control in all reactions. The reaction products were visualized by subjecting them to electrophoresis in 1% agarose in 1 × TBE buffer containing 0.5 μg/mL ethidium bromide.
Statistical analysis
Statistical significance was assessed by Student t test and considered significant at P < .05.
Results
CD4XL in purified T cells followed by anti-CD3 stimulation induces not only CD4 but also CD8 T-cell apoptosis
To examine whether CD4XL affects apoptosis induction in response to CD3 stimulation and to exclude the role of monocytes/macrophages, we prepared purified T cells and then performed cellular stimulation in this population. Following CD4XL, timing of the addition of anti-CD3 mAb was examined in preliminary experiments and the effects of CD28 costimulation were also evaluated. To assess apoptosis induction in each T-lymphocyte subset separately, we employed 2-color immunofluorescence analysis with PerCP-labeled anti-CD8 mAb subsequent to FITC-annexin V staining and evaluated annexin V-bound apoptotic cells within CD4 T cells (CD8− population) as well as in CD8+population.
As summarized in Table 1, we could successfully demonstrate significant T-cell apoptosis induction only under conditions that anti-CD3 was added 24 hours after CD4XL (hereafter referred to simply as [CD4XL/CD3]). Notably, we observed for the first time that CD8 T-cell apoptosis was also induced. We confirmed our previous finding that CD4XL alone conducted in purified T cells (ie, in the absence of monocytes/macrophages) by treatment with Leu3a/GAM was not sufficient to induce apoptosis. Anti-CD3 stimulation alone also did not induce T-cell apoptosis. However, addition of anti-CD3 mAb 24 hours after CD4XL was found to induce significant T-cell apoptosis in both subsets. In addition to annexin V-binding analysis, we confirmed the apoptosis by determining the emergence of sub-G0/G1 fragmented nuclei by propidium iodide staining (data not shown). Timing of CD3 stimulation appeared to be critical because we did not observe significant T-cell apoptosis if it was added 40 minutes after CD4XL. Further, we found that additional CD28 stimulation in conjunction with CD3 stimulation reversed the [CD4XL/CD3]-induced T-cell apoptosis.
[CD4XL/CD3]-induced lymphocyte apoptosis is blocked by soluble Fas or by anti-FasL mAb
To examine the involvement of Fas/FasL in [CD4XL/CD3]-induced T-cell apoptosis, we evaluated the effect of adding soluble Fas (sFas) or anti-FasL mAb 4H9 in interfering with Fas/FasL interaction. As shown in Figure 1, addition of sFas resulted in a dose-dependent inhibition of the [CD4XL/CD3]-induced apoptosis. Addition of anti-FasL mAb 4H9, which has previously been shown to block activation-induced apoptosis in T cells,25 was also found to block apoptosis in a dose-dependent manner. These results indicate that CD95/CD95L interaction is required for the induction of [CD4XL/CD3]-induced T-cell apoptosis. However, neither sFas nor 4H9 blocked apoptosis completely, implicating the operation of other apoptosis-inducing molecules in addition to Fas/FasL.
[CD4XL/CD3]-induced lymphocyte apoptosis is blocked by soluble Fas or by anti-FasL mAb.
Soluble Fas (sFas: closed circles) or 4H9 (closed squares) was added at the indicated concentrations (μg/mL) to the cultures of CD4 cross-linked T cells plus anti-CD3 mAb ([CD4XL/CD3]: open square), and apoptosis was evaluated by annexin V binding. Apoptotic populations of CD4XL alone (open circle) and/or anti-CD3/CD28 mAbs (open triangle) are also indicated. Data represent mean ± SD of 3 experiments. *Significantly (P < .001) different from CD4XL/CD3 samples.
[CD4XL/CD3]-induced lymphocyte apoptosis is blocked by soluble Fas or by anti-FasL mAb.
Soluble Fas (sFas: closed circles) or 4H9 (closed squares) was added at the indicated concentrations (μg/mL) to the cultures of CD4 cross-linked T cells plus anti-CD3 mAb ([CD4XL/CD3]: open square), and apoptosis was evaluated by annexin V binding. Apoptotic populations of CD4XL alone (open circle) and/or anti-CD3/CD28 mAbs (open triangle) are also indicated. Data represent mean ± SD of 3 experiments. *Significantly (P < .001) different from CD4XL/CD3 samples.
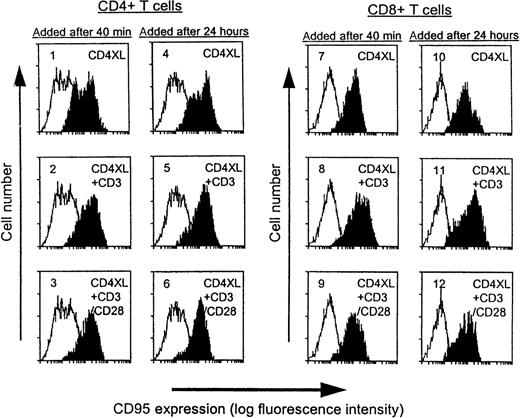
CD4XL alone is sufficient to up-regulate CD95 expression in both CD4 T cells and CD8 T cells
As shown in Figure 2, basal levels of CD95 expression were unchanged in medium-treated controls during the culture period (unshaded histograms), whereas CD4XL treatment resulted in marked Fas up-regulation in both subsets. We examined whether additional CD3 stimulation influenced Fas expression and observed that Fas expression was increased in both subsets by CD3 stimulation, regardless of the timing of addition (panels 2, 5, 8, and 7). CD28 costimulation did not further affect the levels of Fas expression induced by CD4XL plus CD3 stimulation. Collectively, CD4XL was sufficient to up-regulate Fas expression, and the ability of CD28 stimulation to block apoptosis could not be ascribed to regulation of levels of Fas expression.
CD4XL alone is sufficient to up-regulate CD95 expression in both CD4 T cells and CD8 T cells.
PBTs were pretreated with CD4XL at 37°C for 40 minutes or for 24 hours. Thereafter, anti-CD3 mAb was added with or without anti-CD28 mAb and cells were cultured for 18 hours. CD95 expression was analyzed by flow cytometry. Histograms obtained from the indicated conditions are shaded, and histograms from time-matched medium control (unshaded) are also indicated in panels 1 through 12. Data represent 1 of 3 experiments.
CD4XL alone is sufficient to up-regulate CD95 expression in both CD4 T cells and CD8 T cells.
PBTs were pretreated with CD4XL at 37°C for 40 minutes or for 24 hours. Thereafter, anti-CD3 mAb was added with or without anti-CD28 mAb and cells were cultured for 18 hours. CD95 expression was analyzed by flow cytometry. Histograms obtained from the indicated conditions are shaded, and histograms from time-matched medium control (unshaded) are also indicated in panels 1 through 12. Data represent 1 of 3 experiments.
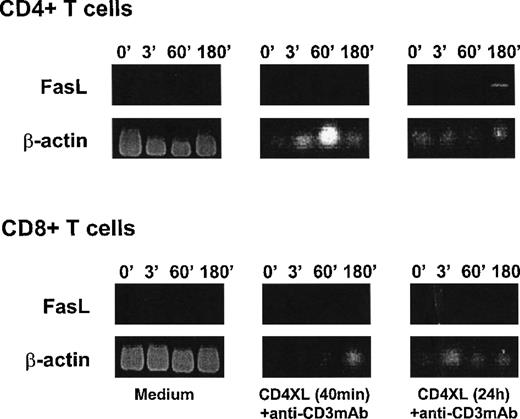
CD3 stimulation induces CD95L mRNA in CD4 T cells after priming with CD4XL
To explore CD95L expression in response to CD3 stimulation, we examined CD95L mRNA expression in purified CD4 T cells and CD8 T cells by RT-PCR. As shown in Figure3, CD3 stimulation 40 minutes after CD4XL did not induce mRNA for CD95L in either CD4 or CD8 T cells in the culture period examined. However, addition of anti-CD3 mAb 24 hours after CD4XL resulted in expression of CD95L mRNA only in CD4 T cells but not in CD8 T cells. These results indicate that CD4XL primes CD4 T cells to express FasL in response to anti-CD3 mAb stimulation. Because the functional interference of CD95/CD95L interaction by addition of sFas or by anti-FasL mAb abrogated [CD4XL-CD3]-induced T-cell apoptosis (Figure 1), this finding also supports the notion that CD4-cross-linked/CD3-stimulated CD4 T cells might gain cytotoxic function by expressing FasL and engage CD95 to induce apoptosis in CD4 and CD8 T cells in this culture system.
CD3 stimulation induces CD95L mRNA in CD4 T cells after priming with CD4XL.
Purified CD4 T cells (top) or CD8 T cells (bottom) were either untreated (medium: left panels) or CD4 cross-linked for 40 minutes (middle panel) or for 24 hours (right panel) and then stimulated with anti-CD3 mAb. Cells were harvested at the indicated times (in minutes) after addition of anti-CD3 mAb and examined for CD95L mRNA by RT-PCR. Figures show agarose gel electrophoresis stained with ethidium bromide for amplified fragments for CD95L and β-actin. Data represent 1 of 2 experiments.
CD3 stimulation induces CD95L mRNA in CD4 T cells after priming with CD4XL.
Purified CD4 T cells (top) or CD8 T cells (bottom) were either untreated (medium: left panels) or CD4 cross-linked for 40 minutes (middle panel) or for 24 hours (right panel) and then stimulated with anti-CD3 mAb. Cells were harvested at the indicated times (in minutes) after addition of anti-CD3 mAb and examined for CD95L mRNA by RT-PCR. Figures show agarose gel electrophoresis stained with ethidium bromide for amplified fragments for CD95L and β-actin. Data represent 1 of 2 experiments.
CD3 stimulation 24 hours after CD4XL down-modulates Bcl-x, but not Bcl-2, in both CD4 T cells and CD8 T cells, and CD28-costimulation prevents Bcl-x down-modulation
Contrary to T-cell lines, such as Jurkat T cells, PBTs are fairly resistant to apoptosis by agonistic anti-Fas antibody even though a substantial number of cells express Fas antigen. In general, a cell's fate of death versus survival can be determined by the balance between pro-apoptotic and anti-apoptotic signals. The Bcl-2 family comprises a number of proteins that play critical roles in the regulation of apoptotic cell death. In particular, both Bcl-2 and Bcl-xL have been shown to prevent apoptosis in response to a wide variety of stimuli.26 We thus examined intracytoplasmic Bcl-x or Bcl-2 protein expression in conjunction with cell-surface staining by flow cytometry. As shown in Figures 4A and 4B, we found that addition of anti-CD3 mAb 24 hours after CD4XL resulted in reduced Bcl-x expression in both CD4 T cells and CD8 T cells (panels 13 in Figure 4A, 4B). Of note, this reduction of Bcl-x expression was not observed if anti-CD3 mAb was added 40 minutes after CD4XL (panels 12 in Figure 4A, 4B), implicating that a certain period is required following CD4XL for the cells to become susceptible to CD3-induced Bcl-x down-modulation. Further, this [CD4XL/CD3]-induced Bcl-x down-modulation was prevented by CD28 costimulation in both subpopulations (panels 15, Figure 4A, 4B). Contrary to Bcl-x expression, the levels of Bcl-2 expression (Figure5) were not significantly affected in all conditions except for a slight reduction in mean fluorescence intensity (MFI) that was observed in CD8 T cells in which anti-CD3 mAb was added 24 hours after CD4XL (Figure 5B, panel 13), but this expression was not reversed by CD28 costimulation (panel 15).
CD3 stimulation of PBTs 24 hours after CD4XL down-modulates Bcl-x in both CD4 T cells and CD8 T cells, and CD28 costimulation prevents Bcl-x down-modulation.
[CD4XL/CD3] PBT cell cultures are showing Bcl-x expression in CD4 T cells (A) and in CD8 T cells (B). Cell-culture conditions are indicated in each panel, and isotype controls are indicated in top panels. Cells were analyzed by flow cytometry for intracytoplasmic Bcl-x expression in conjunction with surface labeling with FITC-anti-CD8 mAb. Broken lines represent the lowermost boundary of cells cultured in medium alone and labeled with anti–Bcl-x Ab. Cells that emerged to the left of this line represent Bcl-x down-modulated cells, and their percentages are indicated in the figure.
CD3 stimulation of PBTs 24 hours after CD4XL down-modulates Bcl-x in both CD4 T cells and CD8 T cells, and CD28 costimulation prevents Bcl-x down-modulation.
[CD4XL/CD3] PBT cell cultures are showing Bcl-x expression in CD4 T cells (A) and in CD8 T cells (B). Cell-culture conditions are indicated in each panel, and isotype controls are indicated in top panels. Cells were analyzed by flow cytometry for intracytoplasmic Bcl-x expression in conjunction with surface labeling with FITC-anti-CD8 mAb. Broken lines represent the lowermost boundary of cells cultured in medium alone and labeled with anti–Bcl-x Ab. Cells that emerged to the left of this line represent Bcl-x down-modulated cells, and their percentages are indicated in the figure.
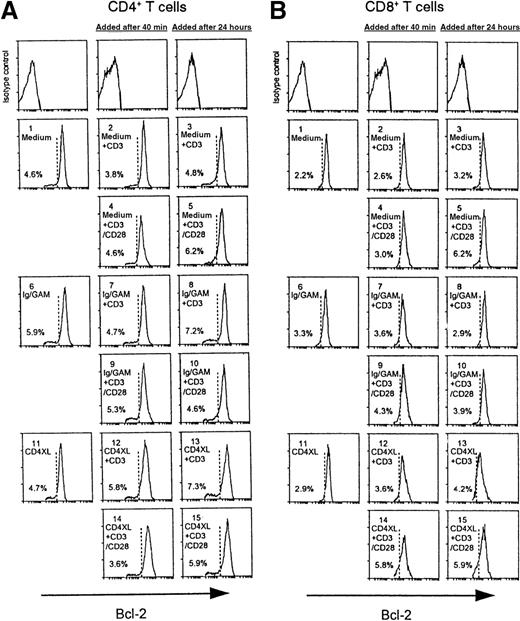
CD3 stimulation of PBTs 24 hours after CD4XL does not affect Bcl-2 expression in CD4 T cells (A) or in CD8 T cells (B).
Values denote the percentage of cells with Bcl-2 down-modulation.
CD3 stimulation of PBTs 24 hours after CD4XL does not affect Bcl-2 expression in CD4 T cells (A) or in CD8 T cells (B).
Values denote the percentage of cells with Bcl-2 down-modulation.
Role of cytokines in [CD4XL/CD3]-induced T-cell apoptosis
We have previously shown that CD4XL performed in PBMCs could lead to IFNγ and TNFα secretion without induction of IL-2 and that these secreted cytokines affected T-cell apoptosis-induction by up-regulating Fas and by increasing the susceptibility to apoptosis.17 We conducted intracytoplasmic cytokine (IFNγ, TNFα and IL-2) staining in conjunction with annexin V binding to analyze cytokine expression and apoptosis simultaneously at the single-cell level. As shown in Figure 6, CD4XL of purified T cells did not induce significant cytokine secretion or annexin V binding; however, addition of anti-CD3 mAb after CD4XL resulted in significant IFNγ and TNFα expression in both subsets but IL-2 was barely induced. Most of the IFNγ- or TNFα-expressing cells were found to bind annexin V, indicating that these cytokine-expressing cells undergo apoptosis, although the majority of apoptotic cells did not secrete IFNγ or TNFα. CD28 costimulation resulted in IL-2 expression without affecting the levels of IFNγ or TNFα but significantly reduced the emergence of annexin V-positive cells. The protection from apoptosis by CD28 costimulation thus appears to be associated with a mechanism involving IL-2 secretion and not as a result of inhibition of the levels of IFNγ or TNFα expression.
[CD4XL/CD3] treatment of PBTs results in induction of TNF and IFNγ in T cells undergoing apoptosis; anti-CD28 stimulation results in IL-2 expression and rescues T cells from apoptosis.
PBTs (1 × 106/mL) were cultured in complete medium, or treated with CD4XL, and were stimulated after 24 hours with anti-CD3 mAb with or without CD28-stimulation in the presence of Brefeldin A. Following a culture period of 18 hours, cells were harvested and labeled with annexin V and anticytokine antibodies for simultaneous assessment of intracytoplasmic cytokine production and apoptosis in CD4 T cells (A) and in CD8 T cells (B). Percentages of positive cells are indicated.
[CD4XL/CD3] treatment of PBTs results in induction of TNF and IFNγ in T cells undergoing apoptosis; anti-CD28 stimulation results in IL-2 expression and rescues T cells from apoptosis.
PBTs (1 × 106/mL) were cultured in complete medium, or treated with CD4XL, and were stimulated after 24 hours with anti-CD3 mAb with or without CD28-stimulation in the presence of Brefeldin A. Following a culture period of 18 hours, cells were harvested and labeled with annexin V and anticytokine antibodies for simultaneous assessment of intracytoplasmic cytokine production and apoptosis in CD4 T cells (A) and in CD8 T cells (B). Percentages of positive cells are indicated.
To further examine the role of induced IFNγ and TNFα in apoptosis induction, we examined the effects of neutralizing antibodies to IFNγ and TNFα in the [CD4XL/CD3]-cell culture system. Addition of anti-IFNγ and TNFα antibodies was found to block [CD4XL/CD3]-induced apoptosis in a dose-dependent manner; both antibodies blocked CD8 T-cell apoptosis more effectively than CD4 T-cell apoptosis (Figure 7).
Neutralizing antibodies to TNF and IFNγ block [CD4XL/CD3]-induced T-cell apoptosis.
CD4XL/CD3 treatment was performed in PBTs in the absence or presence of various concentrations of anti-IFNγ antibody (open circles) or anti-TNFα antibody (closed circles). Data obtained from the simultaneous addition of anti-IFNγ antibody (1.0 μg/mL) and anti-TNFα antibody (3.0 μg/mL) are indicated as open squares. Apoptosis was determined by annexin V labeling. Data represent 2 independent experiments.
Neutralizing antibodies to TNF and IFNγ block [CD4XL/CD3]-induced T-cell apoptosis.
CD4XL/CD3 treatment was performed in PBTs in the absence or presence of various concentrations of anti-IFNγ antibody (open circles) or anti-TNFα antibody (closed circles). Data obtained from the simultaneous addition of anti-IFNγ antibody (1.0 μg/mL) and anti-TNFα antibody (3.0 μg/mL) are indicated as open squares. Apoptosis was determined by annexin V labeling. Data represent 2 independent experiments.
[CD4XL/CD3]-induced apoptosis in CD8 T cells requires physical contact with CD4 cross-linked T cells
Finally we conducted the effector-target cell analysis, utilizing a Transwell cell culture system. As depicted in Figure8, purified CD8 T cells were placed in the upper chamber that was separated from the bottom chamber in which purified CD4 T cells were placed by membrane inserts. Addition of anti-CD3 mAb alone induced IFNγ expression in a substantial fraction of cells in both subsets but did not lead to apoptosis induction (Figure 8, left panels). However, if CD3 mAb was added after CD4XL, although the percentage of IFNγ-expressing cells was unaffected, CD4 T cells in the bottom chamber underwent apoptosis, whereas CD8 T cells in the upper chamber were prevented from apoptosis. These results clearly indicate that [CD4XL/CD3]-treatment drives CD4 T cells to become apoptosis-inducing effector cells and that physical contact is required for apoptosis induction in this system.
Cell-to-cell contact is required for [CD4XL/CD3]-activation-induced CD8 T-cell apoptosis.
CD4 T cells treated with (right panels) or without (left panels) CD4XL were placed below the insert (bottom chamber), and CD8 T cells were placed in the upper chamber. After 24-hour culture, anti-CD3 mAb was added and cells were incubated for an additional 18 hours. Cells were harvested, labeled with annexin V, and stained for intracytoplasmic IFNγ. Percentages of positive cells in each quadrant are indicated. Data represent 1 of 2 experiments.
Cell-to-cell contact is required for [CD4XL/CD3]-activation-induced CD8 T-cell apoptosis.
CD4 T cells treated with (right panels) or without (left panels) CD4XL were placed below the insert (bottom chamber), and CD8 T cells were placed in the upper chamber. After 24-hour culture, anti-CD3 mAb was added and cells were incubated for an additional 18 hours. Cells were harvested, labeled with annexin V, and stained for intracytoplasmic IFNγ. Percentages of positive cells in each quadrant are indicated. Data represent 1 of 2 experiments.
Discussion
Activation of T cells derived from transformed cell lines or hybridomas results in induction of FasL expression and rapid susceptibility to Fas-mediated apoptosis; in primary T lymphocytes, however, the process of activation-induced cell death requires a more prolonged period of time.27-29 Our present study shows that CD3 activation of modified CD4 T cells in which CD4 molecules are pre-cross-linked induces FasL expression in CD4 T lymphocytes and sensitizes both subsets of T cells for accelerated Fas-mediated apoptosis. One of the major observations in this study is that in addition to influencing CD4 T cells, CD4XL in PBTs could alter the susceptibility of CD8 T cells for anti-CD3-activation-induced apoptosis. Although the evidence was circumstantial, sensitization of T cells for apoptosis appears to be mediated by down-modulation of Bcl-x. This notion is supported by the finding that CD3 stimulation provided immediately after CD4XL did not result in apoptosis or in down-modulation of Bcl-x. This finding also indicates that following CD4XL, a certain period of time (approximately 24 hours) is required for the T cells to become susceptible to activation-induced cell death. Further, co-stimulation by CD28-blocked apoptosis as well as Bcl-x down-modulation without affecting the levels of Fas expression. In agreement with this finding, CD28 costimulation has been shown to transmit an apoptosis-blocking signal by enhancing the expression of Bcl-x.30 These results extend previous observations and provide a molecular basis to explain the mechanism of [CD4XL/CD3]-induced T-cell apoptosis. Further, these findings have implications for HIV pathogenesis as HIV gp120 utilizes CD4 as its main receptor and suggest a possible novel role for HIV gp120, that of generating CD4+ cytotoxic T cells.
Apoptosis in CD8 T cells was found to depend on contact with FasL-expressing CD4 T cells. The detection of FasL on CD4 T cells was made by RT-PCR; repeated attempts to demonstrate FasL expression on T cells by flow cytometry using anti-FasL antibodies were unsuccessful, even when cells were treated with matrix metalloproteinase inhibitor KB 8301 (data not shown). This difficulty in demonstrating surface FasL expression on lymphocytes has been previously documented by us10 and by others.27 Possibly, the limit of sensitivity of flow cytometry does not permit detection of FasL on lymphocytes. Cell separation experiments revealed that soluble factors, including IFNγ and TNFα induced from CD4 T cells by CD4XL, were by themselves insufficient to induce apoptosis of CD8 T cells even in combination with CD3 activation in the absence of physical contact between CD4 and CD8 T cells. In this regard, effective CD95 receptor/ligand signaling has been shown to require physical contact between receptor and ligand-bearing cells.31,32 Our previous studies as well have shown that TNFα and IFNγ can make cells more susceptible to apoptosis but are insufficient to induce apoptosis.14 With respect to CD8 T cells, we never observed apoptosis in this subset in our previous studies of CD4XL in PBMCs, in which abundant amounts of TNFα and IFNγ were present in the presence of FasL-expressing monocytes.14,17 18 We thus believe that the effects of these cytokines combined with CD3 stimulation are required for down-regulation of Bcl-x in CD8 T cells, hence making them susceptible to undergo apoptosis.
Apoptosis-blocking abilities of neutralizing antibodies to IFNγ/TNFα indicate that factors that predispose CD4–cross-linked cells to undergo apoptosis could also be ascribed to polarized cytokine secretion (ie, IFNγ/TNFα production) without IL-2 secretion in response to CD3 stimulation. It has been shown that TNFα not only mediates a death signal but also promotes apoptosis by up-regulating Fas.17 Accelerated CD3-mediated T-cell apoptosis has been associated with IFNγ production in the absence of IL-2.33This combination of cytokines has also been shown to promote preferential induction of CD4 CTLs.34 Additionally, CD28 co-stimulation may also block apoptosis via IL-2 secretion, because restoration of IL-2 secretion without affecting the levels of IFNγ/TNFα was observed by CD28 costimulation. Exogenously provided IL-2 has previously been shown to rescue T cells from [CD4XL/CD3]-induced apoptosis.35 IL-2 has also been proposed to function as a modifier of T-cell apoptosis in HIV disease and to contribute to the sustained increases in CD4 counts resulting from administering IL-236 to patients. Rescue of ex vivo culture-induced apoptosis of lymphocytes from HIV-infected patients by exogenous IL-2 has been reported as well.37
With respect to the in vivo relevance of our findings in the context of HIV pathogenesis, our data implicate the following scenario that may be operative in vivo and may serve as a mechanism for death of uninfected T cells in HIV infection. Peripheral lymphoid organs, especially the germinal center of the lymph node, are the major anatomical sites where heavy virus burden exists and where the interaction between HIV and host immune system occurs. The HIV envelope protein gp120-CD4 interaction constitutes the primary interface between the viruses and the T cells. Thus, it is quite likely that CD4 T cells are continuously undergoing CD4XL via an interaction with virions or via envelope proteins expressed on the surface of infected cells. When these CD4 cross-linked T cells encounter antigen-presenting cells in local environments, they receive stimulatory signals through the TCR, thereby giving rise to CD4 CTLs by the mechanism demonstrated here, which, in turn, would kill neighboring T cells. The progressive loss of CD28 by T cells and defective expression of the B7 family of costimulatory molecules in antigen-presenting cells, both of which have been demonstrated in HIV disease, may further impair the in vivo rescue systems for apoptosis and may accentuate the process.1,38,39 In support of this view, features that are characteristically associated with HIV infection (as demonstrated by in situ analysis of lymph nodes) include the increased apoptosis of uninfected cells, predominantly localized at secondary follicles of the lymph node,40,41 and polarized cytokine expression with elevated IFNγ and decreased IL-2.42,43 Moreover, there is a significant increase of FasL-expressing cells with the morphology of macrophages as well as lymphocytes, and the degree of FasL in vivo has been shown to correlate with the degree of apoptosis.9Control of aberrant CD4 signaling as a consequence of reduction of virus burden by antiretroviral treatment may be one mechanism for the marked reduction in apoptosis of CD4 and CD8 T cells following highly active antiretroviral therapy (HAART) in HIV disease.44 45
Acknowledgments
We thank Dr J. D. Mountz (University of Alabama at Birmingham, Birmingham, AL) for hFas-hIgG-pcDNA, MBL Ltd. (Nagoya, Japan) for antihuman FasL mAb, and Dr Y. Ohmoto (Otsuka Pharmaceutical Co, Ltd, Tokushima, Japan) for anti-IFNγ anti-TNFα antibodies.
Supported by National Institutes of Health Grants DA05161 and AI28281 (S.P.) and partly by a grant-by-aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 10180206 to N.O.).
Reprints:Savita Pahwa, Department of Pediatrics, Room 303 Research Bldg, NSUH-NYU, 350 Community Dr, Manhasset, NY 11030; e-mail: spahwa@nshs.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. [CD4XL/CD3]-induced lymphocyte apoptosis is blocked by soluble Fas or by anti-FasL mAb. / Soluble Fas (sFas: closed circles) or 4H9 (closed squares) was added at the indicated concentrations (μg/mL) to the cultures of CD4 cross-linked T cells plus anti-CD3 mAb ([CD4XL/CD3]: open square), and apoptosis was evaluated by annexin V binding. Apoptotic populations of CD4XL alone (open circle) and/or anti-CD3/CD28 mAbs (open triangle) are also indicated. Data represent mean ± SD of 3 experiments. *Significantly (P < .001) different from CD4XL/CD3 samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.195/4/m_bloo01351001x.jpeg?Expires=1769366786&Signature=zH5JTVonDKIr~pa-dCimQsZTb31tJeBarshHjhcnTRd-80iU3CjR~mtBQxfXljnOtI7qUZtJQ5Qe8pL4KaHPuqfaicM0RWFVCyjGnjfm0qrNpbXlt3iez783HZGKcJepoy8cKglBpM15l77BlPGA3-JjuxzS-ZlQjNfiOhp5qGBUfBw1KNW5QNNv54Rk75cHCV~605eppo3407IQ3gbNiroKNoZskrA1Lybq7L7nulScYioBBOhiqiDA4tdGb6drZQMsFfCFDGLdi0qZtc2MVI78d0XkPbX2blObalSY6flMKOm39SiF4lRZo8~3c~wVGcm1oTX86P2k8R3wguBw~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. CD3 stimulation of PBTs 24 hours after CD4XL down-modulates Bcl-x in both CD4 T cells and CD8 T cells, and CD28 costimulation prevents Bcl-x down-modulation. / [CD4XL/CD3] PBT cell cultures are showing Bcl-x expression in CD4 T cells (A) and in CD8 T cells (B). Cell-culture conditions are indicated in each panel, and isotype controls are indicated in top panels. Cells were analyzed by flow cytometry for intracytoplasmic Bcl-x expression in conjunction with surface labeling with FITC-anti-CD8 mAb. Broken lines represent the lowermost boundary of cells cultured in medium alone and labeled with anti–Bcl-x Ab. Cells that emerged to the left of this line represent Bcl-x down-modulated cells, and their percentages are indicated in the figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.195/4/m_bloo01351004x.jpeg?Expires=1769366786&Signature=MVO1DrUFi~Fb3PIzcuKrNAgKR7Q9CyZFy45Jc4XuSjON284JXZJ3ywDvE4CyY-Oo-D-07-5VKP-5Ed2F7sa~RQGQZEHzsAWfoUSl-w~G2XFoaR2IUB-~sy1YOntZaavPnYhF1qCAReoBM~V1gmTXHr4BylNgjSdQUYDocVk7ie7DQHavfw~opQ-D9-k4rmU0g3FVToWEJYdwp9lGHdz0TbZQR0jaxE14MQsUUdjE~~uHbTe3KlUaHvWIZJG1FbRRfCvygYSNTmX-AOX7flb1qI7pwO0kP8C4SlBOsACLQaeZnYkIrmfPOYJ~WLLhZ876TU-3v967GEJmDoNmmuuQCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. [CD4XL/CD3] treatment of PBTs results in induction of TNF and IFNγ in T cells undergoing apoptosis; anti-CD28 stimulation results in IL-2 expression and rescues T cells from apoptosis. / PBTs (1 × 106/mL) were cultured in complete medium, or treated with CD4XL, and were stimulated after 24 hours with anti-CD3 mAb with or without CD28-stimulation in the presence of Brefeldin A. Following a culture period of 18 hours, cells were harvested and labeled with annexin V and anticytokine antibodies for simultaneous assessment of intracytoplasmic cytokine production and apoptosis in CD4 T cells (A) and in CD8 T cells (B). Percentages of positive cells are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.195/4/m_bloo01351006x.jpeg?Expires=1769366786&Signature=jGT~pXnc7MTWUMkLDvNitCrU~ukFlE0sNgg20ODcC4kJtqUCHqwKCKv8iqGp9I5lJrtbo-naGKK6UO3RmCerUidP94SGNBKx4OmvvXiecuJauYGGizfpznh6KTxr~k-VoiBPbqA5gk9cmaMWcFWZQlvCnkL5pkUhQJp0zjSFZMOBUaGYAwraz4D4uJu9oAEZMRb50R3omaNfrO4X0-i4jpDwWHFMPCl06z~ottNdmjICbW4sRACuTOzgO9bYqcHSJOwh7BdiNM-0vR4NCUXRa0~5KEZod-40SnkdsBGdIeRYdyGykKnX7z4ODKf5VYlCQ55DOW31hZsl63pMPLhDHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Neutralizing antibodies to TNF and IFNγ block [CD4XL/CD3]-induced T-cell apoptosis. / CD4XL/CD3 treatment was performed in PBTs in the absence or presence of various concentrations of anti-IFNγ antibody (open circles) or anti-TNFα antibody (closed circles). Data obtained from the simultaneous addition of anti-IFNγ antibody (1.0 μg/mL) and anti-TNFα antibody (3.0 μg/mL) are indicated as open squares. Apoptosis was determined by annexin V labeling. Data represent 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.195/4/m_bloo01351007x.jpeg?Expires=1769366786&Signature=Xs86wG~KvaW1uSVkSh~GVvy-TcOZV1WArjdFqfPT4xpZEhgAyiIQyzB~0NYd753sscdnvhTy1NnQRXIFtlylYR6vS29MZHuOmafh-X3bonLUEPs-x-vqLWsul1NARHViEzGUhQspcSpIqOSupgLPzy0SKG18b5KdGtJqzPt1wKRzMq521GX-11JBF2qGeh6-EVXWHEDOnnBEXEFDOjZlCehqWmkT2JribgNyry9M8KEUozYxG4Ws2vjXbJuzhMqZQaaPMvy-KgzC~to2MRRu9MGu4EgzqInbI0uxBYIvSya1lu01bskjlyqPU0Q~IbFNW2J52HRboLIR3nJWQe-~5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Cell-to-cell contact is required for [CD4XL/CD3]-activation-induced CD8 T-cell apoptosis. / CD4 T cells treated with (right panels) or without (left panels) CD4XL were placed below the insert (bottom chamber), and CD8 T cells were placed in the upper chamber. After 24-hour culture, anti-CD3 mAb was added and cells were incubated for an additional 18 hours. Cells were harvested, labeled with annexin V, and stained for intracytoplasmic IFNγ. Percentages of positive cells in each quadrant are indicated. Data represent 1 of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.195/4/m_bloo01351008x.jpeg?Expires=1769366786&Signature=2tcHRJej-zcVUyhWVDsp2UetfMBJnZGOP-rmhzSCWL2Lh6G24rEdMAYfRv9LgOO5xvX2i-QN2L6DpET7jHU9VYTnqEaTaXSOav9mVkrZnLR375n72drErsCF~PW3N1ZWM~V-hFPntpVbByFWhd6sdx5RGICEzeppXMTnkVCyDc5HFGUOHs6-mNQdJNeCuvOQ76f7TnyHJSLgs0kCI6sILPP34qOtiQFBMHg6u9fY-oGg2Rd49Ri8BBxjNmk3MPeFQFz9IxpMrTDd2aY-C5085DCvH7UxVWugu0rV8m2NKO22iwUypzxXeQT3JEz0htYSmRoYv2QlBXwNdK508c0U1g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
